Neuroferritinopathy is a rare progressive disease showing extrapyramidal symptoms such as chorea, dystonia, or parkinsonism and cognitive impairment as well as an accumulation of iron in the basal ganglia. Low serum ferritin levels and cavitation of the basal ganglia in brain magnetic resonance (MR) imaging are frequently observed in patients with neuroferritinopathy [1]. Mutations in the FTL1 gene encoding the ferritin light chain are the cause of neuroferritinopathy, and four insertional mutations were identified in four separate Asian families [2-5]. Here, we report a Korean patient with neuroferritinopathy who showed cystic lesions in the basal ganglia and a novel mutation in FTL1.
A 43-year-old woman presented with involuntary movement in her arms and mouth. Her symptoms had gradually worsened until she first visited our clinic at age 47. She had no perinatal and developmental problems, and no history of previous neurological and psychiatric illnesses. Her parents and five siblings did not have neurological illness. In a neurological examination, she showed very mild choreic dyskinesia in her shoulders, hands and feet at rest. When she was trying to speak or move her limbs, her limb dyskinesia temporarily worsened and dystonic spasms appeared in her lips and neck. Sometimes, she made a sniffing sound. She showed mild dystonia and mild bradykinesia in her fingers, but she did not show rigidity, ataxia, and gait abnormality. Laboratory studies showed no abnormality in her complete blood count, routine chemistry, thyroid function tests, and autoimmune screening tests. Her serum ceruloplasmin, iron, transferrin, and ferritin (20.9 μg/L; reference 10–122 μg/L) levels were normal. Her brain MR angiography was unremarkable. Meanwhile, her brain MR imaging showed bilateral globus pallidus lesions consisting of three layers with a cystic core, middle, and outer layer. The cystic core exhibited hypointensity in T1-weighted and fluid-attenuated inversion recovery (FLAIR) images. The middle layer surrounding the core was hypointense in T1 and hyperintense in FLAIR images, while the outer layer was hyperintense in T1 and hypointense in FLAIR and susceptibility-weighted images. This hypointensity lesion extended downward to the substantia nigra (Figure 1A). No other cortical or subcortical region showed abnormality. Dopamine transporter positron emission tomography imaging showed a diffuse mild reduction of striatal uptake. Whole genome sequencing and subsequent Sanger sequencing confirmed a novel mutation in exon 4 of FTL1 (c.439_440het_dupG, NM_000146.3), which causes a frameshift (p.D147GfsX34, NP_000137.2) leading to truncation of the ferritin light chain protein (Figure 1B). Her choreic movement partially responded to an oral haloperidol treatment.
Figure 1.
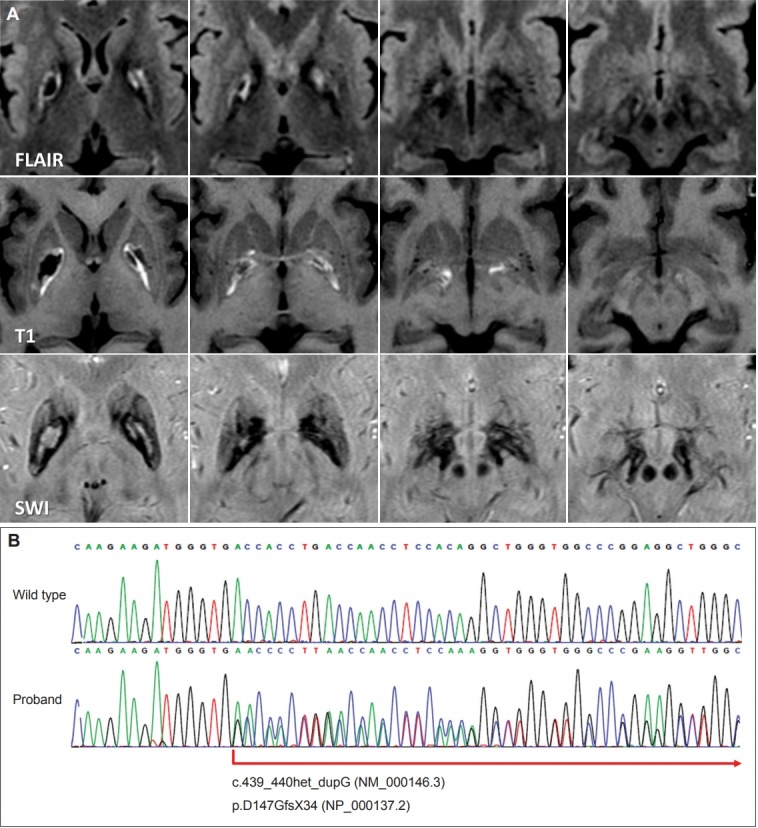
Brain MR images and the DNA sequence of the neuroferritinopathy patient. A: Fluid-attenuated inversion recovery (FLAIR) MR images show a trilamellar cystic lesion in the pallidum. T1-weighted and susceptibility-weighted (SWI) MR images show a cystic core and a surrounding rim of iron deposition. B: Sanger sequencing results of the proband showing a novel frameshift mutation (c.439_440het_dupG, NM_000146.3) in the FTL1 gene.
Until recently, ten mutations in FTL1 were found in more than 90 patients with neuroferritinopathy [5,6]. Except for one missense mutation found in a Portuguese individual with gypsy ancestry, all mutations, including the mutation found in our patient, were insertional mutations in exon 4 that caused a frameshift; therefore, exon 4 is considered to be a mutational hot spot in the FTL1 gene regardless of ethnicity [6]. Patients with FTL1 mutations that cause conformational changes only in helix-E of the ferritin light chain protein were likely to present a more severe phenotype than those with mutations leading to disruption of both helix-D and E [3]. Focal onset of chorea or dystonia in the late 30s and frequent associations with bulbar symptoms such as orolingual dyskinesia are the most typical clinical presentations of the 460InsA mutation, and it is the most frequent type of FTL1 mutation that causes changes in both helix D and E [1]. The FTL1 mutation found in our patient also causes production of both abnormal helix-D and E, and her clinical manifestations closely resemble the typical presentation of an FTL1 mutation producing abnormal helix-D and E.
Although the accumulation of iron can be found in the cerebral and cerebellar cortical surfaces appearing as thin hypointense lines in susceptibility-weighted imaging, referred to as a pencil-lining sign [7], iron accumulation in the subcortical nuclei combined with cystic degeneration in the pallidum and even in the putamen are the most characteristic features in MR imaging, especially in advanced cases [1,6]. Lesions with hyperintensity in T1-weighted and hypointensity in iron-sensitive MR images appear in the basal ganglia, thalamus, substantia nigra, red nucleus, and dentate nucleus in asymptomatic carriers; the pallidal lesion found in advanced patients exhibits a typical cystic core with hypointensity in T1-weighted and FLAIR MR images as well as a surrounding rim with hyperintensity in T1-weighted and hypointensity in iron-sensitive MR images [1]. Interestingly, FLAIR MR images of the current patient exhibited trilamellar intensity around the cystic lesion, which may represent different stages of expanding pathology, consisting of an outer layer with iron deposition, a middle layer with suspicious on-going degenerative process and gliosis, and a cystic core with tissue loss. The FTL1 mutation may cause impaired sequestration of iron by ferritin, thereby leading to the accumulation of free iron, production of free radicals, increased oxidative stress, and neurodegeneration [6]. This hypothetical cascade of pathogenesis may explain the different stages of lesions shown in the MR imaging.
In summary, we found a novel c.439_440het_dupG mutation in exon 4 of FTL1 in a patient presenting with typical clinical manifestations of neuroferritinopathy, and trilamellar cystic degeneration in the FLAIR MR image may be another radiological sign that characterizes neuroferritinopathy.
Acknowledgments
This study was financially supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. 2015R1C1A2A01054507) and the Basic Science Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2017R1A2B2006694). This study was approved by the institutional review board of Gangnam Severance Hospital.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
REFERENCES
- 1.Chinnery PF, Crompton DE, Birchall D, Jackson MJ, Coulthard A, Lombes A, et al. Clinical features and natural history of neuroferritinopathy caused by the FTL1 460InsA mutation. Brain. 2007;130:110–119. doi: 10.1093/brain/awl319. [DOI] [PubMed] [Google Scholar]
- 2.Ohta E, Nagasaka T, Shindo K, Toma S, Nagasaka K, Ohta K, et al. Neuroferritinopathy in a Japanese family with a duplication in the ferritin light chain gene. Neurology. 2008;70:1493–1494. doi: 10.1212/01.wnl.0000310428.74624.95. [DOI] [PubMed] [Google Scholar]
- 3.Kubota A, Hida A, Ichikawa Y, Momose Y, Goto J, Igeta Y, et al. A novel ferritin light chain gene mutation in a Japanese family with neuroferritinopathy: description of clinical features and implications for genotypephenotype correlations. Mov Disord. 2009;24:441–445. doi: 10.1002/mds.22435. [DOI] [PubMed] [Google Scholar]
- 4.Nishida K, Garringer HJ, Futamura N, Funakawa I, Jinnai K, Vidal R, et al. A novel ferritin light chain mutation in neuroferritinopathy with an atypical presentation. J Neurol Sci. 2014;342:173–177. doi: 10.1016/j.jns.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni W, Li HF, Zheng YC, Wu ZY. FTL mutation in a Chinese pedigree with neuroferritinopathy. Neurol Genet. 2016;2:e74. doi: 10.1212/NXG.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi S, Rovida E. Neuroferritinopathy: from ferritin structure modification to pathogenetic mechanism. Neurobiol Dis. 2015;81:134–143. doi: 10.1016/j.nbd.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batla A, Adams ME, Erro R, Ganos C, Balint B, Mencacci NE, et al. Cortical pencil lining in neuroferritinopathy: a diagnostic clue. Neurology. 2015;84:1816–1818. doi: 10.1212/WNL.0000000000001511. [DOI] [PMC free article] [PubMed] [Google Scholar]


