Abstract
Prohibitin 1 (PHB1) is a mitochondrial chaperone whose expression is dysregulated in cancer. In liver cancer, PHB1 acts as a tumor suppressor, but the mechanisms of tumor suppression are incompletely understood. Here we aimed to determine PHB1 target genes to better understand how PHB1 influences liver tumorigenesis. Using RNA-Seq analysis, we found interleukin-8 (IL-8) to be one of the most highly up-regulated genes following PHB1 silencing in HepG2 cells. Induction of IL-8 expression also occurred in multiple liver and nonliver cancer cell lines. We examined samples from 178 patients with hepatocellular carcinoma (HCC) and found that IL-8 mRNA levels were increased, whereas PHB1 mRNA levels were decreased, in the tumors compared with adjacent nontumorous tissues. Notably, HCC patients with high IL-8 expression have significantly reduced survival. An inverse correlation between PHB1 and IL-8 mRNA levels is found in HCCs with reduced PHB1 expression. To understand the molecular basis for these observations, we altered PHB1 levels in liver cancer cells. Overexpression of PHB1 resulted in lowered IL-8 expression and secretion. Silencing PHB1 increased c-Jun N-terminal kinase (JNK) and NF-κB activity, induced nuclear accumulation of c-JUN and p65, and enhanced their binding to the IL-8 promoter containing AP-1 and NF-κB elements. Conditioned medium from PHB1-silenced HepG2 cells increased migration and invasion of parental HepG2 and SK-hep-1 cells, and this was blocked by co-treatment with neutralizing IL-8 antibody. In summary, our findings show that reduced PHB1 expression induces IL-8 transcription by activating NF-κB and AP-1, resulting in enhanced IL-8 expression and release to promote tumorigenesis.
Keywords: c-Jun N-terminal kinase (JNK), NF-κB, migration, invasion, hepatocellular carcinoma, interleukin-8, prohibitin 1
Introduction
Prohibitin 1 (PHB1) is a highly conserved, ubiquitously expressed protein with diverse functions located in multiple cellular compartments. PHB1 was first identified as a mitochondrial chaperone that is essential for function and biogenesis of mitochondria (1). Nuclear PHB1 interacts with many proteins, including retinoblastoma and p53, where it serves as a transcriptional cofactor repressing or activating the transcriptional activities of E2F (2, 3) or p53 (4), respectively. PHB1 was thought to act as a tumor suppressor because its expression was suppressed after partial hepatectomy prior to liver regeneration (5). However, the role of PHB1 in cancer remains controversial because PHB1 is overexpressed in some cancers (6). Nevertheless, PHB1 clearly has a tumor-suppressive role in breast, gastric, and prostate cancers (7, 8). In addition, reduced PHB1 expression occurs in patients with inflammatory bowel disease, a chronic inflammatory condition that predisposes to colon cancer, and overexpression of PHB1 inhibits colitis-associated colon cancer tumorigenesis in mice (9).
Our group reported that hepatic PHB1 expression is reduced during chronic cholestatic injury in both experimental murine models and in humans (10, 11). We also reported that liver-specific Phb1 knockout mice develop hepatocellular carcinoma (HCC)3 (12) and that Phb1 heterozygotes are predisposed to cholestasis-associated cholangiocarcinoma (CCA) (13). We found that hepatic PHB1 expression is down-regulated at the mRNA level in the majority of human patients with HCC and CCA, and reduced PHB1 expression increases the growth of HCC and CCA cells and inversely correlates with tumor growth in the murine CCA model (13). We have identified two potential mechanisms of PHB1's tumor-suppressive effects in the liver: first by negatively regulating the insulin-like growth factor 2 (IGF2)–H19 axis (14) and second by heterodimerizing with MAX to bind and repress E-box–driven gene expression, such as c-MYC (13). A major goal of this work was to identify other targets of PHB1 that are important in liver tumorigenesis.
In the course of our investigation, we uncovered the finding that PHB1 negatively regulates the expression of the pro-tumorigenic interleukin-8 (IL-8) at the transcriptional level. Loss of PHB1 in multiple cancer cell lines increases the expression of IL-8, which in turn increases the migration and invasion properties of these cells. Our findings are confirmed in human HCC samples and suggest that PHB1 plays an important role in suppressing the development of liver cancers by multiple mechanisms.
Results
PHB1 silencing in liver and colon cancer cell lines up-regulates cancer genes
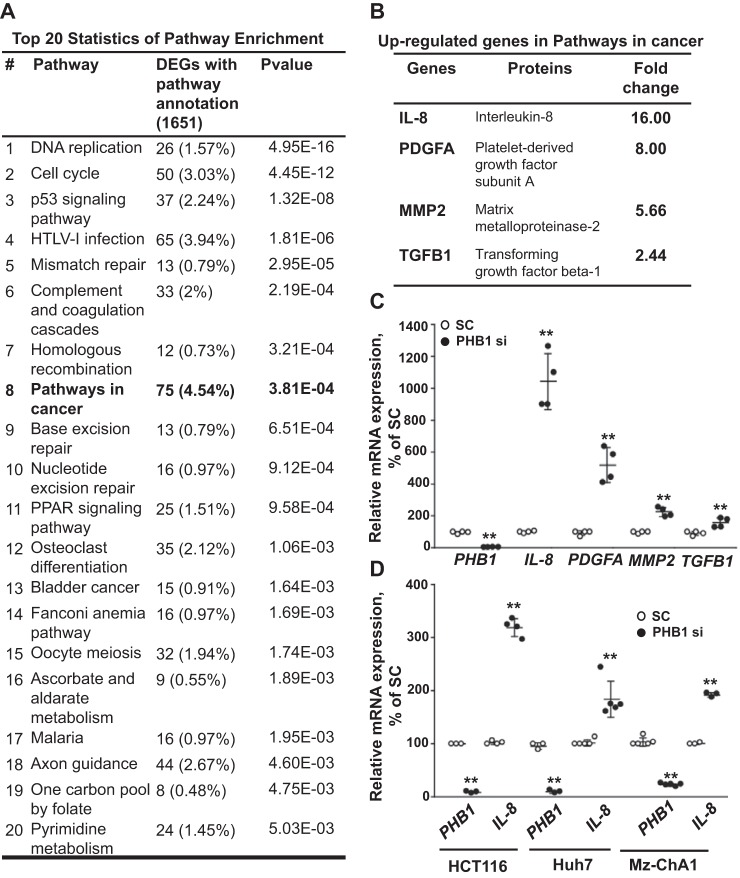
To identify PHB1-regulated genes, PHB1 was silenced in HepG2 cells and then subjected to RNA sequencing. Seventy-two hours of silencing of PHB1 was required to achieve efficient knockdown at the protein level, and no effect was observed on cell viability at this time (Fig. S1, A and B). After pathway enrichment analysis, genes related to cancers were the most highly altered (4.54% of genes) (Fig. 1A). Examining the up-regulated genes in the cancer pathways revealed that IL-8, platelet-derived growth factor subunit A (PDGFA), matrix metalloproteinase-2 (MMP2), and transforming growth factor β-1 (TGFB1) were highly up-regulated (Fig. 1B). These findings were confirmed using real-time PCR in HepG2 cells after 72 h of PHB1 silencing (Fig. 1C). We focused our efforts on IL-8, as it is the most up-regulated gene in the cancer pathways. Silencing PHB1 also raised IL-8 mRNA levels in multiple cancer cell lines of different sources, including HCT116 (colorectal carcinoma), Huh7 (HCC), and Mz-ChA1 (biliary adenocarcinoma) (Fig. 1D and second siRNA shown in Fig. S1C).
Figure 1.
PHB1 silencing up-regulates cancer-related genes in liver and colon cancer cells. A, the top 20 Differentially expressed genes (DEG)-enriched pathways in PHB1-silenced HepG2 cells analyzed by RNA-Seq. B, soluble mediator genes secreted by tumor cells (IL-8, PDGFA, MMP-2, and TGFB1) in the Kyoto Encyclopedia of genes and genomes (KEGG) set pathway in cancer were more than 2-fold up-regulated in PHB1-silenced HepG2 cells. C, quantitative real-time PCR (qRT-PCR) analysis for soluble mediator genes secreted by tumor cells (IL-8, PDGFA, MMP-2, and TGFB1) in PHB1-silenced HepG2 cells. D, IL-8 mRNA expression in PHB1-silenced HCC cells (Huh7), Mz-ChA-1 cells (CCA cell line), and HCT116 cells (colorectal carcinoma cell line). **, p < 0.01 versus respective controls.
Increased IL-8 levels in HCC correlates with poorer patient outcome
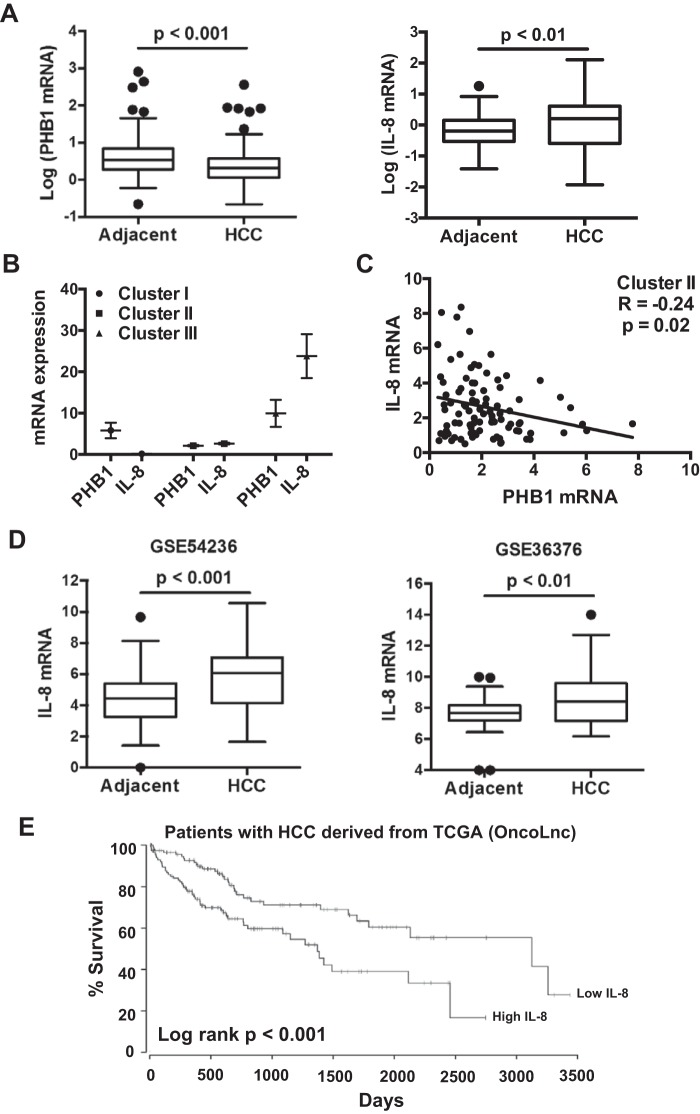
To assess the clinical relevance of PHB1 and IL-8 in HCC, the mRNA levels of PHB1 and IL-8 in 178 patients with HCC were measured. PHB1 mRNA levels were lower in HCC compared with adjacent nontumorous tissue, whereas IL-8 mRNA levels were higher (Fig. 2A). However, there was no correlation between PHB1 and IL-8 mRNA levels when all of the HCC samples were analyzed (p = 0.22) (data not shown). Because the patient samples were likely to be highly heterogeneous, we clustered the patients into three subgroups (subtypes): cluster I with elevated PHB1 but lower IL-8 mRNA levels compared with adjacent NT (58 of 178 patients), cluster II with reduced expression of PHB1 (91 of 178 patients with PHB1 mRNA levels of 28% ± 2% of adjacent NT), and cluster III with higher PHB1 and IL-8 mRNA levels compared with adjacent NT (29 of 178 patients), as shown in Fig. 2B. We examined the correlations between PHB1 and IL-8 expression in each patient subgroup and detected a significant negative correlation between PHB1 and IL-8 mRNA levels in cluster II (p = 0.02), where HCC PHB1 expression was reduced, as shown in Fig. 2C. IL-8 mRNA levels were also elevated compared with adjacent normal samples in multiple gene expression omnibus datasets of HCC (Fig. 2D). Poor patient outcome also correlated with higher IL-8 mRNA levels, as low levels had a 60% chance of survival after 2,500 days compared with a 20% chance of survival in patients with high levels (Fig. 2E).
Figure 2.
PHB1 and IL-8 expression in HCC. A, PHB1 and IL-8 mRNA levels in human HCC and adjacent nontumoral tissues (n = 178). B, HCC samples clustered with the Kmeans algorithm: cluster I with elevated PHB1 and lower IL-8 expression (n = 58), cluster II with reduced PHB1 expression (n = 91), and cluster III with higher PHB1 and IL-8 expression (n = 29). C, Pearson correlation analysis between PHB1 and IL-8 in cluster II. D, IL-8 mRNA levels in HCC compared with adjacent nontumoral tissue in GEO GSE54236 (adjacent, n = 80; HCC, n = 81) and GSE36376 (adjacent, n = 82; HCC, n = 102). E, survival of patients with HCC from the TCGA dataset, classified into IL-8 high tumors (upper 33th percentile, n = 118) or IL-8 low tumors (lower 33th percentile, n = 118).
Loss of PHB1 increases the levels of intracellular and secreted IL-8 in liver cancer cells
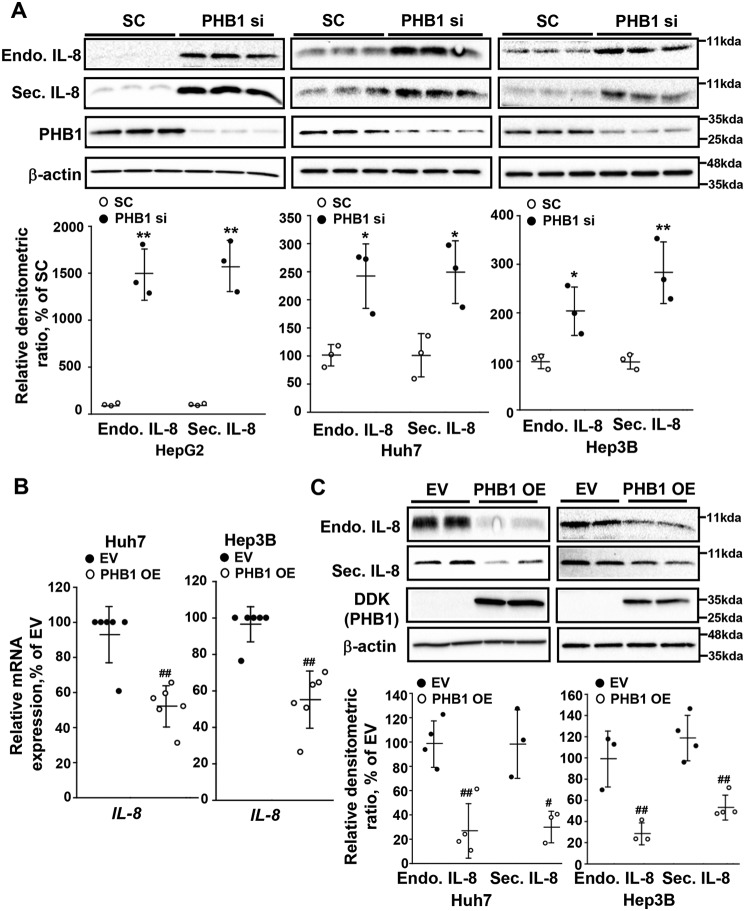
As IL-8 is a cytokine that is secreted from the cell to exert autocrine and paracrine effects on neighboring cells, we examined levels of this protein upon silencing of PHB1 in several liver cancer cell lines. Silencing PHB1 caused an increase in both the intracellular and secreted protein levels of IL-8 compared with the scramble siRNA control in HepG2, Huh7, and Hep3B cell lines (Fig. 3A). Conversely, when PHB1 was overexpressed in Huh7 and Hep3B cells, IL-8 mRNA levels fell by 50–60% (Fig. 3B), whereas intracellular IL-8 protein levels dropped by 75% (Fig. 3C). A significant drop in secreted IL-8 protein (>50%) was also observed in both liver cancer cell lines (Fig. 3C). Overexpression of PHB1 was not explored in HepG2 cells, as basal IL-8 expression is very low (Fig. 3A).
Figure 3.
PHB1 negatively regulates IL-8 expression. A, Western blot analysis of intracellular (Endo) and secreted (Sec) IL-8 in PHB1-silenced HepG2, Huh7, and Hep3B cells. B and C, IL-8 mRNA expression (B) and (C) Western blot analysis of intracellular and secreted IL-8 in PHB1-overexpressing Huh7 and Hep3B cells. All densitometric values were normalized to β-actin. Results are expressed as mean ± S.E. of the mean from at least three independent experiments. *, p < 0.05; **, p < 0.01 versus SC. #, p < 0.05; ##, p < 0.01 versus respective controls. SC, scramble siRNA; PHB1 si, PHB1 siRNA; EV, empty vector; PHB1 OE, PHB1 overexpression vector.
The PHB1 silencing–mediated increase in IL-8 levels is JNK- and NF-κB–dependent
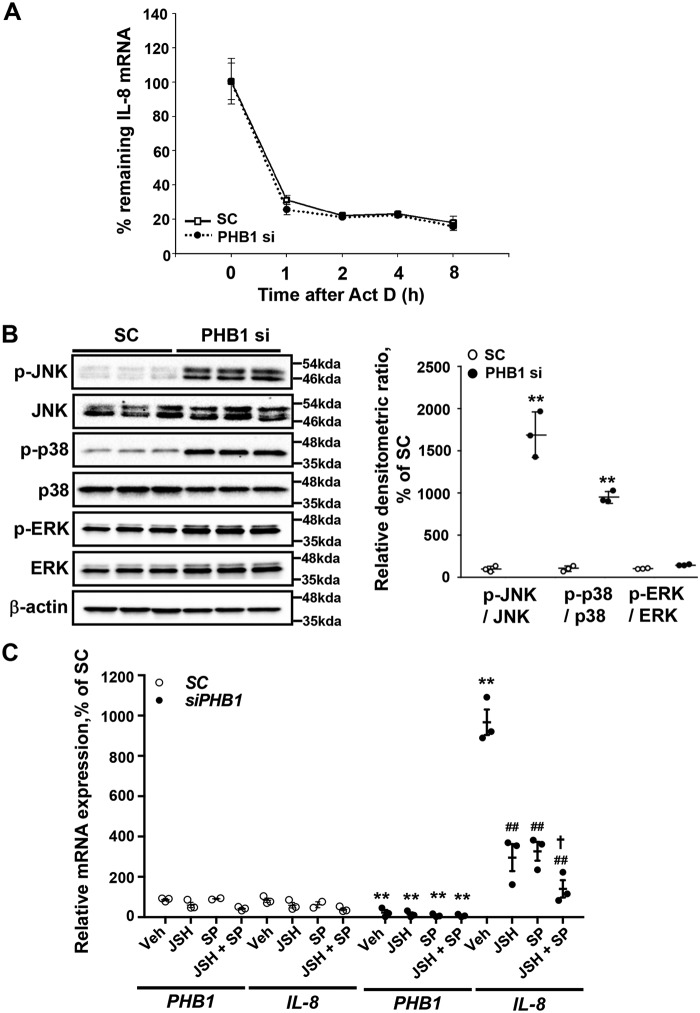
Previous studies have shown that p38 can regulate IL-8 mRNA stability, whereas JNK, NF-κB, and extracellular signal–regulated kinase (ERK) can transcriptionally regulate IL-8 (15–17). Silencing of PHB1 had no effect on IL-8 mRNA stability (Fig. 4A). Silencing PHB1 in HepG2 cells resulted in a marked increase in the levels of phosphorylated JNK (p-JNK) and p38 (p-p38) but a slight increase in total JNK, whereas total p38 was unchanged. This led to a dramatic increase in the p-JNK:JNK and p-p38:p38 ratios (Fig. 4B). Both p-ERK and total ERK levels showed a slight increase so that p-ERK:ERK was unchanged (Fig. 4B). To find out whether the PHB1 silencing–mediated increase in IL-8 expression was due to known IL-8 regulators, inhibitors against p38, NF-κB, and JNK were used in combination with PHB1 silencing to see the effect on IL-8 mRNA levels. Use of any of the inhibitors had no effect on IL-8 mRNA levels when PHB1 was present. Upon PHB1 silencing, IL-8 mRNA levels increased 9-fold, which was unaffected by the p38 inhibitor SB203580 (Fig. S1D). However, when either of the inhibitors against NF-κB (JSH) or JNK (SP) was used, IL-8 induction because of PHB1 silencing was reduced by 40%, but combining JSH with SP resulted in nearly complete inhibition of IL-8 induction (Fig. 4C). This supports that the effect of PHB1 silencing on IL-8 induction is mediated via JNK and NF-κB.
Figure 4.
JNK and NF-κB signaling pathways are required for PHB1 silencing-induced IL-8 expression. A, effect of actinomycin D (Act D) treatment on IL-8 mRNA levels in PHB1-silenced HepG2 cells. B, Western blot analysis of total and phosphorylated JNK, p38, and ERK in PHB1-silenced HepG2 cells. All densitometric values were normalized to β-actin. C, PHB1 and IL-8 mRNA expression in PHB1-silenced HepG2 cells after inhibition of either NF-κB or JNK alone or combined. HepG2 cells were transfected with a negative control or PHB1 siRNA for 24 h, followed by treatment with inhibitors of NF-κB (JSH) or JNK (SP) individually or combined for another 48 h. Results are expressed as mean ± S.E. of the mean from at least three independent experiments. **, p < 0.01 versus respective SC controls; ##, p < 0.01 versus PHB1 siRNA vehicle; †, p < 0.05 versus PHB1 siRNA SP. SC, scramble siRNA; PHB1 si, PHB1 siRNA.
PHB1 silencing induces human IL-8 promoter activity via NF-κB and AP-1 sites
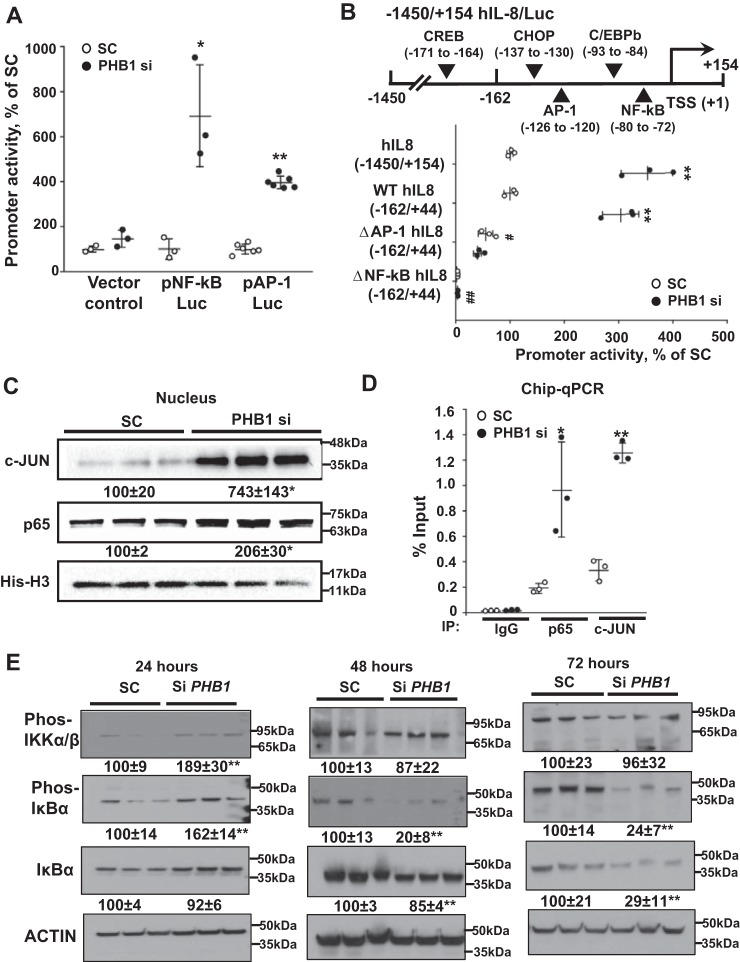
To further elucidate JNK and NF-κB's roles in the up-regulation of IL-8, promoter luciferase reporter constructs of human IL-8 were created. The human IL-8 promoter contains both NF-κB and AP-1 binding sites. Upon silencing of PHB1, the promoter activity of both NF-κB and AP-1 increased 6- and 4-fold, respectively (Fig. 5A). To define which binding sites were involved in the induction of IL-8 expression, HepG2 cells were transfected with PHB1 siRNA, and the effects on the full-length IL-8 promoter (−1450/+154) or a shorter IL-8-promoter (−162/+44) that was WT or mutated in the AP-1 or NF-κB elements were measured. The full-length construct (−1450/+154) and the shorter WT construct (−162/+44) showed comparable increases in promoter activity when PHB1 was silenced, suggesting that the −162/+44 construct contains all essential elements that mediate this induction (Fig. 5B). However, the basal activity was reduced when the AP-1 binding site was mutated and was completely abolished upon mutation of the NF-κB site (Fig. 5B). Silencing PHB1 failed to increase IL-8 promoter activity when either the AP-1 or NF-κB site was mutated (Fig. 5B). To confirm the importance of NF-κB and AP-1, the levels of the p65 and c-JUN were examined in the nuclear fraction of HepG2 cells after PHB1 silencing. A 6-fold increase in c-JUN level was observed in PHB1-silenced cells as well as a 2-fold increase in p65 level (Fig. 5C). ChIP-quantitative PCR also confirmed the increase in both p65 and c-JUN binding to the IL-8 promoter when PHB1 was silenced (Fig. 5D). Elevation in the nuclear p65 level is a result of canonical NF-κB activation, as shown by early phosphorylation of IκB kinase (IKK) and IκBα 24 h after silencing PHB1, followed by a drop in IκBα protein level at 48–72 h (Fig. 5E).
Figure 5.
PHB1 silencing-induced IL-8 promoter activity requires NF-κB and AP-1. A, promoter activity of NF-κB- and AP-1-driven luciferase reporter constructs after PHB1 knockdown. Results are expressed as mean ± S.E. of the mean from at least three independent experiments. *, p < 0.05; **, p < 0.01 versus SC. B, effects of NF-κB and AP-1 sites mutations on PHB1 silencing-induced transcription from the IL-8 promoter. HepG2 cells were transfected with PHB1 siRNA, and effects on IL-8 promoter (1450/+154) activity or reporter activities driven by the human IL-8 promoter (162/+44) WT or mutated in the AP-1 or NF-κB elements were measured. **, p < 0.01 versus respective controls; ##, p < 0.01 versus SC of the WT IL-8 promoter. C, Western blots of c-Jun and p65 in nuclear extracts from PHB1 knockdown HepG2 cells. All densitometric values were normalized to histone H3. D, effects of PHB1 knockdown on p65 and c-Jun binding to regions of the IL-8 promoter, measured by ChIP quantitative PCR. Results are expressed as mean ± S.E. from at least three independent experiments. **, p < 0.01 versus respective controls. SC, scramble siRNA; PHB1 si, PHB1 siRNA. E, Western blots of the NF-κB signaling pathway upon PHB1 knockdown. PHB1 was knocked down between 24 and 72 h in HepG2 cells, and phospho-IKKα/β (Ser-176/180), phospho-IκBα (Ser-32), and IκBα were blotted. All densitometric values were normalized to actin. **, p < 0.01 versus the respective SC control.
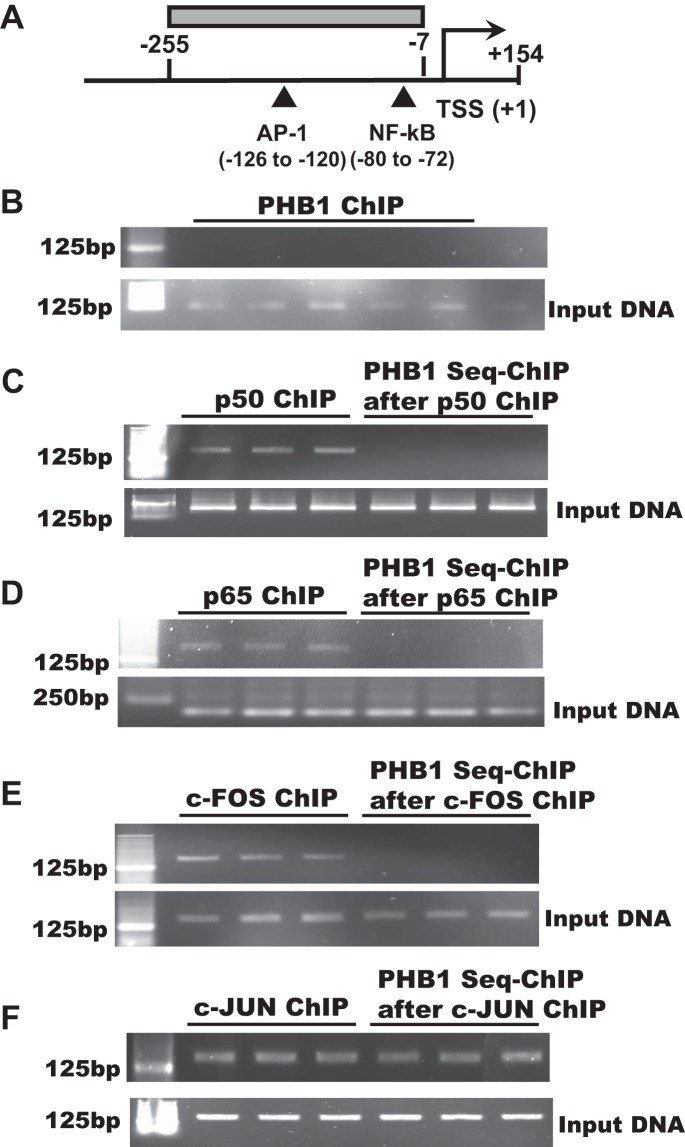
Because PHB1 can act as a transcription co-factor to modulate the activity of p53, E2F, and c-MYC (2, 3, 13), we examined whether it can bind to the IL-8 promoter region containing the AP-1 and NF-κB elements using ChIP and sequential ChIP (Fig. 6A). We found that PHB1 is unable to bind to the DNA by itself (Fig. 6B) or in the presence of p50, p65, or c-FOS (Fig. 6, C–E), but it was able to bind in the presence of c-JUN (Fig. 6F). This raises the possibility that PHB1 could also act as a co-repressor of the AP-1 site.
Figure 6.
PHB1 binds to the IL-8 promoter in the presence of c-JUN but not c-FOS p50 or p65. A, IL-8 promoter region containing the AP-1 and NF-κB sites are shown, as well as the region covered by the primers. HepG2 cells were used for ChIP and seq-ChIP assays as described under “Experimental procedures.” B, on a ChIP assay, PHB1 does not bind directly to the IL-8 promoter spanning the region shown in A. C–F, PHB1 does not bind to the IL-8 promoter in the presence of p50 (C), p65 (D), or c-FOS (E), but it binds in the presence of c-JUN (F).
Mechanisms of PHB1 silencing-induced activation of JNK and NF-κB
Because PHB1 is a well-known mitochondrial chaperone, we next investigated whether mitochondrial dysfunction and increased reactive oxygen species (ROS) could be the underlying mechanism for activation of JNK and NF-κB. Mitochondrial membrane potential and both mitochondrial and total ROS were measured following 48 and 72 h of treatment with PHB1 siRNA or scramble control, and no significant change was observed in these variables (Fig. S1A–S1C). Another possibility is transforming growth factor β–activated kinase 1 (TAK1), which is known to activate JNK, p38, and NF-κB (18). However, PHB1 silencing did not activate TAK1 (indicated by p-TAK1), which was only detectable in the presence of calyculin A (Fig. S2D) (19).
Blocking PHB1 knockdown–induced IL-8 secretion prevents migration and invasion of liver cancer cells
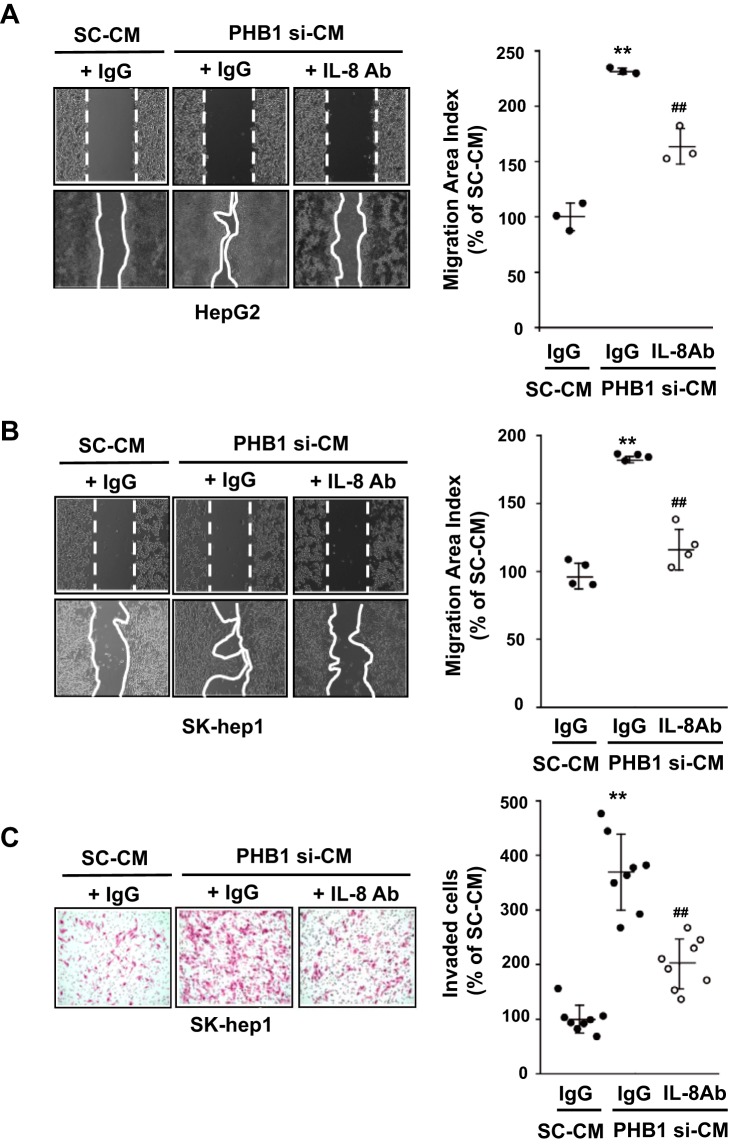
Previous work has shown that IL-8 production from cancer cells increases the migration of adjacent cells (20). To explore whether silencing of PHB1 could affect the migration of parental cells, conditioned medium (CM) from PHB1-silenced HepG2 cells was used on HepG2, a noninvasive epithelial cell line, and SK-hep-1 cells, a highly invasive human hepatic adenocarcinoma cell line with oncogenic mesenchymal stem cell features (21). Both cell lines displayed an increase in cell migration using CM from PHB1-silenced HepG2 cells compared with the scramble control, which was significantly attenuated or inhibited when co-treated with a neutralizing IL-8 antibody (Fig. 7, A and B). Furthermore, the effects of CM on invasion in SK-hep-1 cells was examined, and CM from PHB1-silenced HepG2 cells increased cell invasion, which was attenuated upon co-treatment with the neutralizing IL-8 antibody (Fig. 7C).
Figure 7.
PHB1 knockdown-induced IL-8 secretion promotes migration and invasion of cancer cells. A and B, migration analysis in (A) HepG2 and (B) SK-hep-1 cells treated with CM obtained from HepG2 cells transfected with SC or PHB1 siRNA in the presence of IL-8 antibody or IgG as a negative control. C, invasion assay under the same conditions as in B. Results are expressed as mean ± S.E. of the mean from at least three independent experiments. **, p < 0.01 versus SC-CM; ##, p < 0.01 versus PHB1 si-CM. SC, scramble siRNA; PHB1 si, PHB1 siRNA; SC-CM, conditioned medium obtained from HepG2 cells transfected with SC; PHB1 si-CM, conditioned medium obtained from HepG2 cells transfected with PHB1 siRNA; IL-8 Ab, interleukin-8 antibody.
Discussion
Although PHB1 is best known as a mitochondrial chaperone protein, increasing evidence shows that it has many other important functions, some of which pertain to cancer. Subcellular localization and posttranslational modifications may explain the tumor-regulatory activity of PHB1 in different cell types (14, 22). In particular, mitochondrial and membrane-associated PHB1 in some cancer cells (such as cervical, lung, and bladder) have been shown to confer anti-apoptotic effects and tumorigenesis (23–25). In contrast, nuclear PHB1 exhibits pro-apoptotic or anti-tumorigenic properties. In breast, prostate, osteosarcoma, and colon cancer cells, PHB1 is mainly localized in the nucleus, and PHB1 silencing increases cell proliferation (4, 26–29). We also found that PHB1 is mainly localized in the nucleus in three different HCC cell types, including the HepG2, Huh7, and Hep3B cancer cell lines (Fig. S3). This may be part of the explanation why PHB1 appears to act mainly as a tumor suppressor in the liver (12–14). In the nucleus, PHB1 acts as a transcription co-factor that can either activate (such as p53) or repress (such as E2F1) gene expression (2–4). We have described, in liver cancer cells, two other tumor-suppressive mechanisms, including PHB1 acting as a co-repressor with the CCCT-binding transcription factor CTCF on the imprinting control region to suppress IGF2-H19 (14) and as a heterodimer with MAX to repress E-box–dependent genes, such as c-MYC and MAFG, both oncogenes in the liver (13, 30). In this work, we uncovered additional signaling pathways that are regulated by PHB1 that also affect gene expression, in this case IL-8, and that can contribute to tumorigenesis, invasion, and metastasis when PHB1 expression is lost.
Proinflammatory cytokines play an important role in tumor progression and metastasis, as they are mediators of the paracrine signal between the tumor and the tumor microenvironment (31). IL-8 is a multifunctional CXC chemokine as well as a proinflammatory cytokine that promotes neutrophil chemotaxis and activation (32). IL-8 is produced in both tumor cells and tumor-associated macrophages and plays a critical role in cancer-related inflammation by promoting invasion and metastasis in human cancers (33). Importantly, IL-8 has been reported to be overexpressed and associated with high recurrence rates and short survival in patients with HCC, and its up-regulation promotes HCC invasion and metastasis (34, 35). We identified IL-8 as one of the most up-regulated genes when PHB1 was silenced in HepG2 cells on RNA-Seq and confirmed that this also occurs in multiple other liver cancer cell lines as well as in HCT116 cells, a colon cancer cell line where PHB1 also behaves as a tumor suppressor (Fig. 1D) (29). Silencing PHB1 raised whereas overexpressing PHB1 lowered intracellular and secreted IL-8 levels in liver cancer cells (Fig. 3). We also confirmed that high IL-8 expression occurs in most human HCC, is associated with worse survival, and is inversely associated with PHB1 in a subgroup of patients where PHB1 expression is reduced (Fig. 2). At this time, the mechanisms for heterogenous expression of PHB1 in HCC remain unexplained and will be a focus of future study.
We next focused on elucidating how PHB1 negatively regulates IL-8 at the mRNA level. Increased IL-8 expression can be mediated by activation of the MAPKs ERK, JNK, and p38 as well as NF-κB activation (15–17). JNK activation has been shown to be essential for IL-8 expression through c-JUN (36). p38 activation has been shown to stabilize IL-8 mRNA in HeLa cells (17). We were unable to find any literature regarding direct regulation of p38 or JNK by PHB1, although an increase in JNK activity has been observed in Phb1 knockout livers after bile duct ligation, which was attributed to increased ROS (11). It has been described previously that PHB1 regulates NF-κB nuclear translocation by decreasing importin α3 expression (37). This is consistent with our results showing that PHB1 silencing increases the nuclear content of p65 (Fig. 5C). However, we found that PHB1 silencing turned on the canonical NF-κB activation pathway (Fig. 5E), suggesting that more than one mechanism may be involved in elevating the nuclear p65 level. The available literature suggests that PHB1 positively regulates ERK activity in HeLa cells (6) and rat granulosa cells (39). However, in liver cancer cells, PHB1 silencing had no effect on ERK activity.
Our study showed that, in liver cancer cells, PHB1 silencing activated JNK, p38, and NF-κB. Inhibition of JNK strongly attenuated PHB1 silencing–mediated IL-8 induction, but inhibition of p38 had no effect (Fig. 4). Consistently, PHB1 silencing did not influence IL-8 mRNA stabilization. In addition, inhibiting NF-κB also attenuated IL-8 induction (Fig. 4). When both JNK and NF-κB were inhibited, PHB1 silencing no longer induced IL-8 expression (Fig. 4C). Taken together, our results show that PHB1 silencing increased IL-8 expression at the transcription level by mechanisms that require JNK and NF-κB.
Transcriptional regulation of human IL-8 is mainly by NF-κB and AP-1. The region from +1 to −133 within the 5′-flanking region of the human IL-8 promoter is critical for the transcriptional regulation of IL-8 (40) and contains the binding sites for NF-κB and AP-1. We found that the activity of the construct −162/44-Luc was very similar to the activity of −1450/154-Luc, suggesting that all important elements are contained within −162/44 of the human IL-8 promoter. Consistently, mutation of the AP-1 site lowered the basal promoter activity and prevented IL-8 promoter activation by PHB1 silencing. On the other hand, mutation of the NF-κB site abolished both basal and PHB1 silencing–induced promoter activity. This result suggests that the NF-κB site is essential for the basal activity of the IL-8 promoter and that both AP-1 and NF-κB are required for PHB1 silencing to activate the IL-8 promoter. Indeed, PHB1 silencing activated reporter activity driven by both AP-1 and NF-κB elements, nuclear accumulation of c-JUN and p65, and their binding to the IL-8 promoter (Fig. 5). In addition, we found that PHB1 was able to bind to the IL-8 promoter region in the presence of c-JUN (Fig. 6F), which raises the possibility that PHB1 can also serve as a co-repressor of the AP-1 site.
JNK, p38, and NF-κB are all known to be regulated by oxidative stress, which prompted us to speculate whether silencing PHB1 could have led to mitochondrial dysfunction and increased ROS. However, there was no evidence of increased ROS or mitochondrial dysfunction under the experimental conditions (Fig. S2, A–C). This was somewhat surprising because PHB1 is a well-known mitochondrial chaperone. One possibility may have to do with where PHB1 is mainly localized in the cell under study. The three cell lines we used all seem to have a predominance of PHB1 in the nuclear compartment (Fig. S3), where it may play more of a tumor suppressor role. Thus, increased ROS is not the underlying mechanism for activation of JNK, p38, and NF-κB. Another potential mechanism is TAK1, which has an important role in the regulation of inflammatory disorders, including cancer. and regulates IL-8 expression through the AP-1 and NF-κB pathway (19, 40). However, PHB1 silencing did not affect TAK1 activation (Fig. S2D). Because p-TAK1 was only detectable when cells were treated with calyculin A to block its dephosphorylation (19), it is possible that we may have missed a small up-regulation that was masked by calyculin A. The underlying molecular mechanisms of how PHB1 regulates JNK and p38 remain unclear and will require further investigation.
Tumor-derived IL-8 has been shown to promote the invasion and migration of adjacent tumor cells (41). We also found that conditioned medium from PHB1-silenced HepG2 cells induced migration and invasion of parental HepG2 cells and SK-hep-1 cells; the latter is a highly invasive cell line that has a more mesenchymal feature. Both migration and invasion were significantly attenuated in the presence of neutralizing antibody to IL-8 (Fig. 7), which illustrates that one mechanism for PHB1 loss to promote a more aggressive liver cancer phenotype is through up-regulation and release of IL-8.
In summary, we have identified a novel mechanism by which reduced PHB1 expression increased IL-8 transcription by activating the NF-κB and JNK/AP-1 pathways. Loss of PHB1 leads to increased release of IL-8, which can stimulate cancer cell migration and invasion in an autocrine and paracrine manner. Our findings provide further insight into the molecular mechanisms of IL-8 regulation and added JNK, p38, and NF-κB signaling pathways that are normally suppressed by PHB1 in liver cancer cells.
Experimental procedures
Source of human HCC with adjacent nontumorous specimens
178 HCC specimens and adjacent nontumorous tissues were obtained from Xiangya Hospital (Central South University, Changsha, Hunan, China). These specimens were fresh-frozen samples obtained from patients undergoing surgical liver resection from 2013 to 2017 and were stored in liquid nitrogen in the institutional biobank. All human specimens were obtained with patients' informed consent. The use of human samples was approved by the Institutional Review Board of the Central South University, Xiangya Hospital Authority. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the Institutional Review Board of the Medical Ethical Committee of Xiangya Hospital Central South University.
TCGA dataset from OncoLnc
The graph showing survival analysis was generated using a TCGA dataset from OncoLnc (http://www.oncolnc.org/)4 (38, 42).
Cell culture and treatment with inhibitors of p38, NF-κB, and JNK
Human CCA MzChA-1 and HCC HepG2, Hep3B, and Huh7 cell lines were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 2 mmol/liter l-glutamine. The human colorectal adenocarcinoma HCT116 cell line was cultured in McCoy's 5A medium containing 10% FBS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. The human SK-hep-1 cell line (derived from liver adenocarcinoma with oncogenic mesenchymal stem cells characteristics) (21) was cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 1 mm sodium pyruvate, and 1% nonessential amino acids.
In some experiments, HepG2 cells were treated with inhibitors of p38 (5 μm, SB203580, Cell Signaling Technology, Beverly, MA), NF-κB (10 μm, JSH-23, Sigma), JNK (5 μm, SP600125, Sigma), or a combination of JSH-23 (10 μm) and SP600125 (5 μm) for 48 h alone or in combination with PHB1 silencing as described below. For experiments with calyculin A (Santa Cruz Biotechnology Inc., Paso Robles, CA), 500 nm was added 30 min prior to the end of the experiment.
PHB1 silencing and overexpression in vitro
Cell lines were seeded in 6-well plates for PHB1 overexpression or silencing. Huh7 and Hep3B cells (2 × 105) were forward-transfected with 2 μg of PHB1 overexpression or empty vector (Origene, Rockville, MD) for 48 h using JetPRIME® reagent (Polyplus-transfection, Radnor, PA) according to the manufacturer's instructions. For PHB1 silencing, prevalidated Silencer® select siRNA against human PHB1 (Ambion, S10424) or scramble siRNA control (Thermo Scientific) was reverse-transfected into cells at a dose of 20 nm in 6-well plates using Lipofectamine RNAiMAX transfection reagent (Invitrogen) for 24 to 72 h. A second siRNA for PHB1 (Ambion, S143790) was also tested at 50 nm.
RNA isolation and gene expression analysis
Total RNA was isolated using TRIzol (Invitrogen). Gene expression was assessed using real-time PCR. Total RNA was subjected to reverse transcription using Moloney murine leukemia virus reverse transcriptase (Lucigen, Middleton, WI). TaqMan probes for human PHB1, IL-8, PDGFA, MMP2, and TGFB1 were purchased from Thermo Fisher (Carlsbad, CA). Universal PCR Master Mix was purchased from Bio-Rad. Hypoxanthine phosphoribosyltransferase 1 was used as a housekeeping gene. The thermal profile consisted of an initial denaturation at 95 °C for 3 min, followed by 40 cycles at 95 °C for 3 s and at 60 °C for 30 s. The cycle Ct value of the target genes was normalized to that of the housekeeping gene to obtain the ΔCt. The ΔCt was used to find the relative expression of target genes according to the following formula: relative expression = 2 − ΔΔCt, where ΔΔCt = ΔCt of target genes under the experimental condition − ΔCt of the target gene under control conditions. In some experiments, HepG2 cells were first treated with PHB1 siRNA or scramble control for 48 h, followed by actinomycin D (10 μg/ml, Sigma), and IL-8 mRNA levels were measured up to 8 h after actinomycin D.
RNA-Seq
RNA-Seq and bioinformatics were provided by BGI Americas Corp. (Cambridge, MA).
Protein isolation and Western blot analysis
Nuclear extract isolation for cells was performed using a nuclear extraction kit (Abcam, Cambridge, MA), and total protein extracts from cells were prepared using radioimmune precipitation assay lysis buffer (Abcam) containing a protease and phosphatase inhibitor mixture (Thermo Fisher) and subjected to SDS-PAGE followed by Western blotting according to standard methods (Amersham Biosciences, Waltham, MA). Membranes were probed with antibodies to PHB1 (Thermo Scientific), IL-8 (Abcam), phospho-JNK, JNK, phospho-ERK, ERK, phospho-p38 mitogen-activated protein kinase (MAPK), p38 MAPK, c-JUN, TAK1 and phospho-TAK1, phospho-IKKα/β (Ser-176/180), phospho-IκBα (Ser-32) and IκBα (Cell Signaling Technology, Beverly, MA), and p65 (ProteinTech, Rosemont, IL). To ensure equal loading, membranes were probed with anti-actin or histone H3 antibodies. For ECL detection, the ChemiDocTM XRS+ system (Bio-Rad) was used. Blots were quantified using the ImageJ densitometry program (National Institutes of Health), and test protein expression was normalized to actin (total protein extracts) or histone H3 (nuclear extract) control.
Promoter constructs and luciferase assay
The human IL-8 promoter (hIL-8, −1450/+154) was purchased from Genecopoeia (Rockville, MD). The WT human IL-8 promoter −162/+44 (hIL-8 (-162/+44)-Luc) and hIL-8 (-162/+44)-Luc containing point mutations in the AP-1 and NF-κB DNA binding sites (ΔAP-1 hIL-8 (−162/+44)-Luc, ΔNF-κB hIL-8 (−162/+44)-Luc) were kindly provided by Dr. Marc Hershenson (University of Michigan, Ann Arbor, MI). For NF-κB (TGGGGACTTTCCGC)X5, AP-1 (TGACTAA)X7 reporter constructs, two complementary oligonucleotides containing the multimerized elements were annealed and ligated into the BglII and SalI restriction sites of the pLuc-Mcs plasmid using T4 DNA ligase (New England Biolabs, Ipswich, MA). The promoter constructs and pEZX/SV40 (1 μg) were co-transfected into HepG2 cells with jetPRIME® following the manufacturer's instructions. Luciferase assays were performed 24 h later using the Secrete-Pair dual luminescence assay kit (Genecopoeia) and the Dual-Luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's protocol.
ChIP and Seq-ChIP assay
To examine changes in protein binding to the AP-1 and NF-κB regions of the human IL-8 promoter in an endogenous chromatin configuration following PHB1 knockdown, ChIP assays were done using the EpiTect ChIP OneDay protocol (Qiagen, Valencia, CA). Briefly, DNA was immunoprecipitated by c-JUN or p65 antibody. The final purified DNA was detected by real-time PCR analysis. PCR (186 bp) of the human IL-8 promoter region containing the AP-1 and NF-κB elements used forward primer 5′-AAGAAAACTTTCGTCATACTCCG-3′ and reverse primer 5′-TGGCTTTTTATATCATCACCCTAC-3′ (GenBank accession no. NM_000584.3) (15).
In separate experiments, ChIP and seq-ChIP were done using the manufacturer's suggested protocol from the Pierce agarose ChIP kit (Thermo Fisher). Briefly, for AP-1, DNA was immunoprecipitated by anti-PHB1, anti-c-FOS, and anti-c-JUN antibodies. For NF-κB, DNA was immunoprecipitated by anti-PHB1, anti-p65, and anti-p50 antibodies. The purified DNA was detected by PCR analysis. PCR of the human IL-8 promoter region (GenBank accession no. NM_000584) containing the AP-1 and NF-κB used forward primer 5′-ACTATATCTGTCACATGGTACTATG-3′ (bp −230 to −255) and reverse primer 5′-CATTATGTCAGAGGAAATTCCACG-3′ (−7 to −31 relative to the transcription start site). All PCR products were run on 2% agarose gels. The PCR conditions consisted of an initial denaturation at 94 °C for 3 min, followed by 25 cycles at 94 °C for 30 s, and then annealing and extension at 67 °C for 90 s using the Advantage GC 2 PCR kit (Clontech, Mountain View, CA), in accordance with the suggested protocol.
Total and mitochondrial ROS measurement
Total ROS levels were measured using the 2′,7′-dichlorofluorescin diacetate (DCFDA) kit (ab113851, Abcam) according to the manufacturer's guide. Mitochondrial ROS were measured using the MitoSOXTM Red mitochondrial superoxide indicator (M36008, Invitrogen). Cells were loaded with 5 μm MitoSOX in Hank's balanced salt solution (HBSS) medium for 15 min at 37 °C. After washing cells with warm PBS twice, fluorescence was measured by plate reader at an excitation of 510 nm and emission of 580 nm. All readings were normalized to live cell number.
Mitochondrial membrane potential measurement
Mitochondrial membrane potential was measured using the JC-1 assay kit (ab113850, Abcam). Cells were loaded with 5 μm JC-1 in DMEM for 30 min at 37 °C. After washing cells with warm PBS twice, fluorescence was measured by plate reader at an excitation of 488 nm and emission of 529 and 590 nm. All readings were normalized to live cell number.
Cell viability measurement
Cell viability was measured using trypan blue (Invitrogen) staining.
Migration and invasion assays after treatment with CM
To see the effect of released IL-8, HepG2 cells were first treated with PHB1 siRNA or scramble control for 48 h in the presence of 10% FBS. CM (2 ml) was then used to treat parental HepG2 and SK-hep-1 cells for 24 h with or without anti-IL-8 antibody (2.5 μg/ml, Abcam). The migration assay was measured using the Culture-Insert 2 Well in μ-Dish 35 mm Kit from ibidi (Martinsried, Germany). Briefly, the cells were seeded onto the culture insert of a 24-well plate. After 18 h of plating, the cells were treated with CM from PHB1-silenced HepG2 cells or scrambled HepG2 cells and incubated for 72 h. After 24 h, photographic images were acquired under an inverted microscope (EVOS XL core, Life Technologies), and the migration-occupied area was measured using ImageJ software.
The invasion assay was assessed using the Matrigel Invasion Chamber Kit (Corning, Bedford, MA). Briefly, the cells were placed onto the top insert and treated with CM from PHB1-silenced HepG2 cells or scrambled HepG2 cells. Invaded cells on the bottom of the insert membrane were stained with Diff-QuikTM stain. Invaded cells were counted by photographing the membrane through the microscope. For IL-8 blocking studies in the presence of CM (2 ml), cells were incubated for 24 or 72 h with CM from PHB1-silenced HepG2 cells in the presence of antibodies specific for IL-8 or control IgG and subsequently analyzed for migration and invasiveness.
Statistical analysis
Data are expressed as mean ± S.E. Statistical analysis was performed using Student's t test, analysis of variance, and Fisher's test. For mRNA and protein levels, ratios of genes and proteins to the respective housekeeping densitometric values were compared. Because the HCC patient samples were likely to be highly heterogeneous, we clustered patients with the expression patterns of the genes PHB1 and IL-8 using the Kmean algorithm in MATLAB. The number of clusters was determined by the silhouette method. For survival analysis, log-rank test was used to compare the survival ratio differences between samples with high versus low IL-8 expression. Significance was defined by p < 0.05.
Author contributions
J. W. Y. and S. C. L. conceptualization; J. W. Y., B. M., L. B.-T., T. L., Z. L., H. Y., W. F., J. W., Y. L., E. S., and S. C. L. data curation; J. W. Y., B. M., L. B.-T., T. L., Z. L., H. Y., W. F., J. W., E. S., and S. C. L. formal analysis; J. W. Y. and S. C. L. methodology; J. W. Y., B. M., and L. B.-T. writing-original draft; B. M., L. B.-T., J. W., E. S., J. M. M., and S. C. L. writing-review and editing; Z. L., H. Y., and S. C. L. resources; S. C. L. supervision; S. C. L. funding acquisition; S. C. L. project administration.
Supplementary Material
This work was supported by National Institutes of Health Grant R01CA172086 (to S. C. L., H. P. Y., and J. M. M.), Plan Nacional of I+D SAF2017–88041-R, Innovative Medicines Initiative (IMI) Grant 777377-LITMUS-H2020-JTI-IMI2–2016-09, Instituto de Salud Carlos III Grant CIBERehd MAT-3, and Centers of Excellence Severo Ochoa Grant SEV-2016–0644 (to J. M. M.). J. M. M. is a consultant for Abbott. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party–hosted site.
- HCC
- hepatocellular carcinoma
- CCA
- cholangiocarcinoma
- IL-8
- interleukin-8
- NT
- non-tumorous tissue
- JNK
- c-Jun N-terminal kinase
- ERK
- extracellular signal–regulated kinase
- JSH
- JSH-23
- SP
- SP600125
- IKK
- IκB kinase
- ROS
- reactive oxygen species
- CM
- conditioned medium
- MAPK
- mitogen-activated protein kinase
- Luc
- luciferase
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- seq-ChIP
- sequential ChIP
- TCGA
- the cancer genome atlas
- Ct
- cycle threshold.
References
- 1. Nijtmans L. G., Artal S. M., Grivell L. A., and Coates P. J. (2002) The mitochondrial PHB complex: roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell Mol. Life Sci. 59, 143–155 10.1007/s00018-002-8411-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi D., Lee S. J., Hong S., Kim I. H., and Kang S. (2008) Prohibitin interacts with RNF2 and regulates E2F1 function via dual pathways. Oncogene 27, 1716–1725 10.1038/sj.onc.1210806 [DOI] [PubMed] [Google Scholar]
- 3. Wang S., Fusaro G., Padmanabhan J., and Chellappan S. P. (2002) Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene 21, 8388–8396 10.1038/sj.onc.1205944 [DOI] [PubMed] [Google Scholar]
- 4. Fusaro G., Dasgupta P., Rastogi S., Joshi B., and Chellappan S. (2003) Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J. Biol. Chem. 278, 47853–47861 10.1074/jbc.M305171200 [DOI] [PubMed] [Google Scholar]
- 5. McClung J. K., Danner D. B., Stewart D. A., Smith J. R., Schneider E. L., Lumpkin C. K., Dell'Orco R. T., and Nuell M. J. (1989) Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem. Biophys. Res. Commun. 164, 1316–1322 10.1016/0006-291X(89)91813-5 [DOI] [PubMed] [Google Scholar]
- 6. Rajalingam K., Wunder C., Brinkmann V., Churin Y., Hekman M., Sievers C., Rapp U. R., and Rudel T. (2005) Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat. Cell Biol. 7, 837–843 10.1038/ncb1283 [DOI] [PubMed] [Google Scholar]
- 7. Koushyar S., Jiang W. G., and Dart D. A. (2015) Unveiling the potential of prohibitin in cancer. Cancer Lett. 369, 316–322 10.1016/j.canlet.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 8. Liu T., Tang H., Lang Y., Liu M., and Li X. (2009) MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 273, 233–242 10.1016/j.canlet.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 9. Kathiria A. S., Neumann W. L., Rhees J., Hotchkiss E., Cheng Y., Genta R. M., Meltzer S. J., Souza R. F., and Theiss A. L. (2012) Prohibitin attenuates colitis-associated tumorigenesis in mice by modulating p53 and STAT3 apoptotic responses. Cancer Res. 72, 5778–5789 10.1158/0008-5472.CAN-12-0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang H., Li T. W., Zhou Y., Peng H., Liu T., Zandi E., Martínez-Chantar M. L., Mato J. M., and Lu S. C. (2015) Activation of a novel c-Myc-miR27-Prohibitin 1 circuitry in cholestatic liver injury inhibits glutathione synthesis in mice. Antioxid. Redox Signal. 22, 259–274 10.1089/ars.2014.6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbier-Torres L., Beraza N., Fernández-Tussy P., Lopitz-Otsoa F., Fernández-Ramos D., Zubiete-Franco I., Varela-Rey M., Delgado T. C., Gutiérrez V., Anguita J., Pares A., Banales J. M., Villa E., Caballería J., Alvarez L., et al. (2015) Histone deacetylase 4 promotes cholestatic liver injury in the absence of prohibitin-1. Hepatology 62, 1237–1248 10.1002/hep.27959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ko K. S., Tomasi M. L., Iglesias-Ara A., French B. A., French S. W., Ramani K., Lozano J. J., Oh P., He L., Stiles B. L., Li T. W., Yang H., Martínez-Chantar M. L., Mato J. M., and Lu S. C. (2010) Liver-specific deletion of prohibitin 1 results in spontaneous liver injury, fibrosis, and hepatocellular carcinoma in mice. Hepatology 52, 2096–2108 10.1002/hep.23919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan W., Yang H., Liu T., Wang J., Li T. W., Mavila N., Tang Y., Yang J., Peng H., Tu J., Annamalai A., Noureddin M., Krishnan A., Gores G. J., Martínez-Chantar M. L., et al. (2017) Prohibitin 1 suppresses liver cancer tumorigenesis in mice and human hepatocellular and cholangiocarcinoma cells. Hepatology 65, 1249–1266 10.1002/hep.28964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramani K., Mavila N., Ko K. S., Mato J. M., and Lu S. C. (2016) Prohibitin 1 regulates the H19-Igf2 axis and proliferation in hepatocytes. J. Biol. Chem. 291, 24148–24159 10.1074/jbc.M116.744045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jundi K., and Greene C. M. (2015) Transcription of interleukin-8: how altered regulation can affect cystic fibrosis lung disease. Biomolecules 5, 1386–1398 10.3390/biom5031386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gangwani M. R., and Kumar A. (2015) Multiple protein kinases via activation of transcription factors NF-κB, AP-1 and C/EBP-δ regulate the IL-6/IL-8 production by HIV-1 Vpr in astrocytes. PLoS ONE 10, e0135633 10.1371/journal.pone.0135633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhattacharyya S., Gutti U., Mercado J., Moore C., Pollard H. B., and Biswas R. (2011) MAPK signaling pathways regulate IL-8 mRNA stability and IL-8 protein expression in cystic fibrosis lung epithelial cell lines. Am. J. Physiol. Lung Cell Mol. Physiol. 300, L81–87 10.1152/ajplung.00051.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gui T., Sun Y., Shimokado A., and Muragaki Y. (2012) The roles of mitogen-activated protein kinase pathways in TGF-β-induced epithelial-mesenchymal transition. J. Signal Transduct. 2012, 289243 10.1155/2012/289243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Omori E., Inagaki M., Mishina Y., Matsumoto K., and Ninomiya-Tsuji J. (2012) Epithelial transforming growth factor β-activated kinase 1 (TAK1) is activated through two independent mechanisms and regulates reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 109, 3365–3370 10.1073/pnas.1116188109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernando R. I., Castillo M. D., Litzinger M., Hamilton D. H., and Palena C. (2011) IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 71, 5296–5306 10.1158/0008-5472.CAN-11-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eun J. R., Jung Y. J., Zhang Y., Zhang Y., Tschudy-Seney B., Ramsamooj R., Wan Y. J., Theise N. D., Zern M. A., and Duan Y. (2014) Hepatoma SK Hep-1 cells exhibit characteristics of oncogenic mesenchymal stem cells with highly metastatic capacity. PLoS ONE 9, e110744 10.1371/journal.pone.0110744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng Y. T., Chen P., Ouyang R. Y., and Song L. (2015) Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis 20, 1135–1149 10.1007/s10495-015-1143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sato S., Murata A., Orihara T., Shirakawa T., Suenaga K., Kigoshi H., and Uesugi M. (2011) Marine natural product aurilide activates the OPA1-mediated apoptosis by binding to prohibitin. Chem. Biol. 18, 131–139 10.1016/j.chembiol.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 24. Patel N., Chatterjee S. K., Vrbanac V., Chung I., Mu C. J., Olsen R. R., Waghorne C., and Zetter B. R. (2010) Rescue of paclitaxel sensitivity by repression of Prohibitin1 in drug-resistant cancer cells. Proc. Natl. Acad. Sci. U.S.A. 107, 2503–2508 10.1073/pnas.0910649107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang L., Dong P., Zhang Z., Li C., Li Y., Liao Y., Li X., Wu Z., Guo S., Mai S., Xie D., Liu Z., and Zhou F. (2015) Akt phosphorylates Prohibitin 1 to mediate its mitochondrial localization and promote proliferation of bladder cancer cells. Cell Death Dis. 6, e1660 10.1038/cddis.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng X., Mehta R., Wang S., Chellappan S., and Mehta R. G. (2006) Prohibitin is a novel target gene of vitamin D involved in its antiproliferative action in breast cancer cells. Cancer Res. 66, 7361–7369 10.1158/0008-5472.CAN-06-1004 [DOI] [PubMed] [Google Scholar]
- 27. Shi S. L., Li Q. F., Liu Q. R., Xu D. H., Tang J., Liang Y., Zhao Z. L., and Yang L. M. (2009) Nuclear matrix protein, prohibitin, was down-regulated and translocated from nucleus to cytoplasm during the differentiation of osteosarcoma MG-63 cells induced by ginsenoside Rg1, cinnamic acid, and tanshinone IIA (RCT). J. Cell Biochem. 108, 926–934 10.1002/jcb.22324 [DOI] [PubMed] [Google Scholar]
- 28. Gamble S. C., Odontiadis M., Waxman J., Westbrook J. A., Dunn M. J., Wait R., Lam E. W., and Bevan C. L. (2004) Androgens target prohibitin to regulate proliferation of prostate cancer cells. Oncogene 23, 2996–3004 10.1038/sj.onc.1207444 [DOI] [PubMed] [Google Scholar]
- 29. Guan X., Liu Z., Wang L., Johnson D. G., and Wei Q. (2014) Identification of prohibitin and prohibiton as novel factors binding to the p53 induced gene 3 (PIG3) promoter (TGYCC)(15) motif. Biochem. Biophys. Res. Commun. 443, 1239–1244 10.1016/j.bbrc.2013.12.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu T., Yang H., Fan W., Tu J., Li T. W. H., Wang J., Shen H., Yang H., Xiong T., Steggerda J., Liu Z., Noureddin M., Maldondo S. S., Annamalai A., Seki E., Mato J. M., and Lu S. C. (2018) Mechanisms of MAFG dysregulation in cholestatic liver injury and development of liver cancer. Gastroenterology 10.1053/j.gastro.2018.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mantovani A., Savino B., Locati M., Zammataro L., Allavena P., and Bonecchi R. (2010) The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 21, 27–39 10.1016/j.cytogfr.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 32. Xie K. (2001) Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 12, 375–391 10.1016/S1359-6101(01)00016-8 [DOI] [PubMed] [Google Scholar]
- 33. Joyce J. A., and Pollard J. W. (2009) Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang W., Chen Z., Zhang L., Tian D., Wang D., Fan D., Wu K., and Xia L. (2015) Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology 149, 1053–1067.e14 10.1053/j.gastro.2015.05.058 [DOI] [PubMed] [Google Scholar]
- 35. Ren Y., Poon R. T., Tsui H. T., Chen W. H., Li Z., Lau C., Yu W. C., and Fan S. T. (2003) Interleukin-8 serum levels in patients with hepatocellular carcinoma correlations with clinicopathological features and prognosis. Clin. Cancer Res. 9, 5996–6001 [PubMed] [Google Scholar]
- 36. Krause A., Holtmann H., Eickemeier S., Winzen R., Szamel M., Resch K., Saklatvala J., and Kracht M. (1998) Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 273, 23681–23689 10.1074/jbc.273.37.23681 [DOI] [PubMed] [Google Scholar]
- 37. Theiss A. L., Jenkins A. K., Okoro N. I., Klapproth J. M., Merlin D., and Sitaraman S. V. (2009) Prohibitin inhibits tumor necrosis factor alpha–induced nuclear factor-κ B nuclear translocation via the novel mechanism of decreasing importin α3 expression. Mol. Biol. Cell 20, 4412–4423 10.1091/mbc.e09-05-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anaya J. (2016) OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Science 2, e67 10.7717/peerj-cs.67 [DOI] [Google Scholar]
- 39. Chowdhury I., Thompson W. E., Welch C., Thomas K., and Matthews R. (2013) Prohibitin (PHB) inhibits apoptosis in rat granulosa cells (GCs) through the extracellular signal-regulated kinase 1/2 (ERK1/2) and the Bcl family of proteins. Apoptosis 18, 1513–1525 10.1007/s10495-013-0901-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y., Du Y., Wang H., Du L., and Feng W. H. (2017) Porcine reproductive and respiratory syndrome virus (PRRSV) up-regulates IL-8 expression through TAK-1/JNK/AP-1 pathways. Virology 506, 64–72 10.1016/j.virol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waugh D. J., and Wilson C. (2008) The interleukin-8 pathway in cancer. Clin. Cancer Res. 14, 6735–6741 10.1158/1078-0432.CCR-07-4843 [DOI] [PubMed] [Google Scholar]
- 42. Anaya J., Reon B., Chen W. M., Bekiranov S., and Dutta A. (2015) A pan-cancer analysis of prognostic genes. PeerJ 3, e1499 10.7717/peerj.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.