Abstract
Insulin-induced gene 1 (INSIG1) regulates sterol synthesis by mediating the activation of sterol regulatory element–binding protein (SREBP) and the degradation of the HMG-CoA reductase (HMGCR). INSIG1 is up-regulated during HIV-1 infection, but its role in HIV-1 infection is unknown. In this report, using pseudovirus production, protein overexpression, and gene knockouts, we found that INSIG1 inhibits HIV-1 production by accelerating the degradation of the HIV-1 Gag protein. Unlike the degradation of HMGCR via the E3 ubiquitin ligase autocrine motility factor receptor (AMFR), a process that depends on the proteasome, INSIG1 coordinated with another ligase, translocation in renal carcinoma chromosome 8 (TRC8), and promoted Gag degradation through the lysosome pathway. We conclude that INSIG1 functions as a sentinel responsive to HIV-1 production and inhibits HIV-1 replication by degrading Gag, a process occurring at intracellular membrane sites such as the endoplasmic reticulum and endosomes where both INSIG1 and Gag may be located.
Keywords: virus, virology, viral protein, protein degradation, protein expression, insulin-induced gene 1 (INSIG1), HIV-1 production, TRC8, Gag protein, lysosome pathway degradation, ubiquitin ligase, endosome, ring finger protein 139 (RNF139), HIV replication
Introduction
Viruses rely on their cellular hosts to provide energy and building blocks for successful replication. During viral infection, the virus regulates the sterol synthesis as a strategy to facilitate viral replication and viral budding in lipid rafts (1–3). Infection with the human cytomegalovirus markedly increases the flux from the tricarboxylic acid cycle to the fatty acid biosynthesis pathway, and the inhibition of fatty acid biosynthesis suppresses the replication of human cytomegalovirus (4). In the HIV-1 infection, the presence of the HIV-1 accessory protein Nef in infected cells increases the cellular cholesterol content and remodels intracellular cholesterol trafficking. Nef is capable of binding cholesterol and enhancing cholesterol trafficking to lipid rafts where HIV buds. Nef also redistributes the cholesterol transporter ABCA1, causing a sharp inhibition of cholesterol efflux (5, 6).
Two groups of proteins are involved in the control of cytoplasmic cholesterol synthesis. First, the transcription factors sterol regulatory element–binding proteins (SREBPs)3 play critical roles in this process by regulating more than 30 downstream genes needed for the uptake and synthesis of cholesterol and fatty acids (7–9). Second, the critical enzyme 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMGCR) catalyzes the rate-limiting step in the synthesis of cholesterol (10, 11). Both pathways are regulated by the insulin-induced genes insig1 and insig2 (12–14). INSIG1 exceeds INSIG2 ∼50-fold on the expression level in SV-589 cells and plays more critical roles in cholesterol synthesis (15). As a critical cholesterol synthesis inhibitor, INSIG1 inhibits fatty acid synthesis in two ways. First, in the presence of cholesterol, INSIG1 cooperates with sterols to inhibit the release of the SCAP–SREBP complex from the endoplasmic reticulum (ER) to the Golgi, where the SREBP is cleaved and activated. At the same time, INSIG1 interacts with the HMGCR directly and promotes its degradation via the ubiquitin-mediated proteasome pathway (9, 11, 16).
INSIG1, but not INSIG2, is up-regulated in HIV-1 infection (17). In Nef-containing HIV-1 virus-infected T cells, seven cholesterol enzymes are increased, and they contribute to the Nef-mediated cholesterol accumulation within infected cells. Interestingly, despite the general up-regulation of cholesterol synthesis in infected cells, as a cholesterol synthesis inhibitor, INSIG1 is up-regulated in HIV-1 infection (17). Furthermore, INSIG1 is also up-regulated by another HIV-1 accessory protein Vif (18). Thus, the up-regulation of INSIG1 in HIV-1 infection might not be limited to the regulation of cholesterol synthesis. In contrast, INSIG1 might be up-regulated by cell hosts as a strategy to fight against HIV-1 infection. Therefore, new functions of INSIG1 involved in HIV-1 infection should be explored.
In this study, we found that INSIG1 is up-regulated during the process of HIV-1 production, but not in the process of infection. This observation indicates a role played by INSIG1 inside cells rather than on the plasma membrane. We also found that INSIG1 inhibits the replication of HIV-1 by promoting the degradation of the HIV-1 Gag, a process mediated by the E3 ligase TRC8 via the lysosome pathway. The cholesterol content of the cells is not changed significantly during either process. Therefore, a cholesterol-independent monitoring and regulation of HIV-1 production through the INSIG1–TRC8 E3 ligase system has been revealed.
Results
INSIG1 is up-regulated in HIV-1 production
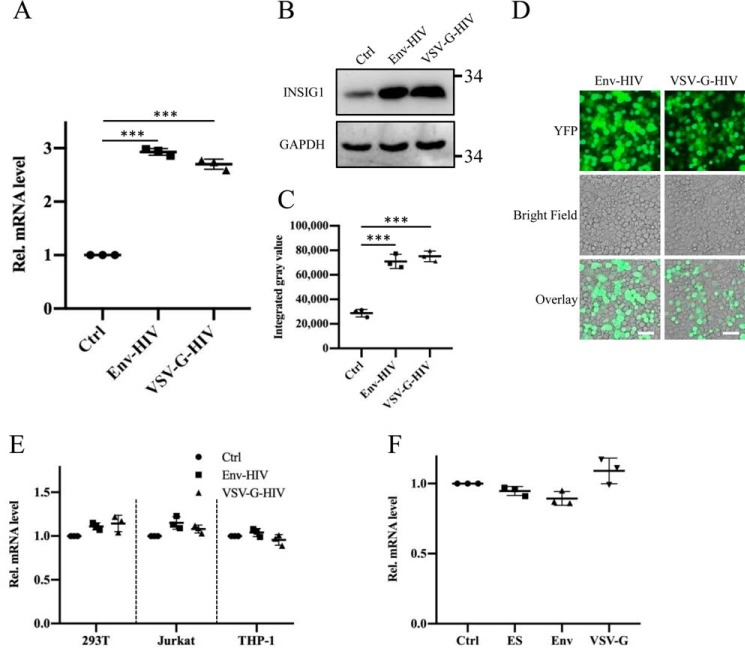
It is known that INSIG1 expression is up-regulated in HIV-1–infected T cells (17), but in which step the INSIG1 is up-regulated during viral replication and the consequence of this up-regulation are not clear. To address this, we used the pseudovirus production system to detect the influence of INSIG1 in the process of HIV-1 production and single-cycle infection. First, in agreement with a previous report (17), using an Env or VSV-G pseudotyped virus production system, we found that INSIG1 was markedly up-regulated in 293T cells during HIV-1 production on both the mRNA and protein levels (Fig. 1, A–C). More than 90% of target cells could be transfected successfully (Fig. 1D). Interestingly, no up-regulation of INSIG1 was observed in a single-cycle infection by the pseudovirus HIV-1 in either 293T or HIV-tropic Jurkat and THP-1 cells (Fig. 1E). The level of INSIG1 was also not changed in a separate expression of HIV Gag-pol, VSV-G, and Env in 293T cells by the transfection of these plasmids individually (Fig. 1F). Therefore, INSIG1 is up-regulated in HIV-1 replication, but this up-regulation depends on the active production of virions within cells rather than a single protein expression. The separate expression of Gag-pol, VSV-G, or Env does not induce INSIG1 up-regulation.
Figure 1.
INSIG1 was up-regulated in HIV-1 production, but not changed in separate expression of HIV Gag-pol, VSV-G, and Env. A, mRNA levels of INSIG1 was up-regulated in both Env and VSV-G pseudotyped HIV-1–producing 293T cells, measured by quantitative RT-PCR. Shown is mean ± S.D. (error bars) from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. B, protein level of INSIG1 in the HIV-producing cells was detected by Western blotting, and it was up-regulated accordingly. C, quantification of INSIG1 in the Western blot analysis in B by ImageJ. The protein levels of INSIG1 were increased by about 2.5 times in the HIV-producing cells. Shown is mean ± S.D. from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. D, the transfection efficiencies of pseudotyped HIV-1 production systems were shown by the fluorescence produced by YFP encoded within NLENY1-ES-IRES. The transfection efficiencies are more than 90% based on YFP-positive cell counting. Scale bars, 50 μm. E, 293T, Jurkat, and THP-1 cells were infected with Env or VSV-G pseudotyped HIV-1, respectively, and the mRNA level of INSIG1 in the infected cells was detected by quantitative RT-PCR. The mRNA level of INSIG1 was not changed by infection of Env or VSV-G pseudotyped HIV-1. F, 293T cells were transfected with the HIV-1 backbone plasmid NLENY1-ES-IRES (ES) and the viral envelope plasmid pSRHS-Env or pVSV-G, respectively, and the mRNA level of INSIG1 in the transfected cells was detected by quantitative RT-PCR. No change in INSIG1 mRNA transcription was observed under these conditions.
Overexpression of INSIG1 inhibits HIV-1 production
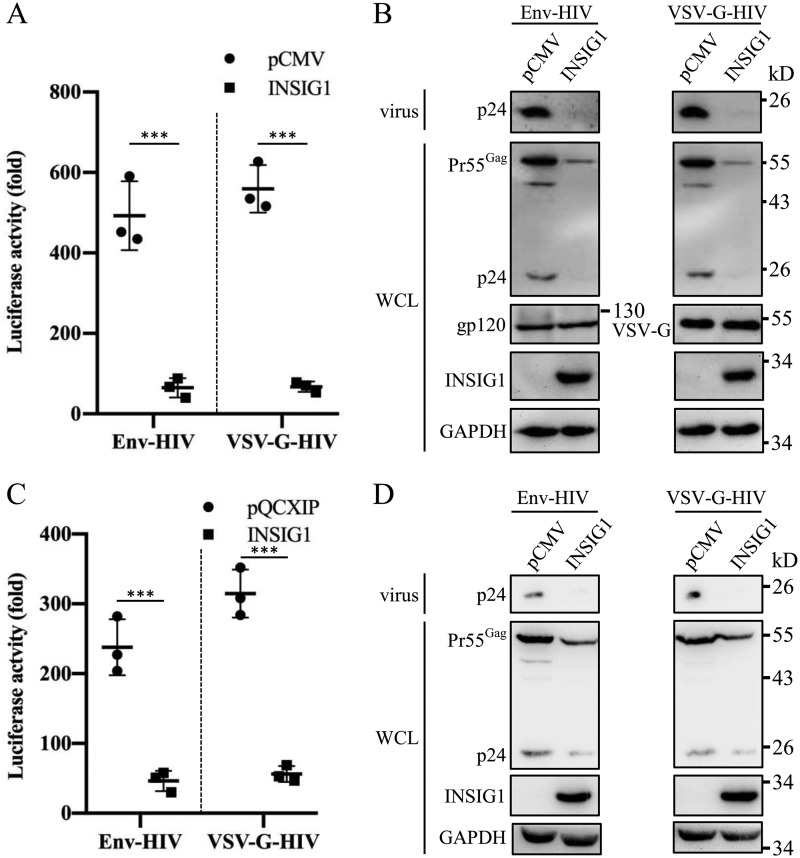
Because the endogenous expression of INSIG1 is responsive to HIV-1 production, we investigated the effect of INSIG1 in HIV-1 production in 293T cells. Compared with the controls, the overexpression of INSIG1 inhibited the production of HIV-1 significantly in both the Env and VSV-G enveloped pseudovirus (Fig. 2A). Western blot analysis showed that in the presence of overexpressed INSIG1, the level of Pr55Gag was significantly reduced within cells, which was accompanied by fewer virions released to the medium. The glycoproteins Env and VSV-G were not affected by INSIG1 overexpression (Fig. 2B).
Figure 2.
The overexpression of INSIG1 inhibited the production of HIV-1 in 293T and Jurkat cells. A, INSIG1 was overexpressed in HIV-1–producing 293T cells, and the supernatant was harvested to infect TZM-bl cells to test the virus titer by luciferase activity assay. Both Env and VSV-G enveloped virus production in 293T were inhibited significantly by INSIG1 expression. Shown is mean ± S.D. (error bars) from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. B, Pr55Gag and Env/VSV-G in the HIV-1–producing cells and p24 in the supernatant in A were detected by Western blotting. Pr55Gag and its cleavage products in cells and p24 in supernatant were significantly reduced by INSIG1. For envelope glycoproteins, both Env and VSV-G were not changed in the INSIG1-overexpressing 293T cells. C, INSIG1 was overexpressed in HIV-1–producing Jurkat cells, and the supernatant was harvested to infect TZM-bl cells to test the virus titer by a luciferase activity assay. Both Env and VSV-G enveloped virus production in Jurkat were inhibited significantly by INSIG1 expression. Mean ± S.D. from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. D, Pr55Gag in the HIV-1–producing cells and p24 in the supernatant in C were detected by Western blotting. Pr55Gag and its cleavage products in cells and p24 in supernatant were significantly reduced by INSIG1.
To confirm these results in HIV-permissive cells, we further investigated the effect of INSIG1 on HIV-1 production in Jurkat cells. Consistent with the results in 293T cells, INSIG1 overexpression significantly inhibited both the Env and VSV-G pseudotyped HIV-1 production and reduced the Pr55Gag level in HIV-1–producing cells (Fig. 2, C and D).
INSIG1 increases the degradation of HIV-1 Gag
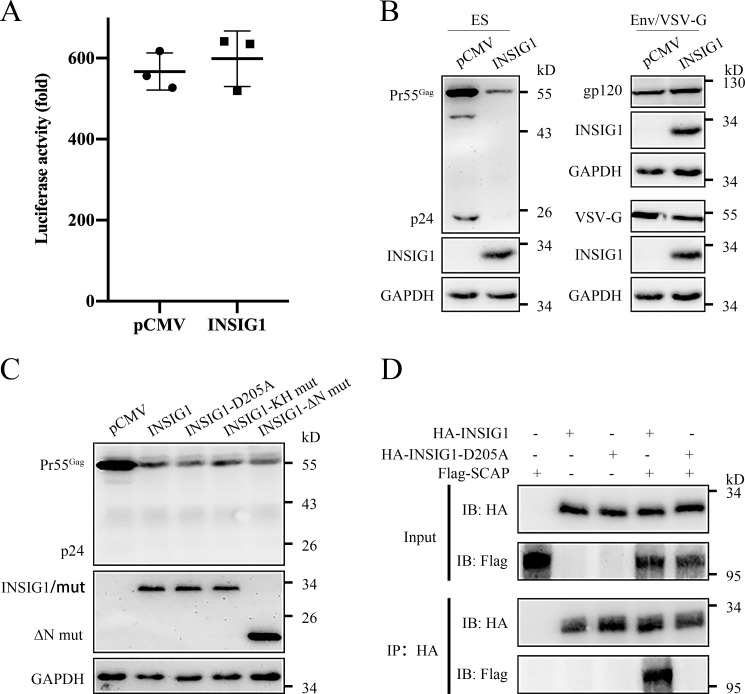
To determine in which step INSIG1 affects HIV-1 production, we investigated whether INSIG1 influences HIV-1 promoter activity. INSIG1 had no obvious effect on HIV-1 LTR promoter activity (Fig. 3A). INSIG1 overexpression reduces the Pr55Gag level in HIV-producing cells, and INSIG1 is well-known to be involved in ubiquitination-mediated protein degradation, including the degradation of HMGCR. These observations reminded us to explore whether INSIG1 is directly involved in HIV-1 Gag degradation, a process independent of HIV-1 assembly. To address this, HIV-1 Gag protein and glycoprotein were expressed separately by the transfection of an NLENY1-ES-IRES (HIV-1 backbone plasmid, encoding all HIV-1 proteins except envelope, abbreviated as ES) or an envelope glycoprotein plasmid (encoding VSV-G or HIV-1 Env) in the presence of INSIG1 in 293T cells. Compared with the control sample, INSIG1 overexpression decreased the protein level of Gag but did not change the protein level of Env and VSV-G (Fig. 3B). This indicates that the effect of INSIG1 on HIV-1 production is Gag-dependent.
Figure 3.
INSIG1 promoted the degradation of HIV-1 Gag. A, INSIG1 had no effect on LTR activity. 293T cells were transfected with LTR-luc (LTR-promoted luciferase expression plasmid), Tat, and β-gal and with or without INSIG1. The measured luciferase activity was normalized by a β-gal assay. Shown is mean ± S.D. (error bars) from three independent experiments. B, expression of ES plasmid (NLENY1-ES-IRES), Env, or VSV-G plasmids, respectively, in the presence of INSIG1 in 293T. The expression of relevant proteins was detected by Western blotting. INSIG1 decreased the protein level of Gag encoded from NLENY1-ES-IRES plasmid but does not change the level of Env and VSV-G. C, pCMV-promoted Gag-pol was expressed in the presence of INSIG1, INSIG1-D205A, INSIG1-KH mut, or INSIG1-ΔN mut in 293T cells. Pr55Gag levels in the transfected cells were detected by Western blotting. WT and three mutant INSIG1s all reduced the protein levels of HIV-1 Gag in 293T cells. D, HA-tagged INSIG1 or INSIG1-D205A was expressed with FLAG-tagged SCAP in 293T cells. 36 h after transfection, the cultures were treated with 5 μg/ml cholesterol for 5 h and harvested, lysed, and immunoprecipitated (IP) with anti-HA mAb to precipitate INSIG1. INSIG1 and SCAP in pellets and supernatants were detected by Western blotting with anti-HA or anti-FLAG antibodies. SCAP was co-immunoprecipitated with WT but not D205A mutant INSIG1.
INSIG1 is a six-transmembrane protein with both N and C termini facing the cytosol (19). INSIG1 has a longer N terminus than INSIG2 (19, 20), and Asp-205 is a critical residue for mediating interaction with the SCAP and HMGCR (21). The C-terminal five residues of INSIG1 (KH motif) form a motif that interacts with the coatomer for COPI-mediated Golgi-to-ER retrieval (22). To address the importance of these structural motifs of INSIG1 in HIV-1 production, we generated the mutation or truncation of these motifs. None of these mutants lost the ability to reduce HIV-1 Gag protein level. This indicates that these motifs are not involved in this HIV-1–related process (Fig. 3C). To determine whether D205A mutation is successful in INSIG1, the mutant was co-immunoprecipitated with SCAP in the presence of cholesterol. As shown in Fig. 3D, SCAP was co-immunoprecipitated with WT but not D205A mutant INSIG1.
Knockout of INSIG1 increases HIV-1 production
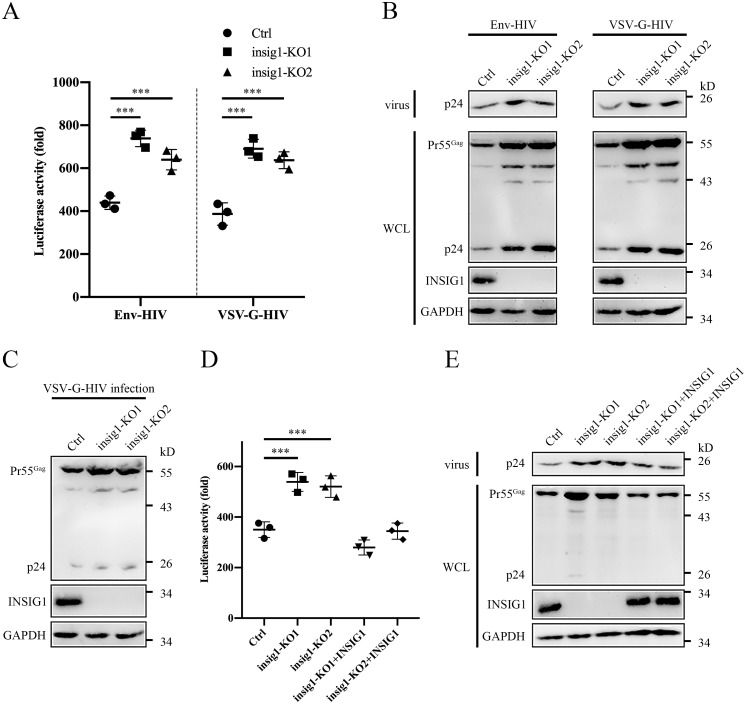
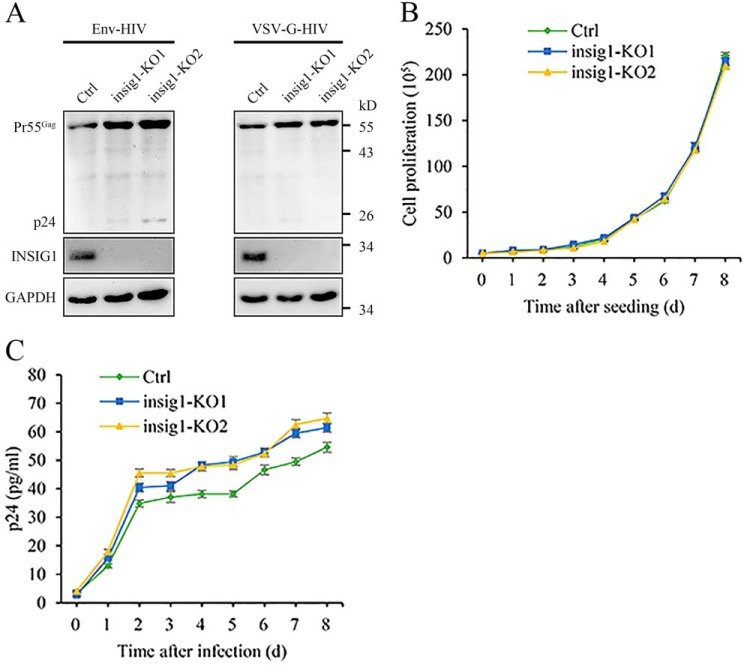
To investigate the influence of endogenous INSIG1 on HIV-1 production, we generated the insig1 knockout 293T cell line by the CRISPR/Cas9 technique. The knockout of insig1 increased HIV-1 production and was associated with the accumulation of Gag at the protein level (Fig. 4, A and B). The protein level of Gag was also increased in the insig1 knockout cells infected by the VSV-G enveloped pseudovirus (Fig. 4C). The reintroduction of INSIG1 with a native promoter and a synonymous encoding sequence in insig1 knockout cells restored the HIV-1 production to WT (Fig. 4, D and E). Therefore, the experiments of both the overexpression and the knockout of INSIG1 support the critical role of INSIG1 in HIV-1 production through the regulation of Gag degradation.
Figure 4.
Knockout of insig1 in 293T increased HIV-1 production. A, Env and VSV-G–pseudotyped HIV-1 was produced in insig1 knockout and control 293T cells. The supernatant was harvested to infect TZM-bl cells to test the virus titer by luciferase activity assay. The HIV-1 production was increased noticeably in insig1 knockout 293T cells. Shown is mean ± S.D. (error bars) from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. B, Pr55Gag in the HIV-1–producing cells and p24 in the supernatant in A were detected by Western blotting. Pr55Gag and its cleavage products in cells and p24 in supernatant were increased accordingly in insig1 knockout 293T cells. C, insig1 knockout and control 293T cells were infected with equal amounts of VSV-G enveloped pseudovirus. Protein level of Pr55Gag was also increased in insig1 knockout cells, as shown by Western blotting. D, INSIG1 with native promoter and synonymous encoding sequence was reintroduced into insig1 knockout cells in the Env pseudotyped HIV-1 production experiment described in A. Reintroduction of INSIG1 restored the inhibition of INSIG1 on HIV-1 production as shown by the luciferase activity assay. Mean ± S.D. from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. E, Western blot analysis of the relevant proteins in D showed that Pr55Gag in the HIV-1–producing cells and p24 in the supernatant were restored by reintroduction of INSIG1 accordingly.
The function of INSIG1 in HIV-1–permissive Jurkat cells
Both the overexpression and the knockout of INSIG1 support the role of this protein in HIV-1 production in 293T cells. To confirm this observation in natural HIV-1–permissive cells further, the insig1 knockout Jurkat cell line was established through the CRISPR/Cas9 technique. As with 293T (Fig. 4), the absence of INSIG1 led to a modest increase of Gag in Jurkat following an infection by Env or VSV-G enveloped pseudovirus (Fig. 5A). The knockout of insig1 had no obvious effect on the growth of Jurkat cells (Fig. 5B). In multiple-round HIV-1 replication using a virus produced from proviral construct pNL4-3, the absence of INSIG1 also increased the amount of virus released to the medium (Fig. 5C). These data further support the role of INSIG1 in HIV-1 replication under physiological conditions.
Figure 5.
Knockout of insig1 in Jurkat increased HIV-1 replication. A, insig1 knockout and control Jurkat cells were infected with equal amounts of Env or VSV-G enveloped pseudovirus. Western blotting detection of infected cells indicated that the protein level of Pr55Gag was increased in insig1 knockout Jurkat cells. B, cell proliferation rate of insig1 knockout Jurkat cells and the control cells. Knockout of insig1 has no obvious effect on the growth of Jurkat cells. The assay was started with 50,000 cells. Data shown are representative of three independent experiments. C, absence of INSIG1 increased the amount of virus released to the medium. insig1 knockout Jurkat cells and the control cells were infected with NL4-3 virus with a titer of 50 pg of p24. Supernatants were harvested at the indicated time, and virus replication was monitored using p24 ELISA. Data shown were representative of three independent experiments.
INSIG1 inhibits HIV-1 production through a TRC8-mediated lysosome pathway
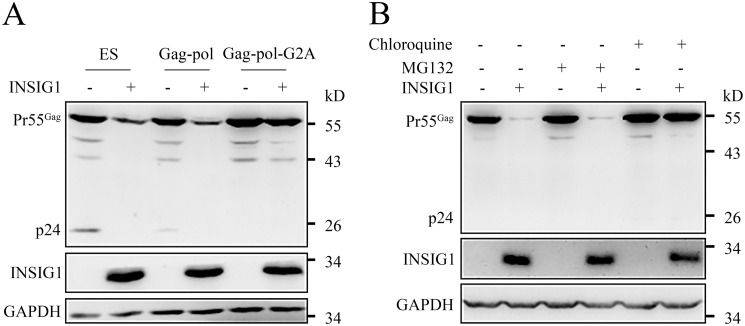
To further interrogate the Gag degradation dependence of INSIG1, we also used the pCMV-promoted Gag-pol expression plasmid (only containing the Gag-pol encoding region, which is short and easy to introduce mutations into, distinguished from the LTR-promoted ES plasmid). A Gag-pol with G2A mutation, which loses its membrane-targeting ability because of the inability to be myristoylated (23), was also tried. As expected, the Gag from pCMV-promoted Gag-pol could still be degraded by INSIG1 (Fig. 6A), in agreement with the observation that INSIG1 has no influence on HIV-1 promoter activity (Fig. 3A). Interestingly, the introduction of the G2A mutation in Gag eliminated this degradation and restored the level of Gag. This indicates that the membrane localization of Gag dependent on Gly-2 myristoylation is necessary for INSIG1-mediated Gag degradation (Fig. 6A).
Figure 6.
The degradation of HIV-1 Gag protein by INSIG1 depended on membrane-targeting ability of Gag and the proper function of cell lysosome. A, 293T cells were transfected with NLENY1-ES-IRES (ES), Gag-pol, or Gag-pol-G2A plasmids, with or without HA-INSIG1. Pr55Gag level in the transfected cells was detected by Western blotting. Pr55Gag in the cells transfected with Gag-pol was also degraded by INSIG1. The introduction of G2A mutation in Gag abolished this degradation and restored the level of Gag. B, 293T cells were co-transfected with INSIG1 and Gag-pol, treated with chloroquine or MG132 at 6 h post-transfection, and cultured for another 24 h for detection. Degradation of Pr55Gag by INSIG1 was inhibited in the presence of chloroquine, but not MG132.
It is known that INSIG1 could form a ubiquitination complex with the E3 ligase autocrine motility factor receptor (AMFR; also named gp78) and regulate the degradation of HMGCR through a proteasome pathway (15, 24, 25). Asp-205 is a juxtamembrane residue critical for the INSIG1 function, the mutation of which will abolish both SCAP–SREBP–mediated and HMGCR-mediated cholesterol regulation. The mutation of Asp-205 did not abrogate the inhibiting capability of INSIG1 in HIV-1 production (Fig. 3C). Accordingly, we suspected that the function of INSIG1 in HIV-1 inhibition might involve other enzymes/pathways. Protein degradation is usually carried out through the proteasome pathway or the lysosome pathway and could be inhibited by the small-molecule chemicals MG132 and chloroquine, respectively (26, 27). To investigate the mechanism of INSIG1 inhibition on HIV-1, MG132 and chloroquine were supplemented in the Gag degradation system. Chloroquine, but not MG132, could efficiently restore the Gag protein level in the presence of INSIG1 (Fig. 6B). Therefore, unlike HMGCR degradation, Gag degradation is controlled by the lysosome system, and another E3 ligase other than AMFR might be involved.
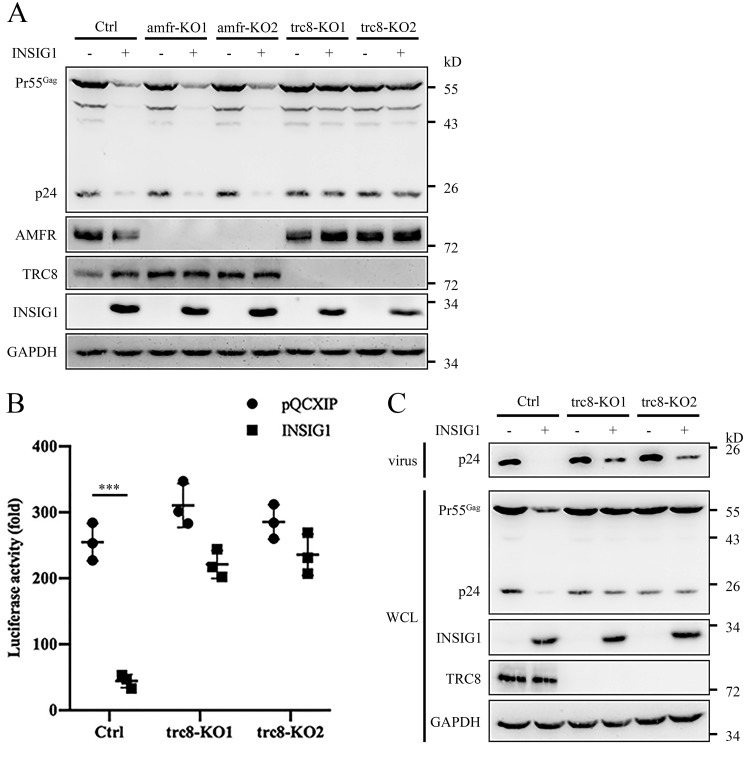
Another E3 ligase, translocation in renal cancer from chromosome 8 (TRC8; also named RNF139), has been reported to interact with INSIG1 and to be involved in the sterol-responsive process (15, 28). Unlike AMFR, which interacts with INSIG1 only, TRC8 could form a complex with both INSIG1 and INSIG2. Considering the partially redundant function of AMFR and TRC8 in the sterol response, we supposed that TRC8 might be the ligase involved in Gag degradation during HIV-1 production. To determine this, the AMFR and TRC8 knockout 293T cells were produced. Indeed, the absence of TRC8, but not AMFR, inhibited the Gag degradation induced by INSIG1 (Fig. 7A).
Figure 7.
INSIG1 inhibited HIV-1 production through the TRC8 pathway. A, amfr knockout, trc8 knockout, and control 293T cells were co-transfected with INSIG1 and Gag-pol plasmids. Pr55Gag level in the transfected cells was detected by Western blotting. Knockout of trc8, but not amfr, restored Pr55Gag level. B, Env pseudotyped HIV-1 was produced in the presence of INSIG1 in trc8 knockout Jurkat cells. After 48 h, the supernatants were harvested to infect TZM-bl cells to test the virus titer by luciferase activity assay. The inhibition of HIV-1 production by INSIG1 was largely hampered in the absence of TRC8. Shown is mean ± S.D. (error bars) from three independent experiments. ***, p < 0.001 compared with the control sample using an independent t test. C, Pr55Gag in the HIV-1–producing cells and p24 in the supernatant in B were detected by Western blotting. The levels of Pr55Gag in cells and p24 in supernatant were largely restored in trc8 knockout cells.
To verify the function of TRC8 in HIV-1 production, a trc8 knockout Jurkat cell line was generated and used to produce pseudotyped HIV-1 in the presence or absence of INSIG1. As expected, the inhibition of HIV-1 production by INSIG1 was largely hampered in the absence of TRC8 in Jurkat cells (Fig. 7, B and C). This observation confirmed that TRC8 is the ligase involved in the inhibition of HIV-1 production by INSIG1.
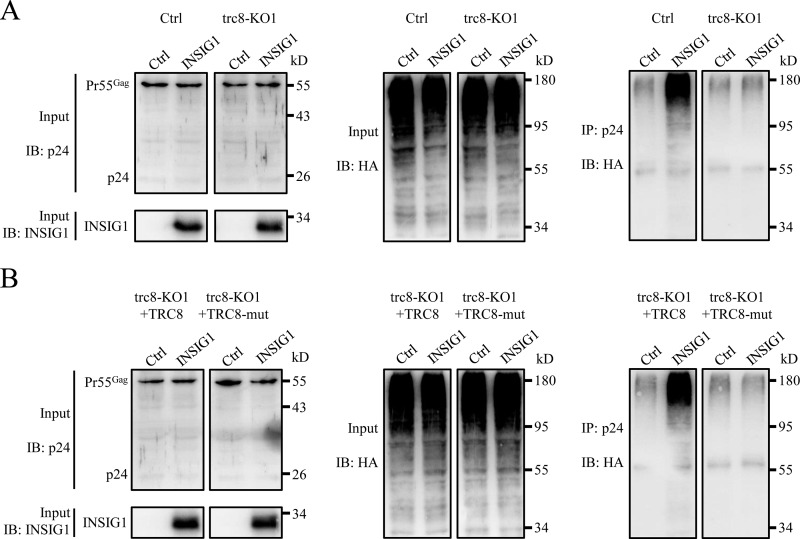
Gag has been reported to be degraded by E3 ligase, including BCA2 (29). The INSIG1–TRC8 ligase complex might degrade Gag in a similar manner. To confirm this, HA-tagged ubiquitin was co-expressed with INSIG1 and NLENY1-ES-IRES in WT or trc8 knockout 293T cells. As shown in Fig. 8A, in the presence of the lysosome inhibitor chloroquine, ubiquitinated Gag could be detected efficiently in the presence of INSIG1. Furthermore, the ubiquitinated Gag proteins was diminished in the trc8 knockout cells, even in the presence of INSIG1 (Fig. 8A). In addition, reintroduction of TRC8, but not its enzymatic defective mutant (TRC8-mut, with 572–574 LRK mutated to AAA), restored the function of INSIG1 on Gag ubiquitination (Fig. 8B). Therefore, INSIG1–TRC8 complex plays a major role in Gag degradation through the lysosome pathway.
Figure 8.
INSIG1 increased the ubiquitination of Gag via TRC8. A, the stable trc8 knockout cell line (trc8-KO1) and control 293T cells were transfected with plasmids of NLENY1-ES-IRES, HA-ubiquitin, together with or without INSIG1. The cells were then treated with chloroquine at 6 h post-transfection and cultured for another 24 h prior to harvesting. Cell lysates were immunoprecipitated with anti-p24 antibody, and corresponding proteins were analyzed by Western blotting. As shown in the right panel, in control cells, but not in trc8 knockout cells, Gag could be more efficiently ubiquitinated in the presence of INSIG1. B, WT TRC8 or its enzymatic defective mutant (TRC8-mut) were supplemented into the experimental system used in A in trc8 knockout cells. Cell lysates were immunoprecipitated with anti-p24 antibody, and corresponding proteins were analyzed by Western blotting. Reintroduction of TRC8, but not its mutant, restored the function of INSIG1 on Gag ubiquitination.
INSIG1 degrades Gag at intracellular membrane sites, including ER and endosomes
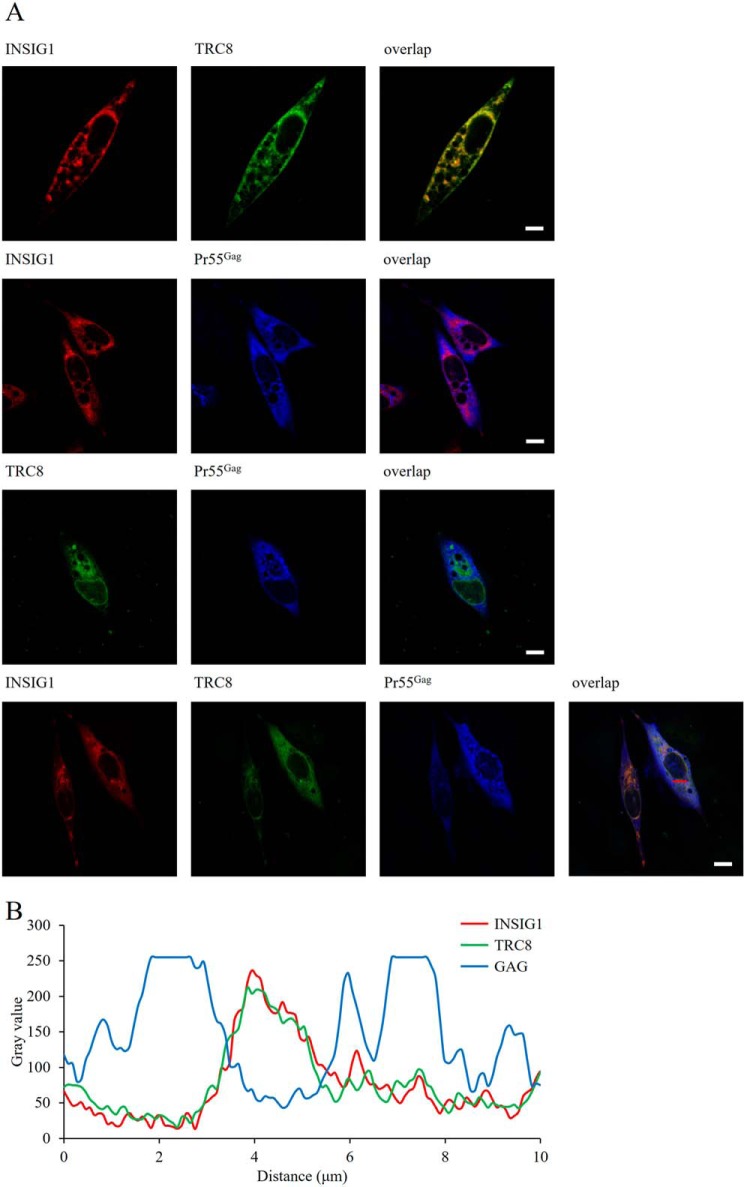
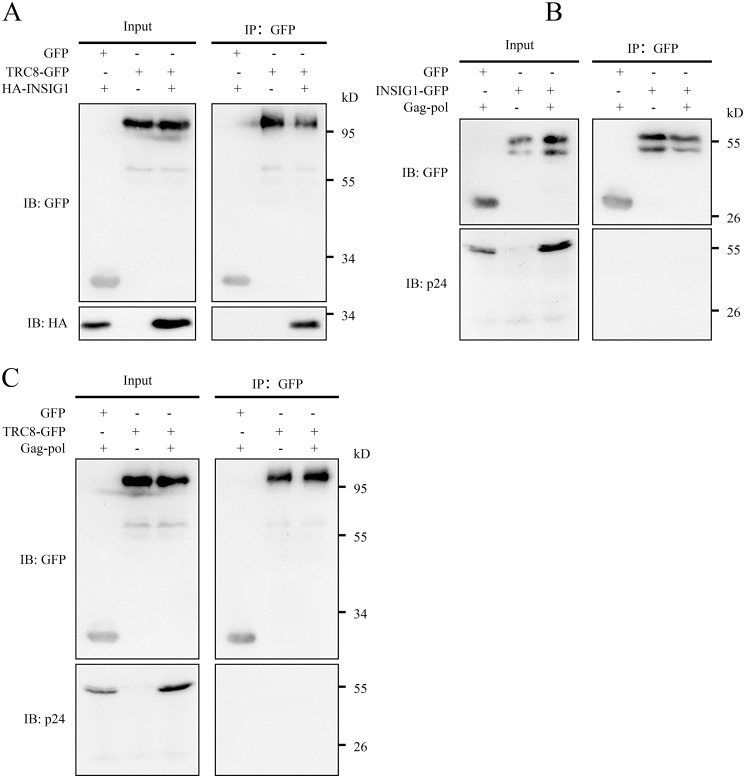
The up-regulation of INSIG1 leads to Gag degradation effectively. INSIG1 and TRC8 are localized on ER membrane and endosomes predominately, and the degradation of Gag is myristoylation-dependent (Fig. 6A). Therefore, we supposed the degradation of Gag by INSIG1–TRC8 should occur at intracellular membrane sites such as ER or endosomes. To determine this, we investigated the co-localization of these proteins by confocal microscopy. As expected, INSIG1 and TRC8 co-localized nicely within the cells. Surprisingly, we could not observe a significant co-localization between INSIG1 and Gag or between TRC8 and Gag (Fig. 9). We also observed that, instead of being co-localized with INSIG1–TRC8, Gag seemed to be located in the sites where INSIG1–TRC8 was absent. Because the fluorescence signal of Gag was very weak when co-expressed with INSIG1–TRC8, the addition of chloroquine was necessary for observing a strong enough fluorescence signal of Gag within the cells. We also found that no direct interaction between INSIG1–TRC8 and Gag could be detected by co-immunoprecipitation (Fig. 10). Therefore, we speculate that the interaction between INSIG1–TRC8 and Gag might be transient, and the quick degradation of Gag resulting from this interaction might limit the detection of direct interaction among these proteins. Of course, currently, we cannot preclude the possibility that more proteins other than INSIG1–TRC8 might be involved in this process.
Figure 9.
Localization of the co-expressed INSIG1, TRC8, and Pr55Gag. A, HeLa cells were transfected with INSIG1-RFP, TRC8-EGFP, or both, together with Gag-pol. Chloroquine was added to inhibit the degradation of Gag. Gag proteins in HeLa cells were immunostained by p24 primary antibody and AMCA-conjugated secondary antibody. Localization of the co-expressed INSIG1, TRC8, and Gag proteins was imaged by confocal laser-scanning microscope. INSIG1 and TRC8 co-localized nicely within cells, but no significant co-localization between INSIG1 and Gag, or between TRC8 and Gag, was observed. Scale bars, 10 μm. B, the co-localization of INSIG1, TRC8, and Gag was measured by scatter plot analysis at the position of the red line in A.
Figure 10.
INSIG1 and TRC8 did not interact with Pr55Gag directly. In 293T cells, TRC8-EGFP was co-transfected with HA-INSIG1 or Gag-pol, and INSIG1-EGFP was co-transfected with Gag-pol. Chloroquine was added to inhibit the degradation of Gag. Cell lysates were immunoprecipitated with GFP-Trap Sepharose and analyzed by Western blotting. INSIG1 was co-immunoprecipitated with TRC8 efficiently (A), but Gag did not co-immunoprecipitate with either INSIG1 (B) or TRC8 (C).
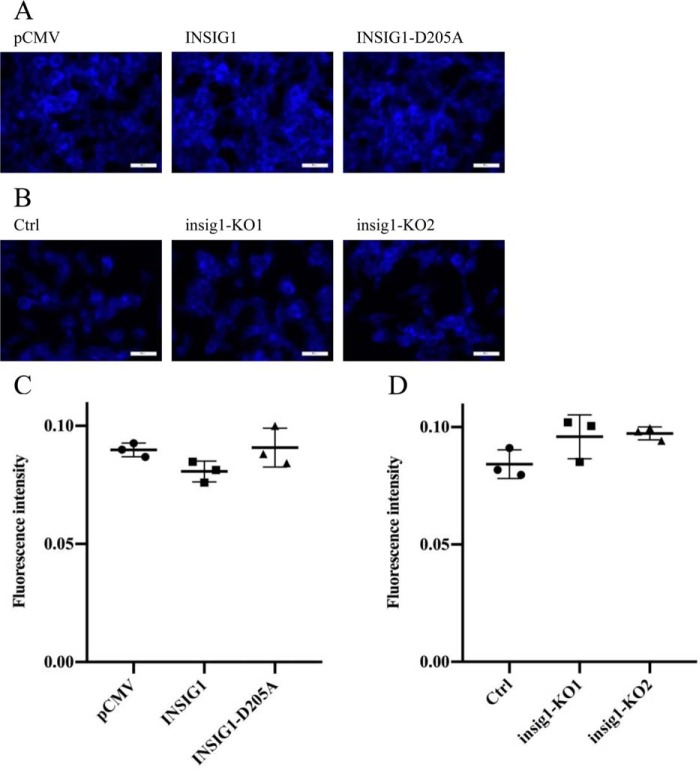
INSIG1 is the regulator of cholesterol synthesis. The critical residue for cholesterol control, Asp-205, is not involved in Gag degradation; therefore, we suspected that the role of cholesterol in the Gag degradation might be unimportant. To determine this, the cholesterol content of the cells was measured by a cholesterol-binding stain filipin in the cells with INSIG1 overexpressed or knocked out. No significant change in cholesterol content could be found in either case. This indicates that cholesterol might not be a critical factor in this process (Fig. 11).
Figure 11.
Presence or absence of INSIG1 did not lead to significant change in cholesterol content in 293T. A, 293T cells were transfected with empty vector, INSIG1, or INSIG1-D205A, and stained with filipin 48 h later. Stained cells were imaged by a fluorescence microscope. Scale bars, 50 μm. B, insig1 knockout 293T cells were stained with filipin. Stained cells were imaged by a fluorescence microscope. Scale bars, 50 μm. C, fluorescence intensity of filipin in stained cells in A was quantified by ImageJ. Shown is mean ± S.D. (error bars) from three independent experiments. D, fluorescence intensity of filipin in stained cells in B was quantified by ImageJ. Shown is mean ± S.D. from three independent experiments.
Discussion
The function of INSIG1 in sterol synthesis has been well-documented as a prominent cholesterol regulator. The RNA level of INSIG1 is markedly up-regulated in HIV-1 infection. This prompted us to study its role in HIV-1 replication. In this study, we found INSIG1 is up-regulated in HIV-1 production on both the mRNA and protein levels, and the overexpression or knockout of INSIG1 reduces or enhances the HIV-1 virion yield, respectively. Our study also revealed that TRC8, the INSIG1-interacting E3 ligase, plays a pivotal role in this process by mediating the degradation of HIV-1 Gag.
INSIG1 could be up-regulated markedly during HIV-1 replication. This response is virion production–dependent but not infection-dependent, based on our observation of the pseudovirus system, which separated the two processes into two independent assays. Furthermore, during HIV-1 production, the expression level of INSIG1 responds to virion generation following the co-expression of Gag-pol and Env, but not Gag-pol itself. Because the backbone plasmid (ES plasmid used here) encodes all HIV-1 proteins except Env, these data indicate that INSIG1 senses and degrades Gag by monitoring enveloped virions rather than single protein, such as Gag and other accessory proteins. It has been reported previously that the accessory proteins Nef and Vif could up-regulate INSIG1 (17, 18). The discrepancy might result from the different cell lines and experimental conditions used in the different laboratories.
To date, the only connection between INSIG1 and viral infection has been reported by Wang et al., who showed that the INSIG1–AMFR E3 complex could bridge the activation of the TANK-binding kinase 1 (TBK1) kinase by ubiquitinating the innate immune adaptor stimulator of interferon genes (STING; also known as MITA, ERIS, or MPYS) (30). INSIG1–AMFR catalyzed the Lys-27–linked polyubiquitination of STING and recruited TBK1 to facilitate its translocation to the perinuclear microsomes. The depletion of AMFR or INSIG1 impaired STING-mediated antiviral gene induction. This process is sensitive to the stimulation of cytosolic viral DNA but not viral RNA. Despite being an RNA virus, HIV-1 RNA usually evades the cell host's innate immune response by multiple tactics, but the viral reverse-transcribed cDNA following HIV-1 entry could be recognized by the host immune system under certain circumstances (31–34). In our study, we measured the expression level of interferons in these cell lines on HIV-1 production and infection. No immune activation could be detected (data not shown); thus, the STING signaling pathway is not triggered here. We also found that TRC8, rather than AMFR, is involved in HIV-1 inhibition. Therefore, a totally different signaling pathway might be involved for HIV-1 inhibition of INSIG1.
Both AMFR and TRC8 interact with INSIG1, and both are involved in fatty acid monitoring inside the cells. TRC8 could bind sterol directly through its SSD (sterol-sensing domain), which is absent in AMFR. Accordingly, TRC8 could sense and react to cholesterol concentration promptly (28). The D205A mutation in INSIG1 could disrupt the interaction between INSIG1 and AMFR, but not the interaction with TRC8. Therefore, D205A mutation does not disturb the degradation of HIV-1 Gag. This suggests that the substrates and the degradation pathway controlled by AMFR and TRC8 are different in cells, despite their overlap in cholesterol control.
HIV-1 Gag could be degraded efficiently by the INSIG1–TRC8 E3 ligase complex, but the direct interaction between Gag and INSIG1–TRC8 could not be identified. One possibility for their interaction might be mediated by cholesterol and other phospholipids, such as the p17-Matrix favored phosphatidylinositide phosphatidylinositol 4,5-bisphosphate, but an unidentified bridging protein could not be precluded at this stage.
INSIG1 and its associated E3 ligases AMFR and TRC8 are located predominantly in ER and endosomes. This was confirmed in our observation by confocal microscopy (Fig. 8). HIV-1 mainly assembled and budded from plasma membrane in most of the cell types except macrophage, in which a budding from multivesicular body (MVB) was also observed (35, 36). By N-terminal myristoylation at Gly-2, HIV-1 Gag could target various membrane systems, including the plasma membrane, MVB, or other endosomes. In this study, we found that the Gag degradation by INSIG1 is N-terminal myristoylation-dependent. This indicates that the association of Gag with the membrane is a prerequisite for its INSIG1-mediated degradation. Therefore, these proteins might accumulate on membrane sites where ubiquitination-dependent Gag degradation occurs.
The overexpression and knockout of INSIG1 slightly changed the cholesterol content in the cells (Fig. 11). In this report, we suggested that INSIG1 inhibited HIV production mainly through TRC8-mediated ubiquitination degradation of HIV Gag, and its function on cholesterol metabolism was not involved in this process. The expression of INSIG1 is especially high in the liver, and its homologous protein INSIG2 is ubiquitously expressed. Therefore, the function of INSIG1 on cholesterol regulation may be compensated by INSIG2 or other proteins in the cells. The other possibility may be that, because we used a stable cell line for insig1 knockout experiments, the cells might modify its metabolic pathway for compensating the loss of INSIG1 for long-term survival.
In summary, our study uncovered a new pathway that is involved in HIV-1 monitoring and inhibition. As an effective sentinel on intracellular membrane, INSIG1 could sense HIV-1 production and inhibit HIV-1 replication by the INSIG1–TRC8 E3 complex–mediated Gag degradation. This process is cholesterol-independent. Considering the importance of INSIG1 in sterol synthesis, this study might provide a new connection between cholesterol and other fatty acid synthesis and HIV-1 infection. This finding might be used for the treatment of HIV-1 infection–induced fatty acid dysfunction in the future.
Experimental procedures
Plasmids and clones
INSIG1 was amplified from a 293T cDNA library. The mutant INSIG1-D205A was generated by site-directed mutagenesis with an aspartic acid 205 to alanine mutation. The KH motif mutant of INSIG1 (INSIG1-KH mut) was generated by site-directed mutagenesis with two amino acid mutations (lysine 273 and histidine 275 to alanines). The N-terminal deletion mutant of INSIG1 with N-terminal residues 1–84 deleted (INSIG-ΔN mut) was amplified from WT INSIG1. INSIG1, INSIG1-D205A, INSIG1-KH mut, and INSIG1-ΔN mut were constructed into a pCMV-3HA plasmid containing a CMV promoter for eukaryotic expression and a 3×HA tag fused at the N terminus for Western blot detection. HA-tagged INSIG1 was also constructed into pQCXIP plasmid for MLV retrovirus packaging. For GFP-Trap co-immunoprecipitation analysis, INSIG1 was constructed into a C-terminally EGFP-tagged eukaryotic expression vector pEGFP-N1. For fluorescence location analysis, INSIG1 was fused with an RFP tag at the C terminus by overlap PCR and constructed into a eukaryotic expression vector, pCI-neo. For the ubiquitination assay, INSIG1 was constructed to pCI-neo (without tag).
TRC8 cDNA was a gift from Dr. Jiahuai Han (Xiamen University, China), the enzymatic defective mutant of TRC8 (TRC8-mut) was generated by site-directed mutagenesis with three amino acid mutations (572LRK574 mutated to AAA) (37). TRC8 was constructed into pEGFP-N1 for GFP-Trap co-immunoprecipitation and fluorescence localization analysis. TRC8 and TRC8 mutant were constructed into pCMV-3HA for expression detection.
Eukaryotic expression plasmid of FLAG-tagged SCAP was purchased from YouBio (Changsha, China).
Target sequences for insig1, amfr, and trc8 knockout were designed and constructed to a CRISPR/Cas9 and single-guide RNA lentivirus delivery vector LentiCRISPR. The target sequences are displayed in Table 1. For reintroduction of INSIG1 into insig1 knockout cells, INSIG1 with synonymous encoding sequence lacking the knockout target site was generated by overlap PCR and promoted by insig1 native promoter for expression.
Table 1.
Target sequences for insig1, amfr, and trc8 knockout
| Genes | Target sequences |
|---|---|
| insig1-KO1 | CCTCGTGCTCTTCTCGGTTG |
| insig1-KO2 | TCCGAGGTGACTGTCGATAC |
| amfr-KO1 | CGGCGAGCCGGACCAGCTAA |
| amfr-KO2 | TCTTCGAGTGAGTGAGAGAC |
| trc8-KO1 | TCTGGAGCACGATGCAGAAC |
| trc8-KO2 | GTCTACTACGTTCGTTCAAC |
The HIV packaging plasmids NLENY1-ES-IRES, pVSV-G, and pSRHS-Env were gifts from Dr. David Levy (University of Alabama, Birmingham, AL) (38). The pCMV-promoted Gag-pol expression plasmid and its membrane targeting–deficient mutant Gag-pol-G2A were gifts from Dr. Wentao Qiao (Nankai University). pCDNA3.1-HA-ubiquitin was a gift from Dr. Min Wei (Nankai University).
Cell culture and transfection
HEK293T, HeLa, TZM-bl, Jurkat, and THP-1 cells were obtained from the American Type Culture Collection (ATCC). 293T, HeLa, and TZM-bl cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, incubated at 37 °C under 5% CO2. Jurkat and THP-1 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium with 10% fetal bovine serum. Cells were incubated at 37 °C, under 5% CO2. Cells were transfected with polyethyleneimine 24 h after seeded when cells were 60% confluent.
To analyze the degradation of Gag proteins induced by INSIG1, cells co-transfected with Gag-pol and INSIG1 were cultured in the medium containing 5 μm MG132 (InvivoGen) or 100 μm chloroquine (InvivoGen) 6 h after transfection, and then cells were harvested for analysis 24 h later.
Quantitative real-time PCR
Cells were rinsed with PBS and lysed by TRIzol (Invitrogen) for 15 min at room temperature. Cell lysate was mixed with chloroform and shaken for 15 s and incubated for another 5 min at room temperature. The extraction was centrifuged for 15 min at 12,000 × g, 4 °C to separate the aqueous phase and organic phase. The upper aqueous phase was transferred to a new tube containing an equal volume of isopropyl alcohol to precipitate the total RNA. The RNA precipitate was collected by centrifugation, washed with 75% ethanol, and dissolved in RNase-free water. Reverse transcription was performed according to the manufacturer's instructions for the RT-PCR kit 5× All-In-One RT MasterMix (Applied Biological Materials Inc., Richmond, Canada). Quantitative RT-PCR was carried out using a two-step real-time PCR kit (SYBR Green, Takara). The primer sequences were as follows: INSIG1-RT-forward, ATCTTTTCCTCCGCCTGGTG; INSIG1-RT-reverse, TCTCCGAGGTGACTGTCGAT. The primers were purchased from AuGCT (Beijing, China). The PCRs were performed by the Mastercycler ep realplex2 (Eppendorf, Germany) using the following procedure: predenature at 95 °C for 2 min; amplification for 40 cycles including 95 °C for 5 s and 60 °C for 30 s. Cycle threshold values were calculated by Realplex version 2.2.
Pseudotyped HIV-1 production, cell-free infection, and luciferase assay
To produce pseudotyped HIV-1 in 293T cells, NLENY1-ES-IRES and pSRHS-Env or pVSV-G plasmids were co-transfected in 293T cells. NLENY1-ES-IRES is derived from a replication-competent NL4-3 HIV-1 clone with envelope gene deficiency and containing an independently promoted YFP reporter gene. Consequently, the packaged virus can infect target cells only one time. To produce HIV-1 in Jurkat cells, first, pQCXIP-VSV-G and pQCXIP-Env were packaged in an MLV retrovirus delivery system to generate VSV-G/Env expressing MLV. Second, VSV-G/Env-pseudotyped HIV-1 and VSV-G/Env expressing MLV were used to infect Jurkat cells simultaneously for the new round of viral production. Virus supernatant was collected after 48 h. Harvested virus was used to infect TZM-bl cells or Jurkat cells. 48 h post-infection, cells were rinsed with PBS and lysed in lysis buffer. Cell lysate was clarified by centrifugation (10,000 × g, 3 min) and mixed with luciferin reagent, and the luciferase activity was measured using a GloMax luminometer following the manufacturer's instructions (Promega).
For the LTR activation assay, 293T cells were transfected with LTR-luc (LTR-promoted luciferase plasmid), Tat, β-gal, with or without INSIG1. The luciferase assay was performed as described above. The data were normalized by a β-gal assay. Cell lysates were mixed with ortho-nitrophenyl-β-galactoside, and the absorbance was read at 410 nm by a BioTek Cytation5 hybrid multimode reader.
HIV infection of insig1 knockout Jurkat cells
293T cells were transfected with the HIV-1 proviral construct pNL4-3 using polyethyleneimine. After 48 h, the supernatants were harvested. The p24 antigen levels in the viral supernatants were measured by HIV-1 p24-antigen capture ELISA. To examine consecutive viral replication, insig1 knockout or control Jurkat cells were infected with 50 pg of p24 antigen of NL4-3 viruses. Spinfection was performed at 300 × g for 2 h at room temperature. Cells were washed with medium three times to remove free virions and then cultured in fresh medium. Supernatants were sampled every day, and p24 antigen production was quantified using ELISA.
Western blotting
Protein levels were analyzed by Western blotting. Cell cultures were harvested 48 h after transfection and treated with NP-40 buffer (Nonidet P-40, 20 mm Tris-HCl, pH 8.0, 137 mm NaCl, 10% glycerol, 2 mm EDTA) for 30 min on ice for lysis. The lysate was boiled at 100 °C for 10 min with SDS loading buffer and analyzed by SDS-PAGE. The protein bands were transferred to 0.45-μm polyvinylidene difluoride membranes, blocked with 5% skim milk, and probed with the primary antibodies anti-HA (Sigma, H3663), anti-FLAG (Sigma, F1804), anti-INSIG1 (Santa Cruz Biotechnology, Inc., sc-25124-R), anti-AMFR (Santa Cruz Biotechnology, sc-166358), anti-TRC8 (Santa Cruz Biotechnology, sc-390347), anti-GAPDH (Santa Cruz Biotechnology, sc-32233), anti-HIV-1 p24 (Millipore, Mab8790), anti-VSV-G (Santa Cruz Biotechnology, sc-66180), and anti-gp120 (from the Chinese Center for Disease Control and Prevention), followed by the corresponding HRP-conjugated secondary detection antibodies: goat anti-mouse IgG-HRP (Sungene Biotech) and goat anti-rabbit IgG-HRP (Sungene Biotech). Immunoreactive proteins were detected by a Tannon-5500 gel imager. All of the Western blots were repeated three times, and a representative result is shown.
Generation of knockout cell lines
The insig1, amfr, and trc8 knockout 293T cell lines and insig1 and trc8 knockout Jurkat cell lines were generated by a lentivirus delivery CRISPR/Cas9 system. Lentivirus was produced by co-transfecting 293T cells with 1 μg of each lentiCRISPR (with single-guide RNA inserted) plasmid, 0.5 μg of pMDLg-pRRE, 0.3 μg of pVSV-G, and 0.2 μg of pCMV-rev and then was used to infect 293T or Jurkat cells. After 24 h following infection, the cells were selected with 2 μg/ml puromycin for 7 days to establish stably transduced cell lines.
In vivo ubiquitination assay
293T cells were transfected with Gag-pol, HA-ubiquitin, and pCI-neo-INSIG1. At 24 h post-transfection, 100 μm chloroquine was added to inhibit the degradation of Gag. Another 24 h later, cell cultures were harvested and lysed with RIPA buffer (50 mm Tris-HCl, pH 8, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS) with gentle end-over-end mixing for 2 h at 4 °C and cleared by centrifugation at 12,000 × g for 10 min at 4 °C. Immunoprecipitation was performed with anti-p24 antibody and Protein A-Sepharose beads (Sangon Biotech, Shanghai, China). The beads were washed four times by gentle end-over-end mixing with 1 ml of RIPA of buffer each time and then were analyzed by Western blotting. Supernatant prior to immunoprecipitation was used as input for comparison.
Fluorescence localization
HeLa cells were transfected with any two or whole three plasmids of INSIG1-RFP, TRC8-EGFP, and Gag-pol. 24 h post-transfection, chloroquine was added to inhibit the degradation of Gag. Gag proteins in HeLa cells were stained by p24 primary antibody and AMCA-conjugated secondary antibody. Gag-pol–transfected cells were fixed with 4% paraformaldehyde at room temperature for 10 min, permeabilized with 0.1% Triton X-100 for 10 min, and incubated with anti-p24 mouse mAb (Millipore, Mab8790) for 2 h at room temperature and then stained with AMCA-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, 715-155-150) for another 2 h. All immunofluorescence images were captured with a Leica TCS SP8 confocal laser-scanning microscope.
Co-immunoprecipitation
293T cells were seeded at 3.5 × 106 cells/100-mm dish. After 24 h, cells were transfected with 2.5 μg of HA-tagged INSIG1 and 2.5 μg of FLAG-tagged SCAP. INSIG1-D205A or empty vector was used for comparison. 36 h after transfection, the cells were treated with 5 μg/ml cholesterol for 5 h and then harvested and lysed with RIPA buffer. Cell lysates were clarified by centrifugation (10,000 × g, 10 min, 4 °C). The supernatant was incubated with anti-HA antibody for 2 h and mixed with protein A–agarose (Sangon Biotech) for 2 h afterward. The protein A beads were washed with RIPA buffer four times and recovered by spinning. INSIG1 and SCAP in pellets and supernatants (input) were analyzed by Western blotting with corresponding antibodies.
For GFP-trap co-immunoprecipitation, 293T cells in 100-mm dishes were transfected with TRC8-EGFP and HA-INSIG1, TRC8-EGFP and Gag-pol, or INSIG1-EGFP and Gag-pol. 24 h after transfection, cells were treated with chloroquine to inhibit Gag degradation. After another 24 h, cells were harvested and treated with RIPA buffer for 1 h on ice for lysis. Cell lysates were centrifuged at 12,000 × g for 10 min at 4 °C. The supernatant (as input) was incubated with GFP-Trap beads (Chromotek, Planegg, Germany) by gentle end-over-end mixing for 2 h at 4 °C. The beads were recovered by spinning at 4 °C and 2000 × g for 5 min and then were washed with RIPA buffer four times before they were used for Western blotting detection.
Filipin staining
293T cells expressing INSIG1 or INSIG1-D205A and insig1 knockout cells were stained with a cholesterol-binding stain, filipin (Sigma, F9765) to measure the cholesterol content of cells. Cells were fixed with 4% fresh paraformaldehyde for 1 h at room temperature, incubated with 1 ml of PBS containing 1.5 mg of glycine for 10 min at room temperature to quench the paraformaldehyde, and stained with 1 ml of filipin working solution for 2 h at room temperature. Cells were rinsed three times with PBS before and after each step. Stained cells were imaged by a Leica DMI3000 B fluorescence microscope. Fluorescence intensity of filipin in stained cells was quantified by ImageJ.
Author contributions
Y. Z. data curation; Y. Z. formal analysis; Y. Z., J. L., and J. M. investigation; Y. Z., J. L., and X. L. methodology; Y. Z. writing-review and editing; X. L. funding acquisition; X. L. writing-original draft.
Acknowledgments
We thank Dr. David Levy (University of Alabama, Birmingham, AL), Dr. Wentao Qiao (Nankai University), and Dr. Min Wei (Nankai University) for plasmids and Dr. Yiming Shao (Chinese Center for Disease Control and Prevention, China) for anti-gp120 antibody.
The authors declare that they have no conflicts of interest with the contents of this article.
- SREBP
- sterol regulatory element–binding protein
- HMG
- 3-hydroxy-3-methylglutaryl
- HMGCR
- HMG-CoA reductase
- ER
- endoplasmic reticulum
- LTR
- long terminal repeat
- ES
- NLENY1-ES-IRES
- AMFR
- autocrine motility factor receptor
- TRC8
- translocation in renal carcinoma chromosome 8
- STING
- stimulator of interferon genes
- TBK1
- TANK-binding kinase 1
- MVB
- multivesicular body
- MLV
- murine leukemia virus
- EGFP
- enhanced GFP
- HRP
- horseradish peroxidase
- AMCA
- aminomethylcoumarin
- RIPA
- radioimmune precipitation assay
- YFP
- yellow fluorescent protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
References
- 1. Suomalainen M. (2002) Lipid rafts and assembly of enveloped viruses. Traffic 3, 705–709 10.1034/j.1600-0854.2002.31002.x [DOI] [PubMed] [Google Scholar]
- 2. Nayak D. P., and Barman S. (2002) Role of lipid rafts in virus assembly and budding. Adv. Virus Res. 58, 1–28 10.1016/S0065-3527(02)58001-5 [DOI] [PubMed] [Google Scholar]
- 3. Chazal N., and Gerlier D. (2003) Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67, 226–237, table of contents 10.1128/MMBR.67.2.226-237.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Munger J., Bennett B. D., Parikh A., Feng X. J., McArdle J., Rabitz H. A., Shenk T., and Rabinowitz J. D. (2008) Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186 10.1038/nbt.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bukrinsky M., and Sviridov D. (2006) Human immunodeficiency virus infection and macrophage cholesterol metabolism. J. Leukocyte Biol. 80, 1044–1051 10.1189/jlb.0206113 [DOI] [PubMed] [Google Scholar]
- 6. Zheng Y.-H., Plemenitas A., Fielding C. J., and Peterlin B. M. (2003) Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. 100, 8460–8465 10.1073/pnas.1437453100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown M. S., and Goldstein J. L. (1997) The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 10.1016/S0092-8674(00)80213-5 [DOI] [PubMed] [Google Scholar]
- 8. Shimano H., and Sato R. (2017) SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat. Rev. Endocrinol. 13, 710–730 10.1038/nrendo.2017.91 [DOI] [PubMed] [Google Scholar]
- 9. Sato R. (2010) Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 501, 177–181 10.1016/j.abb.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 10. Goldstein J. L., and Brown M. S. (1990) Regulation of the mevalonate pathway. Nature 343, 425–430 10.1038/343425a0 [DOI] [PubMed] [Google Scholar]
- 11. Sharpe L. J., and Brown A. J. (2013) Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 288, 18707–18715 10.1074/jbc.R113.479808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sever N., Yang T., Brown M. S., Goldstein J. L., and DeBose-Boyd R. A. (2003) Accelerated degradation of HMG CoA reductase mediated by binding of Insig-1 to its sterol-sensing domain. Mol. Cell 11, 25–33 10.1016/S1097-2765(02)00822-5 [DOI] [PubMed] [Google Scholar]
- 13. Sun L. P., Li L., Goldstein J. L., and Brown M. S. (2005) Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J. Biol. Chem. 280, 26483–26490 10.1074/jbc.M504041200 [DOI] [PubMed] [Google Scholar]
- 14. Goldstein J. L., DeBose-Boyd R. A., and Brown M. S. (2006) Protein sensors for membrane sterols. Cell 124, 35–46 10.1016/j.cell.2005.12.022 [DOI] [PubMed] [Google Scholar]
- 15. Jo Y., Lee P. C. W., Sguigna P. V., and DeBose-Boyd R. A. (2011) Sterol-induced degradation of HMG CoA reductase depends on interplay of two Insigs and two ubiquitin ligases, gp78 and Trc8. Proc. Natl. Acad. Sci. U.S.A. 108, 20503–20508 10.1073/pnas.1112831108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong X. Y., and Tang S. Q. (2010) Insulin-induced gene: a new regulator in lipid metabolism. Peptides 31, 2145–2150 10.1016/j.peptides.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 17. van 't Wout A. B., Swain J. V., Schindler M., Rao U., Pathmajeyan M. S., Mullins J. I., and Kirchhoff F. (2005) Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells. J. Virol. 79, 10053–10058 10.1128/JVI.79.15.10053-10058.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D. Y., Kwon E., Hartley P. D., Crosby D. C., Mann S., Krogan N. J., and Gross J. D. (2013) CBFβ stabilizes HIV Vif to counteract APOBEC3 at the expense of RUNX1 target gene expression. Mol. Cell 49, 632–644 10.1016/j.molcel.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feramisco J. D., Goldstein J. L., and Brown M. S. (2004) Membrane topology of human insig-1, a protein regulator of lipid synthesis. J. Biol. Chem. 279, 8487–8496 10.1074/jbc.M312623200 [DOI] [PubMed] [Google Scholar]
- 20. Yabe D., Brown M. S., and Goldstein J. L. (2002) Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 99, 12753–12758 10.1073/pnas.162488899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gong Y., Lee J. N., Brown M. S., Goldstein J. L., and Ye J. (2006) Juxtamembranous aspartic acid in Insig-1 and Insig-2 is required for cholesterol homeostasis. Proc. Natl. Acad. Sci. U.S.A. 103, 6154–6159 10.1073/pnas.0601923103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma W., and Goldberg J. (2013) Rules for the recognition of dilysine retrieval motifs by coatomer. EMBO J. 32, 926–937 10.1038/emboj.2013.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bryant M., and Ratner L. (1990) Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc. Natl. Acad. Sci. U.S.A. 87, 523–527 10.1073/pnas.87.2.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song B. L., Sever N., and DeBose-Boyd R. A. (2005) Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol. Cell 19, 829–840 10.1016/j.molcel.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 25. Ravid T., Doolman R., Avner R., Harats D., and Roitelman J. (2000) The ubiquitin-proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 275, 35840–35847 10.1074/jbc.M004793200 [DOI] [PubMed] [Google Scholar]
- 26. Lee D. H., and Goldberg A. L. (1998) Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8, 397–403 10.1016/S0962-8924(98)01346-4 [DOI] [PubMed] [Google Scholar]
- 27. Steinman R. M., Mellman I. S., Muller W. A., and Cohn Z. A. (1983) Endocytosis and the recycling of plasma membrane. J. Cell Biol. 96, 1–27 10.1083/jcb.96.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J. P., Brauweiler A., Rudolph M., Hooper J. E., Drabkin H. A., and Gemmill R. M. (2010) The TRC8 ubiquitin ligase is sterol regulated and interacts with lipid and protein biosynthetic pathways. Mol. Cancer Res. 8, 93–106 10.1158/1541-7786.MCR-08-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nityanandam R., and Serra-Moreno R. (2014) BCA2/Rabring7 targets HIV-1 Gag for lysosomal degradation in a tetherin-independent manner. PLoS Pathog. 10, e1004151 10.1371/journal.ppat.1004151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Q., Liu X., Cui Y., Tang Y., Chen W., Li S., Yu H., Pan Y., and Wang C. (2014) The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 10.1016/j.immuni.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 31. Harris R. S., Bishop K. N., Sheehy A. M., Craig H. M., Petersen-Mahrt S. K., Watt I. N., Neuberger M. S., and Malim M. H. (2003) DNA deamination mediates innate immunity to retroviral infection. Cell 113, 803–809 10.1016/S0092-8674(03)00423-9 [DOI] [PubMed] [Google Scholar]
- 32. Altfeld M., and Gale M. Jr (2015) Innate immunity against HIV-1 infection. Nat. Immunol. 16, 554–562 10.1038/ni.3157 [DOI] [PubMed] [Google Scholar]
- 33. Jakobsen M. R., Bak R. O., Andersen A., Berg R. K., Jensen S. B., Tengchuan J., Jin T., Laustsen A., Hansen K., Ostergaard L., Fitzgerald K. A., Xiao T. S., Mikkelsen J. G., Mogensen T. H., and Paludan S. R. (2013) IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc. Natl. Acad. Sci. U.S.A. 110, E4571–E4580 10.1073/pnas.1311669110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao D., Wu J., Wu Y.-T., Du F., Aroh C., Yan N., Sun L., and Chen Z. J. (2013) Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 10.1126/science.1240933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morita E., and Sundquist W. I. (2004) Retrovirus Budding. Annu. Rev. Cell Dev. Biol. 20, 395–425 10.1146/annurev.cellbio.20.010403.102350 [DOI] [PubMed] [Google Scholar]
- 36. Jouve M., Sol-Foulon N., Watson S., Schwartz O., and Benaroch P. (2007) HIV-1 buds and accumulates in “Nonacidic” endosomes of macrophages. Cell Host Microbe 2, 85–95 10.1016/j.chom.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 37. Brauweiler A., Lorick K. L., Lee J. P., Tsai Y. C., Chan D., Weissman A. M., Drabkin H. A., and Gemmill R. M. (2007) RING-dependent tumor suppression and G2/M arrest induced by the TRC8 hereditary kidney cancer gene. Oncogene 26, 2263–2271 10.1038/sj.onc.1210017 [DOI] [PubMed] [Google Scholar]
- 38. Levy D. N., Aldrovandi G. M., Kutsch O., and Shaw G. M. (2004) Dynamics of HIV-1 recombination in its natural target cells. Proc. Natl. Acad. Sci. 101, 4204–4209 10.1073/pnas.0306764101 [DOI] [PMC free article] [PubMed] [Google Scholar]