Abstract
Background
Renal transplantation is the optimal treatment for selected patients with end-stage renal disease (ESRD). However, the survival benefit of renal transplantation among patients with ESRD attributed to granulomatosis with polyangiitis (GPA) is unknown.
Methods
We identified patients from the United States Renal Data System with ESRD due to GPA (ESRD-GPA) between 1995 and 2014. We restricted our analysis to waitlisted subjects to evaluate the impact of transplantation on mortality. We followed patients until death or the end of follow-up. We compared the relative risk (RR) of all-cause and cause-specific mortality in patients who received a transplant versus non-transplanted patients using a pooled logistic regression model with transplantation as a time-varying exposure.
Results
During the study period, 1525 patients were waitlisted and 946 received a renal transplant. Receiving a renal transplant was associated with a 70% reduction in the risk of all-cause mortality in multivariable-adjusted analyses (RR=0.30, 95% CI 0.25 to 0.37), largely attributed to a 90% reduction in the risk of death due to cardiovascular disease (CVD) (RR=0.10, 95% 0.06–0.16).
Discussion
Renal transplantation is associated with a significant decrease in all-cause mortality among patients with ESRD attributed to GPA, largely due to a decrease in the risk of death to CVD. Prompt referral for transplantation is critical to optimise outcomes for this patient population.
INTRODUCTION
Granulomatosis with polyangiitis (GPA) is a small vessel vasculitis associated with renal involvement (ie, glomerulonephritis) in up to 70% of patients. Among patients with renal disease, 20%–25% develop end-stage renal disease (ESRD).1 Renal transplantation is the standard of care for selected patients with ESRD attributed to GPA (ESRD-GPA) given prior studies describing safe and successful transplantation in this population.2–4 GPA and ESRD are associated with premature death, often due to cardiovascular disease (CVD), but the impact of renal transplantation on mortality in this population is unknown.5–7
A prior study demonstrated that renal transplantation is associated with a 68% reduction in the risk of death among patients with ESRD waitlisted for a renal transplant.8 The majority of patients included in that study had ESRD attributable to causes other than glomerulonephritis, such as hypertension and diabetes. However, overall health, comorbidities and life expectancy may differ significantly between patients with ESRD due to GPA and patients with ESRD due to diabetes, hypertension or other causes.9,10
We used a national registry of patients with ESRD-GPA to determine the impact of renal transplantation on survival.
PATIENTS AND METHODS
Data source and study population
The United States Renal Data System (USRDS) is a national registry of patients with ESRD, representing an estimated 94% of patients who receive dialysis or kidney transplantation. Patients who refuse replacement therapy, die prior to enrolment or receive transient dialysis for acute renal failure may not be enrolled. Attending nephrologists are required by law to submit a Medical Evidence Report, which includes the cause of ESRD according to the International Classification of Diseases, ninth revision (ICD-9) codes within 45 days of a patient starting a new ESRD treatment.
To assess the impact of transplantation on survival (primary analysis), we included all patients who fulfilled the following criteria: (1) ESRD attributed to GPA (ICD-9: 446.4); (2) initiated haemodialysis or peritoneal dialysis between 1 January 1995 and 31 December 2014; and (3) waitlisted for a renal transplant between 1 January 1995 and 31 December 2015. We excluded patients who were pre-emptively transplanted without being waitlisted or without receiving haemodialysis or peritoneal dialysis. We restricted our analysis to those patients waitlisted for a renal transplant to minimise confounding by indication given that, generally, younger and healthier patients with higher socioeconomic status and social support are more likely to be waitlisted for a renal transplant.8
Covariates
The following information was extracted from the USRDS and used as covariates, exposures or outcomes: demographics (eg, age, sex, self-reported race); body mass index; relevant comorbidities (eg, diabetes, hypertension, coronary artery disease); initial ESRD therapy modality; waitlisting date; transplant status; date of renal transplant; vital status; date of death; and primary cause of death. Relevant comorbidities reported in the Medical Evidence Report at the onset of ESRD were used to calculate a weighted comorbidity score developed specifically for USRDS data.11
Statistical analysis
The date on which the patient was first waitlisted for a renal transplant was used as the start of follow-up. We determined mortality rates (/1000 patient-years) by allocating time spent prior to a renal transplant to the group of patients who did not receive a renal transplant; 95% CIs for mortality rates were estimated using least-squares means. To avoid immortal time bias, we performed a pooled logistic regression in which first renal transplantation was treated as a time-varying exposure; this approach approximates that of a time-dependent Cox regression.12 Age was used as the time scale. We compared the relative risk (RR) of all-cause mortality and cause-specific mortality (ie, CVD, infection and other causes) among patients who received a renal transplant during the study period to those who did not, after adjusting for relevant covariates. Given the limited number of outcomes of interest in some subgroup analyses, we adjusted for comorbidities using a weighted comorbidity score.11 We performed subgroup analyses, evaluating differences in all-cause mortality with and without transplantation according to sex, age group at ESRD onset and year of ESRD onset. For year of ESRD onset subgroup analyses, the cohort was divided into two subcohorts based on the year of ESRD (1995–2004 and 2005–2014). Patients with ESRD onset during a respective time period who were not waitlisted during that time period were excluded from this subgroup analysis. Cumulative incidence functions were used to compare overall and cause-specific mortality between patients who did and did not receive a transplant during the study period.
All p values were two-sided with a significance threshold of <0.05. Statistical analyses were performed using SAS V.9.4.
Sensitivity analysis
We performed sensitivity analyses to verify the results of our primary analysis. To further address potential confounding by indication (or contraindication) that may occur when patients become too sick or otherwise unsuitable for a renal transplant, we censored patients at the time they were inactivated or removed from the transplant waitlist.13 We expected this to attenuate our results since it introduces informative censoring (ie, censoring for a factor in the causal pathway between being waitlisted and dying). In a separate analysis, we censored patients who received living donor transplant at the time of transplantation since these patients may differ from other patients on the waitlist with regard to potential unmeasured confounding factors.
Data use
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
RESULTS
Baseline characteristics
Between 1995 and 2014, 5929 patients were diagnosed with ESRD attributed to GPA; of these, 1525 patients were waitlisted for a renal transplant during the study period (table 1). The average age at the time of being waitlisted for a renal transplant was 49.5 (±16.4) years. The majority of patients were male (59%) and white (86%). Hypertension was the most common comorbidity (67%). Haemodialysis was the most common (86%) first modality of renal replacement therapy. Of the 1525 patients waitlisted for a renal transplant, 946 received a renal transplant during the study period. The average age at the time of transplant was 48.4 (±17.0) years. Deceased donor transplantations were performed in 56% of cases, and living donor transplantations were performed in 44% of cases.
Table 1.
Baseline features of patients with end-stage renal disease due to granulomatosis with polyangiitis (ESRD-GPA)
| GPA-ESRD | Waitlisted | Transplanted during study period | |
|---|---|---|---|
| N | 5929 | 1525 | 946 |
| Age at ESRD, years (%) | |||
| <40 | 12.6% | 27.9% | 33.9% |
| 40–49 | 9.9% | 18.2% | 19.2% |
| 50–59 | 16.9% | 26.6% | 24.4% |
| ≥60 | 60.6% | 27.3% | 22.4% |
| Male (N, %) | 3367 (57%) | 906 (59%) | 560 (59%) |
| Body mass index | 26.8 (±6.7) | 27.0 (±6.5) | 26.7 (±6.4) |
| Race | |||
| White | 5305 (90%) | 1312 (86%) | 825 (87%) |
| Black | 385 (7%) | 132 (9%) | 72 (8%) |
| Other | 239 (4%) | 81 (5%) | 51 (5%) |
| Hispanic | 536 (9%) | 191 (13%) | 106 (11%) |
| Comorbidities | |||
| Diabetes | 852 (14%) | 134 (9%) | 68 (7%) |
| Hypertension | 4014 (68%) | 1010 (67%) | 602 (64%) |
| COPD | 507 (9%) | 48 (3%) | 20 (2%) |
| CAD | 686 (12%) | 67 (5%) | 42 (5%) |
| PVD | 303 (5%) | 35 (2%) | 21 (2%) |
| CHF | 859 (15%) | 94 (6%) | 43 (5%) |
| CVA | 265 (5%) | 36 (2%) | 24 (3%) |
| Other cardiac disease | 151 (3%) | 20 (2%) | 16 (2%) |
| Tobacco | 227 (4%) | 48 (3%) | 20 (2%) |
| Cancer | 306 (5%) | 38 (3%) | 20 (2%) |
| Comorbidity score | 1.1 (±1.8) | 0.5 (±1.2) | 0.4 (±1.1) |
| First modality | |||
| Transplant* | 128 (2%) | - | - |
| Haemodialysis | 5328 (90%) | 1305 (86%) | 798 (84%) |
| Peritoneal dialysis | 450 (8%) | 220 (14%) | 148 (16%) |
Patients transplanted prior to dialysis were excluded from the transplantation analyses.
CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular attack; MPA, microscopic polyangiitis; PVD, peripheral vascular disease.
All-cause mortality
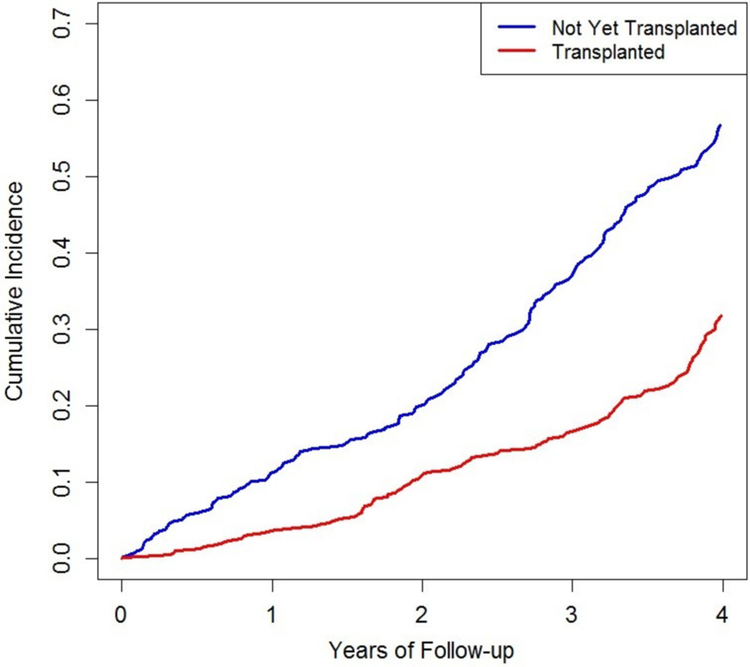
During follow-up, 438 patients died. Among those who received a renal transplant (n=946), there were 199 deaths (table 2). Among those who did not receive a renal transplant (n=579), there were 239 deaths. The mortality rate (95% CI) among those who received a renal transplant was 29.3 (25.5–33.6)/1000 patient-years in contrast to 65.5 (57.7–74.3)/1000 patient-years among those who did not receive a renal transplant (p<0.001). In multivariable-adjusted analyses, renal transplantation was associated with a 70% reduction in the risk of death (figure 1; RR=0.30, 95% CI 0.25 to 0.36).
Table 2.
All-cause mortality according to transplant status among waitlisted patients with end-stage renal disease (ESRD) due to granulomatosis with polyangiitis
| N | Total follow-up | Deaths | Mortality rate (/1000 patient-years, 95% CI) | unadjusted RR (95% CI) | Age-adjusted, sex-adjusted, enrolment-adjusted RR (95% CI)* | Fully adjusted RR (95% CI)† | |
|---|---|---|---|---|---|---|---|
| Overall | 1525 | 10 456 | 438 | 41.9 (38.1 to 46.0) | |||
| Transplanted | 946 | 6804 | 199 | 29.3 (25.5 to 33.6) | 0.39 (0.33 to 0.46) | 0.30 (0.25 to 0.36) | 0.30 (0.25 to 0.36) |
| Not transplanted | 579 | 3651 | 239 | 65.5 (57.7 to 74.3) | Ref | Ref | Ref |
| Age | |||||||
| <40 years | 448 | 3481 | 71 | 20.4 (16.2 to 25.7) | |||
| Transplanted | 344 | 2816 | 39 | 13.9 (10.1 to 19.0) | 0.32 (0.20 to 0.50) | 0.22 (0.14 to 0.35) | 0.19 (0.12 to 0.31) |
| Not transplanted | 104 | 666 | 32 | 48.1 (34.0 to 68.0) | Ref | Ref | Ref |
| 40–49 years | 284 | 2119 | 75 | 35.4 (28.2 to 44.4) | |||
| Transplanted | 188 | 1453 | 35 | 24.1 (17.3 to 33.6) | 0.33 (0.22 to 0.52) | 0.22 (0.14 to 0.37) | 0.25 (0.15 to 0.41) |
| Not transplanted | 96 | 667 | 40 | 60.0 (44.0 to 81.8) | Ref | Ref | Ref |
| 50–59 years | 407 | 2723 | 130 | 47.7 (40.2 to 56.7) | |||
| Transplanted | 232 | 1679 | 57 | 34.0 (26.2 to 44.0) | 0.43 (0.31 to 0.60) | 0.23 (0.16 to 0.34) | 0.21 (0.14 to 0.32) |
| Not transplanted | 175 | 1045 | 73 | 69.9 (55.5 to 87.9) | Ref | Ref | Ref |
| ≥60 years | 417 | 2302 | 168 | 73.0 (62.7 to 84.9) | |||
| Transplanted | 213 | 1256 | 74 | 58.9 (46.9 to 74.0) | 0.62 (0.47 to 0.82) | 0.35 (0.26 to 0.48) | 0.38 (0.27 to 0.52) |
| Not transplanted | 204 | 1046 | 94 | 89.9 (73.4 to 110.0) | Ref | Ref | Ref |
| Sex | |||||||
| Male | 923 | 6268 | 274 | 43.7 (38.8 to 49.2) | |||
| Transplanted | 577 | 4292 | 127 | 29.6 (24.9 to 35.2) | 0.38 (0.31 to 0.48) | 0.28 (0.23 to 0.36) | 0.30 (0.23 to 0.37) |
| Not transplanted | 346 | 1976 | 147 | 74.4 (63.3 to 87.5) | Ref | Ref | Ref |
| Female | 633 | 4358 | 170 | 39.0 (33.6 to 45.3) | |||
| Transplanted | 400 | 2911 | 78 | 26.8 (21.5 to 33.5) | 0.39 (0.29 to 0.51) | 0.33 (0.25 to 0.45) | 0.33 (0.24 to 0.45) |
| Not transplanted | 233 | 1447 | 92 | 63.6 (51.8 to 78.0) | Ref | Ref | Ref |
| Calendar year of ESRD onset* | |||||||
| 1995–2004 | 560 | 2217 | 96 | 43.3 (35.5 to 52.9) | |||
| Transplanted | 347 | 1290 | 25 | 19.4 (13.1 to 28.7) | 0.16 (0.10 to 0.27) | 0.16 (0.10 to 0.26) | 0.17 (0.10 to 0.29) |
| Not transplanted | 213 | 897 | 71 | 79.1 (62.7 to 99.9) | Ref | Ref | Ref |
| 2005–2014 | 890 | 4230 | 121 | 28.6 (23.9 to 34.2) | |||
| Transplanted | 478 | 2097 | 27 | 12.9 (8.8 to 18.8) | 0.24 (0.16 to 0.37) | 0.25 (0.16 to 0.38) | 0.25 (0.16 to 0.38) |
| Not transplanted | 412 | 2121 | 94 | 44.3 (36.2 to 54.3) | Ref | Ref | |
Excluded patients who were not waitlisted prior to the end of time period censoring.
Fully adjusted models adjust for sex, age, ESRD year, comorbidity score, white/non-white, first modality. RR. relative risk.
Figure 1.
Cumulative incidence of all-cause death according to transplant status among waitlisted patients with end-stage renal disease due to granulomatosis with polyangiitis.
Our results were similar across the subgroups, including sex, age at ESRD onset and year of ESRD onset (table 2). As expected, the greatest benefit was identified in those patients under the age of 40 years (RR=0.19, 95% CI 0.12 to 0.31). There was a slight attenuation in the mortality benefit associated with transplantation in the most recent decade (2005–2014) compared with the preceding decade (1995–2004).
Cause-specific mortality
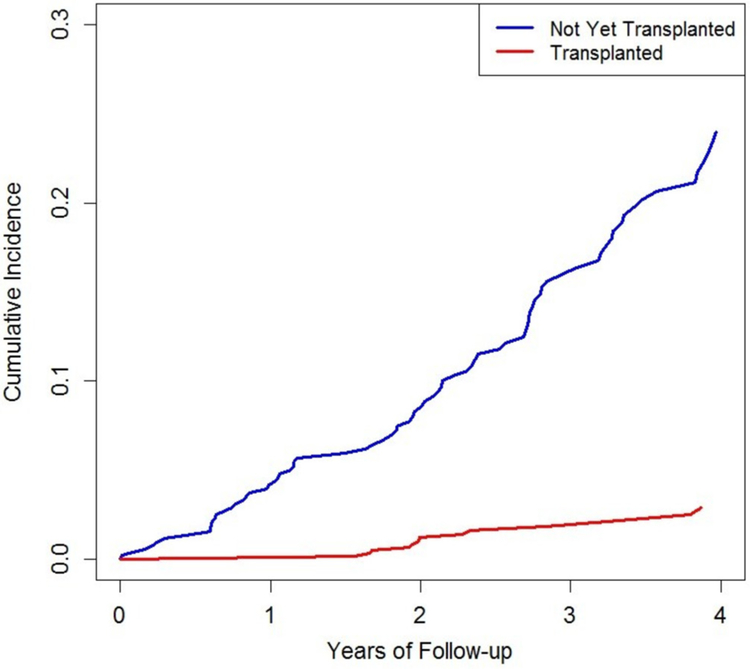
In multivariable-adjusted analyses, patients who received a transplant had a 90% lower risk of death due to CVD than non-transplanted patients (RR=0.10, 95% 0.06–0.16; table 3, figure 2). There were also significant reductions in the risk of death due to infection (RR=0.55, 95% CI 0.31 to 0.97) and other causes (RR=0.48, 95% CI 0.29 to 0.79). The most frequent other causes identified included withdrawal from dialysis (n=19, 14% of other causes) and malignancy (n=24, 9% of other causes). Because of the few number of malignancies, fully adjusted analyses were not possible but in age-adjusted and sex-adjusted analyses, there was no difference in the risk of malignancy (RR=1.44, 95% CI 0.59 to 3.51).
Table 3.
Cause-specific mortality according to transplant status among waitlisted patients with end-stage renal disease due to granulomatosis with polyangiitis
| Deaths | unadjusted RR (95% CI) | Age-adjusted, sex-adjusted, enrolment-adjusted RR (95% CI) | Fully adjusted RR (95% CI) | |
|---|---|---|---|---|
| Cardiovascular death | ||||
| Transplanted | 23 | 0.13 (0.08 to 0.20) | 0.11 (0.07 to 0.18) | 0.10 (0.06 to 0.16) |
| Not transplanted | 97 | Ref | Ref | Ref |
| Infection death | ||||
| Transplanted | 24 | 0.49 (0.28 to 0.84) | 0.52 (0.29 to 92) | 0.55 (0.31 to 0.97) |
| Not transplanted | 29 | Ref | Ref | Ref |
| Other death | ||||
| Transplanted | 152 | 0.46 (0.27 to 0.76) | 0.46 (0.27 to 0.77) | 0.48 (0.29 to 0.79) |
| Not transplanted | 113 | Ref | Ref | Ref |
RR, relative risk.
Figure 2.
Cumulative incidence of death due to cardiovascular disease according to transplant status among waitlisted patients with end-stage renal disease due to granulomatosis with polyangiitis.
Sensitivity analyses
To verify the findings in our primary analysis, we performed two sensitivity analyses. First, we censored patients who were inactivated or removed from the waitlist (n=192). Common reasons for being inactivated or removed included being too sick or medically unsuitable for a renal transplant. The survival benefit associated with transplantation was attenuated in this analysis (RR=0.54, 95% CI 0.44 to 0.66) which was expected because we introduced informative censoring (ie, those who were more likely to die were preferentially eliminated from the non-transplanted group). Second, we censored living donor transplant recipients and found a similar survival benefit as in our primary analysis (RR=0.45, 95% CI 0.37 to 0.55).
DISCUSSION
In this nationwide study of patients with ESRD due to GPA, renal transplantation was associated with a dramatic reduction in the risk of death, especially due to CVD. Previous studies had reported good graft and patient survival in smaller cohorts of renal transplant recipients who had ESRD due to antineutrophil cytoplasmic antibody-associated vasculitis (AAV).3,4,10,14
However, no study has evaluated the relative survival benefit of renal transplantation in this population.
Wolfe et al first reported the significant survival benefit associated with renal transplantation in patients with ESRD from a variety of causes, the minority of whom had glomerulonephritis.8 Indeed, patients with ESRD resulting from GPA are often different from patients with ESRD due to diabetes or hypertension, especially with regard to comorbidities and other organ involvement by GPA that may impact their potential to be waitlisted and overall survival with or without a renal transplant.9 Moreover, patients with GPA typically have a history of immunosuppression and may be immunosuppressed at the time of renal transplantation which could impact outcomes following renal transplantation. Despite these differences, we found that the survival benefit of renal transplantation among patients with GPA was similar to that previously described in the general population of patients with ESRD.8,15 In those studies, RR of death due to CVD associated with transplantation was not reported. The impact of contemporary post-transplant immunosuppression may influence the risk of GPA flares which might prevent further organ damage and improve overall survival.
Moreover, our findings indicate that much of the improvement in mortality is due to a reduction in death due to CVD. CVD is the most common cause of death in ESRD, in general, and transplantation is known to decrease the risk of CVD death in this general population.16 Due to differences in methods, we cannot directly compare our findings with regard to CVD mortality to prior studies.16 However, to our knowledge, this is the first study in AAV to demonstrate that a specific intervention can significantly reduce the risk of death through an impact on cardiovascular death. Previous studies have found an increased risk of CVD among patients with AAV but it is unclear what portion of CVD in this population is mediated by chronic kidney disease, steroid exposure and/or the inflammatory state associated with AAV.7 Post-transplant immunosuppression may also have a beneficial effect with regard to CVD risk. The impact of CVD risk reduction strategies in AAV has not been previously studied.
This study has important implications for the management of AAV (eg, GPA) patients with advanced renal involvement. First, providers, regardless of specialty (eg, rheumatology, nephrology, pulmonary), should consider referring patients to a renal transplant centre for evaluation and do so early in their disease course. Pre-emptive transplantation performed before any dialysis is required is associated with improved outcomes relative to even a 6-month period of dialysis.16 Of note, patients can accrue time on the transplantation waiting list once the estimated glomerular filtration rate (eGFR) is <20 mL/min/1.73 m2; in other words, a patient does not need to be on dialysis to be waitlisted for a renal transplant. However, many transplant centres recommend referrals of patients whose eGFR is >20 (eg, 25–30 mL/min/1.73 m2), to allow sufficient time for recipient and live donor evaluations and to increase the chance that a pre-emptive transplant can be arranged. Moreover, there are few absolute contraindications to organ transplantation and referring potential candidates to a transplant centre is necessary to determine a patient’s candidacy for renal transplantation, regardless of age or comorbidities (eg, malignancy, CVD).
Second, CVD is a common cause of death among waitlisted patients, suggesting that CVD risk assessment and modification may further improve survival in this population. Notably, a prior randomised controlled trial comparing statin therapy versus placebo in patients with ESRD on haemodialysis found no benefit associated with statin therapy.17 It is unclear if these results can be extrapolated to patients with ESRD attributable to immune-mediated conditions (eg, GPA) or those on the waitlist. Future studies might evaluate factors responsible for the dramatic reduction in the risk of death due to CVD following renal transplantation. Possible explanations include physiological differences between filtration through a functioning kidney as opposed to across a dialysis membrane18 and/or differences in the management of patients prior to and after a renal transplant.
There are several strengths of this study related to our data source and study design. In particular, the USRDS is a nationwide registry that captures nearly all patients with ESRD in the USA. The diagnosis of GPA was made and reported by attending nephrologists as per legal requirements associated with documentation for medical benefits associated with having ESRD. Previous studies have used similar methods.3,19 We also designed our study to limit the potential biases associated with confounding by indication as well as immortal time bias by restricting our analysis to waitlisted patients and treating renal transplantation as a time-varying exposure, respectively.8 Generally, people waitlisted for a renal transplant share many common features (eg, generally good overall health, younger age, higher socioeconomic status and strong social support) which would otherwise be potentially impactful confounders in a study that included all patients with ESRD.
Our study has certain limitations. The USRDS enrols patients when they reach ESRD but does not include details regarding the history of GPA. As such, we cannot address how certain factors, such as time between GPA onset and transplantation, ANCA type and titre or immunosuppression exposure, may affect outcomes. The ICD-9 code for MPA is not specific for that condition, and we therefore only included GPA patients with ESRD in this study. While the ICD-9 code for GPA is more specific, validated diagnostic criteria could not be applied to confirm each diagnosis. Additionally, we do not have details regarding ANCA titres in these patients at the time of renal transplantation. The impact of positive ANCA testing at the time of transplantation and duration of remission prior to transplantation on transplant outcomes remains controversial.20,21 Generally, it is recommended that vasculitis be in remission at the time of transplantation (with or without a negative ANCA test) and this remission has lasted for at least 12 months prior to transplantation.20
In summary, in this national cohort study of patients with ESRD due to GPA, we found that renal transplantation is associated with a significant survival benefit, largely due to a dramatic reduction in the risk of death due to CVD. Routine management of GPA patients with advanced chronic kidney disease should include an evaluation for renal transplant eligibility.
Acknowledgements
The authors appreciate the support of the staff of the United States Renal Data System.
Funding This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests ZSW has received funding from a Scientist Development Award from the Rheumatology Research Foundation, a Fund for Medical Discovery Award from the Executive Committee on Research at Massachusetts General Hospital, and an NIH Loan Repayment Award.
Patient consent Not required.
Ethics approval The IRB has determined that this activity does not meet the definition of human subjects research. The investigators conducting this research will not obtain data through an intervention or interaction with individual subjects or identifiable private information about living individuals. This study was exempted from the Partner’s HealthCare Institutional Review Board because it only used deidentified data.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data collected by the United States Renal Data System (USRDS) are extensive and maintained by the National Institutes of Health. Details regarding the data collected can be reviewed at this website: https://www.usrds.org/
REFERENCES
- 1.Moiseev S, Novikov P, Jayne D, et al. End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant 2017;32:248–53. [DOI] [PubMed] [Google Scholar]
- 2.Hruskova Z, Geetha D, Tesar V. Renal transplantation in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2015;30(Suppl 1):i159–63. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Gill J, Shangguan M, et al. Outcomes of renal transplantation in recipients with Wegener’s granulomatosis. Clin Transplant 2011;25:380–7. [DOI] [PubMed] [Google Scholar]
- 4.Geetha D, Eirin A, True K, et al. Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis: a multicenter experience. Transplantation 2011;91:1370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US renal data system 2009 annual data report. Am J Kidney Dis 2010;55(Suppl 1):A6–A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace ZS, Lu N, Unizony S, et al. Improved survival in granulomatosis with polyangiitis: A general population-based study. Semin Arthritis Rheum 2016;45:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviña-Zubieta JA, Mai A, Amiri N, et al. Risk of myocardial infarction and stroke in patients with granulomatosis with polyangiitis (Wegener’s): a population-based study. Arthritis Rheumatol 2016;68:2752–9. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725–30. [DOI] [PubMed] [Google Scholar]
- 9.O’Shaughnessy MM, Montez-Rath ME, Lafayette RA, et al. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol 2015;10:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruskova Z, Stel VS, Jayne D, et al. Characteristics and outcomes of granulomatosis with polyangiitis (Wegener) and microscopic polyangiitis requiring renal replacement therapy: results from the European renal association-European dialysis and transplant association registry. Am J Kidney Dis 2015;66:613–20. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Huang Z, Gilbertson DT, et al. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 2010;77:141–51. [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino RB, Lee ML, Belanger AJ, et al. Relation of pooled logistic regression to time dependent cox regression analysis: the Framingham heart study. Stat Med 1990;9:1501–15. [DOI] [PubMed] [Google Scholar]
- 13.Shafi S, Zimmerman B, Kalil R. Temporary inactive status on renal transplant waiting list: causes, risk factors, and outcomes. Transplant Proc 2012;44:1236–40. [DOI] [PubMed] [Google Scholar]
- 14.Göçeroğlu A, Rahmattulla C, Berden AE, et al. The Dutch Transplantation in Vasculitis (DUTRAVAS) Study: outcome of renal transplantation in antineutrophil cytoplasmic antibody-associated glomerulonephritis. Transplantation 2016;100:916–24. [DOI] [PubMed] [Google Scholar]
- 15.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol 2005;16:1859–65. [DOI] [PubMed] [Google Scholar]
- 16.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002;74:1377–81. [DOI] [PubMed] [Google Scholar]
- 17.Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009;360:1395–407. [DOI] [PubMed] [Google Scholar]
- 18.Bhatti NK, Karimi Galougahi K, Paz Y, et al. Diagnosis and management of cardiovascular disease in advanced and end-stage renal disease. J Am Heart Assoc 2016;5:e003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace ZS, Zhang Y, Lu N, et al. Improving mortality in end-stage renal disease due to granulomatosis with polyangiitis from 1995 to 2014. Arthritis Care Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran S, Little MA. Renal transplantation in antineutrophil cytoplasmic antibody-associated vasculitis. Curr Opin Rheumatol 2014;26:37–41. [DOI] [PubMed] [Google Scholar]
- 21.Little MA, Hassan B, Jacques S, et al. Renal transplantation in systemic vasculitis: when is it safe? Nephrol Dial Transplant 2009;24:3219–25. [DOI] [PubMed] [Google Scholar]