Abstract
Forced normalization is the development of psychiatric symptoms in a patient experiencing remission of seizures. We present a case of Lennox Gastaut syndrome in which forced normalization developed after vagus nerve stimulation was stopped.
The patient had drug resistant epilepsy and failed anti-seizure drugs, vagus nerve stimulation, and a partial callosotomy.
The patient had multiple types of seizures including drop attacks, absences, and tonic–clonic seizures. He tried vagus nerve stimulation for two years without success. Forced normalization developed after the vagus nerve stimulator was turned off.
This is the first case to our knowledge to describe forced normalization after turning off the vagus nerve stimulator.
Abbreviations: EEG, electroencephalography; ASD, anti-seizure drug; VNS, vagus nerve stimulation; LGS, Lennox Gastaut syndrome; FN, forced normalization; ACTH, adrenocorticotropic hormone
Keywords: Forced normalization, Lennox Gastaut syndrome, Vagus nerve stimulation
Highlights
-
•
Forced normalization (FN) is the development of psychiatric symptoms in a patient experiencing remission of seizures
-
•
FN should be on the differential diagnosis in patients presenting with new onset psychosis and seizure freedom
-
•
To our knowledge this is the first case that reports the presence of forced normalization after VNS is turned off
1. Introduction
Forced normalization (FN) is an intriguing phenomenon characterized by the emergence of psychiatric disturbances following the establishment of seizure control or reduction in epileptiform activity on the EEG in a patient with previously uncontrolled epilepsy [1]. The mechanism of FN remains unclear. Theories include alteration in the inhibitory mechanisms of the limbic system after gaining seizure control; a true biological antagonism between seizures and psychosis; ongoing status epilepticus in the limbic system; propagation of epileptiform discharges along unusual pathways, as well as electrical kindling [2]. The phenomenon is more common in patients with intellectual disability and drug-resistant epilepsy, such as those with Lennox Gastaut syndrome (LGS) [3]. Here we discuss a complex case of LGS in which FN developed after vagus nerve stimulation (VNS) was stopped.
2. Case report
A 31-year-old male patient with a diagnosis of LGS suffered drug-resistant epilepsy since the age of 20 months. Aside from moderate cognitive delay, he did not have any other medical conditions. Family history was significant for depression in his mother and two great uncles who committed suicide. The patient experienced different types of seizures including tonic–clonic, tonic, atonic and atypical absence seizures. Several inter-ictal EEGs showed burss and fragments of generalized slow spike–wave. A brain MRI was normal. A number of anti-seizure medications failed to control his epilepsy including ACTH, zonisamide, phenytoin, valproic acid, topiramate, lorazepam, nitrazepam, clonazepam, clobazam, levetiracetam, lamotrigine and carbamazepine. He also had an unsuccessful trial of cannabidiol. The patient had an anterior corpus callosotomy at age 18 years, which worsened his cognitive function and did not benefit his seizures. Two years later, he began to experience increased frequency of seizures and regular visits to the emergency department. At this time, he was experiencing between 5 and 10 seizures per week (mostly drop attacks and absences) and frequent clusters. Due to the malignant evolution of his seizures he underwent implantation of a vagus nerve stimulator at age 23.
Treatment with VNS helped to improve the severity of seizures but only for six months. The output current reached 2 mA with significant hoarseness. The efficacy was lost after six months of treatment with VNS. Due to no improvement and potential concerns of seizure severity worsening and hoarseness the family requested to stop the stimulation after two years of treatment. Two weeks after the VNS was stopped and without any other change in his regimen, he presented to the emergency department with new onset psychosis. He started hallucinating, laughing randomly, and reacting in the absence of stimuli. He became agitated, aggressive, and restless and presented mood swings, paranoia, persecutory delusions, echolalia and echopraxia. His anti-seizure medications at this time included carbamazepine 500 mg twice daily, lamotrigine 175 mg twice daily and levetiracetam 1250 mg twice daily. Quetiapine 25 mg at night was started in hospital to help manage the psychosis. The patient was neurologically intact with no focal deficits on exam. Serum metabolic, infective and inflammatory workups were unremarkable. Serum carbamazepine levels were therapeutic at 37.8 μmol/L. He was admitted to the psychiatry ward and the dose of quetiapine was increased with slight relief of his psychotic symptoms. Shortly after discharge his seizures returned. During this period of seizures his behavioral symptoms were less severe and easier to manage. Two weeks later he experienced extreme escalation of behavior with concurrent seizure cessation. For several years the patient continued to cycle between periods of seizure activity without behavioral disturbance, and periods of psychosis in the absence of seizures (period of 4 to 6 weeks). The patient had numerous EEGs and portable EEG recordings of 24 h in duration. During the periods of psychosis (Fig. 2), the EEGs were unremarkable with rare bursts of generalized spike wave. During periods of seizure activity (Fig. 1), the EEG would show very frequent bursts of generalized slow spike wave (2.5 to 3 Hz), lasting between 4 and 10 s and without clear lateralization of onset and offset. Before the VNS was stopped the EEGs were always very active, showing frequent bursts of GSW. The patient is currently 31 years old and living in a long-term care facility. His anti-seizure medications include carbamazepine 400 mg in the morning, 400 mg in the afternoon and 800 mg in the evening, lamotrigine 175 mg twice daily, clobazam 10 mg twice daily, lacosamide 150 mg twice daily and cannabidiol. His VNS was switched off due to an expired battery. He continues to have 10 to 15 seizures per week but is no longer experiencing periods of psychosis.
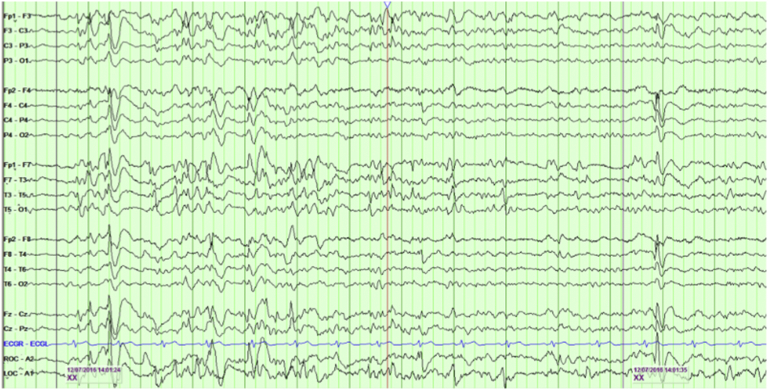
Fig. 2.
Shows a wakefulness sample during a period of psychosis (bipolar montage).
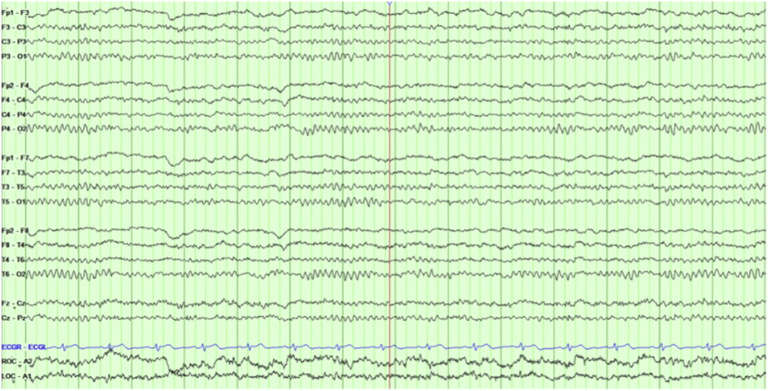
Fig. 1.
This page shows the presence of bursts of generalized spike–wave in an awake sample (bipolar montage). The sample was taken during a week of frequent seizures.
3. Discussion
Our patient met the full criteria for FN [4] including: 1) established diagnosis of epilepsy; 2) subacute behavioral disturbance; 3) complete cessation of seizures for at least one week; 4) dissolution of the spikes assessed in a 60-min awake EEG recording, and 5) recent change in therapeutic regimen (turning off the VNS). Some patients have been reported with new onset of psychiatric symptoms after VNS; although most cases can be attributed to treatment with VNS [5], [6], a few of them do not meet the criteria for FN [7], [8]. It seems VNS increases alertness and reduce sedation, which is independent from seizure control. Acutely increased alertness and decreased sedation may manifest in some patients as a psychosis-like state. Patients with severe intellectual disability may be specifically sensitive for the development of these symptoms.
It is remarkable that after turning off VNS, a therapy that had no substantial benefit to his seizures since implantation, the patient became seizure free. It might be possible that the VNS aggravated seizures and when the device was turned off, seizures were suddenly controlled, triggering a FN syndrome. In that case, this would not be the first report of seizure aggravation with VNS. Koutroumanidis et al. reported severe seizure worsening in a young woman with drug-resistant occipital lobe epilepsy using VNS. Her seizure activity promptly and completely subsided after the stimulator was turned off [9]. Similarly, Arhan et al. reported a case in a child with severe seizure aggravation and status epilepticus [10]. Both cases occurred at a high level of current output (> 2.0 mA). Seizure aggravation after the increase in the current output of VNS might be the result of continuous synchronization of brain networks, facilitating the propagation of previously dormant or inactive potential epileptogenic foci. During the time that the VNS was on in this patient there were some concerns of seizure severity worsening, although the VNS was stopped because of side effects. In Table 1 we can see the available cases in the literature where VNS produced exacerbation of seizures (Table 1).
Table 1.
VNS leading to seizure aggravation.
| Author/year | Patient | Seizures | Epilepsy | Time to seizure aggravation from implantation | Stimulus output current (mA) | Increase in usual seizures | New motor seizures | Recovery after turned off |
|---|---|---|---|---|---|---|---|---|
| Koutroumanidis 2000 | 20 yo female | FA, FIA, F-TCS | Left occipital | 4 monthsa | 2.5 | Yes | Yes | 2 days |
| Murr 2011 | 51 yo male | CPS, SG-TCS | Left frontal | 3 days | 0.25b | No | Yes | 2 days |
| Arhan 2018 | 12 yo girl | CPS, SG-TCS, DA | Left parieto-occipital | 3 monthsc | 2.0 | No | Yesd | 1 day |
Abbreviations: FA: focal aware seizures, FIA: focal impaired awareness seizures, F-TCS: focal to bilateral tonic-clonic seizures, DA: drop attacks.
2 days after increasing output from 2.25 mA to 2.5 mA.
Increasing seizures directly related to increase in the output (especially at 0.5 mA).
1 day after increasing output from 1.5 mA to 2.0 mA.
Status epilepticus.
There was a question as to whether the patient's psychiatric symptoms could be explained by the fact that he was taking levetiracetam, however levetiracetam was removed and the cycles between seizures and psychotic symptoms persisted. Patients with LGS have a tendency to develop chronic psychosis that worsens over time, although for many years he never presented psychiatric symptoms [11]. Finally, it cannot be ruled out that our patient was under the influence of a possible schizophrenic break suffered several years prior, never diagnosed as such, which could have been triggered by the stressful stimulus that was caused by cessation of the VNS. While it is possible that our patient experienced the abrupt onset of long-term psychotic symptoms, it is rare for patients with LGS to have complete seizure freedom and normal EEG for any prolonged period of time [11]. Finally, we do not believe that the callosotomy had any impact in the development of psychiatric symptoms as it was done when the patient was 18 years old and he did not develop psychiatric symptoms until age 25 when the VNS was stopped.
4. Conclusion
Although psychosis and behavioral disturbance are common with the progression of LGS, FN should be in the differential diagnosis of patients presenting with new-onset psychosis and concomitant seizure freedom. This is the first case to our knowledge that reports the presence of FN after VNS was turned off. We hypothesize that when the VNS was turned off the patient became seizure-free, triggering psychosis. Meanwhile, we cannot exclude other new mechanisms producing forced normalization, such as the stimulation and modification of brain structures that may occur with VNS.
Acknowledgments and disclosures
Dr. Téllez-Zenteno receives grants from the University of Saskatchewan, the Royal University Hospital Foundation in Saskatoon, Saskatchewan, and the Saskatchewan Health Research Foundation. Dr. Lee, Dr. Denton, Dr. Ladino, Dr. Vitali and Karen Waterhouse have nothing to disclose. None of the authors have any conflict of interest to declare.
Statement of authorship
SL and LDL drafted the manuscript. AD, LDL, and KW were involved in the critical review of the manuscript. AV and JFTZ were the physicians involved with the case. All authors approved the final version of the manuscript.
Ethics in publishing
SL and LDL drafted the manuscript. AD, LDL, and KW were involved in the critical review of the manuscript. AV and JFTZ were the physicians involved with the case. All authors approved the final version of the manuscript. All the authors follow the ethical standards of Elsevier.
References
- 1.Landolt H. Some clinical EEG correlations in epileptic psychoses (twilight states) Electroencephalogr Clin Neurophysiol. 1953;5:121. [Google Scholar]
- 2.Wolf P. Acute behavioural symptomatology at disappearance of epileptiform EEG abnormality: paradoxical or forced normalization. In: Smith D., Trieman D., Trimble M.R., editors. Neurobehavioural problems in epilepsy. Raven; New York City, NY: 1991. pp. 127–142. [PubMed] [Google Scholar]
- 3.Loganathan M.A., Enja M., Lippmann S. Forced normalization: epilepsy and psychosis interaction. Innov Clin Neurosci. 2015;12(5–6):38–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnamoorthy E.S., Trimble M.R. Forced normalization: clinical and therapeutic relevance. Epilepsia. 1999;40(10):S57–S64. doi: 10.1111/j.1528-1157.1999.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumer D., Davies K., Alexander A., Morgan S. Major psychiatric disorders subsequent to treating epilepsy by vagus nerve stimulation. Epilepsy Behav. 2001;2:466–472. doi: 10.1006/ebeh.2001.0249. [DOI] [PubMed] [Google Scholar]
- 6.Gatzonis S.D., Stamboulis E., Siafakas Angelopoulos E., Georgaculias N., Sigounas E. Acute psychosis and EEG normalization after vagus nerve stimulation. J Neurol Neurosurg Psychiatry. 2000;69:278–279. doi: 10.1136/jnnp.69.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Herdt V., Boon P., Vonck K., Goossens L., Nieuwenhuis L., Paemeleire K. Are psychotic symptoms related to vagus nerve stimulation in epilepsy patients? Acta Neurol Belg. 2003;103(3):170–175. [PubMed] [Google Scholar]
- 8.Adán J., Escosa M., Ayuso-Mateos J.L. Vagus nerve stimulation and psychosis. A single case report. Actas Esp Psiquiatr. 2005;33(2):130–134. [PubMed] [Google Scholar]
- 9.Koutroumanidis M., Hennessy M.J., Binnie C.D., Polkey C.E. Aggravation of partial epilepsy and emergence of new seizure type during treatment with VNS. Neurology. Sep 26 2000;55(6):892–893. doi: 10.1212/wnl.55.6.892. [DOI] [PubMed] [Google Scholar]
- 10.Arhan E., Serdaroğlu A., Hirfanoğlu T., Kurt G. Aggravation of seizures and status epilepticus after vagal nerve stimulation therapy: the first pediatric case and review of the literature. Childs Nerv Syst. 2018;34(9):1799–1801. doi: 10.1007/s00381-018-3806-x. [DOI] [PubMed] [Google Scholar]
- 11.Hancock E.C., Cross J.H. Treatment of Lennox–Gastaut syndrome. Cochrane Database Syst Rev. 2013;2 doi: 10.1002/14651858.CD003277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]