Abstract
Elucidating chromatin’s 3D shape is critical to understanding its function, but the fine structure of chromatin domains remains poorly resolved. In a recent report in Nature, Boettiger et al. (2016) visualize chromatin in super-resolution, gaining unprecedented insight into chromatin architecture.
High-throughput sequencing technologies have enabled the study of chromatin and genes at base-pair resolution. However, putting this information into the context of a three-dimensionally organized nucleus has been more challenging. One of the fundamental unsolved riddles in genome biology is how the chromatin fiber and chromatin domains are organized in 3D space. This question has long gone unanswered because light microscopy has insufficient resolution to clearly discern the chromatin fiber, and electron microscopy, while sufficiently powerful in theory, requires staining and contrasting methods that mask the fine structure of chromatin.
Boettiger et al. (2016) have taken advantage of two game-changing breakthroughs in imaging to tackle this pivotal issue in the field. First, they use super-resolution microscopy, which enables visualization of chromatin structure at resolution beyond that of the light used to generate the image (Rust et al., 2006). Second, they utilize a groundbreaking method, referred to as Oligopaints, which uses complex, custom-designed DNA oligonucleotide libraries to generate fluorescence in situ hybridization (FISH) probes that are able to detect any region in the genome with high efficiency and specificity (Beliveau et al., 2012, 2015). For their study, Boettiger et al. selected 46 genomic domains in the Drosophila genome based on their transcriptional activity and epigenetic status, and analyzed a total of 81 regions and sub-regions within these domains, ranging from 10 to 500 kb in size.
A simple, but unanswered, question has been how the activity state of a genome region relates to its structure. Boettiger et al. classified each analyzed region as active, inactive, or repressed, based on gene transcription, histone modifications, and binding of Polycomb, a well-characterized complex associated with repressed chromatin (Bickmore, 2013), and systematically measured the volume and radius of gyration of loci in all three types of chromatin. In line with previous lower-resolution studies, they find that active regions are the least densely packaged, repressed regions the most densely packaged, and inactive regions fall somewhere in between.
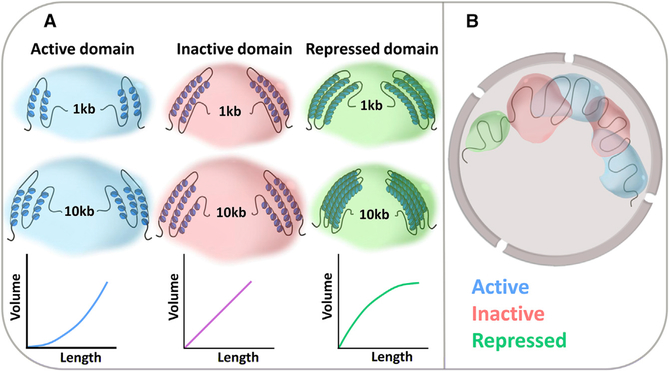
Boettiger et al. (2016) then analyzed in more detail the organization of heterochromatic genome regions silenced by the repressive Polycomb complex. Biochemical studies have previously shown that Polycomb-repressed heterochromatin is denser than euchromatin (Francis et al., 2004) and that Polycombsilenced regions form distinct domains within the nucleus (Cmarko et al., 2003). It has also been known that polymerization of the Polycomb complex, mediated by the SAM (sterile alpha motif) domain of Polyhomeotic (Ph), is required for efficient gene silencing (Robinson et al., 2012) and for formation of the repressive chromatin domain (Isono et al., 2013). The high-precision measurements gathered by Boettiger et al. elaborate on these data and point to Polycomb self-association as a driving force in the organization of the chromatin fiber in repressed domains. The smoking gun to suggest this model is a particularly low scaling constant in Polycomb-repressed regions, meaning that longer Polycomb-repressed regions are denser than shorter regions and that subdomains including only parts of a Polycomb-repressed region have the same volume as the entire region (Figure 1A). This behavior strongly suggests that the dense folding of Polycomb-repressed regions is mediated by long-range interactions, which result in packing of multiple subdomains into the same space, rather than by short-range local interactions, which would increase the density of each subdomain. In support of this interpretation, a set of knockdown experiments confirmed that all of the characteristic chromatin features of Polycomb domains, including condensation and the low scaling constant, require the presence of the self-associating component Ph.
Figure 1. Different Transcriptional States Are Associated with Distinct 3D Organization of Chromatin.
(A) Transcriptionally active chromatin domains are less condensed than inactive or repressed domains and become less dense with increasing domain length. Repressed chromatin shows a reverse behavior, with longer domains becoming more condensed.
(B) Active domains frequently intermingle with other active and inactive domains, whereas Polycomb-repressed regions are spatially isolated from all other domains.
One of the most novel and important results reported in this study is the investigation of how distinct chromatin domains intermingle (Figure 1B). The authors find that active and inactive blocks of chromatin frequently intermix, but repressed regions do not overlap with their neighbors, regardless of their activity. These observations suggest that while repressed regions form distinct subcompartments within the nucleus, the physical separation between active and inactive regions is far less stringent, and their regulation may be mediated through more local interactions.
The fact that Boettiger et al. were able to confirm and clarify the mechanisms of Polycomb function demonstrates the strength and promise of their approach and draws attention to the power of imaging-based techniques to make sense of and enhance biochemical data. The most crucial advantage of imaging-based techniques as a complement to biochemical approaches is the fact that they are, by nature, single-cell experiments. As such, while biochemical studies reveal average trends over an entire population, imaging techniques provide important insight into variability within a cell population. In fact, patterns of variation underlie one of the most suggestive results in this study. The authors observe higher region-to-region variation in the density of active regions compared to inactive and repressed ones. This variation increases with the size of the genomic region and correlates roughly with RNA transcription levels and binding density of the global chromatin organizer protein CTCF. The variation in density between active regions and the extent of intermingling between inactive and active regions suggests that the dynamics of active regions are controlled predominantly by transcriptional activity, and at a much shorter genomic distance, than those for Polycomb-repressed regions. These findings lay the foundation for future studies on the variation in the structure of active chromatin between cells and individual regions and on the relationship between structure and transcription at individual transcription sites. With the methodology described in this study, both of these aspects of chromatin structure are now amenable to experimental investigation.
The genome era brought with it huge promises—and left us with equally large questions. Chromatin state and structure is the physical reality of gene regulation that underlies function and phenotype. It is safe to predict that we will see increasing use of imaging methods to study genome organization, because these approaches are able to directly probe chromatin structure. Imaging modalities are already capable of determining cell-to-cell variation, and, as super-resolution microscopy and powerful tools like Oligopaints become more common, they will be applied to probe the physical structure of the genome with increasing resolution. These improvements, particularly in combination with biochemical approaches, will allow us to answer questions about the fine-scale structure of the genome on a single-cell level and will greatly improve our understanding of the genome by building a much-needed bridge from genome sequence to phenotype.
REFERENCES
- Beliveau BJ, Joyce EF, Apostolopoulos N, Yilmaz F, Fonseka CY, McCole RB, Chang Y, Li JB, Senaratne TN, Williams BR, et al. (2012). Proc. Natl. Acad. Sci. USA 109, 21301–21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau BJ, Boettiger AN, Avendan˜o MS, Jungmann R, McCole RB, Joyce EF, Kim-Kiselak C, Bantignies F, Fonseka CY, Erceg J, et al. (2015). Nat. Commun 6, 7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore WA (2013). Annu. Rev. Genomics Hum. Genet 14, 67–84. [DOI] [PubMed] [Google Scholar]
- Boettiger AN, Bintu B, Moffitt JR, Wang S, Beliveau BJ, Fudenberg G, Imakaev M, Mirny LA, Wu CT, and Zhuang X (2016). Nature 529, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cmarko D, Verschure PJ, Otte AP, van Driel R, and Fakan S (2003). J. Cell Sci 116, 335–343. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Kingston RE, and Woodcock CL (2004). Science 306, 1574–1577. [DOI] [PubMed] [Google Scholar]
- Isono K, Endo TA, Ku M, Yamada D, Suzuki R, Sharif J, Ishikura T, Toyoda T, Bernstein BE, and Koseki H (2013). Dev. Cell 26, 565–577. [DOI] [PubMed] [Google Scholar]
- Robinson AK, Leal BZ, Chadwell LV, Wang R, Ilangovan U, Kaur Y, Junco SE, Schirf V, Osmulski PA, Gaczynska M, et al. (2012). J. Biol. Chem 287, 8702–8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MJ, Bates M, and Zhuang X (2006). Nat. Methods 3, 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]