Abstract
Provitamin A cassava clones were analysed for starch yield and critical starch quality attributes, to understand possible applications in the food industry. Total carotenoids content in the test clones ranged from 0.03-11.94 μg g-1 of fresh root. Starch yield ranged from 8.4-33.2 % and correlated negatively (r = -0.588, P < 0.001) with carotenoids content. Amylose content (16.4–22.1%) didn't differ significantly (P ≤ 0.05) among the cassava clones. Meanwhile, total carotenoid content had significant negative correlations (P ≤ 0.05) with starch pasting temperature, peak time, setback viscosities and peak area. The reduced peak time and pasting temperatures in high-carotenoid cassava signifies reduction in energy requirements in yellow-fleshed roots when compared to white-fleshed cassava. This attribute is desirable for the food industry as it would reduce the overall cost of processing the cassava. Furthermore, final viscosities of starch from carotenoid-rich cassava were lower than those of white-fleshed roots, making provitamin A cassava suitable for soft food processing.
Keywords: Biochemistry, Nutrition, Food analysis
1. Introduction
Vitamin A deficiency (VAD) is as a major health challenge among vulnerable communities, especially children under the age of five years and pregnant women, in low-income countries that are dependent on starchy food staples (WHO, 2009). In fact, VAD has severe health consequences, including irreversible blindness and compromised body immunity, leading to increased morbidity and mortality from preventable diseases (Semba, 1998). This makes VAD one of the leading acute health burdens to families, societies and governments.
Globally, different strategies have been employed and/or initiated to address VAD, and these include: a) fortification of foods to improve the overall quality of diet (Das et al., 2013); 2) dietary diversification that involves an increased supply and consumption of micronutrient rich foods (Villar, 2015); 3) dietary supplementation to be taken as pills, capsules or liquids (Dickinson et al., 2012); and 4) biofortification, that involves systematic plant breeding schemes coordinated by the HarvestPlus project (Pfeiffer, 2010). Remarkable successes with these approaches have been registered in some communities. For instance, it was observed that supplementation with vitamin A could reduce child mortality by up to 30% (Mora et al., 2008). However, vitamin A supplementation and food fortification programmes are difficult to sustain in rural communities of developing countries due to limited and/or dysfunctional social infrastructures. Therefore, of all the above approaches, biofortification has proven to be the most impactful, as it easily penetrates to the hard-to-reach grassroots communities where social infrastructures are often under developed and poverty levels very high.
Cassava (Manihot esculenta Crantz) is an important staple food for more than 800 million people worldwide (Howeler et al., 2013) and over 500 million sub-Saharan Africa. The crop is a major source of starch (Sánchez et al., 2009) and the roots can be consumed fresh or processed into several composite food products. Cassava is particularly important for food security because it can be harvested throughout the year, grows well with minimum input, it's easily propagated using stem cuttings and adaptes well to a number of harsh environments (Ogola and Mathews, 2011; Burns et al., 2012). As such, global cassava production has more than doubled in the past 40 years from 118 million to over 250 million tons (FAO, 2015), with an annual increment of about 10% (Howeler et al., 2013). Therefore, biofortification of cassava for enrichment with provitamin A should have a widespread nutritional impact on vitamin A intake.
Based on this premise, the National Root Crops Programme of Uganda initiated a breeding objective focusing on genetic improvement of cassava for β-carotene (provitamin A). It is envisioned that deployment of such carotenoids-rich cassava varieties can help alleviate dietary Vitamin A deficiency. However, prior to deployment of cassava varieties for either food and/or industry, an understanding of starch yield, composition and quality is important. It suffices t note that starch has a wide range of applications in confectionary industry; however, its rational use depends on its inherent physicochemical and functional properties (Nuwamanya et al., 2011). For instance, amylose content influences starch resistance to digestion (Faulks, 2003) while the starch pasting properties influence the amount of energy required to process starch, stability of the starch during processing as well as the stability of the products (Crosbie and Ross, 2007; Raphael et al., 2011). Therefore, this study was undertaken to determine the yield, composition and properties of the starch from improved provitamin A cassava breeding clones in Uganda. Information generated would aid the deployment of provitamin A cassava in the flourishing food and/or feed industry.
2. Materials and methods
2.1. Plant materials and evaluation site
A total of 37 improved pro-vitamin A cassava clones were selected for this study; of these, 26 clones had varying root pigmentation between cream and deep yellow, while 11 clones had white-fleshed roots. The clones were established at National Crops Resources Research Institute (NaCRRI), Namulonge. The trial was laid out in in a randomized complete block design (RCBD) trial with three replications. NaCRRI is located within the Lake Victoria crescent at 32°37′36.0″E and 0°31′13.7″N, 1134 m above sea level.
Each plot comprised of 12 plants established at a spacing of 1 m × 1m. Blocks were separated by 2 meter alleys to limit inter-plot interference. Standard agronomic practices were followed and plants were harvested for analyses at 12 months after planting (MAP). At harvest, 6 plants were sampled in each plot and all roots on the plant were picked from each plant and assessed for pigmentation of the parenchyma, using the qualitative 1–6 colour standard scale developed by the International Center for Tropical Agriculture (CIAT), where 1 = white, 2 = light cream, 3 = cream, 4 = light yellow, 5 = yellow, 6 = deep yellow. After pigmentation assessment, at least 8 mature roots were selected from each plot, labelled, placed on dry ice and transported immediately to the laboratory, to avoid loss of moisture and subsequent changes in starch yield.
2.2. Quantification of total carotenoids
Total carotenoids content (TCC) was analysed on fresh samples using iCheckTM carotene kit developed by BioAnalyt Laboratory (http://www.bioanalyt.com). Procedures used from previous studies (Esuma et al., 2016) were adopted for TCC quantification. Briefly, three whole roots from each plot were sampled, peeled, washed under running water, wiped with a paper towel and cut longitudinally into four quarters. Opposite quarters were selected from each root, pooled and chopped into small pieces. About 5 g of the homogenous root sample was pounded and ground into a smooth and fine paste using a mortar and pestle. To aid sample grinding, 20 ml of distilled water were added gradually, and the resulting solution transferred into a 50 ml calibrated tube. Consequently, tube contents were shaken thoroughly and 0.4 ml of the homogenized solution injected into the iExTM CAROTENE vial using a syringe. Thereafter, vials were placed on a solid surface for approximately five minutes, shaken again and allowed to stand until two solution phases were evident inside the vial (a distinct upper phase and a turbid lower phase). At this point, absorbance of the vial content (the upper solution phase) was measured at 450 nm using the iCheckTM CAROTENE device. TCC was calculated as:
where Vs = volume of solution transferred to the 50 ml calibrated tube, Ws = weight of sample (in this case 5 g), and A = absorbance of the iExTM CAROTENE vial content at a wavelength of 450 nm. TCC analysis was conducted in a dark room to minimize losses resulting from oxidation due to exposure to light.
2.3. Quantification of starch yield
Starch was extracted according to Nuwamanya et al. (2010b). Four tubers from each plot were peeled, washed under running water and cut longitudinally into halves. Halves from each root were heaped together and chopped into small cubes, using a knife. About 200 g of the cubes were weighed off, mixed in 200–500 mL of 1 M sodium chloride and homogenized using a blender (Waring, New Hartford, USA). The mixture was stirred and filtered using a triple cheese (muslin) cloth. The filtrate was allowed to stand for one hour to facilitate starch sedimentation and the top liquid decanted and discarded. Starch was then washed in 200 ml of distilled water, followed by centrifugation at 3,000 g for 10 min. Finally, the starch was air-dried at 55 °C on aluminum plates in an air-forced oven. Dry weights were taken for the extracted starch samples using an electronic weighing scale (GraviTekTM, China). Thereafter, starch yield (%) was computed as a ration of dry starch weight to fresh root sample weight and multiplied by 100. The dried starch was stored in air-tight containers for subsequent analyses.
2.4. Determination of starch properties
2.4.1. Amylose content
Amylose content was estimated according to the protocol described by Nuwamanya et al. (2015). To remove impurities, 100 mg of starch (extracted in 2.3 above) was dispersed into 1 ml of hot ethanol. After 2 minutes, ethanol was decanted and starch gelatinized by heating in 5 ml of 0.1 M sodium hydroxide in a water bath at 80 °C for 30 minutes. A 0.1 ml aliquot was then obtained from the gelatinized solution and treated with an equal volume of 0.1 M acetic acid. This was followed by dilution with 4.6 ml of distilled water and staining with 0.2 ml of 10 % iodine/KI solution. Absorbance of the resultant stained solution was read in a spectrophotometer at 620 nm to determine the concentration of amylose. Percentage amylose content was determined using an amylose standard from Sigma Aldrich.
2.4.2. Water binding capacity
Water binding capacity was determined using the method described by Nuwamanya et al. (2011). Briefly, an aqueous starch suspension was made by dissolving 1 g of starch in 20 ml of water. This was agitated for 1 hour on an orbital shaker and centrifuged for 10 min at 2200 rpm. The free water was decanted and wet starch drained for 10 min and weighed on an electronic weighing scale (GraviTekTM, China). Water bound to starch was obtained in grams as the difference between weight of wet and dry starch.
2.4.3. Starch pasting properties
Starch pasting properties were determined using a Rapid Visco Analyser (RVA-4500, Perten Instruments, Australia) equipped with Thermocline for Windows Software, using Standard profile 1. Three grams of starch (extracted in 2.3 above) were weighed into RVA canister and a volume equal to 25 g of distilled water added. The RVA test profile was held constant at 50 °C, 960 rpm mixing speed for 10 seconds. The mixing speed was decreased to 160 rpm and the temperature held at 50 °C for an extra 50 seconds. The temperature was steadily increased to 95 °C within 4 minutes and held constant at 95 °C for 2.5 min and steadily lowered back to 50 °C in 3 min and 48 seconds. The final viscosity was recorded after 13 minutes.
2.5. Statistical data analysis
All data generated were analyzed using Minitab software (version 16, © 2010 Minitab Inc. State College, Pennsylvania) to determine extent of variations in root quality attributes of provitamin A clones, and whether or not clone differences exist. Normality of data were tested using the Anderson-Darling test. For normally distributed variables (P-values > 0.05), means were obtained for each of the clones and analysis of variance (ANOVA) used to assess differences among the clones at 5% level of significance. For non-normally distributed variables (P-values ≤ 0.05), Kruskal-Wallis test was used to separate the medians and differences among the clones were tested at 5% level of significance. Relationships among different root attributes were analyzed using Pearson correlation and significance of the correlation was determined at 5%.
3. Results and discussions
This study was undertaken to determine starch yield, composition and properties of starches from improved provitamin A cassava in Uganda. This was envisioned to generate information on effect of carotenoid biofortification on other important root quality traits. This would in turn guide strategic deployment of provitamin A cassava in the feed and/or food industry. Keenly, this study focused on five root quality taits: total carotenoid content, starch yield, amylose content, water binding capacity and pasting properties.
3.1. Total carotenoids content
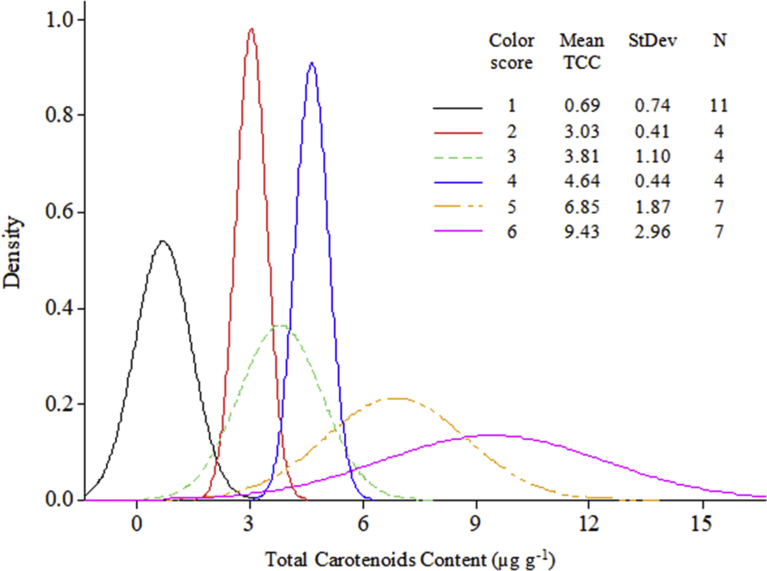
Significant increments in TCC were observed with increase in intensity of root parenchyma pigmentation. White-root clones had the least amounts of TCC in roots (mean = 0.69 μg g-1) while deep yellow roots had the highest mean carotenoids content (9.43 μg g-1) (Fig. 1). Roots with colour scores 2 (light cream), 3 (cream) and 4 (light yellow) did not vary significantly in total carotenoids content (P < 0.05). Generally, TCC in white-fleshed clones ranged from 0.03-2.36 μg g-1 of the fresh root. In the provitamin A clones, TCC ranged from 2.72-3.63 μg g-1 in the light cream clones; 2.17–4.48 μg g-1 in the cream clones; 4.05–5.11 μg g-1 in the light yellow clones; 3.30–8.83 μg g-1 in the yellow clones and 3.41–11.94 μg g-1 in the deep yellow clones (Supplementary Table 1). A strong positive correlation (r = 0.87, P < 0.001) was observed between root parenchyma pigmentation and TCC. This implies that most of the yellow pigmentation in cassava is due to carotenoids content.
Fig. 1.
Variation in total carotenoids in the test clones comprising of both white-fleshed and provitamin A clones. Mean TCC = total carotenoids content; StDev = standard deviation; N = number of colnes analysed; colour scores 1 = white; 2 = light cream; 3 = cream; 4 = light yellow; 5 = yellow; and 6 = deep yellow.
3.2. Relationship between carotenoids content and starch yield
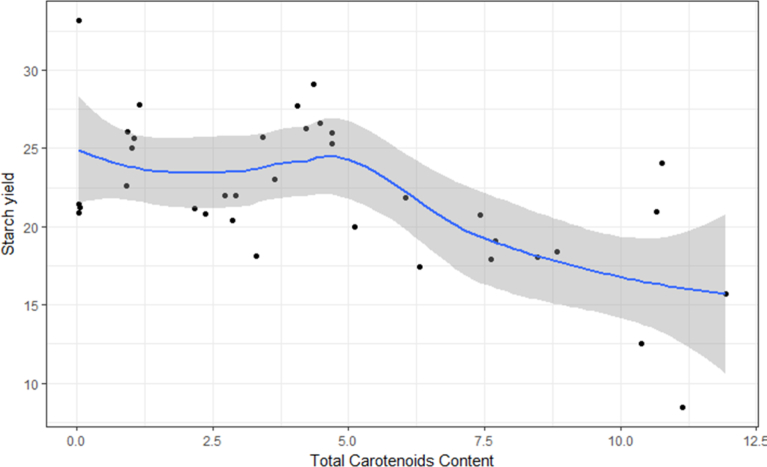
Starch yield ranged from 8.4 - 33.2% and varied significantly (P ≤ 0.05) between clones; with a general reduction in mean starch yield of clones that exhibited total carotenoids >5 μg g-1 (Fig. 2). Certainly, both starch and TCC biosynthesis pathways require substantial energy. It is thus very likely that this negative association could have resulted from the higher utilization of glucose for carotenoid synthesis. In addition, synthesis of phytoene, a carotenoid precursor in plants, requires isopentenyl pyrophosphate (IPP). The IPP is synthesised either from acetyl-CoA or from pyruvate and glyceraldehyde-3-phosphate (Cunningham and Gantt, 1998), all of which are obtained from metabolism of glucose. The increased utilization of glucose for carotenoid synthesis could impact on the amount of glucose available for starch synthesis. However, two clones (UG10F2P479 and UG10F2P622, Supplementary Table 1) had high carotenoids content (>10 μg g-1) and retained high starch yields (>20%; Fig. 2) and such clones would be of interest for further evaluation for purposes of deployment to farmers.
Fig. 2.
Variation in starch yield (%) with total carotenoids content (μg g−1) in the test provitamin A cassava clones.
3.3. Relationship between carotenoids content and starch properties
3.3.1. Amylose content
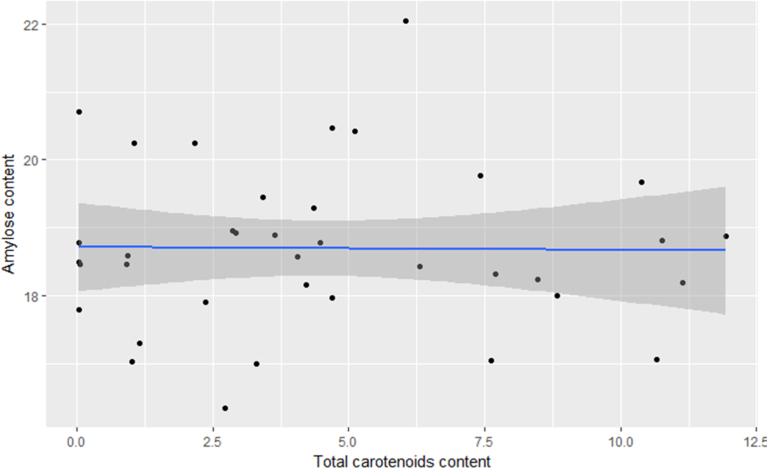
Amylose content ranged from 16.4 - 22.1% in the tested clones (Fig. 3). Non-significant variations were observed in amylose contents of clones at different pigmentation levels. Therefore, any changes in cassava starch composition in terms of amylose content may not be attributed to carotenoids biofortification. The observed range of amylose content is comparable to that of 17–20% and 19–25% reported by Nuwamanya et al. (2010a,b), respectively, in white-flesh cassava varieties and their progenies in Uganda. Elevated levels of amylose (i.e., >25%) tend to be resistant to digestion and thus offer some health benefits to humans. On the other hand, low levels of amylose (i.e., <10%) open up new vistas for cassava's utility in food industry. These are key motivating factors for undertaking routine amylose content quantification in elite cassava breeding clones.
Fig. 3.
Variation in starch amylose content (%) with total carotenoids content (μg g−1) in the test provitamin A cassava clones.
3.3.2. Water binding capacity
Significant differences (P ≤ 0.05) for water binding capacity were observed among the different cassava clones (Table 1). It was generally observed that clones of pigmentation score 3, had starch characterized by lower water binding capacities. However, correlation between TCC and water binding capacity was not significant at 5% (Table 2). This suggests that water binding capacity of starch is not affected by carotenoids content. Water binding capacity depicts the ability of starch to absorb water and hold it even after treatment with external forces (Laufenberg and Schulze, 2009). During food processing, binding of water ensures hydration and increases firmness of dough. Results from this study indicate that carotenoids biofortification may not affect water-binding ability of starch and hence, may not influence firmness of dough during processing.
Table 1.
Variation in water binding capacity and starch pasting properties amongst provitamin A cassava clones.
| Colour score | TCCs (μg g−1) | WBCt (g/g) | Pku (cP) | TRv (cP) | BDw (cP) | Fvx (cP) |
|---|---|---|---|---|---|---|
| 1 | 0.92 | 1.11 ± 0.11a | 7038.1 ± 1032.3a | 2460.9 ± 185.5a | 4577.2 ± 860.7a | 3408.1 ± 280.4a |
| 2 | 2.89 | 1.19 ± 0.01a | 6736.6 ± 138.9a | 2235.1 ± 101.5bc | 4501.5 ± 38.0a | 3108.5 ± 218.8b |
| 3 | 4.29 | 0.94 ± 0.09b | 6215.0 ± 1130.6a | 2048.1 ± 140.1c | 4166.9 ± 1171.9a | 2666.1 ± 133.2c |
| 4 | 4.70 | 1.09 ± 0.03a | 6298.0 ± 654.1a | 2336.3 ± 252.8ab | 3961.8 ± 410.5a | 3232.0 ± 361.1ab |
| 5 | 7.42 | 1.09 ± 0.10a | 6886.9 ± 504.8a | 2367.3 ± 161.6ab | 4519.6 ± 469.0a | 3143.1 ± 214.7b |
| 6 | 10.66 | 1.10 ± 0.07a | 7061.9 ± 492.3a | 2500.1 ± 121.4a | 4561.7 ± 463.4a | 3293.3 ± 132.1ab |
| LSDy | 0.10 | 927.7 | 199.6 | 818.5 | 283.4 | |
| CVz (%) | 7.9 | 11.4 | 7.1 | 15.5 | 7.4 | |
Table 2.
Pearson correlation coefficients among the various starch attributes.
| CSa | SYb | TCCc | PKd | TRe | BDf | FVg | SBh | PTi | PTej | PAk | WBCl | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SYb | -0.518 | |||||||||||
| 0.001 | ||||||||||||
| TCCc | 0.894 | -0.588 | ||||||||||
| 0.000 | 0.000 | |||||||||||
| PKd | -0.008 | -0.455 | -0.049 | |||||||||
| 0.963 | 0.005 | 0.773 | ||||||||||
| TRe | 0.076 | -0.379 | 0.040 | 0.650 | ||||||||
| 0.654 | 0.022 | 0.814 | 0.000 | |||||||||
| BDf | -0.033 | -0.417 | -0.070 | 0.972 | 0.451 | |||||||
| 0.846 | 0.011 | 0.681 | 0.000 | 0.005 | ||||||||
| FVg | -0.136 | -0.181 | -0.117 | 0.566 | 0.865 | 0.396 | ||||||
| 0.424 | 0.291 | 0.490 | 0.000 | 0.000 | 0.015 | |||||||
| SBh | -0.347 | 0.141 | -0.267 | 0.243 | 0.360 | 0.173 | 0.780 | |||||
| 0.035 | 0.412 | 0.110 | 0.148 | 0.028 | 0.307 | 0.000 | ||||||
| PTi | -0.474 | 0.260 | -0.339 | -0.078 | 0.082 | -0.117 | 0.334 | 0.518 | ||||
| 0.003 | 0.125 | 0.040 | 0.646 | 0.629 | 0.489 | 0.043 | 0.001 | |||||
| PTej | -0.441 | 0.618 | -0.480 | -0.419 | -0.247 | -0.415 | -0.025 | 0.261 | 0.524 | |||
| 0.006 | 0.000 | 0.003 | 0.010 | 0.141 | 0.011 | 0.882 | 0.119 | 0.001 | ||||
| PAk | -0.160 | -0.247 | -0.254 | 0.876 | 0.692 | 0.813 | 0.663 | 0.369 | 0.042 | -0.247 | ||
| 0.343 | 0.146 | 0.129 | 0.000 | 0.000 | 0.000 | 0.000 | 0.025 | 0.805 | 0.141 | |||
| WBCl | -0.109 | -0.075 | -0.065 | 0.376 | 0.230 | 0.369 | 0.364 | 0.389 | 0.218 | -0.137 | 0.415 | |
| 0.521 | 0.665 | 0.701 | 0.022 | 0.171 | 0.025 | 0.027 | 0.017 | 0.195 | 0.419 | 0.011 | ||
| Amm | 0.082 | 0.147 | -0.001 | -0.116 | 0.140 | -0.180 | 0.152 | 0.108 | -0.017 | 0.005 | -0.106 | -0.048 |
| 0.630 | 0.393 | 0.994 | 0.495 | 0.407 | 0.287 | 0.369 | 0.526 | 0.919 | 0.977 | 0.531 | 0.777 |
Significant correlations of importance are presented in bold. aColour score, bStarch yield, cTotal carotenoids content, dPeak viscosity, eTrough viscosity, fBreakdown viscosity, gFinal viscosity, hSetback viscosity, iPeak time, jPasting temperature, kPeak area, lWater binding capacity and mAmylose content.
3.3.3. Starch pasting properties
Pasting properties of starch are of particular importance in the food industry because they determine the most appropriate utility of starches by the industry. The properties of importance for this study were the pasting temperature, peak time, viscosity at peak, breakdown, trough and setback, final viscosity and the peak area.
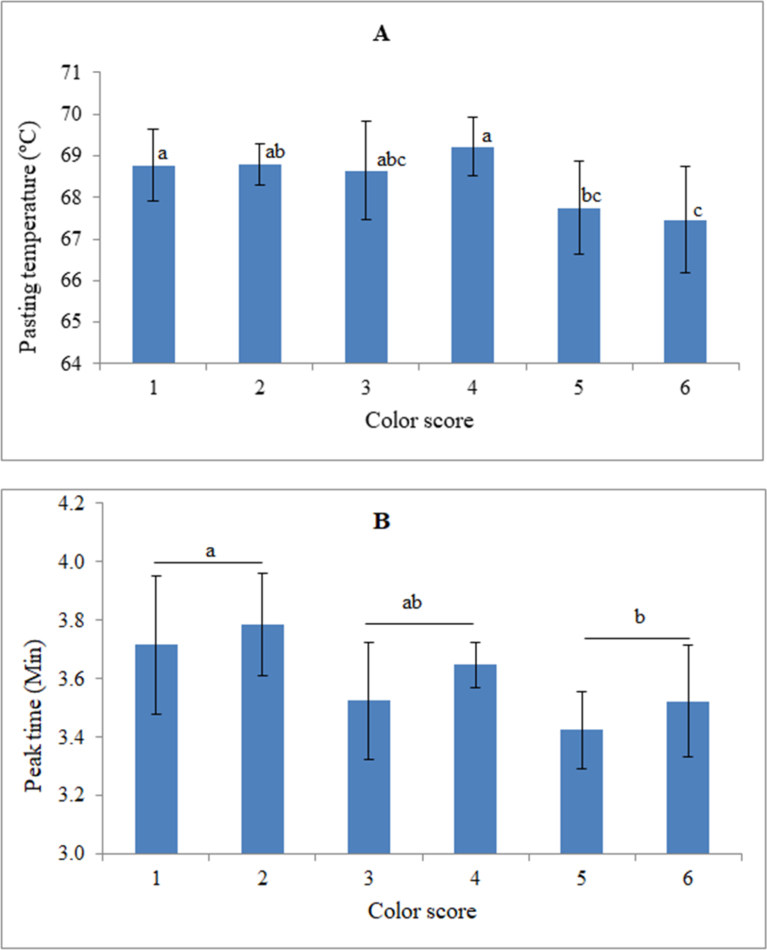
Pasting temperature of starches for clones used in this study ranged from 65.75-70.10 °C, with highly provitamin A clones (color score 5 and 6) exhibiting significantly lower pasting temperatures than the rest of the clones (Fig. 4A). A significant negative correlation was also observed between total carotenoids content and pasting temperature (r = -0.48, P = 0.003) (Table 2). The low pasting temperatures imply that the starch crystals from carotenoids-rich cassava reached maximum swelling much earlier than those from white-fleshed clones. This finding implies that high carotenoids-rich cassava easily form pastes. This property would qualify pro-vitamin A cassava clones for the food industry, as they would be amenable to processing due to low energy requirements. The reduced pasting temperatures in clones with high total carotenoids content may be attributed to deposition of phosphate monoesters in starch. Phosphate monoesters were earlier reported to be deposited in starch (Eliasson, 2004) during carotenoids biosynthesis and have been linked to reduction in gelatinization temperatures of sweet potato starches (Jane et al., 1999). Starch phosphorylation is dependent on various enzymes especially glucan dikinase and phosphoglucan dikinase (Eliasson, 2004; Ritte et al., 2006), which are activated during carotene biosynthesis.
Fig. 4.
Variation in pasting properties of different provitamin A cassava clones, during the RVA heating phase. A = pasting temperature versus provitamin A colour score; B = peak time versus provitamin A colour score. Scores 1 = white; 2 = light cream; 3 = cream; 4 = light yellow; 5 = yellow; and 6 = deep yellow. Different superscripts on each column chart indicate significant differences between means (P ≤ 0.05).
From the observations in amylose content (Fig. 3, Table 2), no significant relationship was noted between amylose content and TCC (r = 0.001, P = 0.994). Therefore, any differences in starch physio-chemistry brought about by carotenoids may be attributed to change in structure and not composition. The pasting temperature is strongly related to the ability of starch granules to take up water (Crosbie and Ross, 2007). It is also indicative of the minimum temperature required to cook or process a sample. This would imply that cassava clones with high TCC may require less cooking and processing time than the white-fleshed varieties. These attributes increase the competitiveness of provitamin A cassava.
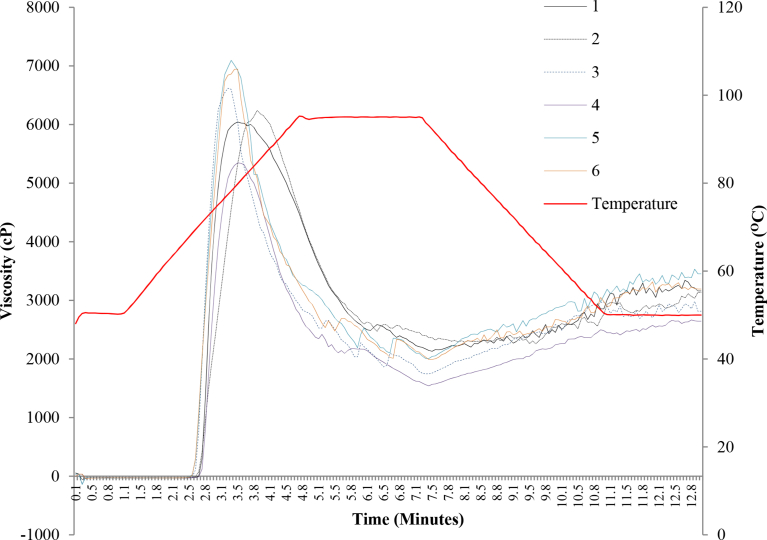
Another attribute closely related to the pasting properties of starch is the peak time, which is the time taken to attain maximum swelling of starch granules. Peak time of starches from the different cassava clones ranged from 3.3 - 4.1 minutes (Fig. 4B) and varied significantly (P ≤ 0.05) among clones of different pigmentations. Peak times of the starches generally reduced with increase in carotenoids content (Figs. 4B and 5B). Similarly, a significant negative correlation was observed between peak time and carotenoids content (r = -0.40, P = 0.040) (Table 2). As discussed above, the reduction in peak time would imply that carotenoids-rich cassava may require less processing time than the white-fleshed cassava and hence requiring less energy for processing into food.
Fig. 5.
General pasting curves of selected provitamin A cassava clones. Scores 1 = white; 2 = light cream; 3 = cream; 4 = light yellow; 5 = yellow; and 6 = deep yellow.
Peak viscosity, defined as the highest viscosity attained by starch slurry during heating, ranged from 5259 - 8346 cP in the white-fleshed root clones and 4750–7647 cP in the provitamin A clones. Non-significant differences were observed among the peak viscosities of the clones at varying levels of pigmentation (Table 1). Peak viscosity may be associated with starch crystalline structure and amylopectin branch chain distribution. During gelatinization, crystallinity of starch is lost and amylose leaches out of amylopectin, hence leading to increase in viscosity. High peak viscosities were earlier attributed to weak granular structure of starch (Raphael et al., 2011) that leads to easy leaching of amylose. These findings imply that carotenoids biofortification of cassava may not affect the starch granular structure.
Trough viscosity, which is the lowest viscosity obtained during the cooling phase, ranged from 1909-2662 cP (Table 1). Correlations between trough viscosity and carotenoids content were non-significant. Trough viscosity is dependent on the total starch available during pasting and presence of compounds such as soluble amylose and proteins in the starch (Raphael et al., 2011). This should imply that carotenoids biofortification may not affect the availability of starch or compounds in the starch during pasting.
Meanwhile, breakdown viscosity ranged from 3116-5761 cP in the white-fleshed root clones and 2679–5529 cP in the provitamin A clones. Breakdown viscosity reflects the ability of the starch to withstand shear stress while holding at maximum temperature, hence making it a vital parameter in food industry. Starches with high breakdown viscosities are therefore inferior in industry (Raphael et al., 2011) due to the rapid fall in their viscosities under shear. No significant differences were observed between breakdown viscosities of starches from cassava clones at varying levels of pigmentation. This should imply that carotenoids biofortification does not affect the ability of starches to withstand shear during processing.
Final viscosities; generally referred to as cold paste viscosities (Crosbie and Ross, 2007) ranged from 2829-3734 cP in starches from white-fleshed clones (Supplementary Table 1), with a mean final viscosity of 3408.1 cP (Table 1). Meanwhile, final viscosities of starches from pro-vitamin A clones ranged from 2485-3509 cP (Supplementary Table 1). The cold paste viscosities were significantly lower in pro-vitamin A clones with score 3, while starches from white-fleshed clones had the highest final viscosities (Table 1).
Increase in viscosity after cooling has been attributed to retrogradation and incomplete thermal mixing of the viscous test sample (Crosbie and Ross, 2007). Retrogradation is linked to realignment of amylose and amylopectin chains after heat-cool phases (Wang et al., 2015) and is an irreversible phenomenon. Findings from this study imply that cooked pro-vitamin A cassava roots may have lower retrogradation percentages than white-fleshed cassava roots. This would make carotenoids-rich cassava better suited for making foods with soft texture such as confectionery products and baby foods. Indeed, this would be advantageous for making provitamin A composite foods for children to aid alleviate vitamin A deficiency in children, a prevalent problem in most communities that primarily depend on cassava.
Values for TCC (non-normally distributed) are presented as medians for each colour score category. Values for other attributes are presented as Mean ± standard deviation. Values with the same superscript in a column are not significantly different (P = 0.05). cP = centipoise, sTotal carotenoids Content, tWater binding capacity, uPeak viscosity, vTrough viscosity, wBreakdown viscosity, xFinal viscosity, yLeast significant difference, zCoefficient of variation.
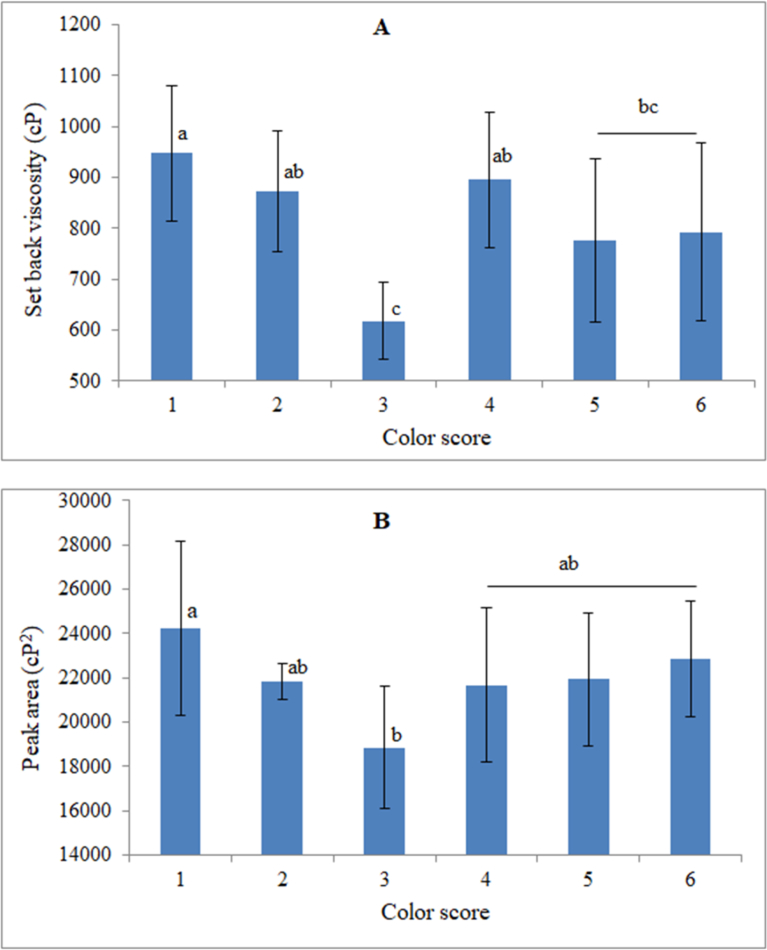
Another vital property of starch is the setback viscosity, measured as the difference between the final and the trough viscosity. During setback, there is re-crystallization of starch granules after the heating stages. The setback viscosity varied significantly (P ≤ 0.05) among cassava clones. Indeed, setback viscosities ranged from 685 to 1106 cP in the white-fleshed clones and from 534 to 1010 cP for the pro-vitamin A clones. Even though clones with pigmentation score 3 exhibited the lowest setback viscosities, there was a general reduction in setback viscosities as carotenoids levels increased, with a significant negative correlation registered between setback viscosity and TCC (r = -0.35, P = 0.035). Setback viscosity depends largely on the increase in viscosity of liquids as they are cooled (Crosbie and Ross, 2007) and is a reflection of starch thermodynamic behavior and stability during processing. Low setback viscosities are indicative of stability of starches during processing (Raphael et al., 2011), an important parameter in the food industry. In this regard, starches from carotenoids-rich cassava would be preferred for industrial food processing due to stability of their pastes during processing.
Peak area, another important pasting property of starch defined as the area under the viscosity curve from gelatinization to breakdown was also evaluated. Provitamin A cassava clones had significantly (P ≤ 0.05) lower peak areas than the white-fleshed clones (Fig. 6). Conjointly, reduced pasting temperatures and peak area, would signify that starches from provitamin A cassava are easier to liquefy, hence reducing the overall energy cost for processing.
Fig. 6.
Variation in pasting properties of different cassava clones during the RVA holding and cooling phases. A = Setback viscosity versus colour score; B = Peak area versus colour score. Scores 1 = white; 2 = light cream; 3 = cream; 4 = light yellow; 5 = yellow; and 6 = deep yellow. Different superscripts on each column chart indicate significant differences between means (P ≤ 0.05).
4. Conclusions
From the datasets generated from this study, two conclusions are deducted. First, an increase in TCC in cassava beyond 5 μg g-1 was associated with reduced starch yield. Second, reductions in pasting temperature, peak time, setback viscosity and peak area were associated with higher TCC clones. Overall, this would imply that less energy may be required to process provitamin A cassava, and thus qualifying its use in the food industry. Such interventions could potentially help mitigate vitamin A deficiency that is prevalent in most cassava growing communities. These findings open up new exiting opportunities for cassava biofortification research.
Declarations
Author contribution statement
Robert Sezi Kawuki: Conceived and designed the experiments.
Ephraim Nuwamanya: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Evans Atwijukire: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Joseph Ffuna Hawumba: Performed the experiments.
Yona Baguma: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Enoch Wembabazi: Analyzed and interpreted the data.
Williams Esuma: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by funds from the Programme for Emerging Agricultural Research Leader (PEARL) that was coordinated by Cornell University through the Next Generation Cassava Breeding Project (https://www.nextgencassava.org).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank the support staff at National Crops Resources Research Institute (NaCRRI), Uganda, who helped in sample processing and data generation. Specifically, we thank Rose Agwang, Jane Aol, Michael Ahimbisibwe, Arnold Katungisa and John Robert Oba for their assistance.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Burns A.E., Gleadow R.M., Zacarias A.M., Cuambe C. E. o., Miller R.E., Cavagnaro T.R. Variations in the chemical composition of cassava (Manihot esculenta Crantz) leaves and roots as affected by genotypic and environmental variation. J. Agric. Food Chem. 2012;60(19):4946–4956. doi: 10.1021/jf2047288. [DOI] [PubMed] [Google Scholar]
- Crosbie G.B., Ross A.S. American Association of Cereal Chemists, Inc (AACC); 2007. The RVA Handbook. [Google Scholar]
- Cunningham F., Jr., Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1998;49(1):557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- Das J.K., Salam R.A., Kumar R., Bhutta Z.A. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Syst. Rev. 2013;2(1):67. doi: 10.1186/2046-4053-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A., Bonci L., Boyon N., Franco J.C. Dietitians use and recommend dietary supplements: report of a survey. Nutr. J. 2012;11(1):14. doi: 10.1186/1475-2891-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson A.-C. CRC Press; 2004. Starch in Food: Structure, Function and Applications. [Google Scholar]
- Esuma W., Herselman L., Labuschagne M.T., Ramu P., Lu F., Baguma Y., Kawuki R.S. Genome-wide association mapping of provitamin A carotenoid content in cassava. Euphytica. 2016;212(1):97–110. [Google Scholar]
- FAO . Food Agricultural Organization of the United Nations; 2015. Food Outlook Global Food Markets. [Google Scholar]
- Faulks R.M. Starch: resistant starch. In: Ceballero B., editor. Encyclopedia of Food Science and Nutrition. 2003. pp. 5579–5583. [Google Scholar]
- Howeler R.L., Thomas N., Holst Sanjuán K., Sanjuán K.H., Quirós H., Isebrands J., Corrales González V.G. FAO; Roma (Italia): 2013. Save and Grow: Cassava. A Guide to Sustainable Production Intensification. [Google Scholar]
- Jane J., Chen Y., Lee L., McPherson A., Wong K., Radosavljevic M., Kasemsuwan T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76(5):629–637. [Google Scholar]
- Laufenberg G., Schulze N. Vol. 2. 2009. A modular strategy for processing of fruit and vegetable wastes into value-added products; pp. 286–353. (Handbook of Waste Management and Co-product Recovery in Food Processing). [Google Scholar]
- Mora J.R., Iwata M., von Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 2008;8(9):685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwamanya E., Baguma Y., Atwijukire E., Acheng S., Alicai T. Effect of cassava brown streak disease (CBSD) on cassava (Manihot esculenta Crantz) root storage components, starch quantities and starch quality properties. Int. J. Plant Physiol. Biochem. 2015;7(2):12–22. [Google Scholar]
- Nuwamanya E., Baguma Y., Emmambux N., Rubaihayo P. Crystalline and pasting properties of cassava starch are influenced by its molecular properties. Afr. J. Food Sci. 2010;4(1):008–015. [Google Scholar]
- Nuwamanya E., Baguma Y., Emmambux N., Taylor J., Patrick R. Physicochemical and functional characteristics of cassava starch in Ugandan varieties and their progenies. J. Plant Breed Crop Sci. 2010;2(1):001–011. [Google Scholar]
- Nuwamanya E., Baguma Y., Wembabazi E., Rubaihayo P. A comparative study of the physicochemical properties of starches from root, tuber and cereal crops. Afr. J. Biotechnol. 2011;10(56):12018–12030. [Google Scholar]
- Ogola J., Mathews C. Adaptation of cassava (Manihot esculenta) to the dry environments of Limpopo, South Africa: growth, yield and yield components. Afr. J. Agric. Res. 2011;6(28):6082–6088. [Google Scholar]
- Pfeiffer W.H. Paper Presented at the 19th World Congress of Soil Science. 2010. HarvestPlus: developing and delivering micronutrient-dense crops. [Google Scholar]
- Raphael M., Yona B., Stephen K., Nuwamanya N., Patrick R., Settumba M., Samuel K. Functional properties of starches on the East African market. Afr. J. Food Sci. 2011;5(10):594–602. [Google Scholar]
- Ritte G., Heydenreich M., Mahlow S., Haebel S., Kötting O., Steup M. Phosphorylation of C6-and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett. 2006;580(20):4872–4876. doi: 10.1016/j.febslet.2006.07.085. [DOI] [PubMed] [Google Scholar]
- Sánchez T., Salcedo E., Ceballos H., Dufour D., Mafla G., Morante N., Jaramillo G. Screening of starch quality traits in cassava (Manihot esculenta Crantz) Starch Staerke. 2009;61(1):12–19. [Google Scholar]
- Semba R.D. The role of vitamin A and related retinoids in immune function. Nutr. Rev. 1998;56(1):S38–S48. doi: 10.1111/j.1753-4887.1998.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Villar J.L. TWN; 2015. Tackling Hidden Hunger: Putting Diet Diversification at the Centre. [Google Scholar]
- Wang S., Li C., Copeland L., Niu Q., Wang S. Starch retrogradation: a comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015;14(5):568–585. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2009. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995-2005: WHO Global Database on Vitamin A Deficiency.http://www.who.int/iris/handle/10665/44110 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.