Abstract
Creutzfeldt–Jakob disease (CJD) presents with seizures as an early symptom in only approximately 3% of cases. These seizures often present as nonconvulsive status epilepticus (NCSE) or epilepsia partialis continua (EPC). Here, we describe a case of probable sporadic CJD (sCJD) in an 83-year-old man whose manifest an unusual presentation of left-hand tonic seizures without evolution to EPC, as well as brain MRI findings interpreted as peri-ictal changes, which led to an initial misdiagnosis of focal epilepsy.
Keywords: Creutzfeldt–Jakob disease, Seizure, EEG, MRI
Highlights
-
•
The patient presented initially with complex partial seizures and the right-sided PLEDs EEG pattern.
-
•
Peri-ictal changes interpreted from brain MRI led clinicians to treat seizures with anti-seizure drugs, but ineffective.
-
•
The patient was later diagnosed with probable sporadic Creutzfeldt-Jakob disease.
1. Introduction
Creutzfeldt–Jakob disease (CJD) arises from the accumulation of prion protein (PrPsc) in the brain, which leads to progressive and fatal neurodegeneration. Clinically, CJD predominantly manifests as progressive dementia accompanied by pyramidal, extrapyramidal, and visual signs. Psychiatric symptoms occasionally occur [1], [2], and seizures are uncommon as an early symptom of sporadic CJD (sCJD). Nonspecific signs and symptoms point to the difficulty of distinguishing CJD from other diseases characterized by rapidly progressive cognitive impairment. The periodic sharp wave complexes (PSWC) on electroencephalography (EEG), hyperintensity on diffusion-weighted imaging (DWI) of brain magnetic resonance imaging (MRI) of the cerebral cortex (cortical ribboning) and striatum, and elevated 14-3-3 protein in CSF are helpful in the diagnosis of sCJD. However, atypical clinical presentations with ambiguous laboratory findings may still cause misdiagnosis.
2. Case report
An 83-year-old retired male farmer from eastern Taiwan presented with one month of a mild headache, behavioural changes, cognitive impairment, and irritability. According to the patient, his hypertension and hyperlipidaemia are regularly being followed up, and he had no history of head injuries or epilepsy. His family described the patient as sometimes appearing absent-minded and exhibiting strange behavior, such as mistaking the newspaper for his clothing. The family also reported visual hallucinations and progressive memory impairment. Investigation results from prior consultation at a nearby regional hospital included an EEG revealing "seizure activity" and a brain MRI showing possible post-ictal changes. Two weeks after his first consultation, he came to our neurology outpatient department for a second opinion and was diagnosed with focal epilepsy. He was started on levetiracetam 1000 mg/day. Ten days later, the patient experienced a sudden onset of irritable mood followed by loss of consciousness and was brought to the emergency room (ER). In the ER, the patient regained consciousness and found his left hand weak with a Medical Research Council grade of 3/5. Acute stroke was suspected, but brain computed tomography showed no intracerebral haemorrhage. Based on the provisional diagnosis of seizure disorder with post-ictal left hand weakness, the patient was hospitalized. Considering that levetiracetam may have caused or exacerbated his irritability, his anti-seizure drug regimen was switched to gabapentin 1200 mg/day plus clobazam 20 mg/day.
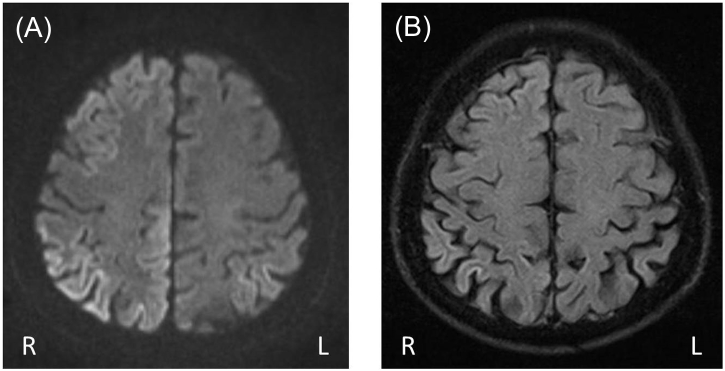
To evaluate the seizure etiology, an examination of the patient's CSF was conducted and yielded a white blood cell count of 0 cells/μL, a protein level of 26.9 mg/dL, and a glucose level of 86 mg/dL (the serum glucose level was 122 mg/dL). The complete blood count and blood chemistry were normal, and the thyroid function was also normal. Venereal Disease Research Laboratory, Epstein Barr Virus serology, and tumor marker tests were all negative. The brain MRI showed gyriform hyperintensity bilaterally in the frontal, parietal, temporal, and occipital lobes, with the hyperintensity being more prominent on the right side in DWI (Fig. 1). The differential diagnosis of the brain MRI findings included peri-ictal changes, encephalitis, and hypoxic encephalopathy.
Fig. 1.
(A) Diffusion-weighted MRI (DWI) showing gyriform hyperintensity bilaterally in frontal, parietal, temporal, and occipital lobes, with the hyperintensity being more prominent on the right side. (B) Fluid-attenuated inversion recovery (FLAIR) image showing hyperintensity in the right parietal and temporal cortex.
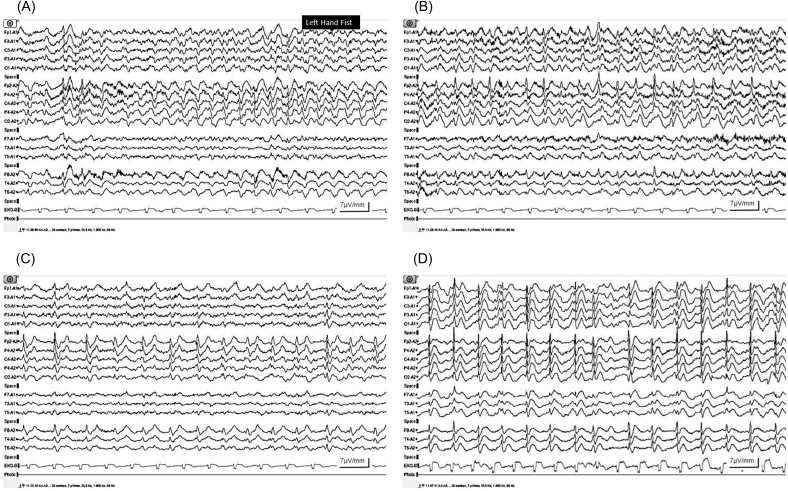
Despite the change in anti-seizure drugs, the patient remained agitated and experienced frequent focal tonic seizures of the left hand with impaired awareness. The stereotypic seizure was characterized by the patient making a fist with his left hand, which was sometimes followed by left arm stiffness; was associated with mental confusion; and had a duration ranging from 152 to 195 s. The seizure frequency was 18–30 seizures per day. An ictal EEG showed rhythmic epileptiform discharges arising from the right hemisphere and then spreading to the contralateral hemisphere (Fig. 2A, B). An interictal EEG showed right-sided lateralized periodic discharges (LPDs) with maximum amplitude in the right mid-frontal region (Fig. 2C). Treatment with zonisamide 200 mg/day was added to the anti-seizure drug regimen, and the gabapentin dose was increased to 1800 mg/day; however, no improvement was found, and the patient gradually developed clouding of consciousness and dysarthria. The follow-up EEG nine days later showed PSWC with right predominance and recurrence every 0.8–1.2 s (Fig. 2D). While the seizure frequency was reduced with the addition of levetiracetam 2500 mg/day, the level of consciousness worsened to lethargy, and the PSWC pattern was not attenuated. After two weeks of aggressive but ineffective treatment with anti-seizure drugs, pulse therapy was given for possible autoimmune encephalitis. However, the patient showed no clinical improvement. Elevated 14-3-3 protein in the CSF was confirmed, and the patient was later diagnosed with probable sCJD. Tests for both protease-resistant prion protein and prion protein gene (PRNP) were not performed. The patient died within three months of the onset of symptoms.
Fig. 2.
(A) Ictal EEG showing rhythmic discharges arising from the right hemisphere, and (B) then spreading to the contralateral hemisphere. (C) Interictal EEG showing right-sided lateralized periodic discharges (LPDs) with maximum amplitude in the right mid-frontal region. (D) Follow-up EEG nine days later showed periodic sharp wave complexes (PSWC) with right predominance and recurrence every 0.8–1.2 s.
3. Discussion
3.1. Seizure and EEG findings
Epileptic seizures as the initial presenting feature of sCJD have been reported in only 3% of cases [3], with such seizures often presenting as nonconvulsive status epilepticus (NCSE) [4], [5], myoclonus, and epilepsia partialis continua (EPC) [6], [7], [8], [9]. Hence, our patient's presentation of early focal tonic seizures and no evolution to EPC is particularly rare, and this unusual presentation led to the misdiagnosis of focal epilepsy after it was combined with the radiographic findings interpreted as peri-ictal changes.
The PSWC EEG pattern is commonly seen in CJD cases, with a sensitivity and specificity of 64% and 91%, respectively [3]. Lateralized or focal periodic sharp waves have been reported, especially early in the course of CJD. Partial seizure activity and PLEDs in early CJD might indicate that PrPSc accumulation can have a focal or regional origin in the brain. Although the mechanism by which PrPSc causes neuronal damage and seizures remains unknown, the rare seizure presentation and different phenotypes of CJD would be associated with the modulation of PRNP, which may influence the susceptibility to seizure, and variable PrPSc strains which show distinctive clinical and histopathological properties [10], [11], [12]. The relationships among different PrPSc strains, variable PRNP, and seizures should be studied further to increase our understanding of this topic.
3.2. Psychiatric symptoms
Psychiatric symptoms including paranoia, visual hallucinations, and aggression occasionally occur during the initial phase of sCJD [1], [2]. However, mood and behaviour disturbances also occur in seizure disorders, as part of ictal clinical patterns or post-ictal psychosis. Thus, such psychiatric presentations can quickly be taken as compatible with a seizure disorder diagnosis if focal seizures present as an early symptom of sCJD [13]. Isolated visual disturbances at disease onset are the peculiar characteristic of the Heidenhain variant of CJD, which often shows occipito-parietal hyperintensity on DWI or FLAIR imaging [14]. In this case, the early symptoms of cognitive impairment, seizure, psychiatric symptoms, and visual hallucinations, combined with brain MRI showing diffuse hyperintensity bilaterally in the frontal, parietal, and temporal and occipital lobes, seemed less likely to indicate the Heidenhain variant.
3.3. MRI findings
The typical findings of brain MRI in sCJD are hyperintensity in the cerebral cortex (known as the cortical ribbon sign), striatum, or thalamus on DWI or FLAIR imaging [1], [15]. Peri-ictally, brain MRI changes include hyperintense signal changes on FLAIR images and DWI due to vasogenic and cytotoxic edema. Peri-ictal MRI abnormalities and EEG findings often show topographic concordance, and the image changes entirely or partially resolve over weeks to months [16], [17]. Our literature review yielded a case of NCSE erroneously diagnosed as CJD because similar MRI changes were seen in CJD and peri-ictal states [18]. In our case, we cannot conclusively state whether the MRI findings were due to seizure activity or CJD pathology. Nonetheless, this case illustrates that, in the context of sCJD with early seizures, peri-ictal changes interpreted from MRI may lead clinicians astray when making treatment decisions.
4. Conclusion
sCJD should be considered as a differential diagnosis if a patient presents with focal seizures and a rapid progression of cognitive dysfunction, even in cases in which brain MRI is initially interpreted as indicating peri-ictal changes.
Disclosure
The authors report no conflict of interest.
Ethical statement
This is a retrograde study, and ethics approval (NYMUH IRB No. 2017A024) was obtained according to our institutional standards.
Originality and plagiarism: Pei-Shan Hsiao, Yuan-Ming Lee, Ping-Huang Tsai.
Data access and retention: Fu-Sin Chu, Chao-Lin Lee, Fang-Chun Liu.
Disclosure and conflicts of interest: The authors report no conflict of interest.
Contributor Information
Pei-Shan Hsiao, Email: 10813@ymuh.ym.edu.tw.
Yuan-Ming Lee, Email: 10811@ymuh.ym.edu.tw.
Fu-Sin Chu, Email: 11332@ymuh.ym.edu.tw.
Chao-Lin Lee, Email: 11316@ymuh.ym.edu.tw.
Fang-Chun Liu, Email: 16225@ymuh.ym.edu.tw.
Ping-Huang Tsai, Email: phtsai@ymuh.ym.edu.tw.
References
- 1.Mead S., Rudge P. CJD mimics and chameleons. Pract Neurol. 2017;17:113–121. doi: 10.1136/practneurol-2016-001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerr I., Poser S. Clinical diagnosis and differential diagnosis of CJD and vCJD. With special emphasis on laboratory tests. Apmis. 2002;110:88–98. doi: 10.1034/j.1600-0463.2002.100111.x. [DOI] [PubMed] [Google Scholar]
- 3.Wieser H.G., Schindler K., Zumsteg D. EEG in Creutzfeldt–Jakob disease. Clin Neurophysiol. 2006;117:935–951. doi: 10.1016/j.clinph.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa P.S., Bensalem-Owen M.K., Fee D.B. Sporadic Creutzfeldt–Jakob disease presenting as nonconvulsive status epilepticus case report and review of the literature. Clin Neurol Neurosurg. 2010;112:537–540. doi: 10.1016/j.clineuro.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Lapergue B., Demeret S., Denys V., Laplanche J.L., Galanaud D., Verny M. Sporadic Creutzfeldt–Jakob disease mimicking nonconvulsive status epilepticus. Neurology. 2010;74:1995–1999. doi: 10.1212/WNL.0b013e3181e39703. [DOI] [PubMed] [Google Scholar]
- 6.Donmez B., Cakmur R., Men S., Oztura I., Kitis A. Coexistence of movement disorders and epilepsia partialis continua as the initial signs in probable Creutzfeldt–Jakob disease. Mov Disord. 2005;20:1220–1223. doi: 10.1002/mds.20502. [DOI] [PubMed] [Google Scholar]
- 7.Lee K., Haight E., Olejniczak P. Epilepsia partialis continua in Creutzfeldt–Jakob disease. Acta Neurol Scand. 2000;102:398–402. doi: 10.1034/j.1600-0404.2000.102006398.x. [DOI] [PubMed] [Google Scholar]
- 8.Parry J., Tuch P., Knezevic W., Fabian V. Creutzfeldt–Jakob syndrome presenting as epilepsia partialis continua. J Clin Neurosci. 2001;8:266–268. doi: 10.1054/jocn.2000.0845. [DOI] [PubMed] [Google Scholar]
- 9.Taskiran A., Tezer F.I., Saygi S. Epilepsia partialis continua as the presenting symptom in probable sporadic Creutzfeldt–Jakob disease. Epileptic Disord. 2011;13:82–87. doi: 10.1684/epd.2011.0400. [DOI] [PubMed] [Google Scholar]
- 10.Aguzzi A., Heikenwalder M., Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552. doi: 10.1038/nrm2204. [DOI] [PubMed] [Google Scholar]
- 11.Gambetti P., Kong Q., Zou W., Parchi P., Chen S.G. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–239. doi: 10.1093/bmb/66.1.213. [DOI] [PubMed] [Google Scholar]
- 12.Striebel J.F., Race B., Chesebro B. Prion protein and susceptibility to kainate-induced seizures: genetic pitfalls in the use of PrP knockout mice. Prion. 2013;7:280–285. doi: 10.4161/pri.25738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rees J.H., Smith S.J., Kullmann D.M., Hirsch N.P., Howard R.S. Creutzfeldt–Jakob disease presenting as complex partial status epilepticus: a report of two cases. J Neurol Neurosurg Psychiatry. 1999;66:406–407. doi: 10.1136/jnnp.66.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baiardi S., Capellari S., Ladogana A., Strumia S., Santangelo M., Pocchiari M. Revisiting the Heidenhain variant of Creutzfeldt–Jakob disease: evidence for prion type variability influencing clinical course and laboratory findings. J Alzheimers Dis. 2015;50:465–476. doi: 10.3233/JAD-150668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitali P., Maccagnano E., Caverzasi E., Henry R.G., Haman A., Torres-Chae C. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011;76:1711–1719. doi: 10.1212/WNL.0b013e31821a4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cianfoni A., Caulo M., Cerase A., Della Marca G., Falcone C., Di Lella G.M. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol. 2013;82:1964–1972. doi: 10.1016/j.ejrad.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Xiang T., Li G., Liang Y., Zhou J. A wide spectrum of variably periictal MRI abnormalities induced by a single or a cluster of seizures. J Neurol Sci. 2014;343:167–172. doi: 10.1016/j.jns.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Albanese M., Placidi F., Romigi A., Schirinzi T., Liguori C., Marchi A. Symptomatic nonconvulsive status epilepticus erroneously suggestive of sporadic Creutzfeldt–Jakob disease. J Neurol Sci. 2015;348:274–276. doi: 10.1016/j.jns.2014.11.012. [DOI] [PubMed] [Google Scholar]