Abstract
Objective
To clarify the specificity of the ‘hot cross bun’ sign (HCBS) for multiple system atrophy (MSA) in adult cerebellar ataxia or parkinsonism.
Methods
The radiologic information systems at an academic center and affiliated veterans’ hospital were queried using the keywords ‘hot cross bun,’ ‘pontocerebellar,’ ‘cruciate,’ ‘cruciform,’ ‘MSA,’ ‘multiple system atrophy,’ and ‘multisystem atrophy.’ Scans were reviewed by a neurologist and neuroradiologist to identify the HCBS. Subjects with the HCBS were reviewed by 2 neurologists to identify the most likely etiology of the patient’s neurologic symptoms.
Results
Eleven cases were identified. Etiologies included MSA (4 probable, 2 possible), hereditary cerebellar ataxia (3/11), probable dementia with Lewy bodies (1/11), and uncertain despite autopsy (1/11).
Conclusion
MSA was the most common etiology. However, 5 of the 11 patients did not have MSA. The most common alternate etiology was an undefined hereditary cerebellar ataxia (3/11).
Keywords: Multiple system atrophy, olivopontocerebellar atrophy, magnetic resonance imaging, cerebellar ataxia
The ‘hot cross bun’ sign (HCBS) is a radiologic finding describing a cruciform T2 hyperintense signal on axial MRI of the pons. It is thought to represent gliosis of pontocerebellar fibers and is most commonly observed in patients with the cerebellar subtype of multiple system atrophy (MSA-C) but has also been reported in patients with spinocerebellar ataxia (SCA) 1, 2, 3, 7, and 8, progressive multifocal leukoencephalopathy, paraneoplastic cerebellar degeneration from a burnedout testicular tumor, leptomeningeal metastases from breast cancer, bilateral middle cerebral peduncle infarction, cerebrotendinous xanthomatosis, fragile X tremor ataxia syndrome (FXTAS) and variant Creutzfeldt-Jakob disease [1-7]. No study has clarified the specificity of the HCBS for MSA in adult cerebellar ataxia or parkinsonism.
MATERIALS & METHODS
The radiologic information systems at a single academic institution and an affiliated veterans hospital were queried to identify brain MRI radiology reports from January 2000 to February 2017 containing the terms ‘hot cross bun,’ ‘pontocerebellar,’ ‘cruciate,’ ‘cruciform,’ ‘MSA,’ ‘multiple system atrophy,’ and ‘multisystem atrophy.’ A movement-disorder neurologist reviewed the MRI reports or scans and excluded all subjects that did not have the HCBS or a description consistent with it. A neuroradiologist reviewed the scans that were not excluded to confirm the presence of the HCBS.
The medical records of all included subjects were reviewed separately by 2 movement-disorder neurologists to determine the most likely causative etiology of the HCBS. Cases not consistent with a typical presentation of MSA with adult (age of onset > 30 years of age) cerebellar ataxia or parkinsonism were excluded. If there was a diagnosis from autopsy or genetic testing that accounted for the patient’s clinical picture, this was accepted as the etiology. The medical record was reviewed for the clinical, laboratory, and imaging data that might support a specific etiology. Parkinsonism was defined as bradykinesia with rigidity, tremor, or postural instability. A cerebellar syndrome was defined as gait ataxia with cerebellar dysarthria, limb ataxia, or cerebellar oculomotor dysfunction. If the information was not documented, it was assumed to be absent. These data were used to determine if the subject met the criteria for probable or possible MSA according to the Second Consensus Statement [8]. Both neurologists then met to review each case and agree upon a neurological diagnosis as the etiology of the HCBS based upon the documented clinical data.
The study was approved by the Oregon Health & Science University (eIRB #16731) and Portland Veterans Administration (MIRB #4050) Joint Institutional Review Board. A waiver of Informed consent process and authorization was obtained.
RESULTS
The initial keyword search identified 119 patients whose radiology reports contained at least one of the search keywords. Further review of these radiology reports and the associated MR images found that 10 of 119 patients had MR imaging positive for the HCBS. Two of 10 patients with a HCBS were not consistent with adult cerebellar ataxia or parkinsonism and were excluded. These consisted of a childhood case of congenital olivopontocerebellar atrophy and an adult case that presented with cognitive impairment. Three cases with the HCBS discovered during routine clinical practice were included after the database search had taken place. Ultimately, 11 cases were included in the analysis. Figure 1 outlines the process utilized for subject inclusion.
Figure 1.
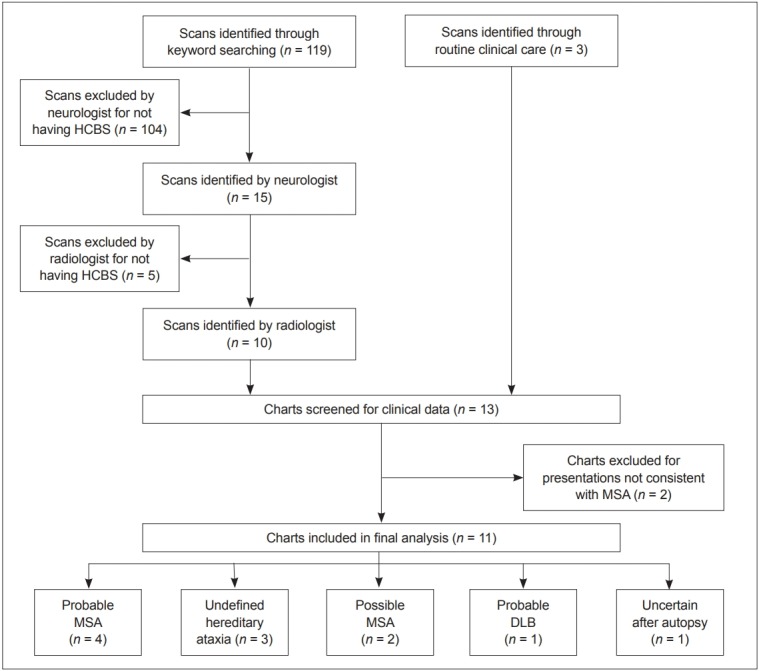
Subject inclusion process. HCBS: ‘hot cross bun’ sign, MSA: multiple system atrophy, DLB: dementia with Lewy bodies.
Brain tissue with pathology analysis was available for only 1 case. In life, this patient (case 2) had freezing of gait, rigidity, sialorrhea, and dysphagia suggestive of MSA; however, at autopsy there was only mild loss of pigmented neurons in the substantia nigra and negative staining for alpha-synuclein. There were no glial cytoplasmic inclusions. The case was reviewed by a neuropathologist who confirmed that the autopsy was not consistent with MSA. Genetic testing was negative for SCA1, 2, 3, 6, 7, 8, 10, and 17, dentatorubral-pallidoluysian atrophy, and Friedrich’s ataxia. This subject’s diagnosis was uncertain despite autopsy.
Genetic testing was performed in 2 of the remaining cases and was negative in both (cases 5 and 11). Patient 5 developed dysarthria and gait imbalance at age 36. Her oldest son and brother had global developmental delay, and her youngest son and niece had epilepsy. Testing for SCA1, 2, 3, 6, and 7 was negative. Due to urinary urgency and orthostatic hypotension, she fulfilled the clinical criteria for possible MSA. However, since the young age of onset and positive family history were atypical of MSA, her clinician suspected an undefined SCA, and this case was labeled as ‘undefined hereditary ataxia’. Cases 10 and 11 were 2 slowly progressive young-onset progressive cerebellar ataxias with family histories supporting an autosomal dominant inheritance pattern. Patient 10 did not have genetic testing, and patient 11 was negative for SCA1, 2, 3, 6, 8, and 17, but due to high clinical suspicion, both of these cases were diagnosed as ‘undefined hereditary ataxia’.
Patient 3 developed progressive cognitive decline and hallucinations at age 60. There was no orthostatic hypotension, erectile dysfunction, dysphagia, or urinary frequency or incontinence. Parkinsonism with antecollis was noted at age 64. At age 65, he had fluctuating cognitive impairment and scored 18/30 on Mini-Mental State Examination. He fulfilled the clinical criteria for probable dementia with Lewy bodies.
Of the remaining 6 cases, 4 met the clinical criteria for ‘probable MSA’ and 2 for ‘possible MSA’. Figure 2 displays axial T2 sequences of the pons of the non-MSA subjects with the ‘hot cross bun’ sign. Table 1 summarizes the clinical characteristics of the subjects with the HCBS.
Figure 2.
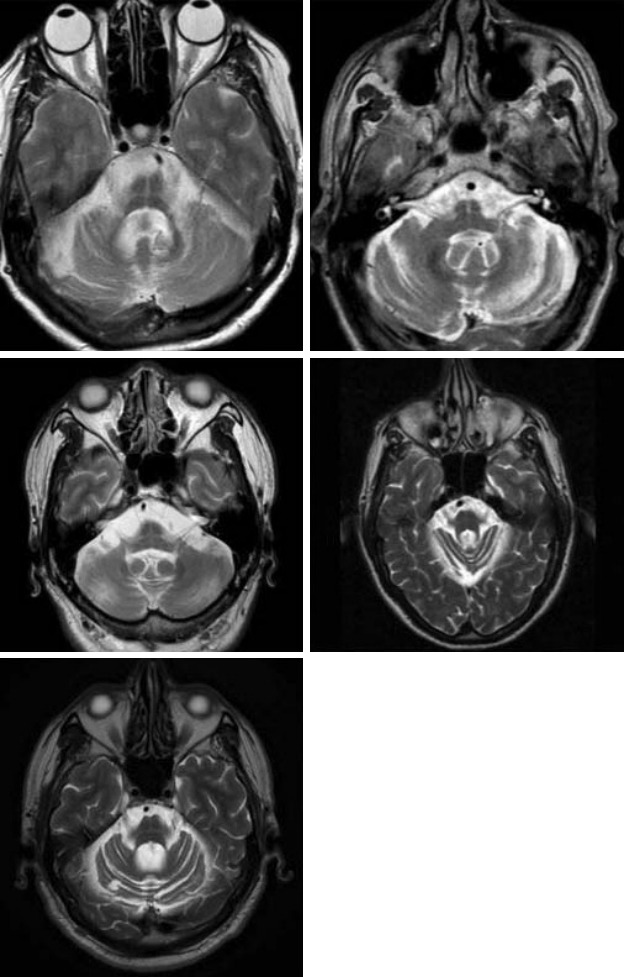
Axial T2 sequences of the pons of the non-MSA subjects with the ‘hot cross bun’ sign. MRI scans of cases 2, 3, 5, 10, and 11 are represented in the top left, top right, middle left, middle right, and bottom left images, respectively. MSA: multiple system atrophy.
Table 1.
Clinical characteristics of 11 subjects with the ‘hot cross bun’ sign
| ID | Sex | Age at onset (years) | Current age (years) | Time to MRI (years after symptom onset) | Parkinsonism | Cerebellar signs | Urinary symptoms | Erectile dysfunction | Orthostasis | Levodopa response | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 55 | 60 | 2 | Yes | Yes | Frequency | Not documented | Not documented | No | Possible MSA |
| 2 | F | 59 | Dead at 71 | 9 | No | Yes | Incontinence | N/A | Not documented | N/A | Uncertain after autopsy |
| 3 | M | 60 | 65 | 5 | Yes | No | None | No | No | No | Probable DLB |
| 4 | M | 61 | 66 | 3 | Yes | No | Retention | Yes | Yes | Yes | Possible MSA |
| 5 | F | 36 | 40 | 2 | No | Yes | Urgency | N/A | Yes | N/A | Undefined hereditary ataxia |
| 6 | F | 58 | 69 | 8 | No | Yes | Incontinence | N/A | No | No | Probable MSA |
| 7 | F | 55 | 68 | 3 | No | Yes | Incontinence | N/A | Yes | N/A | Probable MSA |
| 8 | F | 53 | Dead at 65 | 4 | Yes | Yes | Incontinence and retention | N/A | Yes | No | Probable MSA |
| 9 | F | 66 | 68 | 0 | Yes | Yes | Incontinence | N/A | No | Yes | Probable MSA |
| 10 | M | 33 | 79 | 33 | No | Yes | None | Not documented | Not documented | N/A | Undefined hereditary ataxia |
| 11 | M | 30 | 68 | 36 | No | Yes | None | Yes | Not documented | N/A | Undefined hereditary ataxia |
MSA: multiple system atrophy, DLB: dementia with Lewy bodies.
DISCUSSION
Current literature describes the HCBS as ‘highly specific for MSA.’ [9] This may lead clinicians to prematurely diagnose patients with MSA based upon the presence of an HCBS. In our sample, the HCBS clearly influenced the clinician to code a diagnosis of MSA in 3 of 11 cases.
Fiber tractography in a clinically probable MSA-C case with the HCBS has shown decreased volume of fiber bundles corresponding to the corticospinal tracts, transverse pontocerebellar fibers, pons, and cerebellum compared to a healthy subject [10]. We hypothesized that any cause of pontocerebellar degeneration would result in the HCBS and that MSA would be the most common, but not the only, etiology of this sign.
We included all subjects presenting clinically with adult cerebellar ataxia or parkinsonism at our centers found to have the HCBS, with the aim of clarifying the specificity of the sign for MSA in a sample with a conceivable age range and initial symptomatology for MSA. Six of the 11 subjects met the Second Consensus Criteria for probable or possible MSA. Although only 1 of the 5 non-MSA patients had an autopsy, the remaining subjects had clinical presentations that were very atypical of MSA. This suggests that the HCBS is not as specific for MSA as has been described. However, it is worth noting that 3/5 of the non-MSA patients had symptom onset in their 30s, and of the patients with age of onset > 50 years, 6/8 were diagnosed with probable or possible MSA.
There are some limitations to this study. There were no genetically confirmed ataxia cases. Of the 3 cases labeled as ‘hereditary ataxia,’ the etiologies were not specifically defined, though the onset in the 4th decade of slowly progressive cerebellar ataxias with autosomal dominant family histories strongly supports an undefined hereditary ataxia. Similarly, none of the MSA cases were pathologically confirmed, and the diagnoses were made solely based on the Second Consensus Criteria. Without genetic or postmortem verification, there may have been some misdiagnoses in the clinically diagnosed cases.
In addition, the keyword search could only search internal MRI reports, and cases of the HCBS that had MRIs reviewed by outside radiologists prior to referral to our center may have been missed. This was likely a significant factor limiting the sample size of our study.
In our sample, the HCBS was not specific for MSA, with only 6/11 of the cases fulfilling the clinical criteria for probable or possible MSA. A larger study with pathologic confirmation needs to be performed to further clarify the specificity of the HCBS for MSA.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Lee YC, Liu CS, Wu HM, Wang PS, Chang MH, Soong BW. The ‘hot cross bun’ sign in the patients with spinocerebellar ataxia. Eur J Neurol. 2009;16:513–516. doi: 10.1111/j.1468-1331.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 2.Jain RS, Nagpal K, Tejwani S. ‘Hot-cross bun’ and ‘inverse trident sign’ in progressive multifocal leukoencephalopathy with HIV seropositivity. Neurol India. 2014;62:341–342. doi: 10.4103/0028-3886.137032. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa H, Kawada N, Taniguchi A, Odachi K, Mizutani A, Asahi M, et al. Paraneoplastic neurological syndrome due to burned-out testicular tumor showing hot cross-bun sign. Acta Neurol Scand. 2016;133:398–402. doi: 10.1111/ane.12469. [DOI] [PubMed] [Google Scholar]
- 4.Pan Z, Yang G, Yuan T, Wang Y, Pang X, Gao Y, et al. ‘Hot cross bun’ sign with leptomeningeal metastases of breast cancer: a case report and review of the literature. World J Surg Oncol. 2015;13:43. doi: 10.1186/s12957-015-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roh SY, Jang HS, Kim YH. Hot cross bun sign following bilateral pontine infarction: a case report. J Mov Disord. 2013;6:37–39. doi: 10.14802/jmd.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RS, Sannegowda RB, Agrawal A, Hemrajani D, Jain R, Mathur T. ‘Hot cross bun’ sign in a case of cerebrotendinous xanthomatosis: a rare neuroimaging observation. BMJ Case Rep. 2013;2013:bcr2012006641. doi: 10.1136/bcr-2012-006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamm C, Healy DG, Quinn NP, Wüllner U, Moller JC, Schols L, et al. The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain. 2005;128(Pt 8):1855–1860. doi: 10.1093/brain/awh535. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasahara S, Miki Y, Kanagaki M, Kondo T, Yamamoto A, Morimoto E, et al. “Hot cross bun” sign in multiple system atrophy with predominant cerebellar ataxia: a comparison between proton density-weighted imaging and T2-weighted imaging. Eur J Radiol. 2012;81:2848–2852. doi: 10.1016/j.ejrad.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Loh KB, Rahmat K, Lim SY, Ramli N. A Hot Cross Bun sign from diffusion tensor imaging and tractography perspective. Neurol India. 2011;59:266–269. doi: 10.4103/0028-3886.79143. [DOI] [PubMed] [Google Scholar]


