Highlights
-
•
Pigment production and accumulation is dependent of high C:N ratios in F. oxysporum and A. chevaleri.
-
•
Red pigment content of F. oxysporum in terms of Absorbance units per gram of biomass increased in 191% through use of blue light. Different light wavelengths stimulate synthesis of additional pigments in A. chevalieri with highest accumulation in red and UV-light.
-
•
Stimulation of pigment production in co-culture is species – specific, being only accomplished in A. chevalieri. With a rise higher that 500% of a pigment obtained in green light.
Abbreviations: AU/Bgr, (absorbance units per gram of biomass)
Keywords: Fungal pigments, C:N ratio, LED light, Co-culture
Abstract
In addition to plant-derived, fungal pigments have become an alternative in respect to synthetic ones. Besides Monascus sp., several pigment-producing fungi do not have culture conditions well-established yet. In this research, media composition, light wavelength and co-culture were evaluated, results were reported in Absorbance Units per gram of biomass (AU/Bgr). For Fusarium oxysporum a C:N ratio above 7 was advantageous, using both complex and defined media; blue LED light increased the AU/Bgr value from 18013 to 344; co-culture did not enhance pigment production. In Aspergillus chevalieri a high C:N ratio with glucose as carbon source was ideal. When exposing cultures to light, UV and red light gave the highest pigmentation; moreover, differential UV-VIS spectra in all wavelengths suggested production of additional pigments. Particularly a pigment observed when cultured in green light was also found in co-culture with yeast and there was an improvement of AU/Bgr value of 52549%. This is the first report regarding light effect and co-culture for these fungi, as well as C:N ratio for A. chevalieri.
1. Introduction
The use of synthetic or artificial colors is a frequent subject of debate led by safety regulatory entities; this concern has caused the extensive study of artificial colors regarding their effect on health. The European Food and Safety Authority (EFSA) started a general re-evaluation in 2009 after which, by examining the consumption safety thresholds, they decided to lower the acceptable dairy intake (ADI) of the colorants quinoline yellow (E104), sunset yellow (E110) and ponceau 4R (E124) [[1], [2], [3]]; showing the increase in restrictions concerning the use of some dyes. Given this situation, the fundamental problem to be solved is to deliver a new group of natural pigments. The successful marketing of natural pigments from plant source as food coloring reflects the presence and importance of niche markets [4]; however, it must be outlined that the main bottleneck of natural color production from vegetable origin is that obtainment takes longer, and production is subject to environmental conditions.
Filamentous fungi instead, present more advantages since they have a greater ability to be produced independently of field conditions, such is the case of species of Monascus sp. that synthesize yellow, orange, purple and red pigments [5]. Some strains of this genera have already been cultured in rice and in defined media of glucose and monosodium glutamate yielding 89,3 and 64 abs/g dry product respectively [6,7]. In addition, nowadays fungi have started to become a reality in the market of natural colors. Beta-carotene from Blakeslea trispora is approved by the EFSA and production reaches concentrations of almost 17 g/L; this fungus as well has been cultured employing several carbon sources such as glucose, lactose and vegetable oils [8,9].
Despite this scenario, for other pigment-producing fungal species, studies on the optimization of production are not available yet; added to this, given that most of this type of metabolites are not growth associated, the study of induction by factors that allow increasing their yield becomes necessary.
Among these scarcely studied fungi Aspergillus chevalieri (or teleomorphs in Eurotium sp.) and Fusarium oxysporum are promising strains and reports of pigment production in submerged culture are very limited. There are some works dealing with pigment production in submerged culture for other strains belonging to Fusarium sp. such as Fusarium verticillioides and Fusarium moniliforme. In the case of A. chevalieri and related species, literature mainly focuses on characterization of compounds in the fractionated extracts obtained when culturing in standard media as PDA or Malt extract-yeast extract-sucrose [[10], [11], [12]] in search of bio-activities [[13], [14], [15], [16], [17]].
Some species of the genera Fusarium sp. like F. verticilloides, F.fujikuroi, and F. oxysporum produce a series of pigments among which bikaverin, norbikaverin, and other compounds like nectriafurone and O-demethylanhydrofusarubrin are found [18]. Bikaverin, a pigment produced by F. oxysporum under acidic conditions, is considered by some authors as a naphtoquinone [19]. Others consider it a tetracyclic benzoxanthone resulting from the synthesis performed by a multifunctional class I polyketide synthase, followed by subsequent group modifications mediated by mono-oxygenase and a methyltransferase [20,21].
For Aspergillus chevalieri and related species as A. glaucus, A.cristatus, and A. ruber pigment production has been reported, specifically hydroxi-anthraquinones. Physcion or parietin, asperflavin, questin, erytroglaucin, and glycosilated anthraquinones can be found among these compounds [15]. Additional to these ones, a type of molecules with a benzaldehyde core can be found as well, having also an isporene unit and lateral chains of 7 carbons, among these, compounds like flavoglaucin, auroglaucin, dihydroauroglaucin, and tetrahydroaurogaucin can be found. These compounds present differences in the level of unsaturation of its lateral chain [16].
These two strains are promissory sources of pigments for diverse reasons, one is their biological activity. In respect to bikaverin from Fusarium oxysporum, so far, it has not been defined a clear role of the substance in plant pathogenicity [22,23]. In contrast to this, the purpose of biosynthesis of these compound might be related with inhibition of microorganisms in a saprophytic phase since it has already been reported that the compound has antibacterial and anti-parasitic activity. There is as well one work that highlights its potential as an antioxidant in neuronal cell lines [[23], [24], [25]].
Regarding pigments of Aspergillus chevaleri and related species, antioxidant activity of benzil derivatives has been well documented as well as the antimicrobial activity of their anthraquinones, and in fact, this fungi can be found in a fermented food in Japan named Katsuobushi [10,26,27].
The second reason of their potential is that they can also be considered as new alternatives to existing natural pigments of yellow and red hue; in terms of its stability, it is known that natural colors like curcumin and betacyanins are unstable [28,29]. So far there are no research articles regarding the stability of the pigments produced by these two fungi; however, it is well-known that anthraquinones are heat [30,31] and light stable [32]. It is also known that yellow hue anthraquinones are produced by Aspergillus chevalieri and related species [27,33,34]. There is also ongoing research in the Biotechnology research group concerning stability assays of the red pigment of F. oxysporum using castor oil as a vehicle finding almost the same level of heat stability as carmine (antraquinone lake emulsion) and improved light stability (data not included in this study).
The metabolites produced by these two strains are compounds of secondary metabolism, it is well-known that many of the genes clusters coding for secondary metabolites are just expressed under specific conditions and that some standard laboratory maintenance protocols do not trigger these pathways [35]. For this reason is of great importance the study of the effect of nutritional, biotic and abiotic factors [[36], [37], [38], [39], [40]] in this context is necessary to evaluate the effect of variables as carbon to nitrogen ratio, complex or defined media, stimulation through interaction with microorganisms and exposure to different light wavelengths [41]. Therefore, this paper aims to determine culture conditions for improving pigment production in the filamentous fungi Fusarium oxysporum and Aspergillus chevalieri through nutritional, biotic and abiotic stimuli.
2. Materials and methods
2.1. Strain maintenance and inoculum preparation
Strains of Fusarium oxysporum and Aspergillus chevalieri were obtained from a collection of the Biotechnology research group and cultured in PDA for 2 weeks at 23 °C + 1 °C.
In each fungus, for preculture, a modification of the section inoculum preparation found in the method M38-A2 of the CLSI was performed [42], 12 fragments of mycelium of 1 cm Φ were macerated sequentially until disintegration of the mycelial circles; 40 ml of saboreaud liquid medium were added to the macerated biomass with an adjusted pH of 5,6 for F. oxysporum, and A. chevalieri. Each blend was syringe filtered through a holder with a 50 μm mesh; the filtrate was then disposed in 50 ml conic tubes and used to inoculate seed cultures; pre-inoculum was carried in saboreaud medium. The flasks were left at 24 °C ± 1 °C and 100 rpm with a basal light irradiance of 0,715 W/m2; through preliminary studies, it could be determined that the proper incubation times for each fungus were 36 h for F. oxysporum and 120 h for A. chevalieri. For all assays performed, 8 ml of mycelial suspension were used as inoculum (10% of total working volume) equivalent to 0,2 g/L dry weight for F. oxysporum and 0,26 g/L for A. chevalieri.
2.2. Culture media assay
Culture was undertaken with 80 ml of medium. Evaluated media had a base salt composition consisting of (in g/L): MgSO4.7H2O - 0,02, KCl - 0,01 KH2PO4 - 0,03 and NaNO3 - 0,08 [43]; In addition, a pH of 4,0 was established for F. oxysporum; and of 5,5 for A. chevalieri; composition of the media is shown in Table 1, Table 2. The incubation time was 15 days for F. oxysporum [44,45] and 18 days for A. chevalieri [17,26,27,46] with the same incubation conditions used in the inoculum; triplicates were done for all conditions.
Table 1.
C and N content for several media in F. oxysporum.
| C:N Ratio | Culture Media |
|---|---|
| C:N 3 | M15Y5 |
| C:N 2 | M15Y7,5 |
| C:N 1,5 | M15Y10 |
| C:N 6 | M30Y5 |
| C:N 4 | M30Y7,5 |
| C:N 3 | M30Y10 |
| C:N 9 | M45Y5 |
| C:N 6 | M45Y7,5 |
| C:N 4,5 | M45Y10 |
| C:N 7,03 | BFL 20 |
| C:N 7,03 | BFL 30 |
| C:N 7,03 | BFL 40 |
Table 2.
C and N content for several media in A. chevalieri.
| C:N Ratio | Culture Media |
|---|---|
| C:N 10 | G10Y1 |
| C:N 3,3 | G10Y3 |
| C:N 2 | G10Y5 |
| C:N 20 | G20Y1 |
| C:N 6,6 | G20Y3 |
| C:N 4 | G20Y5 |
| C:N 30 | G30Y1 |
| C:N 10 | G30Y3 |
| C:N 6 | G30Y5 |
| C:N 7,03 | BFL 20 |
| C:N 7,03 | BFL 30 |
| C:N 7,03 | BFL 40 |
M: Maltodextrin Y: Yeast extract: BFL: Barley Flour.
G: Glucose Y: Yeast extract.
2.3. Influence of LED light assay
The fungi were inoculated in 15 flasks for each selected media, incubation conditions were the same as in the culture media assay and five wavelengths were evaluated using light emitting diodes: Blue, green, red, white and UV-A light; a light irradiance of 9 + 0,5 W/m2 was established for the first four wavelengths and for UV light the value was set at 0,05 + 0,005 W/m2.
2.4. Co-culture assay
2.4.1. Bacteria and yeast seed culture
Strains of P. fluorescens and K. marxianus were maintained in Pseudomonas P Agar and Malt extract-YPD Agar correspondingly. For seed culture, these were grown in the same media without agar. Incubation conditions were 130 rpm and 30 °C for P. fluorescens and 150 rpm and 37 °C for K. marxianus.
2.4.2. Co-culture
The fungi were inoculated in 24 flasks for each selected media, incubation conditions were the same as in the culture media assay; each half was then used for co-culture with the soil dwelling bacteria P. fluorescens or the yeast K. marxianus; days 6th, 7th and 8th were established for the start of co-incubation in F. oxysporum; in the case of A. chevalieri it was performed on the 3rd, 4th and 5th day. Final optical density of 0,05 was established for each microorganism (0,0125 g/L dry weight for bacteria and 0,175 g/L for yeast).
2.5. Biomass quantification
The content in the flasks was filtrated using cellulose filter paper and wet biomass was freeze-dried for 3 days at −50 °C and 20 mTorr (EYELA-FDU-1110・2110 freeze dryer).
2.6. Pigment extraction
Dry biomass was processed in an IKA® - A 11 basic analytical mill; and later pulverized in a mortar, to obtain a particle size of 0,3 mm. 100 mg of biomass and 250 mg of 0,5 mm glass beads were weighed into 1,5 ml PFA (Perfluoroalkoxy-alkane) vials. Finally, 0,7 ml of dichloromethane was added and extraction took place in a digital disruptor-genie (Scientific Industries ™) at 3000 rpm for 8 min. After a cycle of extraction, tubes were centrifuged at 13000 rpm, and colored supernatant collected; the process in the disruptor was repeated three times with clean solvent.
2.6.1. Estimation of produced pigments
Extracts were filtered with a 0,45 μm PTFE syringe filter unit, (SLCR025 EMD-Millipore) and the volume of filtered extract was recorded before making dilutions or stocking undiluted extracts in glass vials (to discard bias of solvent volatility) The estimation of pigment production in terms of absorbance units was calculated according to formula 1.
Formula 1. Absorbance Units
Where:
AU: Absorbance Units
Vfl: Filtered Volume
Vr: Read Volume in spectrophotometer
With this information, the yield of Absorbance units per biomass (AU/Bgr) was obtained.
The different extracts were taken to a quartz microplate to be read at 520 nm and 395 nm, for Fusarium oxysporum and Aspergillus chevalieri respectively in the culture media experiment. For the experiments of LED light and the co-culture effect, scans of the extracts in the spectrophotometer were done in order to take into consideration the presence of other pigments whose production have been stimulated under these diverse conditions.
2.7. Statistical analysis
Logarithm transformation was applied to the data of C:N ratio in Fusarium oxysporum and media type in Aspergillus chevalieri, this allowed the analysis of data by one way-ANOVA. Untransformed data for LED light effect was analyzed in the same manner; and finally the co-culture effect by two way-ANOVA; only in Fusarium oxysporum; which was not possible for Aspergillus chevalieri since several pigments occurred merely in specific conditions. Significant differences were considered for those sets of data with p-value < 0,05.
3. Results
3.1. Media composition and C:N ratio
Through literature revision and the spectrophotometric scan of the dichloromethane crude extract of F. oxysporum obtained from cell disruption, it can be inferred that the red pigment produced is bikaverin, with a λmax of 520 nm [44,47].
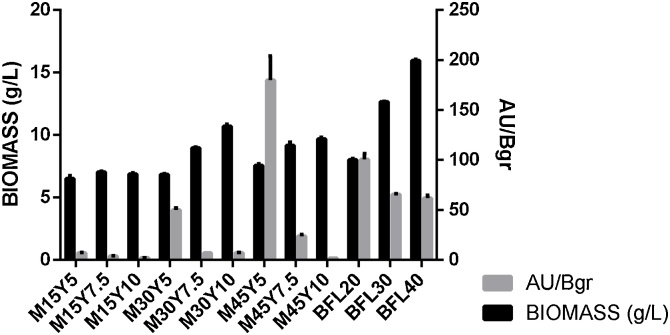
The effect of media composition as well as the C:N ratio could be determined (Fig. 1); there is satisfactory stimulation of pigment production if there is a high C:N ratio and the concentration of the nitrogen source is ≤ 5 g/L. Since the p-value for media type is > 0,05, there is no statistical difference between chemically defined media and complex media; nevertheless, p-value is < 0,05 for the factor C:N ratio (Table 3).
Fig. 1.
Biomass and Pigment production in MY and BFL media for F. oxysporum.
Table 3.
p-value for different treatments.
| FACTOR | p – value |
|
|---|---|---|
| F. oxysporum | A. chevalieri | |
| C:N ratio | 3,439 e-14* | 2,2 e-16 |
| Media Type | 0,912 | 1,267 e-4 |
| LED Light | 4,217 e-13* | n/aa |
| Microorganism | 1,607 e-06* | n/aa |
| Co-culture Day | 0,07056 | n/aa |
Not applicable.
In the present research, it is possible to prove that the complex media BFL 40 with a C:N ratio of 7,03 that gave the highest biomass concentration did not give the highest AU/Bgr value. In contrast, the best result of pigment production was achieved in the M45Y5 media with a C:N ratio of 9 but with lower biomass (7,65 g/L- Fig. 1), showing that bikaverin production is not necessarily proportional to biomass production. This media was selected for further assays.
Concerning Aspergillus chevalieri, spectrophotometric scan and literature give some insight in the production of a yellow pigment, probably tetrahydroauroglucin a benzyl derivative which λmax is of 396 nm [10].
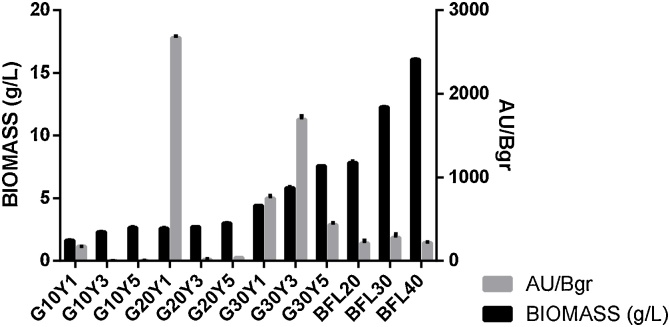
The major difference in respect to Fusarium oxysporum was that, in this case, a complex media composed of barley flour did not give a high pigment content (Fig. 2); this can also be supported by both p-values which are lower than <0,05 (Table 3). Behavior regarding high C:N ratio remains in defined media with the best results presented in ratios of 10, 20 and 30 respectively. The highest AU/Bgr value was achieved with G20Y1 media; nevertheless, biomass concentration was not above 2,58 g/L; this media was selected for further assays.
Fig. 2.
Biomass and Pigment production in GY and BFL media for A. chevalieri.
3.2. Wavelength effect
Taking into consideration that there was no proportional relation between biomass concentration and pigment production in culture media assay, wavelength assay was executed as another approach not only to stimulate pigment biosynthesis and accumulation but also biomass production.
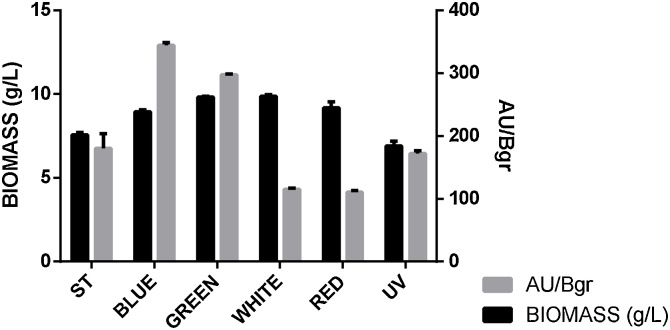
Through our experimentation, we found that pigment biosynthesis was improved in F. oxysporum when using blue and green light in respect to the AU/Bgr obtained in the media M45Y5 with standard light irradiance (Fig. 3). In our case, production was improved in a 191,2%, along with a significant statistical difference (Table 3). Biomass concentration in blue and green light was also higher (8,8 and 9,72 g/L respectively).
Fig. 3.
Biomass and Pigment production in different LED light for F. oxysporum.
For A. chevalieri, a positive effect of light was encountered; in this case, resulting in the production of more than one pigment. This inference was established after performing the spectrophotometric scan of the dichloromethane mycelial extract as well as the changes in the hue obtained in this crude extract (Supplementary File, Fig. A and B), which allowed finding a wide peak different from the initial one obtained in the culture media assay and in the case of blue light the presence of two peaks.
Red and UV light managed to improve the results obtained in terms of AU/Bgr value (Table 4), where the value increased more than two times with red light and more than three times with UV light. Biomass content increased in blue, green and white light respectively. The fact that there is no significant increase of AU/Bgr value in blue light might be related with a higher molecule diversity since the spectra for this condition gave the widest peaks (Supplementary File, Fig. A)
Table 4.
Biomass and Pigment production in different LED light for A. chevalieri.
| λmax | LED | BIOMASS | AU/Bgr |
|---|---|---|---|
| 360 nm | Blue | 9,95 + 0,92 | 2750,68 + 451,30 |
| 455 nm | Blue | 9,95 + 0,92 | 2343,57 + 113,05 |
| 435 nm | Green | 7,77 + 0,96 | 2526,71 + 341,63 |
| White | 9,69 + 0,94 | 1746,83 + 108,03 | |
| 430 nm | Red | 2,85 + 0,15 | 7306,59 + 80,46 |
| UV | 3,02 + 0,03 | 7976,46 + 438,23 |
3.3. Co-culture effect
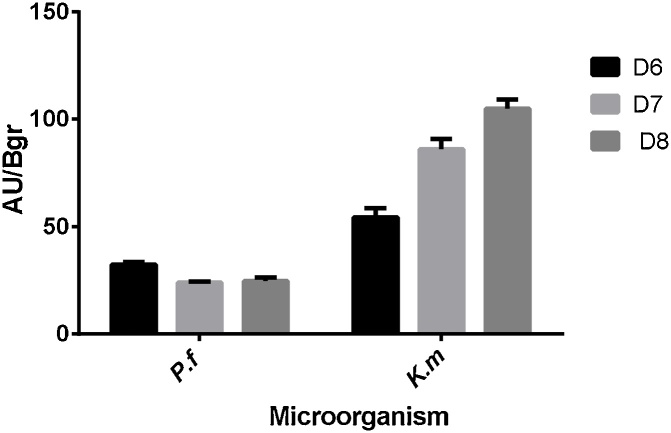
Co-culture with P.fluorescens had no effect on pigment production by F. oxysporum, on the contrary, it considerably diminished production when comparing AU/Bgr value with the one obtained in the culture media with standard light conditions. Regarding the assay with yeast, it was noticed that pigment production did not decrease drastically (Fig. 4), AU/Bgr for day 8 was almost the same as the one obtained in media with standard light conditions. This demonstrates that the type of microorganism used in co-culture has a negative statistical effect (Table 3).
Fig. 4.
Pigment production in co-culture for F. oxysporum with bacteria or yeast.
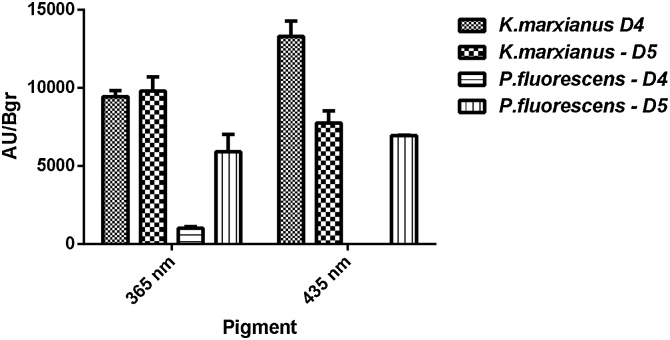
For A. chevalieri presence of a pigment with λmax of 365 nm was observed, there was also an increment of a pigment with λmax of 435 nm in comparison to green light where it was initially found. Elicitation occurred when co-cultured with P.fluorescens at day 5 and for K. marxianus at day 4 and 5 (Fig. 5); this demonstrates that for this fungi microorganism type as well as starting time of co-incubation influence concentration and differential production of pigments.
Fig. 5.
Pigment production in co-culture for A. chevalieri with bacteria or yeast.
There were higher AU/Bgr values for experiments performed with K. marxianus; it is possible then that P. fluorescens can produce compounds with a slight activity against A. chevalieri as well. In the case of co-culture with yeast, the competition mechanism performed by A. chevalieri might be directed preferably to avoid yeast from consuming carbon source and other nutrients. This implies that metabolism in A. chevalieri has a turnover with preference for metabolite production intended to stop yeast growth; with biomass reduced to less than 2,60 g/L (data not shown), which can be supported by the fact that in the experiment with green light, a lower AU/Bgr was obtained but with higher biomass content.
4. Discussion
4.1. Media composition and C:N ratio
In general, for all fungi, it was observed that a C:N ratio equal or above 7 induced pigment production; however, this occurred only with defined simple media for A. chevalieri while in F. oxysporum this took place for both defined and complex media. Regarding the effect of the nitrogen source in pigment production, some research shows the preference for organic nitrogen sources in biosynthesis (except for glutamine) over nitrates and ammonium salts [48]; especially if these are as complex as yeast extract; in our case the presence of sodium nitrate did not have a negative effect given its low concentration.
Other authors have performed as well assays evaluating culture media composition for extracellular red pigment production in other species of Fusarium sp. as F. fujikuroi, in which it has been found that high proportions of carbon to nitrogen and that organic nitrogen sources are beneficial for pigment accumulation (Measurement at 500 nm) [49]; another example is the decrease in bikaverin biosynthesis for an overproducing mutant of F.fujikuroi with rising concentration of soy bean meal [50], showing as well that biomass and pigment production are not directly correlated. Also, other works with F. moniliforme show that the highest production takes place in media with high carbon and low nitrogen concentration such as potato dextrose and malt extract broth. (Measurement at 500 nm) with an AU of 1,5/ml [51,52].
For many secondary metabolites of the genera Aspergillus sp. down-regulation of some pathways for secondary metabolism occurs when glucose is used [53]; but not in the case of this research. In the future it would be important to determine why in this specific case production took place with the use of glucose instead of using a complex media with diverse polysaccharides; however, results concerning C:N in other species of Aspergillus sp. are coherent with those obtained in the present research; Casas-López and collaborators found the highest production of lovastatin from A. terreus in a medium with a C:N ratio of 41,3; nevertheless, this was achieved with a disaccharide [54].
4.2. Wavelength effect
Regarding light effect, increase in production was observed in F. oxysporum mainly with blue light; whereas for A. chevalieri increase was noted with red and UV light, showing that there are several photoreceptors and specific transcription factors involved and that these vary with fungal species and metabolic route. In general, this outcome is concordant with the work of other authors in which for five filamentous fungi tested, satisfactory yields were obtained with red and blue light; also with data of Monascus sp. that presents the highest absorbance units for the intracellular extract when exposed to red light [36]
When comparing the results obtained by the latter author, specifically for Fusarium verticilIiodes with the ones obtained in this work for F. oxysporum there is difference since it can be seen that the highest intracellular yield is achieved with red light instead of blue light [55]. It is feasible to suggest that in the cited research work other red pigments different from bikaverin were taken into consideration. (Since the specific wavelength at which the extract was read is not specified), or that response to light can significantly vary among species of the same genera.
The results seen in F. oxysporum with different wavelengths support the idea that bikaverin biosynthesis is light-regulated. It is important to outline that various research have found that production of this pigment in related species, such as F. fujikuroi, was improved in mutants of the DASH-cryphtochrome, white collar complex as well as velvet complex when compared to the wild type under low nitrogen conditions [[56], [57], [58]]. Such findings can indicate, that, blue light induction can be regulated by the photoreceptor VIVID [59]. For the case of green light, opsin-related proteins OpsA and CarO might be related in stimulation. Regarding white, red and UV light some hypothesis can arise; one, that the photoreceptor for the red wavelength is not involved in bikaverin biosynthesis; concerning white light, since it is the mixture of all wavelengths, possibly does not activate a specific photoreceptor, and secondly UV light might have had an effect in metabolism which can be reflected in lower biomass and pigment production.
In the case of Aspergillus chevalieri UV and red light yielded the highest AU/Bgr numbers; a scan of the extracts showed that a molecule absorbing at a λmax of 430 nm (in CHCl3) can correspond to parietin which λmax reported in methanol is 433 nm [60,61] and 431 nm in ethanol [62]. The enhancement of pigmentation with red and UV wavelength can shed light on the involvement of photoreceptors in the biosynthesis of this compound; phytochrome (red light); and photolyase CryA (UVA light). In this context, it is necessary to highlight that UV light might have induced some stress since it has already been reported that rise in parietin production has occurred in Xanthoria parietina with increasing exposure to UV light. The main pigment could have served in a feasible manner as a photo-protector or as an antioxidant in A. chevalieri. In Blakeslea trispora oxidative stress caused an increase in carotenoid content, therefore it is reasonable to propose that other fungal pigments serve this function [[63], [64], [65], [66]].
Nonetheless, it is important to mention that blue light, in addition, has two absorption maxima with λmax of 360 nm and 455 nm respectively, this second one shows a wider peak with a noticeable decrease in absorption mostly evident above 530 nm. This suggests the presence of several orange-red pigments within the extract that have already been reported for the teleomorph Eurotium chevalieri as well as some close species as E. repens, E. rubrum, E. cristatum, and the anamorph A. glaucus; some of these are erythroglaucin with λmax of 465 nm, 493 nm, 510 nm, 523 nm in ethanol [67,68], catenarin with a λmax of 463 nm in ethanol [27]. There are not enough reports that expose pigments with λmax near to 360 nm, there is only research that presents coloured compounds as Neoechinulin B or TMC-120 derivative present in Eurotium sp. [69]. Overall, the results obtained are in line with others exposed for other fungal isolates such as the case of some marine fungi, in which production of secondary metabolites is given in a wavelength specific manner with satisfactory results obtained using red and blue light [70].
4.3. Co-culture effect
Through co-culture only pigment production was improved in A. chevalieri employing bacteria and yeast as well, nevertheless displaying differences in AU/Bgr. For F. oxysporum, although there were differences between bacteria and yeast, no enhancement in AU/Bgr was observed regarding production in standard media; this proves that this approach at an interaction level for F. oxysporum is species specific. Possible explanations are: The increase in pH caused by the addition of bacteria culture to the flasks caused a down-regulation of the bikaverin pathway, since production is favoured under acidic conditions [47]; alternatively, that the C:N ratio could have been altered as a result of a faster consumption rate of the carbon source characteristic of bacterial growth kinetics compared to the fungi. Diminished production can also be a consequence of the antifungal molecules synthesized by P. fluorescens; with activity against this fungi [71].
The above does not imply that this fungus cannot present a response to other microorganisms since it has been reported in another species of the genera, as F. tricinctum, a different profile of metabolites when co-cultured with B. subtilis [72].
Molecules observed in A. chevalieri have λmax of 365 nm and 435 nm; identity for the first compound (365 nm) cannot be secured; nevertheless, it is feasible that neoechinulin B or TMC 120 derivative 2 [69] are present in the extract. The second λmax might correspond to emodin, also found when stimulated with green light, this last compound is an anthraquinone with well-known activity against some gram-positive bacteria [27,[73], [74], [75]] and has shown antifungal effects to yeast like C. albicans, and C. neoformans [76]. Reports of negative effects are limited in gram negative bacteria, and until now, only described in Haemophilus parasuis [77]. These findings are reasonable considering that antimicrobial activity has been reported for some metabolites of Eurotium sp. [17,26] and its anamorphs (Aspergillus sp.); therefore, accumulation of compounds in this research might be destined to later secretion in order to inhibit competing microorganisms; however, this must be confirmed with further experimentation evaluating pure emodin from wasted media of A. chevalieri. Other experiments in progress not included in this work with biomass extracts of this fungi have shown an IC50 (Inhibitory concentration) of 17,5 ppm against Staphylococcus aureus (Unpublished data).
In the present work, yeast stimulates higher accumulation of pigment in A. chevalieri; other research in which pigment of a strain of Penicillium sp. could only be yielded through co-cultivation with Candida tropicalis exemplifies another positive outcome [78].
In other species such as A. fumigatus meroterpenoid production was induced when co-cultured with S. rapamycinicus, showing slight activity against this bacteria [79]; in contrast accumulation of metabolites for A. terreus did not occur when co-cultured with two Streptomyces sp. [80]. This confirms that stimulation can be species-specific. Equally, increment in synthetized desipeptides has been found in teleomorphs such as Emericella sp. [81] which displayed moderate activity against S. aureus.
5. Conclusions
C:N ratios equal to or higher than 7 stimulate high pigment production in F. oxysporum, while for A. chevalieri ideal C:N ratios are among 10–20.
Blue and green light favour increment in bikaverin production in F. oxysporum while several pigments can be produced in A. chevaileri when stimulated with different light wavelengths.
Induction of pigment production by means of co-culture with bacteria and yeast is species-specific, it was not observed for F. oxysporum, but it could be found in A. chevalieri.
This work allows considering other approaches for the biotechnological production of pigments besides the study of culture media, further work can be performed to optimize the value of light irradiance or photoperiod instead of continuous exposure. Also, for F. oxysporum and other fungi, if required, evaluation of several strains of bacteria or yeast in a culture collection (preferably biosafety I) can be performed in search of the specific interaction that enhances pigment biosynthesis.
Acknowledgments
Special acknowledgments to the Administrative Department of Science and Technology from Colombia−COLCIENCIAS (Governmental Entity) which funded the project code: 111556934264 contract no. 0023-2013 (call 569) and permitted the development of this work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00308.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.E.P. on F.A. and N.S. added to F. (ANS), Scientific Opinion on the re-evaluation of Quinoline Yellow (E104) as a food additive 1. EFSA J. 2009;7:1–40. [Google Scholar]
- 2.E.P. on F.A. and N.S. added to F. (ANS), Scientific Opinion on the re-evaluation of Sunset Yellow FCF (E110) as a food additive. EFSA J. 2009;7:1–44. [Google Scholar]
- 3.E.P. on F.A. and N.S. added to F. (ANS), Scientific Opinion on the re-evaluation of Ponceau 4R (E 124) as a food. EFSA J. 2009;7:1–39. [Google Scholar]
- 4.Dufossé L., Fouillaud M., Caro Y., Mapari S.A.S., Sutthiwong N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014;26:56–61. doi: 10.1016/j.copbio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Blanc P.J., Loret M.O., Santerre A.L., Pareilleux A., Prome D., Prome J.C., Laussac J.P., Goma G. Pigments of Monascus. J. Food Sci. 1994;59:862–865. [Google Scholar]
- 6.De Carvalho J.C., Oishi B.O., Pandey A., Soccol C.R. Biopigments from Monascus: Strains Selection, Citrinin Production and Color Stability. Brazilian Arch. Biol. Technol. 2005;48:885–894. [Google Scholar]
- 7.Mukherjee G., Singh S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochem. 2011;46:188–192. [Google Scholar]
- 8.Papaioannou E.H. Substrate contribution on carotenoids production in Blakeslea trispora cultivations. Food Bioprod. Process. 2009;88:305–311. [Google Scholar]
- 9.Nanou K., Roukas T. Waste cooking oil: A new substrate for carotene production by Blakeslea trispora in submerged fermentation. Bioresour. Technol. 2016;203:198–203. doi: 10.1016/j.biortech.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 10.Miyake Y., Ito C., Itoigawa M., Osawa T. Antioxidants produced by Eurotium herbariorum of filamentous fungi used for the manufacture of karebushi, dried bonito (Katsuobushi) Biosci. Biotechnol. Biochem. 2009;73:1323–1327. doi: 10.1271/bbb.80887. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa Y., Morimoto K.Y., Hamasaki T. Metabolites of Eurotium species, their antioxidative synergism with tocopherol properties. J. Food Sci. 1985;50:1742–1744. [Google Scholar]
- 12.Ishikawa Y., Morimoto K., Hamasaki T. Flavoglaucin, a metabolite of Eurotium chevalieri, its antioxidation and synergism with tocopherol. J. Am. Oil Chem. Soc. 1984;61:1864–1868. [Google Scholar]
- 13.Li D.L., Li X.M., Li T.G., Dang H.Y., Wang B.G. Dioxopiperazine alkaloids produced by the marine mangrove derived endophytic fungus Eurotium rubrum. Helv. Chim. Acta. 2008;91:1888–1893. [Google Scholar]
- 14.Kijjoa A., Lima R., Vasconcelos M., Pinto M., Almeida A., Dethoup T., Singburaudom N. The in vitro anticancer activity of the crude extract of the sponge-associated fungus Eurotium cristatum and its secondary metabolites. J. Nat. Pharm. 2010;1:25. [Google Scholar]
- 15.Li D.L., Li X.M., Wang B.G. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: Structural elucidation and DPPH radical scavenging activity. J. Microbiol. Biotechnol. 2009;19:675–680. [PubMed] [Google Scholar]
- 16.Gao J., León F., Radwan M.M., Dale O.R., Husni A.S., Manly S.P., Lupien S., Wang X., Hill Ra., Dugan F.M., Cutler H.G., Cutler S.J. Benzyl derivatives with in vitro binding affinity for human opioid and cannabinoid receptors from the fungus Eurotium repens. J. Nat. Prod. 2011;74:1636–1639. doi: 10.1021/np200147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanokmedhakul K., Kanokmedhakul S., Suwannatrai R., Soytong K., Prabpai S., Kongsaeree P. Bioactive meroterpenoids and alkaloids from the fungus Eurotium chevalieri. Tetrahedron. 2011;67:5461–5468. [Google Scholar]
- 18.Mapari S.A., Meyer A.S., Thrane U., Frisvad J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009;8:24. doi: 10.1186/1475-2859-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medentsev A.G., Arinbasarova A.Y., Akimenko V.K. Biosynthesis of naphthoquinone pigments by fungi of the genus Fusarium. Appl. Biochem. Microbiol. 2005;41:503–507. [PubMed] [Google Scholar]
- 20.Kjaer D., Pedersen C., Lock D.B., Smith J.R. Bikaverin and Norbikaverin, Benzoxanthentrione Pigments of Gibberella fujikuroi. J. Chem. Soc. 1971:2792–2797. doi: 10.1039/j39710002792. [DOI] [PubMed] [Google Scholar]
- 21.Limón M.C., Rodríguez-Ortiz R., Avalos J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 2010;87:21–29. doi: 10.1007/s00253-010-2551-1. [DOI] [PubMed] [Google Scholar]
- 22.a Bell A., Wheeler M.H., Liu J., Stipanovic R.D., Puckhaber L.S., Orta H. United States Department of Agriculture-Agricultural Research Service studies on polyketide toxins of Fusarium oxysporum f sp vasinfectum: potential targets for disease control. Pest Manag. Sci. 2003;59:736–747. doi: 10.1002/ps.713. [DOI] [PubMed] [Google Scholar]
- 23.Son S.W., Kim H.Y., Choi G.J., Lim H.K., Jang K.S., Lee S.O., Lee S., Sung N.D., Kim J.C. Bikaverin and fusaric acid from Fusarium oxysporum show antioomycete activity against Phytophthora infestans. J. Appl. Microbiol. 2008;104:692–698. doi: 10.1111/j.1365-2672.2007.03581.x. [DOI] [PubMed] [Google Scholar]
- 24.Nirmaladevi D., Venkataramana M., Chandranayaka S., Ramesha A., Jameel N.M., Srinivas C. Neuroprotective effects of Bikaverin on H2O 2-Induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014;34:973–985. doi: 10.1007/s10571-014-0073-6. [DOI] [PubMed] [Google Scholar]
- 25.Deshmukh R., Mathew A., Purohit H.J. Characterization of antibacterial activity of bikaverin from Fusarium sp. HKF15. J. Biosci. Bioeng. 2014;117:443–448. doi: 10.1016/j.jbiosc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Gao J., Radwan M.M., León F., Wang X., Jacob M.R., Tekwani B.L., Khan S.I., Lupien S., Hill R.A., Dugan F.M., Cutler H.G., Cutler S.J. Antimicrobial and antiprotozoal activities of secondary metabolites from the fungus Eurotium repens. Med. Chem. Res. 2012;29:997–1003. doi: 10.1007/s00044-011-9798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anke H., Kolthoum I., Zähner H., Laatsch H. Metabolic products of microorganisms. 185. The anthraquinones of the Aspergillus glaucus group. I. Occurrence, isolation, identification and antimicrobial activity. Arch. Microbiol. 1980;126:223–230. doi: 10.1007/BF00409924. [DOI] [PubMed] [Google Scholar]
- 28.Esatbeyoglu T., Huebbe P., Ernst I.M.A., Chin D., Wagner A.E., Rimbach G. Curcumin-from molecule to biological function. Angew. Chemie Int. Ed. 2012;51:5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 29.Scotter M.J. 2015. Colour Additives for Foods and Beverages. Cambridge. [Google Scholar]
- 30.Fernández-López J.A., Angosto J.M., Giménez P.J., León G. Thermal stability of selected natural red extracts used as food colorants. Plant Foods Hum. Nutr. 2013;68:11–17. doi: 10.1007/s11130-013-0337-1. [DOI] [PubMed] [Google Scholar]
- 31.Harris R.M. A primer on colorful additives. Color. Technol. Plast. 1999:1–12. [Google Scholar]
- 32.Sakunpak A., Sirikatitham A., Panichayupakaranant P. Preparation of anthraquinone high-yielding Senna alata extract and its stability. Pharm. Biol. 2009;47:236–241. [Google Scholar]
- 33.Gessler N.N., Egorova A.S., Belozerskaya T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013;49:85–99. [Google Scholar]
- 34.Caro Y., Anamale L., Fouillaud M., Laurent P., Petit T., Dufosse L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: an overview. Nat. Products Bioprospect. 2012;2:174–193. [Google Scholar]
- 35.a Brakhage A., Schroeckh V. Fungal secondary metabolites - strategies to activate silent gene clusters. Fungal Genet. Biol. 2011;48:15–22. doi: 10.1016/j.fgb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Miyake T., Mori A., Kii T., Okuno T., Usui Y., Sato F., Sammoto H., Watanabe A., Kariyama M. Light effects on cell development and secondary metabolism in Monascus. J. Ind. Microbiol. Biotechnol. 2005;32:103–108. doi: 10.1007/s10295-005-0209-2. [DOI] [PubMed] [Google Scholar]
- 37.Castrillo M., Avalos J. Light-mediated participation of the VIVID-like protein of Fusarium fujikuroi VvdA in pigmentation and development. Fungal Genet. Biol. 2014;71:9–20. doi: 10.1016/j.fgb.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Heydt M., Rüfer C., Raupp F., Bruchmann A., Perrone G., Geisen R. Influence of light on food relevant fungi with emphasis on ochratoxin producing species. Int. J. Food Microbiol. 2011;145:229–237. doi: 10.1016/j.ijfoodmicro.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Netzker T., Flak M., Krespach M.K., Stroe M.C., Weber J., Schroeckh V., Brakhage A.A. Microbial interactions trigger the production of antibiotics. Curr. Opin. Microbiol. 2018;45:117–123. doi: 10.1016/j.mib.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Sarkar A., Funk A.N., Scherlach K., Horn F., Schroeckh V., Chankhamjon P., Westermann M., Roth M., a Brakhage A., Hertweck C., Horn U. Differential expression of silent polyketide biosynthesis gene clusters in chemostat cultures of Aspergillus nidulans. J. Biotechnol. 2012;160:64–71. doi: 10.1016/j.jbiotec.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Tisch D., Schmoll M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010;85:1259–1277. doi: 10.1007/s00253-009-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CLSI . 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard-Second Editon. [Google Scholar]
- 43.Zapata P.A., Rojas D.F., Atehortua L. Production of biomass, polysaccharides, and ganoderic acid using non-conventional carbon sources under submerged culture of the lingzhi or reishi medicinal mushroom, Ganoderma lucidum (W.Curt.:Fr.)P. Karst. (Higher Basidiomycetes) Int. J. Med. Mushrooms. 2012;14:197–203. doi: 10.1615/intjmedmushr.v14.i2.80. [DOI] [PubMed] [Google Scholar]
- 44.Studt L., Wiemann P., Kleigrewe K., Humpf H.U., Tudzynski B. Biosynthesis of fusarubins accounts for pigmentation of Fusarium fujikuroi perithecia. Appl. Environ. Microbiol. 2012;78:4468–4480. doi: 10.1128/AEM.00823-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shukla R., Chand S., Srivastava A.K. Batch kinetics and modeling of gibberellic acid production by Gibberella fujikuroi. Enzyme Microb. Technol. 2005;36:492–497. [Google Scholar]
- 46.Gomes N.M., Dethoup T., Singburaudom N., Gales L., Silva A.M.S., Kijjoa A. Eurocristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochem. Lett. 2012;5:717–720. [Google Scholar]
- 47.Wiemann P., Willmann A., Straeten M., Kleigrewe K., Beyer M., Humpf H.U., Tudzynski B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: genes, their function and regulation. Mol. Microbiol. 2009;72:931–946. doi: 10.1111/j.1365-2958.2009.06695.x. [DOI] [PubMed] [Google Scholar]
- 48.Silveira S.T., Daroit D.J., Sant’Anna V., Brandelli A. Stability modeling of red pigments produced by Monascus purpureus in submerged cultivations with sugarcane bagasse. Food Bioprocess Technol. 2013;6:1007–1014. [Google Scholar]
- 49.Boonyapranai K., Tungpradit R., Lhieochaiphant S., Phutrakul S. Optimization of submerged culture for the production of naphthoquinones pigment by Fusarium verticillioides. Chiang Mai J. Sci. 2008;35:457–466. [Google Scholar]
- 50.Lale G.J., Gadre R.V. Production of bikaverin by a Fusarium fujikuroi mutant in submerged cultures. AMB Express. 2016 doi: 10.1186/s13568-016-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanly Pradeep F., Shakila Begam M., Palaniswamy M., Pradeep B.V. Influence of culture media on growth and pigment production by Fusarium moniliforme KUMBF1201 isolated from paddy field soil. World Appl. Sci. J. 2013;22:70–77. [Google Scholar]
- 52.Stanly Pradeep F., Pradeep B.V. Optimization of pigment and biomass production from Fusarium moniliforme under submerged fermentation conditions. Int. J. Pharm. Pharm. Sci. 2013;5:526–535. [Google Scholar]
- 53.Ruiz B., Chávez A., Forero A., García-Huante Y., Romero A., Sánchez M., Rocha D., Sánchez B., Rodríguez-Sanoja R., Sánchez S., Langley E. Production of microbial secondary metabolites: regulation by the carbon source. Crit. Rev. Microbiol. 2010;36:146–167. doi: 10.3109/10408410903489576. [DOI] [PubMed] [Google Scholar]
- 54.Casas López J.L., Sánchez Pérez J.A., Fernández Sevilla J.M., Acién Fernández F.G., Molina Grima E., Chisti Y. Production of lovastatin by Aspergillus terreus: effects of the C:N ratio and the principal nutrients on growth and metabolite production. Enzyme Microb. Technol. 2003;33:270–277. [Google Scholar]
- 55.Velmurugan P., Lee Y.H., Venil C.K., Lakshmanaperumalsamy P., Chae J.C., Oh B.T. Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J. Biosci. Bioeng. 2010;109:346–350. doi: 10.1016/j.jbiosc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Estrada A.F., Avalos J. The White Collar protein WcoA of Fusarium fujikuroi is not essential for photocarotenogenesis, but is involved in the regulation of secondary metabolism and conidiation. Fungal Genet. Biol. 2008;45:705–718. doi: 10.1016/j.fgb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Wiemann P., Brown D.W., Kleigrewe K., Bok J.W., Keller N.P., Humpf H.U., Tudzynski B. FfVel1 and fflae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol. Microbiol. 2010;77:972–994. doi: 10.1111/j.1365-2958.2010.07263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castrillo M., García-Martínez J., Avalos J. Light-dependent functions of the Fusarium fujikuroi CryD DASH cryptochrome in development and secondary metabolism. Appl. Environ. Microbiol. 2013;79:2777–2788. doi: 10.1128/AEM.03110-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Idnurm A., Verma S., Corrochano L.M. A glimpse into the basis of vision in the kingdom Mycota. Fungal Genet. Biol. 2010;47:881–892. doi: 10.1016/j.fgb.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marković Z., Manojlović N., Zlatanović S. Electronic absorption spectra of substituted anthraquinones and their simulation using ZINDO / S method. Comput. Mech. 2008;2:73–79. [Google Scholar]
- 61.Duca D., Farrugia C. Development and validation of HPLC-PDA assay method of Frangula emodin. Mediterr. J. Chem. 2016;5:374–386. [Google Scholar]
- 62.Räisänen R., Björk H., Hynninen P.H. Two-dimensional TLC separation and mass spectrometric identification of anthraquinones isolated from the fungus Dermocybe sanguinea. Zeitschrift Fur Naturforsch. - Sect. C J. Biosci. 2000;55:195–202. doi: 10.1515/znc-2000-3-410. [DOI] [PubMed] [Google Scholar]
- 63.Solhaug K.A., Gauslaa Y., Nybakken L., Bilger W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003;158:91–100. [Google Scholar]
- 64.Solhaug K.A., Gauslaa Y. Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ. 2004;27:167–176. [Google Scholar]
- 65.Larsson P., Večeřová K., Cempírková H., Solhaug K.A., Gauslaa Y. Does UV-B influence biomass growth in lichens deficient in sun-screening pigments? Environ. Exp. Bot. 2009;67:215–221. [Google Scholar]
- 66.Nanou K., Roukas T. Stimulation of the biosynthesis of carotenes by oxidative stress in Blakeslea trispora induced by elevated dissolved oxygen levels in the culture medium. Bioresour. Technol. 2011;102:8159–8164. doi: 10.1016/j.biortech.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Suemitsu R., Iwai J., Kawaguchi K., Haitani N., Kitagawa N. Isolation and identification of erythroglaucin (1,4,5-Trihydroxi-7-methoxy-2- methylanthraquinone) from the Mycelium of Alternaria porri (Ellis) Ciferri. Agric. Biol. Chem. 1977;41:2289–2290. [Google Scholar]
- 68.Engstrom G.W., Stenkamp R.E., Mcdorman D.J., Jensen L.H. Spectral identification, X-ray structure determination, and iron-chelating capability of erythroglaucin, a red pigment from Aspergillus ruber. J. Agric. Food Chem. 1982;30:304–307. [Google Scholar]
- 69.Slack G.J., Puniani E., Frisvad J.C., Samson R.A., Miller J.D. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009;113:480–490. doi: 10.1016/j.mycres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 70.Cai M., Fang Z., Niu C., Zhou X., Zhang Y. Light regulation on growth, development, and secondary metabolism of marine-derived filamentous fungi. Folia Microbiol. (Praha) 2013;58:537–546. doi: 10.1007/s12223-013-0242-x. [DOI] [PubMed] [Google Scholar]
- 71.Upadhyay A., Srivastava S. Phenazine-1-carboxylic acid is a more important contributor to biocontrol Fusarium oxysporum than pyrrolnitrin in Pseudomonas fluorescens strain Psd. Microbiol. Res. 2011;166:323–335. doi: 10.1016/j.micres.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 72.Ola A.R.B., Thomy D., Lai D., Proksch P. 2013. Inducing Secondary Metabolite Production by the Endophytic Fungus Fusarium tricinctum through Coculture with Bacillus subtilis. [DOI] [PubMed] [Google Scholar]
- 73.Shan B., Cai Y.Z., Brooks J.D., Corke H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008;109:530–537. [Google Scholar]
- 74.Chukwujekwu J.C., Coombes P.H., Mulholland D.A., van Staden J. Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis. S. Afr. J. Bot. 2006;72:295–297. [Google Scholar]
- 75.Basu S., Ghosh A., Hazra B. Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn., and Lantana camara Linn.: isolation of emodin and physcion as active antibacterial agents. Phyther. Res. 2005;19:888–894. doi: 10.1002/ptr.1752. [DOI] [PubMed] [Google Scholar]
- 76.Agarwal S.K., Singh S.S., Verma S., Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000;72:43–46. doi: 10.1016/s0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 77.Li L., Song X., Yin Z., Jia R., Li Z., Zhou X., Zou Y., Li L., Yin L., Yue G., Ye G., Lv C., Shi W., Fu Y. The antibacterial activity and action mechanism of emodin from Polygonum cuspidatum against Haemophilus parasuis in vitro. Microbiol. Res. 2016;186–187:139–145. doi: 10.1016/j.micres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 78.Hailei W., Zhifang R., Ping L., Yanchang G., Guosheng L., Jianming Y. Improvement of the production of a red pigment in Penicillium sp. HSD07B synthesized during co-culture with Candida tropicalis. Bioresour. Technol. 2011;102:6082–6087. doi: 10.1016/j.biortech.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 79.König C.C., Scherlach K., Schroeckh V., Horn F., Nietzsche S., a Brakhage A., Hertweck C. Bacterium induces cryptic meroterpenoid pathway in the pathogenic fungus Aspergillus fumigatus. Chembiochem. 2013;14:938–942. doi: 10.1002/cbic.201300070. [DOI] [PubMed] [Google Scholar]
- 80.Chen H., Daletos G., Abdel-Aziz M.S., Thomy D., Dai H., Brötz-Oesterhelt H., Lin W., Proksch P. Inducing secondary metabolite production by the soil-dwelling fungus Aspergillus terreus through bacterial co-culture. Phytochem. Lett. 2015;12:35–41. [Google Scholar]
- 81.Oh D.-C., Kauffman C.A., Jensen P.R., Fenical W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007;70:515–520. doi: 10.1021/np060381f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.