Abstract
The antibiotic trimethoprim is frequently used to manage Burkholderia infections, and members of the resistance-nodulation-division (RND) family of efflux pumps have been implicated in multidrug resistance of this species complex. We show here that a member of the distinct Escherichia coli multidrug resistance B (EmrB) family is a primary exporter of trimethoprim in Burkholderia thailandensis, as evidenced by increased trimethoprim sensitivity after inactivation of emrB, the gene that encodes EmrB. We also found that the emrB gene is up-regulated following the addition of gentamicin and that this up-regulation is due to repression of the gene encoding OstR, a member of the multiple antibiotic resistance regulator (MarR) family. The addition of the oxidants H2O2 and CuCl2 to B. thailandensis cultures resulted in OstR-dependent differential emrB expression, as determined by qRT-PCR analysis. Specifically, OstR functions as a rheostat that optimizes emrB expression under oxidizing conditions, and it senses oxidants by a unique mechanism involving two vicinal cysteines and one distant cysteine (Cys3, Cys4, and Cys169) per monomer. Paradoxically, emrB inactivation increased resistance of B. thailandensis to tetracycline, a phenomenon that correlated with up-regulation of an RND efflux pump. These observations highlight the intricate mechanisms by which expression of genes that encode efflux pumps is optimized depending on cellular concentrations of antibiotics and oxidants.
Keywords: antibiotic, reactive oxygen species (ROS), transcription factor, gene regulation, multidrug transporter, oxidative stress, bacterial virulence, efflux pump, EmrB, MarR, multidrug resistance
Introduction
Bacteria experience diverse environmental challenges during host infection. To survive, they must withstand host-derived stresses, such as changes in temperature, pH, generation of reactive oxygen species (ROS),3 and nutrient scarcity. In addition, they may encounter antibiotics, disinfectants, and other harmful chemicals. To proliferate in the face of such challenges, bacteria employ diverse transcription factors, which respond to the environmental changes and modulate gene expression. Multiple antibiotic resistance regulator (MarR) family transcriptional regulators often participate in regulating expression of virulence genes in response to environmental cues (1). First characterized in Escherichia coli K-12, MarR was shown to play critical roles in conferring resistance to antibiotics, household disinfectants, and oxidative stress (2). Members of the protein family named for E. coli MarR are ubiquitous in bacterial species, and the most common mode of transcriptional regulation involves binding to the intergenic DNA between the gene encoding the MarR homolog and divergently or adjacently oriented genes, thereby hindering access of RNA polymerase to the promoter. DNA binding by a given MarR family protein may be modified upon binding to small-molecule ligands or oxidants, a result of which is altered expression of genes in its regulon (1).
Many pathogenic Burkholderia species exhibit inherent resistance to a wide range of antibiotics and disinfectants (3). Efflux systems involved in antibiotic resistance include the resistance-nodulation-division (RND) efflux systems and the E. coli multidrug resistance B (EmrB) family transporters (3–5). Extensively studied pathogens include Burkholderia pseudomallei, a soil saprophyte causing melioidosis, and its clonal derivative Burkholderia mallei, a host-adapted pathogen, which is the causative agent of glanders (6). Both are categorized as category B priority pathogens because of the low infectious dose, capability for aerosol transmission, and resistance to antibiotics (3). By contrast, Burkholderia cenocepacia is an opportunistic pathogen that primarily infects immunocompromised patients (7). Burkholderia thailandensis is particularly closely related to B. pseudomallei and B. mallei, and these species are founding members of the B. pseudomallei (Bpc) group (8, 9). B. thailandensis is much less virulent despite conservation of many genes involved in virulence (10).
One of the very early host responses to a bacterial infection is the production of ROS. For example, NADPH oxidase generates superoxide radicals (O2˙̄), which give rise to H2O2. H2O2 may in turn oxidize ferrous iron to generate highly reactive hydroxyl radicals (OH•) (11, 12). ROS can target bacterial DNA, protein, or membrane lipids and thus can attenuate bacterial survival and proliferation in the host environment (13). Transition metals, such as Cu(II) and Zn(II), can also cause microbial poisoning when in excess (14). In addition, Cu(II) can participate in the Fenton reaction and cause elevated intracellular ROS production.
Bacterial responses to ROS by transcriptional regulators such as MarR family proteins have been described, including in Burkholderia species (15, 16); oxidation of cysteine residues, usually by reversible formation of disulfide linkages, leads to conformational changes that allow such transcriptional regulators to promote expression of genes involved in detoxification of ROS or repair of ROS-mediated damage (17). The redox-active Cu(II) has also been implicated in modification of MarR proteins by inducing formation of disulfide linkages between separate dimers, but with distinct outcomes (15, 18).
B. thailandensis encodes 12 annotated MarR homologs, all of which are conserved in B. pseudomallei and B. mallei (19). One of these is encoded by BTH_I0021, which is upstream of a gene encoding an EmrB/QacA family transport protein. This genomic locus is conserved among pathogenic species such as B. pseudomallei, B. mallei, and B. cenocepacia (19). Based on the identified roles of this MarR family protein in oxidant sensing and control of a transporter, we have assigned the name OstR (oxidant-sensing transport regulator). We report here differential and OstR-dependent expression of BTH_I0022 encoding the EmrB family transporter under reducing and oxidizing conditions and that OstR employs a novel response to oxidants involving vicinal cysteines. Notably, disruption of BTH_I0022 confers sensitivity to trimethoprim, an antibiotic commonly used in combination with sulfamethoxazole to treat Burkholderia infections. Unexpectedly, disruption of BTH_I0022 also confers increased resistance to tetracycline, a resistance that correlates with up-regulation of an RND efflux pump.
Results
Conserved genomic locus encoding OstR and EmrB
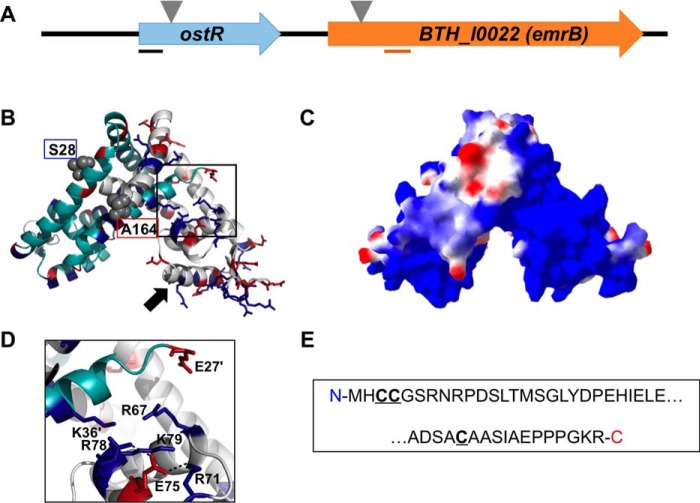
The gene encoding OstR is immediately upstream of BTH_I0022, which encodes an EmrB/QacA family drug resistance transporter (Fig. 1A). A model of OstR was predicted using SwissModel and 4AIK (Yersinia pseudotuberculosis RovA (20)) as a template, one of the structures with highest sequence identity to OstR (28%). This model shows the expected homodimer, which consists of a dimerization region formed by N- and C-terminal α1, α5, and α6 helices from both subunits and a DNA-binding region that includes recognition helices α4/α4′ (arrow; Fig. 1B). In addition, each monomer contains N- and C-terminal extensions beyond the conserved core fold that is represented in the modeled structure, with the core fold spanning residues Ser28–Ala164 (Fig. 1B; terminal residues in gray space-filling representation). The electrostatic surface potential of the OstR core was calculated by Swiss PDB Viewer. Notably, one lobe of the dimeric protein is predicted to be highly positively charged (Fig. 1C). The other lobe features a more usual distribution of charges, being electropositive surrounding the recognition helix and the wing regions, a common feature that would promote interaction with the negatively charged cognate DNA. The unusual surface charge distribution within one protein lobe appears to derive from a constellation of five Arg and Lys residues, which is not balanced by equivalent negative charge (Fig. 1D); salt bridges between Glu75 and Arg71/Arg78 are predicted, whereas the side chain of Glu27′ is predicted to be >8 Å from the other positively charged side chains (Lys36′, Arg67, and Lys79). Another notable feature of the OstR sequence is the presence of three cysteine residues per monomer, two vicinal cysteines (Cys3 and Cys4) in the N-terminal extension that precedes the core MarR fold and Cys169 in the C-terminal extension (Fig. 1E). With six cysteines per dimer, regulation of OstR function by oxidation would be possible.
Figure 1.
Burkholderia thailandensis OstR. A, ostR-emrB genomic locus. Transposon insertion is indicated by inverted triangles. Lines below the arrows illustrating open reading frames represent the positions of PCR amplicons used for quantitative RT-PCR. B, predicted model of OstR based on template 4AIK, created by SwissModel (in automated mode; GMQE 0.55) and visualized by PyMOL. One monomer is shown in cyan and the other is in gray, with positively charged residues in blue and negatively charged amino acids in red. Serine at position 28 and alanine at position 164 (marking the range of amino acids included in the model) are shown in space-filling representation. Black arrow, DNA recognition helix. C, electrostatic surface potential was calculated using Swiss PDB Viewer (red, negative; blue, positive). D, magnified image of the positively charged patch. E, N- and C-terminal extensions of OstR that are not included in the modeled structure, with cysteines in boldface type and underlined.
OstR regulates emrB differentially in response to oxidants
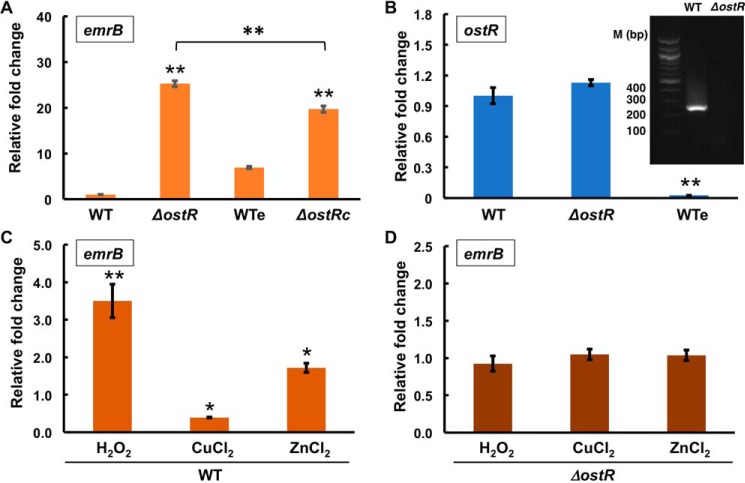
To assess whether OstR participates in regulation of emrB, we obtained and verified B. thailandensis E264 mutant strains in which ostR or emrB genes were disrupted by the insertion of transposons at position 98 of ostR and position 89 of emrB open reading frames, respectively, to generate ΔostR and ΔemrB strains (Fig. 1A) (21). Relative transcript levels of emrB were measured in both WT and ΔostR strains. emrB was up-regulated 25.2 ± 0.7-fold in the ΔostR strain (Fig. 2A), suggesting that OstR is a repressor of emrB. Complementation of ΔostR was attempted using plasmid-encoded ostR. Because a gentamicin-resistant derivative of the broad host range vector pBBR1-MCS5 was used for this purpose, WT cells were transformed with empty pBBR1-MCS5 (generating strain WTe) to control for any effects of gentamicin on gene expression. However, restoration of WT levels of emrB expression was not achieved in ΔostR transformed with pBBR1-MCS5-ostR (ΔostRc) and grown with gentamicin, only an ∼20% reduction in expression compared with WTe. To address the possible reason, expression of ostR was determined in WTe grown with gentamicin and found to be significantly reduced (Fig. 2B). Consistent with a gentamicin-mediated reduction in ostR expression, emrB expression was ∼7-fold higher in WTe compared with WT cells (Fig. 2A). This suggests that gentamicin negatively regulates the expression of ostR, a consequence of which is increased emrB expression.
Figure 2.
Regulation of gene expression. A, relative transcript level of emrB in WT, ΔostR, WTe (containing empty vector), and ΔostRc (complemented with ostR) strains. -Fold changes are reported relative to the reference gene encoding glutamate synthase large subunit (BTH_I3014/BTH_RS27550) and normalized to expression in WT cells, except for expression in ΔostRc, which is reported relative to WTe. Error bars, S.D. values of three biological replicates. B, relative -fold change in transcript level of ostR in ΔostR strain calculated by the 2−ΔCT method relative to the reference gene and normalized to expression in WT cells. The gel shows expression of ostR in WT and ΔostR strains. Lane 1, 100-bp DNA marker. C and D, relative abundance of transcript level of the gene encoding EmrB in the presence of 2 mm H2O2, CuCl2, and ZnCl2 in WT and ΔostR strains. The transcript levels were calculated using 2−ΔΔCT relative to the reference gene and normalized to expression in the corresponding unsupplemented cultures. Error bars, S.D. of three individual experiments. Asterisks represent statistically significant differences in expression compared with WT cells, except as indicated in A, based on a Student's t test (*, p < 0.05; **, p < 0.001).
Many MarRs are autoregulatory (1). To determine whether any autoregulation of ostR by OstR occurs, we analyzed expression of the gene fragment that is upstream of the point of transposon insertion (position 98 of the ostR ORF). There was no significant difference in expression between WT and ΔostR, indicating that expression of ostR is not regulated by OstR (Fig. 2B). By contrast, analysis of ostR expression using primers, which amplify a fragment that is downstream of the transposon insertion, showed no expression in ΔostR consistent with transposon-mediated gene disruption (Fig. 2B, gel inset); combined with the ∼25-fold increase in emrB expression in ΔostR, this experiment also shows that emrB is expressed under the control of its own promoter.
To determine the effect of oxidants on the expression of emrB, WT and ΔostR strains were grown to mid-log phase followed by incubation with a 2 mm concentration of the oxidant H2O2 or CuCl2 for 30 min. RNA was isolated, and cDNA was made using gene-specific primers for analysis of relative transcript levels by quantitative RT-PCR. In the presence of 2 mm H2O2, emrB expression was increased 3.5 ± 0.4-fold, whereas incubation with 2 mm CuCl2 resulted in significant reduction in emrB expression (0.4 ± 0.01-fold; Fig. 2C). To determine whether redox-inactive metals also regulate emrB expression, we assessed Zn2+-dependent transcriptional regulation of emrB. Gene expression analysis revealed an ∼2-fold increase in emrB expression in the presence of 2 mm ZnCl2. No significant change in emrB expression was observed in the ΔostR strain in the presence of oxidants or ZnCl2, suggesting that the observed changes in gene expression in WT cells depend on OstR (Fig. 2D).
Gene disruption affects sensitivity to antibiotics and oxidants
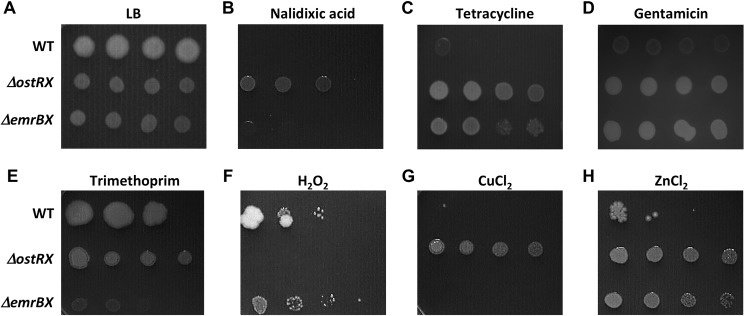
For clues to the substrate for EmrB, the WT, ΔostRX, and ΔemrBX strains (in which antibiotic resistance cassettes were removed from transposons) were serially diluted and spotted on LB-agar plates containing antibiotics. It was previously reported that expression of the corresponding B. cepacia EmrB protein (named BcrA) in E. coli resulted in resistance to tetracycline and nalidixic acid (22). Nalidixic acid was seen to be toxic to WT cells and to ΔemrBX, whereas the ΔostRX strain was more resistant (Fig. 3B). A minimal inhibitory concentration (MIC) was estimated by growing the respective strains in liquid culture containing serially diluted antibiotics; this analysis indicated a similar MIC for WT and ΔemrBX cells, whereas the MIC for ΔostRX cells was higher (Table 1). The increased resistance exhibited by ΔostRX would be consistent with up-regulation of emrB and more efficient export of nalidixic acid.
Figure 3.
Gene deletions alter resistance to antibiotics and oxidants. In each panel, the top row shows WT, the middle row shows ΔostRX, and the bottom row shows ΔemrBX (strains deleted for antibiotic resistance cassettes within transposons). Spots represent 10-fold serial dilutions of the indicated strains; the doubling time of mutant strains (∼60 min) is greater than that of WT cells (∼26 min). A, LB. B, nalidixic acid (10 μg/ml). C, tetracycline (1 μg/ml). D, gentamicin (150 μg/ml). E, trimethoprim (50 μg/ml). F, treatment with 5 mm H2O2. G, 5 mm CuCl2. H, 2 mm ZnCl2.
Table 1.
MIC in LB medium
| B. thailandensis E264 derivative | Concentration |
|||
|---|---|---|---|---|
| Nalidixic acid | Tetracycline | Gentamicin | Trimethoprim | |
| μg/ml | ||||
| WT | 64 | 2 | 2,000 | 64 |
| ΔostRX | 256 | 8 | 32,000 | >1,000 |
| ΔemrBX | 64 | 4 | 16,000 | 16 |
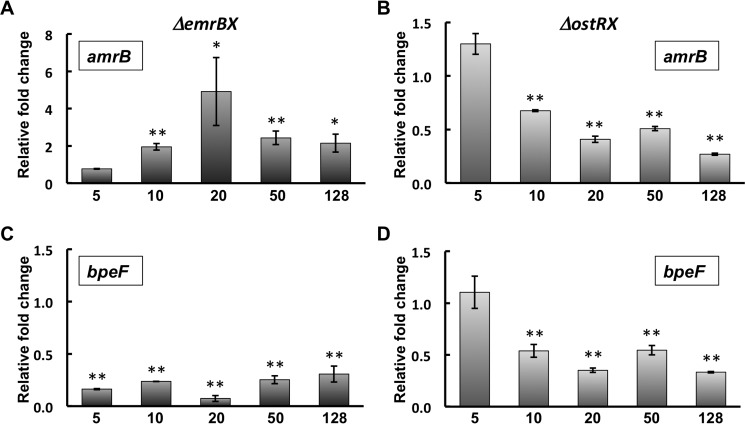
Resistance to tetracycline was likewise elevated in the ΔostRX strain, consistent with export through EmrB; however, resistance was also increased in the ΔemrBX strain (Fig. 3C and Table 1). Expression of two operons encoding RND efflux systems (BpeEF-OprC and AmrAB-OprA) was previously reported to be induced by the tetracycline derivative doxycycline (23); a possible explanation for the increased resistance to tetracycline in the ΔemrBX strain is therefore that failure to export tetracycline through EmrB might lead to elevated intracellular concentrations and induction of one or both of these RND efflux systems. We therefore grew ΔemrBX and ΔostRX strains in the presence of different concentrations of tetracycline and measured expression of amrB and bpeF (Fig. 4). Expression of amrB was greater in ΔemrBX cells grown with at least 10 μg/ml tetracycline, whereas expression of bpeF was reduced. In contrast, expression of both amrB and bpeF was reduced in ΔostRX cells grown with tetracycline. This is consistent with the inference that the increased resistance to tetracycline characteristic of ΔemrBX cells is due to induction of the operon that encodes AmrAB-OprA.
Figure 4.
Expression of genes encoding AmrAB-OprA and BpeEF-OprC efflux pumps in the presence of tetracycline. A and B, expression of amrB (BTH_I2444) in ΔemrBX and ΔostRX, respectively. C and D, expression of bpeF (BTH_II2105) in ΔemrBX and ΔostRX, respectively. Expression is reported relative to unsupplemented cultures. Asterisks represent statistically significant differences in expression compared with unsupplemented cultures based on Student's t test (*, p < 0.05; **, p < 0.001). Horizontal axes identify the concentration of tetracycline (in μg/ml). Note that the ordinate scale for A differs from that in B–D. Error bars, S.D.
High concentrations of gentamicin were tolerated by WT cells, with a further increase in resistance in ΔostRX cells (Fig. 3D and Table 1). Because increased resistance was also observed in ΔemrBX cells, one possibility is that failure to export gentamicin by EmrB may result in induction of an alternate exporter, as seen for tetracycline. Notably, EmrB appears to be a primary efflux system for trimethoprim; at a concentration of the antibiotic where growth of WT and ΔostRX was largely unaffected, as estimated by plate assays, growth of ΔemrBX was severely compromised (Fig. 3E). Consistent with EmrB being important for trimethoprim efflux, the MIC for ΔemrBX cells was lower than the MIC for WT cells (Table 1).
Because emrB expression was sensitive to redox state (Fig. 2C), the strains were exposed to either 5 mm H2O2 or CuCl2 and plated on LB-agar plates. Whereas the ΔemrBX strain exhibited similar sensitivity to either oxidant as WT cells, ΔostRX was more sensitive to H2O2 but less sensitive to CuCl2 (Fig. 3, F and G). This differential sensitivity is intriguing, and it parallels the opposite effects of either oxidant on emrB expression, suggesting that gene regulation by oxidized OstR depends on the identity of the oxidant. Exposure to ZnCl2, however, resulted in a phenotype similar to that observed on treatment with tetracycline and gentamicin (Fig. 3H).
Interaction of OstR with Zn2+ and oxidants in vitro
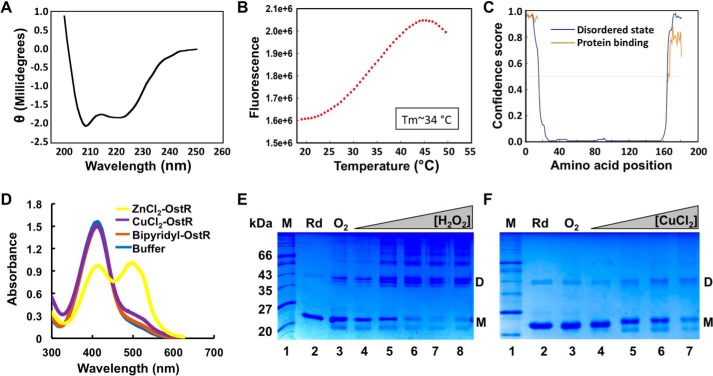
OstR was examined in vitro, focusing on responses to the inducers of differential emrB expression in vivo. The gene encoding OstR was cloned, and the protein was expressed in E. coli and purified using a nickel-nitrilotriacetic acid–agarose column to apparent homogeneity (Fig. 5 (E and F), lanes 2). The calculated molecular mass of monomeric OstR is ∼21 kDa. Far-UV CD spectroscopy showed that the predicted secondary structure composition of OstR is about 37% α-helix, 25% β-sheet, and 38% random coils (Fig. 5A). By comparison, the structure of Deinococcus radiodurans HucR, which also has an N-terminal extension beyond the conventional MarR fold, reveals ∼55% α-helix, a helical content that is also reflected in its CD spectrum; the lower α-helical content for OstR may reflect that its unique N- and C-terminal extensions are not helical (24, 25). The secondary structure of OstR was predicted using the PSIPRED Protein Analysis Workbench (26); disorder prediction using DISOPRED3 revealed that the extensions beyond the core MarR fold represent intrinsically disordered regions and that they likely participate in protein interaction, both with high confidence scores (Fig. 5C).
Figure 5.
Characterization of OstR. A, far-UV CD spectrum of OstR. Ellipticity is presented in machine units (millidegrees). B, thermal denaturation of OstR determined by differential scanning fluorometry. Fluorescence intensity reflects binding of SYPRO Orange to hydrophobic regions of denatured protein as a function of temperature. C, prediction of intrinsically disordered regions (blue) and likelihood of disordered regions participating in protein interactions (orange). The y axis reflects confidence score, with values >0.5 considered relevant. D, release of metal ion from denatured OstR determined by absorbance of PAR. Absorbance at 416 nm corresponds to uncomplexed PAR, whereas absorbance at 520 nm reflects formation of PAR–metal ion complex. E and F, OstR oxidation by H2O2 (E) and CuCl2 (F). In both images, the left lanes show protein marker (kDa), lanes 2 show protein incubated with DTT (Rd; species migrating at ∼43 kDa is residual oxidized protein), and lanes 3 show air-oxidized protein (O2). E, lanes 4–8, increasing concentration of H2O2 (10 μm to 2 mm). F, lanes 4–7, increasing concentration of CuCl2 (10 μm to 2 mm).
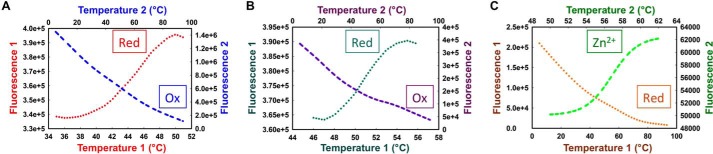
A thermal stability assay in which SYPRO Orange was used as a fluorescent reporter of protein unfolding as a function of temperature showed that reduced OstR exhibited a one-step melting transition with a relatively low Tm = 34.1 ± 0.1 °C (Fig. 5B). Protein stability was found to increase in the presence of DNA (Tm = 39.3 ± 0.2 °C) and Zn2+ (Tm = 37.2 ± 0.1 °C) or upon oxidation (Tm ∼39 °C; Table S1). Incubation of OstR with bipyridyl prior to measurement of thermal stability did not affect the Tm, indicating that purified OstR had no metal ions already bound. To verify binding to metal ions, OstR was incubated with 2 mm ZnCl2 followed by treatment with 4-(2-pyridylazo) resorcinol (PAR). The metallochromic indicator PAR chelates zinc ions released from the protein upon its denaturation, which results in an absorbance peak at ∼520 nm, whereas uncomplexed PAR has an absorbance maximum at 416 nm (27). As shown by the marked absorbance at 520 nm, OstR binds Zn2+ (Fig. 5D).
Each OstR monomer harbors three cysteine residues, all within the N- and C-terminal extensions (Fig. 1E). Reduced OstR was therefore incubated with increasing concentration of H2O2 and CuCl2, and oxidation products were analyzed by electrophoresis on SDS-polyacrylamide gels. When oxidized with H2O2, OstR formed dimers and oligomeric species as well as intramolecular disulfide bonds, as indicated by a species with a faster migration than reduced monomeric protein. In addition, OstR formed three dimeric forms with distinct mobility, likely reflecting different disulfide-bonded species (Fig. 5E).
OstR was also oxidized by CuCl2. Whereas the appearance of multiple dimeric species was more evident on H2O2-mediated oxidation, intramolecular disulfide bonds and oligomeric species were also seen upon oxidation with CuCl2.
OstR binds the emrB promoter
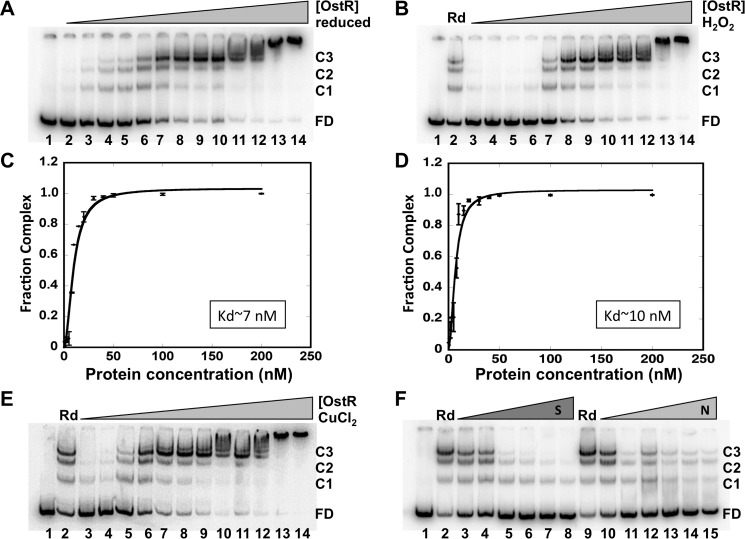
Electrophoretic mobility shift assays (EMSAs) were performed to determine OstR binding upstream of emrB. Reduced OstR bound the 146-bp DNA spanning ostR and emrB forming multiple complexes (Fig. 6A). The apparent dissociation constant Kd was 6.7 ± 0.8 nm with Hill coefficient nH = 1.9 ± 0.3, indicating positive cooperativity of binding (Table S2 and Fig. 6C). At lower protein concentration, the faster-migrating complex C1 was visible, whereas the slower-migrating complex C3 remained predominant across a greater range of concentration. Nonspecific binding may occur at even higher protein concentration, leading to slower migration of the complexes. To address whether OstR bound specifically to the emrB promoter, increasing concentration of competitor DNA was added in the reaction with specific labeled DNA and a constant concentration of the protein. Specific unlabeled DNA competed efficiently for OstR binding (Fig. 6F, lanes 3–8). Nonspecific DNA did not compete as efficiently for OstR binding to its cognate DNA (Fig. 6F, lanes 10–15; note that the highest concentration of specific competitor DNA represents a 250-fold excess over specific labeled DNA, whereas the highest concentration of the 5,368-bp pET28b represents an almost 2,000-fold excess).
Figure 6.
OstR binds emrB promoter DNA. A, EMSA showing reduced OstR binding emrB promoter DNA (0.2 nm). DNA was titrated with increasing concentration of OstR (1–200 nm; lanes 2–14). Lane 1, free DNA only. Free DNA and protein–DNA complex are shown as FD and C1–C3, respectively, at the right. B and E, EMSA showing oxidized OstR binding emrB promoter DNA. Reactions in lanes 1 contained free DNA only, and reactions in lanes 2 contained reduced OstR. Lanes 3–14, operator DNA (0.2 nm) titrated with increasing concentration (1–200 nm) of oxidized protein (oxidized with 2 mm H2O2 and 5 μm CuCl2, respectively). Normalized complex fraction for reduced OstR (C) and normalized complex fraction for H2O2-oxidized OstR (D) are plotted as a function of OstR concentration. S.D. values of three replicates are represented as error bars. F, EMSA showing OstR (5 nm) bound to 0.2 nm labeled operator DNA challenged with increasing concentration of specific unlabeled 146-bp operator DNA (0.2–50 nm, lanes 3–8) or nonspecific DNA pET28b (0.2–10 nm, lanes 10–15). The reaction in lane 1 contained free DNA only; the reactions in lanes 2 and 9 contained no competitor DNA.
OstR was oxidized with 2 mm H2O2, and DNA binding was visualized by EMSA (Fig. 6, B and D). The affinity of H2O2-oxidized OstR for cognate DNA was not markedly different from that of reduced protein (Kd = 9.7 ± 1.1). Incubation of increasing concentration of 2 mm CuCl2-oxidized OstR with emrB promoter DNA also showed no significant change in DNA-binding affinity, Kd = 6.5 ± 1.0 (Fig. 6E and Table S2).
Substitution of cysteine residues leads to altered binding affinity for promoter DNA
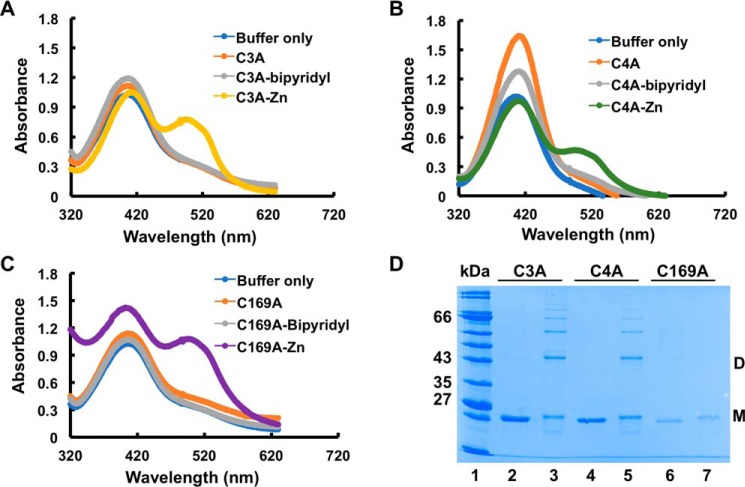
To determine the role of cysteines in oxidant sensing by OstR, we replaced each cysteine with alanine and created three individual cysteine mutants (OstR-C3A, OstR-C4A, and OstR-C169A). Each variant was expressed in E. coli and purified to apparent homogeneity (Fig. 7, lanes 2). The yield of OstR-C169A mutant was lower compared with the other variants, with precipitation observed after purification, indicating that the absence of Cys169 may cause instability or improper folding.
Figure 7.
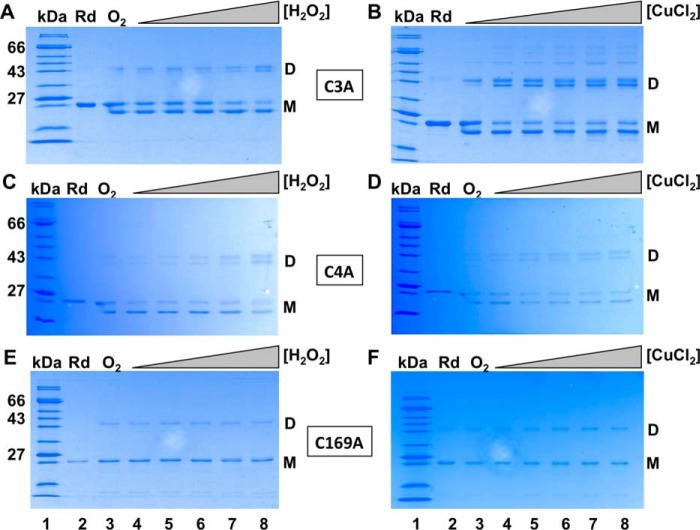
Oxidation of OstR cysteine variants by H2O2 and CuCl2. A, C, and E, OstR-C3A. OstR-C4A and OstR-C169A oxidized with increasing concentration of H2O2 (10 μm to 2 mm). B, D, and F, OstR-C3A. OstR-C4A and OstR-C169A oxidized with increasing concentration of CuCl2 (10 μm to 2 mm). Lanes 1, protein marker (kDa); lanes 2, reduced protein (Rd); lanes 3, air-oxidized protein (O2), except for B, in which air-oxidized protein is not shown.
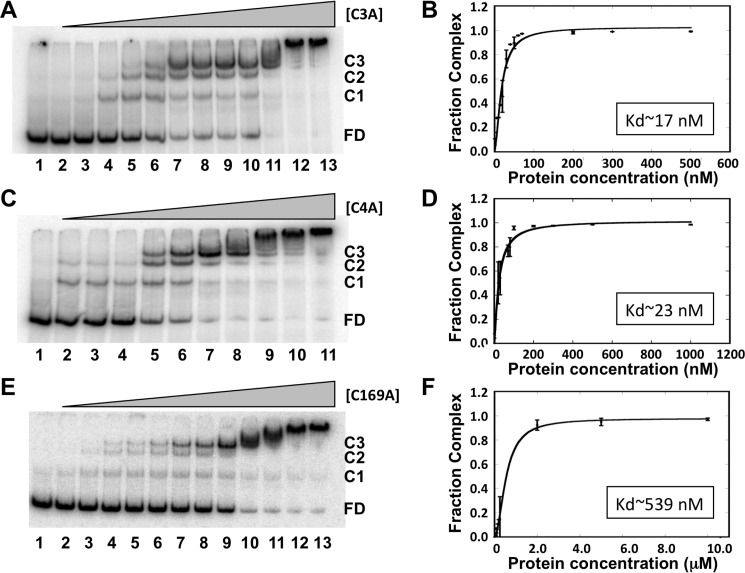
Increasing concentrations of OstR-C3A, OstR-C4A, or OstR-C169A were incubated with 146-bp emrB promoter DNA followed by analysis of protein–DNA complexes by EMSA (Fig. 8, A, C, and E). OstR-C3A showed a modest reduction in DNA-binding affinity with Kd = 17.5 ± 2.3 and Hill coefficient, nH = 1.6 ± 0.3 (Fig. 8B). OstR-C4A exhibited a comparable reduction in DNA-binding affinity with Kd = 23.4 ± 3.2 and nH = 1.2 ± 0.3 (Fig. 8D). Replacing Cys169 with alanine, however, caused DNA binding to be significantly impaired, with Kd = 539 ± 58 nm and nH = 1.9 ± 0.2 (Fig. 8E and Table S2). Because the cysteine residues all reside in extensions beyond the core MarR fold, these data suggest that modulation of these extensions leads to structural rearrangements that impact the DNA-binding region, an impact that implies an interaction between the extensions and the protein core.
Figure 8.
Cysteine variants bind DNA with reduced affinity. A, OstR-C3A binding emrB promoter DNA. DNA (0.2 nm) was titrated with increasing concentration of OstR-C3A (1–200 nm; lanes 2–13). Lane 1, free DNA only. Free DNA and protein–DNA complex are shown as FD and C1–C3, respectively, at the right. C and E, EMSA showing binding of OstR-C4 and OstR-C169A to promoter DNA, respectively. B, D, F, normalized complex fraction for OstR-C3A, OstR-C4A, and OstR-C169A, respectively, plotted as a function of protein concentration. Error bars, S.D. of three replicates.
Cys169 participates in intramolecular disulfide bond formation
The role of individual cysteines in disulfide bond formation was investigated by incubation of OstR variants with increasing concentration of H2O2 (Fig. 7, A, C, and E) or CuCl2 (Fig. 7, B, D, and E), followed by analysis by SDS-PAGE. All variants were readily oxidized on exposure to air (lanes 3). Intramolecular disulfide bonds were seen for both OstR-C3A and OstR-C4A, as evidenced by a product that migrated faster than reduced, monomeric OstR (Fig. 7, A–D). By contrast, such faster-migrating monomeric species were absent with oxidized OstR-C169A (Fig. 7, E and F), suggesting that Cys169 is required for intramolecular disulfide bond formation. Oxidized OstR-C3A and OstR-C4A also formed two prominent dimeric species, whereas oxidized WT OstR featured three dimeric species and oxidized OstR-C169A formed only one. Higher-order oligomeric species were observed in the presence of CuCl2, but not in the case of H2O2-mediated oxidation.
All cysteines play a structural role
Differential scanning fluorometry showed that OstR-C3A and OstR-C4A are thermally more stable (Tm = 44.7 ± 0.1 and 50.6 ± 0.1 °C, respectively) than WT OstR (Fig. 9A (red dotted line) and Fig. 7B (blue dotted line), respectively). The addition of oxidants (H2O2 or CuCl2), however, resulted in significant destabilization of these mutant variants, as reflected in high initial fluorescence and the absence of a melting transition (Fig. 9, A (blue dashed line) and B (purple dashed line), respectively, and Table S3). Upon binding to ligands such as DNA or zinc, both OstR-C3A and OstR-C4A variants showed significant increase in thermal stability (Table S3). By contrast, OstR-C169A exhibited a high initial fluorescence and a decrement in fluorescence as a function of temperature and no identifiable melting transition (Fig. 9C, orange dotted line). Such a pattern of interaction with SYPRO Orange suggests that OstR-C169A exposes hydrophobic residues, indicating improper folding and a feature reminiscent of molten globule formation. The addition of oxidant had no effect on this pattern. In the presence of zinc, OstR-C169A behaved as a globular protein with a clear melting transition (Fig. 9C, green dashed line). Incubation of OstR-C169A with DNA also resulted in protein stabilization (Table S3), consistent with the ability of this protein variant to bind DNA (albeit with low affinity; Fig. 8 (E and F)). That substitution of either cysteine impacted thermal stability is consistent with the inference that both N- and C-terminal extensions contact the protein core.
Figure 9.
Differential scanning fluorometry showing thermal stability of cysteine variants. Fluorescence intensity reflects binding of SYPRO Orange to hydrophobic regions of denatured protein as a function of temperature. A, reduced OstR-C3A (red dotted line; left and bottom axes) and H2O2 oxidized OstR-C3A (blue dashed line; right and top axes). B, reduced OstR-C4A (teal dotted line; left and bottom axes) and H2O2-oxidized OstR-C4A (purple dashed line; right and top axes). C, reduced OstR-C169A (orange dotted line; left and bottom axes) and OstR-C169A bound to Zn2+ (green dashed line; right and top axes).
All three cysteine variants bind to zinc
The Zn2+-mediated changes in thermal stability of OstR cysteine variants suggested metal binding. All OstR variants were incubated with 2 mm ZnCl2, followed by denaturation and measurement of released Zn2+ by chelation to PAR. Significant absorbance peaks at ∼520 nm were observed for all three variants, indicating release of Zn2+ from denatured proteins (Fig. 10, A–C). This suggests that none of the three cysteines were required to coordinate Zn2+ in OstR. Based on this information, we opted to assess whether Zn(II) binding modulated cysteine oxidation in vitro. The OstR variants were incubated with 2 mm ZnCl2 for 30 min, followed by oxidation with 12 mm H2O2 for another 30 min. SDS-PAGE analysis showed that both OstR-C3A and OstR-C4A formed intramolecular disulfide bonds, dimeric species, and higher-order oligomers (Fig. 10D, lanes 3 and 5, respectively). The OstR-C169A variant also formed dimers (faint dimeric species in Fig. 10D, lane 7) upon oxidation, but due to the presence of both metal and oxidant, the protein precipitated significantly. That Zn2+ did not preclude cysteine oxidation is consistent with the interpretation that neither Cys3, Cys4, nor Cys169 is required for Zn2+ coordination.
Figure 10.
All cysteine variants bind Zn2+. A–C, release of Zn2+ from denatured OstR-C3A, OstR-C4A, and OstR-C169A, respectively, determined by absorbance of Zn2+ in complex with PAR (peak absorbance at 520 nm). D, identified cysteine mutants were incubated with 2 mm ZnCl2 for 30 min followed by treatment with 12 mm H2O2 for another 30 min (lanes 3, 5, and 7). Lanes 2, 4, and 6, Zn2+-treated OstR-C3A, OstR-C4A, and OstR-C169A, respectively.
Discussion
Antibiotic efflux by EmrB
Trimethoprim inhibits dihydrofolate reductase, and it is frequently used in combination with sulfamethoxazole (as co-trimoxazole) to treat Burkholderia infections. In B. pseudomallei, the RND efflux pump BpeEF-OprC has been shown to extrude trimethoprim (28). Our data identify the EmrB drug transporter encoded by BTH_I0022 as another primary transporter of trimethoprim, as evidenced by the increased sensitivity imposed by its inactivation (Fig. 3E and Table 1).
Expression of the B. cepacia EmrB ortholog (BcrA) in E. coli resulted in increased resistance to nalidixic acid and tetracycline (trimethoprim was not tested) (22). The emrB expression is increased in the ΔostRX strain that shows enhanced resistance to both nalidixic acid and tetracycline (Fig. 3 (B and C) and Table 1), consistent with facilitated efflux. However, we cannot rule out that genes encoding other drug transporters are up-regulated as a result of inactivation of ostR, thereby conferring or contributing to the observed resistance. Because inactivation of the emrB gene did not result in increased sensitivity to nalidixic acid, we also infer that other mechanisms for efflux of this compound exist.
By contrast, the increased resistance to tetracycline in the ΔemrBX strain points to a more intricate cross-talk between efflux systems. The tetracycline derivative doxycycline was previously shown to induce expression of two RND efflux systems, AmrAB-OprA at intermediate concentrations of doxycycline, followed by BpeEF-OprC at very high drug concentrations (23). It was also noted that the addition of an RND inhibitor resulted in a 2–3-fold increase in expression of amrB, an overexpression that was associated with elevated resistance to certain antibiotics. Consistent with these observations, we find that amrB expression is induced in ΔemrBX cells grown with tetracycline, an induction that does not occur in ΔostRX cells. This is consistent with the interpretation that failure to export tetracycline through EmrB leads to induction of amrB (Fig. 4). Taken together, these observations point to a complex mechanism for induction of optimal efflux systems, which should be considered when attempting to restore multidrug resistance by controlling activity of specific efflux pumps.
Redox-dependent differential regulation of emrB by OstR
OstR represses expression of emrB and modulates its expression under conditions of oxidative stress (Fig. 2). Preferred binding of OstR to the emrB promoter is consistent with the observed regulation. Reduced and H2O2-oxidized OstR bind to emrB promoter DNA with comparable affinity (Fig. 6, A–D), yet the presence of oxidant affects gene expression (Fig. 2C). Changes in gene expression due to features other than altered DNA-binding affinity of the cognate transcription factor have been reported previously. For example, the redox-sensitive MarR homolog HypR in Bacillus subtilis binds to DNA with similar affinity regardless of redox state, but only oxidized HypR activates target gene expression (29). Similarly, PecS from the plant pathogen Pectobacterium atrosepticum binds to promoter DNA with comparable affinity at pH 7.4 and 8.3, but it represses gene expression only at alkaline pH; in the case of PecS, the differential ability to repress gene activity was ascribed to its pH-dependent ability to alter DNA topology (30). Reduced and oxidized OstR may likewise impose distinct conformational changes in promoter DNA that manifest as differential ability to compete with RNA polymerase for binding.
OstR also senses Cu2+, which results in further repression of emrB (Fig. 2C). The opposite effects of H2O2 and Cu2+ suggest that the different oxidants result in different OstR oxidation products and therefore distinct effects on gene expression; such opposite effects also manifest in the distinct changes in sensitivity to these oxidants imposed by deletion of ostR, consistent with differential control of genes in the OstR regulon (Fig. 3, F and G). Reduced and Cu2+-oxidized OstR also bind to promoter DNA with equivalent affinity (Table S2). By comparison, the B. thailandensis MarR homolog BifR, which is also subject to Cu2+-mediated oxidation, forms a dimer of dimers when oxidized and acts as a “superrepressor” to attenuate gene expression further even though oxidation has little effect on DNA-binding affinity (15). Taken together, our data suggest that reduced OstR represses emrB and that it operates as a rheostat in the presence of oxidants to optimize emrB expression.
Modification of cysteines affects stability of OstR
Oxidation of OstR and the Cys-to-Ala mutants results in formation of dimeric species (Figs. 5 (E and F) and 7). As evidenced by the formation of an oxidized species that migrates faster than reduced, monomeric OstR, we also infer that Cys169 can participate in intramolecular disulfide linkages with either Cys3 or Cys4 (Fig. 7). The presence of three dimeric species upon oxidation of WT protein (two dimeric species with OstR-C3A and OstR-C4A and one dimer with oxidized OstR-C169A) could be explained by the formation of intermolecular disulfide bonds involving either Cys3 or Cys4 from two OstR dimers alone (as seen for OstR-C169A) or in combination with intramolecular disulfide linkages between Cys169 and either Cys3 or Cys4, resulting in oxidized species with distinct mobilities.
Differential scanning fluorometry reveals that OstR-C169A exposes hydrophobic residues (Fig. 9C), suggesting that it is intrinsically unfolded or that it exists in a molten globule state (25); considering that a closely packed protein conformation with a distinct melting transition is restored in the presence of Zn2+ or DNA, we speculate that it may be the latter. By contrast, OstR-C3A and OstR-C4A mutants are thermally more stable compared with WT OstR (Tables S1 and S3). That thermal stability is altered as a result of either mutation indicates that neither N- nor C-terminal extensions are disordered; rather, they are docked to the core fold of the protein (consistent with the prediction for participation in protein interaction; Fig. 5C).
Notably, oxidation of both OstR-C3A and OstR-C4A leads to a fluorescence profile similar to that observed for reduced OstR-C169A, indicating disruption of the protein fold (Fig. 9, A and B). The unusual electrostatic surface potential that characterizes one lobe of OstR in which positively charged residues are predicted to be clustered (Fig. 1, C and D) affords a clue to the observed destabilization of cysteine mutants. We propose that the C-terminal extension interacts with this positive patch and that a thiolate anion of Cys169 is required to neutralize excess positive charges; electrostatic interactions that stabilize the thiolate anion would lower the pKa of cysteine compared with its intrinsic pKa of ∼8.5 (31). In the absence of Cys169, electrostatic repulsion would result in a disruption of the compact protein fold. This model also explains the need for the vicinal cysteines in the N-terminal extension and the observed destabilization that occurs on oxidation of OstR-C3A and OstR-C4A; upon formation of an intramolecular disulfide bridge between either Cys3 or Cys4 and Cys169, the Cys169 thiolate is lost. Protein stability, therefore, requires the thiolate form of the neighboring N-terminal cysteine, rationalizing why oxidation of an OstR variant with only one N-terminal cysteine would lead to a loss of the protein fold.
The addition of ZnCl2 increases thermal stability of WT OstR and all cysteine variants (Tables S1 and S3), and the PAR assay supports the conclusion that OstR binds Zn2+ (Figs. 5D and 10 (A–C)). Because Zn2+-binding restores a compact fold to OstR-C169A, one possibility is that it coordinates residue(s) in the N-terminal extension and near the positively charged patch (Fig. 1, D and E), thereby promoting association of the N-terminal vicinal cysteines (which are preceded by His) with the positively charged patch, supplying a required negative charge.
Taken together, our data identify B. thailandensis OstR as a novel oxidant-sensitive regulator of the downstream gene encoding an EmrB family transporter. Its unique N- and C-terminal extensions contact the core fold of the protein, in the process modulating protein stability and responses to oxidants. Substrates for EmrB include commonly used antibiotics; whereas inactivation of emrB leads to trimethoprim sensitivity, it results in markedly increased resistance to tetracycline, a resistance that correlates with induced expression of the RND efflux pump AmrAB-OprA, illustrating the intricate mechanisms by which expression of genes encoding efflux pumps is optimized depending on cellular concentrations of inducing antibiotic.
Experimental procedures
Protein modeling and purification
PyMOL was used to visualize the model of OstR, which was generated by homology modeling using SwissModel in automated mode and Protein Data Bank entry 4AIK as template (selected as allowing the most residues of OstR to be included in the model).
The gene encoding B. thailandensis E264 OstR was amplified from genomic DNA using primers OstR_tran_Fw and OstR_tran_Rv containing NdeI and EcoRI restriction sites, respectively (Table S4). The PCR product was cloned into pET28b after restriction digestion with NdeI and EcoRI to express N-terminal His6-tagged protein. The construct was verified by sequencing and then transformed into E. coli BL21(DE3). Cells were grown until mid-exponential phase at 37 °C in LB with 50 μg/ml kanamycin, followed by induction using 1 mm isopropyl β-d-1-thiogalactopyranoside. After 1 h, cells were pelleted at 4 °C and stored at −80 °C. Cell pellets were thawed, and cells were resuspended in wash buffer (50 mm sodium phosphate (pH 7.0), 250 mm NaCl, 5% glycerol). Lysozyme (final concentration 1 mg/ml) and 2 μl of DNase I with 50 μl of 10× DNase I reaction buffer were added to each 5-ml cell suspension, which was incubated at 4 °C for 60 min. Lysate was obtained by centrifugation at 10,000 × g for 1 h at 4 °C and incubated with 1 ml of HIS-Select nickel affinity beads (previously washed with two volumes of distilled H2O and one volume of wash buffer) at 4 °C for 1 h. The beads were then transferred to a gravity flow column to elute protein by the addition of increasing concentrations of imidazole (10–200 mm). Pure protein fractions were detected by SDS-PAGE, pooled, and concentrated using Amicon centrifugal filter units (Millipore). Protein was dialyzed overnight against 50 mm sodium phosphate (pH 7.0), 250 mm NaCl, 10% glycerol, and 5 mm 2-mercaptoethanol. Concentrations were determined based on the absorbance at 280 nm, using a calculated extinction coefficient of 7,450 m−1 cm−1. To create cysteine-to-alanine substitutions, overhanging primers (Table S4) were used to amplify the whole plasmid harboring the ostR gene, reactions were incubated with DpnI, and the DNA was transformed into E. coli TOP10 (Invitrogen); plasmids were sequenced for verification. Mutant proteins were purified as described above.
To determine secondary structure of the protein, the far-UV CD spectrum was measured using a Jasco J-815 CD spectrophotometer (32). In brief, 0.2 mg/ml protein in CD buffer (20 mm sodium phosphate (pH 7.0), 20 mm NaCl, 0.8% glycerol, and 1.5 mm DTT) was used to measure ellipticity. A quartz cuvette with 0.1-cm path length was used after equilibrating protein the sample at room temperature. The K2D program from Dichroweb was used to calculate secondary structure of the protein (33–35). The fitness of data was determined by the normalized root mean square deviation value at a range of 0.094–0.110.
To determine oligomeric states upon oxidation, WT protein and cysteine mutants were treated with increasing concentration of the oxidants (H2O2 and CuCl2) on ice for 20 min followed by termination of the reaction by adding Laemmli sample buffer without 2-mercaptoethanol. Samples were analyzed by SDS-PAGE after heating at 90 °C for 8 min, followed by staining with Coomassie Brilliant Blue.
DNA binding
To determine protein–DNA interaction, an EMSA was performed. Increasing concentration of OstR was incubated on ice for 30 min with 146-bp ostR-emrB intergenic DNA amplified from B. thailandensis E264 genomic DNA with primers BTH_22int_Fw and BTH_22int_Rv (Table S4) and 5′-end–labeled using [γ-32P]ATP and T4 polynucleotide kinase. Protein and DNA were mixed in binding buffer containing 20 mm Tris-HCl (pH 8.0), 50 mm NaCl, 0.1 mm EDTA, and 0.1 mm DTT. Pre-run 8% polyacrylamide gels were used to separate free DNA and protein–DNA complex, followed by phosphorimaging using a Typhoon PhosphorImager (GE Healthcare). Densitometric data were quantified by ImageQuant version 5.1 and are presented based on an average of three replicates ± S.D. KaleidaGraph version 4.0 (Synergy Software) was used to calculate the equilibrium dissociation constant (Kd) by fitting the data to the equation, fmax × (PnH/Kd)/(1 + PnH/KdnH), where fmax is maximal saturation, P is OstR concentration, and nH is the Hill coefficient. EMSA was performed also at 37 °C.
To determine the effect of oxidants on protein–DNA binding, increasing concentrations of oxidized OstR (previously oxidized with 2 mm H2O2 or 5 μm CuCl2) were incubated with intergenic DNA, and EMSAs were performed as described above. To assess the specificity of DNA binding, protein and labeled DNA was titrated with increasing concentrations of unlabeled specific 146-bp operator DNA or with nonspecific unlabeled DNA (pET28b), followed by EMSA.
Thermal stability assay
3 μm protein was added to thermal stability assay buffer (50 mm Tris (pH 8.0), 50 mm NaCl) along with SYPRO Orange (5×). Increasing temperature from 5 to 94 °C (in 1 °C increments) was used in an Applied Biosystems 7500 real-time PCR system for protein unfolding, which was measured by fluorescence of SYPRO Orange bound to the hydrophobic core of denatured protein. Corrected fluorescence emission was obtained by subtracting control values. SigmaPlot version 9 was used for determination of melting temperature by regression analysis using a four-parameter sigmoidal equation. The Tm was calculated from the averages of three technical replicates obtained from each of three independent experiments and is presented as mean ± S.D.
Metal binding
Proteins were incubated with 50 mm 2,2′-bipyridyl at 4 °C to remove any bound metals. Bipyridyl-treated proteins were dialyzed overnight against buffer A (50 mm Tris-HCl (pH 8.0), 250 mm NaCl, 2 mm 2-mercaptoethanol, and 10% glycerol) and then incubated with 1 mm ZnCl2 on ice for 30 min and again dialyzed overnight against buffer A to remove unbound metal. Proteins were denatured by adding 1% SDS in assay buffer (20 mm Tris-HCl (pH 7.5), 50 mm sodium chloride) followed by heating at 90 °C for 10 min. 100 μm PAR was added to each sample and buffer control, and an Agilent 8453 spectrophotometer was used to measure absorbance from 320 to 630 nm. Fluorescence data are reported as the mean of three technical replicates from representative experiments.
Confirmation of transposon mutants and removal of antibiotic resistance cassettes
ostR (BTH_I0021) and emrB (BTH_I0022) were disrupted by the insertion of transposon T8 at position 98 of the ostR ORF to generate strain BTH_I0021–153:ISlacZ-hah-Tc and T23 at position 89 of emrB (strain BTH_I0022–124::ISlacZ-PrhaBo-Tp/FRT). Transposon inserted mutants (ΔostR and ΔemrB) were obtained from the Manoil laboratory and grown on LB-agar plates with 80 μg/ml tetracycline and 50 μg/ml trimethoprim, respectively (21). Single colonies were grown overnight after inoculating in LB medium with the respective antibiotics. To verify correct insertion of transposons, PCR was performed using primers BTH_21_Fw, BTH_22int_Fw, and LacZ_148 (Table S4).
Transposon insertion mutants ΔemrB and ΔostR harbor trimethoprim and tetracycline antibiotic cassettes, respectively. Plasmids pFLPe4 or pCRE3 (a generous gift from H. Schweizer) were used to remove the antibiotic resistance cassettes from ΔemrB or ΔostR, respectively (36, 37). In brief, Flp/Cre recombinase-encoding pFLPe4 or pCRE3 plasmid was transformed into E. coli TOP10 cells. Tri-parental mating was performed to transfer plasmids harboring Flp/Cre recombinase into ΔemrB or ΔostR stains, respectively. Selection was done on LSLB/kanamycin (for pFlpe4) or LSLB/ampicillin (for pCRE3) plates at 30 °C (where LSLB denotes low-salt LB). To induce flp or cre expression, selected colonies were streaked on LSLB/kanamycin/rhamnose (for pFlpe4) or LSLB/ampicillin/rhamnose (for pCRE3) plates. Corresponding antibiotic-sensitive clones were grown at 37 °C to induce loss of the temperature-sensitive pFlpe4/pCRE3. Removal of antibiotic cassettes was confirmed by PCR using primers (Tra_delE_Fw, Tra_delE_Rv; Cre_del21_Fw, Cre_del21_Rv; Table S4). ΔemrB and ΔostR strains in which antibiotic resistance cassettes were removed were named ΔemrBX and ΔostRX.
Genetic complementation
ostR with 170 bp upstream of the coding sequence was amplified using primers OstR_XbaI_Fw and OstR_KpnI_Rev (Table S4), which contain XbaI and KpnI sites, respectively. Digested PCR product was then cloned into the gentamicin-resistant broad host range cloning vector pBBR1-MCS5 (38). The construct was transformed into E. coli TOP10 (Invitrogen), and plasmid was verified by sequencing.
Tri-parental mating was performed to transfer plasmid harboring ostR to the B. thailandensis E264 ΔostR strain. Conjugation was performed by mixing overnight cultures of donor (E. coli TOP10 containing pBBR1-MCS5 plasmid harboring ostR, grown in the presence of 80 μg/ml gentamicin), recipient (ΔostR grown in the presence of 80 μg/ml tetracycline), and helper strain (HB101(pRK2013:Tn7)) in a ratio of 1:1:2. Residual antibiotics were removed by centrifuging the cells and washing pellets with 1.0 ml of LB four times. The pellets were then resuspended in 50 μl of LB and spotted on a preheated LB agar plate. After 12 h of incubation, cells were scraped off and resuspended in 1.0 ml of LB, and serial dilutions were spotted on LB-agar plates with 80 μg/ml tetracycline, 250 μg/ml gentamicin, and 8 μg/ml chloramphenicol. Individual colonies were screened to verify trans-conjugants by PCR with primers Con_pBBR_XbaI and Con_pBBR_KpnI (Table S4). The complemented strain is referred to as ΔostRc. Empty plasmid pBBR1-MCS5 without the ostR gene was similarly transferred to WT B. thailandensis, and the strain was referred to as WTe.
Gene expression analysis
WT and mutant strains were grown overnight, followed by a 1:100 dilution of each culture in fresh LB media. Cultures were grown until A600 of ∼0.5, and cells were harvested by centrifugation and washed with ice-cold diethyl pyrocarbonate–treated water. To determine the effect of oxidants (H2O2 or CuCl2) and ZnCl2 on gene expression, cells were grown until A600 of ∼0.5 and treated with 2 mm H2O2, 2 mm CuCl2, or 2 mm ZnCl2 for 30 min, followed by harvesting and washing with diethyl pyrocarbonate–treated water. The effect of tetracycline on gene expression in ΔostRX and ΔemrBX strains was determined after growing the cells overnight with tetracycline (5, 10, 20, 50, and 128 μg/ml). Cells were stored at −80 °C. The Illustra RNAspin Mini Isolation kit (GE Healthcare) was used to isolate total RNA. Any genomic DNA contamination was removed by Turbo DNase (Ambion) treatment, and PCR was performed using fresh RNA samples to verify the absence of genomic DNA. NanoDrop (Thermo Scientific) was used to measure the concentration of RNA. cDNA was prepared using gene-specific primers (Table S4) and 1 μg of RNA with 1 mm MgCl2, 1 mm dNTP, 10 units of avian myeloblastosis virus reverse transcriptase (New England Biolabs) in 1× avian myeloblastosis virus reverse transcriptase buffer. The reaction mixture was incubated at 42 °C for 60 min. The qPCR was performed using a QuantStudio 6 Flex Real-Time PCR system and SYBR Green I (Sigma) to detect gene expression. Data were normalized to the reference gene glutamate synthase large subunit, which was amplified using primers GluSyn_qPCR_Fw and GluSyn_qPCR_Rv and expressed as 2−ΔCT. The comparative CT method (2−ΔΔCT) was used to analyze the effect of antibiotics, oxidants, and ZnCl2 on gene expression. All expression analyses were performed in biological triplicates and are presented as mean ± S.D.
Plate assay and MIC determination
Sensitivity to antibiotics and oxidants was assessed by plate assays. Overnight cultures of WT and mutant strains were diluted 1:100 using LB medium and grown at 37 °C to A600 ∼0.6. To assess the susceptibility to antibiotics, 5 μl of 10-fold serial dilutions were spotted on LB-agar plates containing the desired antibiotic (nalidixic acid, tetracycline, gentamicin, or trimethoprim). To determine the response to oxidants, 1 ml of each culture was incubated with 5 mm H2O2 or CuCl2 for 30 min at 37 °C, followed by spotting 5 μl of 10-fold serial dilutions on LB-agar plates. Plates were incubated at 37 °C for 18–22 h.
MICs were estimated by preparing 2-fold dilutions of antibiotics in LB in 96-well microtiter plates. Overnight cultures were freshly diluted 1:100 in LB and grown to exponential phase; an equal volume of culture was added to the microtiter wells at an A600 of ∼0.6. Cell growth was determined visually after incubation at 37 °C for 24 h.
Author contributions
A. S. and A. Grove conceptualization; A. S. formal analysis; A. S. and A. Grove validation; A. S., B. W. G., and A. Gupta investigation; A. S. visualization; A. S., A. Gupta, and A. Grove methodology; A. S. and A. Grove writing-original draft; A. S., A. Gupta, and A. Grove writing-review and editing; A. Grove supervision; A. Grove funding acquisition.
Supplementary Material
Acknowledgments
We thank H. Schweizer (University of Florida), for providing plasmids pFLPe4 and pCRE3, Z. Stewart for participation at early stages of this project, and M. Newcomer for use of the Nanodrop.
This work was supported by National Science Foundation Grant MCB-1714219 (to A. Grove) and by a donation from P. Campbell in support of undergraduate research. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1–S4.
- ROS
- reactive oxygen species
- EmrB
- E. coli multidrug resistance B
- EMSA
- electrophoretic mobility shift assay
- MarR
- multiple antibiotic resistance regulator
- MIC
- minimal inhibitory concentration
- OstR
- oxidant-sensing transport regulator
- RND
- resistance-nodulation-division
- PAR
- 4-(2-pyridylazo) resorcinol
- LB
- lysogeny broth
- LSLB
- low-salt LB
- qPCR
- quantitative PCR.
References
- 1. Deochand D. K., and Grove A. (2017) MarR family transcription factors: dynamic variations on a common scaffold. Crit. Rev. Biochem. Mol. Biol. 52, 595–613 10.1080/10409238.2017.1344612 [DOI] [PubMed] [Google Scholar]
- 2. Cohen S. P., Levy S. B., Foulds J., and Rosner J. L. (1993) Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J. Bacteriol. 175, 7856–7862 10.1128/jb.175.24.7856-7862.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhodes K. A., and Schweizer H. P. (2016) Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 28, 82–90 10.1016/j.drup.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piddock L. J. (2006) Multidrug-resistance efflux pumps: not just for resistance. Nat. Rev. Microbiol. 4, 629–636 10.1038/nrmicro1464 [DOI] [PubMed] [Google Scholar]
- 5. Saier M. H., Jr (2016) Transport protein evolution deduced from analysis of sequence, topology and structure. Curr. Opin. Struct. Biol. 38, 9–17 10.1016/j.sbi.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Titball R. W., Burtnick M. N., Bancroft G. J., and Brett P. (2017) Burkholderia pseudomallei and Burkholderia mallei vaccines: are we close to clinical trials? Vaccine 35, 5981–5989 10.1016/j.vaccine.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 7. Scoffone V. C., Chiarelli L. R., Trespidi G., Mentasti M., Riccardi G., and Buroni S. (2017) Burkholderia cenocepacia infections in cystic fibrosis patients: drug resistance and therapeutic approaches. Front. Microbiol. 8, 1592 10.3389/fmicb.2017.01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu Y., Kim H. S., Chua H. H., Lin C. H., Sim S. H., Lin D., Derr A., Engels R., DeShazer D., Birren B., Nierman W. C., and Tan P. (2006) Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 6, 46 10.1186/1471-2180-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahl J. W., Vazquez A. J., Hall C. M., Busch J. D., Tuanyok A., Mayo M., Schupp J. M., Lummis M., Pearson T., Shippy K., Colman R. E., Allender C. J., Theobald V., Sarovich D. S., Price E. P., et al. (2016) The effects of signal erosion and core genome reduction on the identification of diagnostic markers. MBio 7, e00846–16 10.1128/mBio.00846-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haraga A., West T. E., Brittnacher M. J., Skerrett S. J., and Miller S. I. (2008) Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect. Immun. 76, 5402–5411 10.1128/IAI.00626-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang F. C. (2011) Antimicrobial actions of reactive oxygen species. MBio 2, e00141–11 10.1128/mBio.00141-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keyer K., Gort A. S., and Imlay J. A. (1995) Superoxide and the production of oxidative DNA damage. J. Bacteriol. 177, 6782–6790 10.1128/jb.177.23.6782-6790.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imlay J. A. (2013) The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 10.1038/nrmicro3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemire J. A., Harrison J. J., and Turner R. J. (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 11, 371–384 10.1038/nrmicro3028 [DOI] [PubMed] [Google Scholar]
- 15. Gupta A., Fuentes S. M., and Grove A. (2017) Redox-sensitive MarR homologue BifR from Burkholderia thailandensis regulates biofilm formation. Biochemistry 56, 2315–2327 10.1021/acs.biochem.7b00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pande A., Veale T. C., and Grove A. (2018) Gene regulation by redox-sensitive Burkholderia thailandensis OhrR and its role in bacterial killing of Caenorhabditis elegans. Infect. Immun. 86, e00322–00318 10.1128/IAI.00322-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antelmann H., and Helmann J. D. (2011) Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 14, 1049–1063 10.1089/ars.2010.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hao Z., Lou H., Zhu R., Zhu J., Zhang D., Zhao B. S., Zeng S., Chen X., Chan J., He C., and Chen P. R. (2014) The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat. Chem. Biol. 10, 21–28 10.1038/nchembio.1380 [DOI] [PubMed] [Google Scholar]
- 19. Gupta A., Pande A., Sabrin A., Thapa S. S., Gioe B. W., and Grove A. (2019) MarR family transcription factors from Burkholderia species: hidden clues to control of virulence-associated genes. Microbiol. Mol. Biol. Rev. 83, e00039–18 10.1128/MMBR.00039-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quade N., Mendonca C., Herbst K., Heroven A. K., Ritter C., Heinz D. W., and Dersch P. (2012) Structural basis for intrinsic thermosensing by the master virulence regulator RovA of Yersinia. J. Biol. Chem. 287, 35796–35803 10.1074/jbc.M112.379156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher L. A., Ramage E., Patrapuvich R., Weiss E., Brittnacher M., and Manoil C. (2013) Sequence-defined transposon mutant library of Burkholderia thailandensis. MBio 4, e00604–13 10.1128/mBio.00604-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wigfield S. M., Rigg G. P., Kavari M., Webb A. K., Matthews R. C., and Burnie J. P. (2002) Identification of an immunodominant drug efflux pump in Burkholderia cepacia. J. Antimicrob. Chemother. 49, 619–624 10.1093/jac/49.4.619 [DOI] [PubMed] [Google Scholar]
- 23. Biot F. V., Lopez M. M., Poyot T., Neulat-Ripoll F., Lignon S., Caclard A., Thibault F. M., Peinnequin A., Pagès J. M., and Valade E. (2013) Interplay between three RND efflux pumps in doxycycline-selected strains of Burkholderia thailandensis. PLoS One 8, e84068 10.1371/journal.pone.0084068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bordelon T., Wilkinson S. P., Grove A., and Newcomer M. E. (2006) The crystal structure of the transcriptional regulator HucR from Deinococcus radiodurans reveals a repressor preconfigured for DNA binding. J. Mol. Biol. 360, 168–177 10.1016/j.jmb.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 25. Deochand D. K., Perera I. C., Crochet R. B., Gilbert N. C., Newcomer M. E., and Grove A. (2016) Histidine switch controlling pH-dependent protein folding and DNA binding in a transcription factor at the core of synthetic network devices. Mol. Biosyst. 12, 2417–2426 10.1039/C6MB00304D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones D. T., and Cozzetto D. (2015) DISOPRED3: precise disordered region predictions with annotated protein-binding activity. Bioinformatics 31, 857–863 10.1093/bioinformatics/btu744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kushwaha A. K., Deochand D. K., and Grove A. (2015) A moonlighting function of Mycobacterium smegmatis Ku in zinc homeostasis? Protein Sci. 24, 253–263 10.1002/pro.2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar A., Chua K. L., and Schweizer H. P. (2006) Method for regulated expression of single-copy efflux pump genes in a surrogate Pseudomonas aeruginosa strain: identification of the BpeEF-OprC chloramphenicol and trimethoprim efflux pump of Burkholderia pseudomallei 1026b. Antimicrob. Agents Chemother. 50, 3460–3463 10.1128/AAC.00440-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palm G. J., Khanh Chi B., Waack P., Gronau K., Becher D., Albrecht D., Hinrichs W., Read R. J., and Antelmann H. (2012) Structural insights into the redox-switch mechanism of the MarR/DUF24-type regulator HypR. Nucleic Acids Res. 40, 4178–4192 10.1093/nar/gkr1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deochand D. K., Meariman J. K., and Grove A. (2016) pH-dependent DNA distortion and repression of gene expression by Pectobacterium atrosepticum PecS. ACS Chem. Biol. 11, 2049–2056 10.1021/acschembio.6b00168 [DOI] [PubMed] [Google Scholar]
- 31. Poole L. B. (2015) The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 80, 148–157 10.1016/j.freeradbiomed.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta A., and Grove A. (2014) Ligand-binding pocket bridges DNA-binding and dimerization domains of the urate-responsive MarR homologue MftR from Burkholderia thailandensis. Biochemistry 53, 4368–4380 10.1021/bi500219t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Micsonai A., Wien F., Kernya L., Lee Y. H., Goto Y., Réfrégiers M., and Kardos J. (2015) Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 112, E3095–E3103 10.1073/pnas.1500851112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whitmore L., and Wallace B. A. (2004) DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 32, W668–W673 10.1093/nar/gkh371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitmore L., and Wallace B. A. (2008) Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89, 392–400 10.1002/bip.20853 [DOI] [PubMed] [Google Scholar]
- 36. Choi K. H., Mima T., Casart Y., Rholl D., Kumar A., Beacham I. R., and Schweizer H. P. (2008) Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomallei. Appl. Environ. Microbiol. 74, 1064–1075 10.1128/AEM.02430-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia E. C. (2017) Burkholderia thailandensis: genetic manipulation. Curr. Protoc. Microbiol. 45, 4C.2.1–4C.2.15 10.1002/cpmc.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kovach M. E., Elzer P. H., Hill D. S., Robertson G. T., Farris M. A., Roop R. M. 2nd, and Peterson K. M. (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166, 175–176 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.