Abstract
From 2009–2018, 10 consecutive patients with Wilms tumors and bilateral nephroblastomatosis, who had completed standard therapy, were provided a maintenance chemotherapy regimen consisting of vincristine and dactinomycin every 3 months for 12 months in order to prevent an early metachronous Wilms tumor. One patient (10%) with Beckwith Wiedemann syndrome developed a new tumor, without anaplasia. There were no significant toxicities reported during maintenance. All patients are currently alive with no evidence of disease. Further investigations are recommended to determine the utility of this approach.
Keywords: Wilms Tumor, Nephroblastomatosis, Chemotherapy
Introduction
Nephrogenic rests are premalignant abnormal renal foci, and can be either intralobar or perilobar.1,2 These lesions are identified in 42%−45% of Wilms tumors overall and in 90–94% of bilateral Wilms tumors.3,4 Intralobar rests are associated with young age, genitourinary anomalies, and constitutional WT1 mutations.3–5 Perilobar nephrogenic rests are associated with female gender, hemihypertrophy, and Beckwith-Wiedemann syndrome.3–5 Nephroblastomatosis, or the presence of multiple nephrogenic rests, increases the risk of developing a metachronous Wilms tumor.1,2
Treatment of a second de novo Wilms tumor in patients previously treated for Wilms tumor is challenging. Repeating treatment or altering the regimen can compound toxicities. Some previous studies have investigated the prevention of Wilms tumor in patients with predisposing lesions and shown a decrease in the incidence of Wilms tumor.6,7 However, none of these studies have evaluated the effectiveness of chemotherapy in preventing a second metachronous Wilms tumor after treatment of the initial tumor.
In 2001, we treated a patient with Beckwith-Wiedemann syndrome and bilateral nephroblastomatosis who developed hepatoblastoma at 4 months, unilateral Wilms tumor at 16 months, and bilateral Wilms tumors at 36 months. In order to forestall further tumors and minimize remaining renal tissue loss, the patient was given vincristine and dactinomycin every 3 months for 18 months. He remained tumor-free for 34 months, when another unilateral Wilms tumor was resected, followed by radiation and chemotherapy treatment. This patient has been tumor-free for the last 13 years. Based on this index case, we began to treat all patients with Wilms tumor and bilateral nephroblastomatosis using a maintenance chemotherapy regimen consisting of vincristine and dactinomycin every 3 months for 12 months in hope of reducing the likelihood of developing a second metachronous Wilms tumor.
Methods
We conducted a retrospective analysis of our experience with administering maintenance chemotherapy for children with Wilms tumors and bilateral nephroblastomatosis. After standard of care therapy for their initial Wilms tumor, patients with radiographic or pathologic evidence of bilateral nephroblastomatosis were recommended to receive dactinomycin (0.045 mg/kg) and vincristine (2 mg/m2) every 3 months for the 12 months following standard chemotherapy.
With institutional review board approval, we searched our database for all patients with Wilms tumor treated from January 2009 to January 2018 who had bilateral nephroblastomatosis and began the maintenance chemotherapy regimen following standard therapy for the primary Wilms tumor. Patients were considered to have bilateral nephroblastomatosis if they had radiographic or pathologic evidence of nephroblastomatosis or extensive nephrogenic rests in their residual renal tissue after resection of their primary Wilms tumor. Patient demographics, clinical data, outcomes (tumor recurrence, secondary de novo Wilms tumor development, and survival), and treatment toxicities associated with maintenance chemotherapy were evaluated. This retrospective review was approved by our institutional review board. Statistical analysis was completed using OriginPro 2018 (OriginLab Corporation, Northampton, MA).
Results
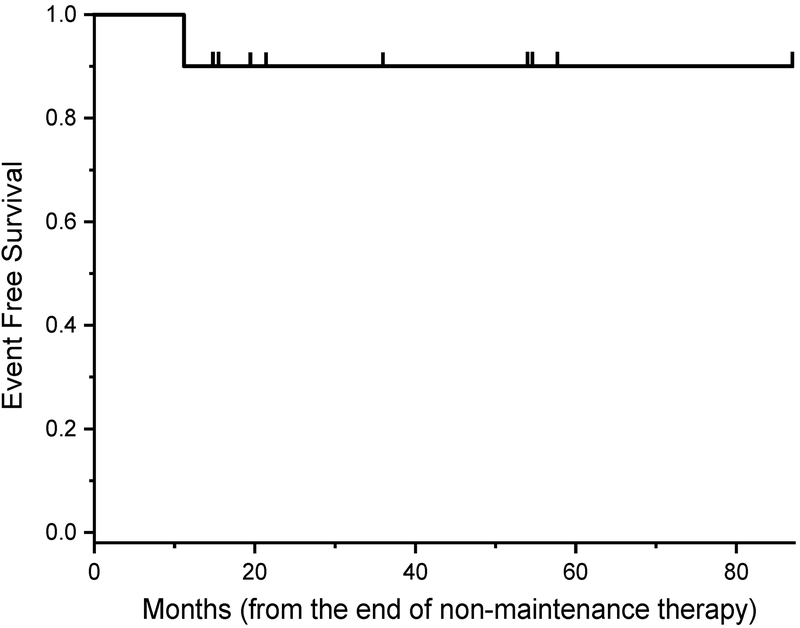
From 2009–2018, 10 consecutive patients with 19 Wilms tumors and bilateral nephroblastomatosis were treated with dactinomycin and vincristine every 3 months for 1 year after standard therapy for their primary Wilms tumors. One patient received 2 additional doses of maintenance chemotherapy per family request. The majority of patients were female (n=9/10; 90%) with bilateral (n=9/10; 90%), favorable-histology (n=18/19; 95%), stage I (n=15/19; 79%) tumors (Table 1). All residual normal kidneys revealed radiographic and pathologic (except one case which was not biopsied) evidence of nephroblastomatosis with 5 (50%) patients exhibiting perilobar nephrogenic rests, 2 (20%) with interlobar rests, and 3 (30%) with insufficient information. Beckwith-Wiedemann syndrome was identified in 1 patient (10%) and isolated hemihypertrophy was identified in 2 other patients (20%). The majority of Wilms tumors were treated with a partial nephrectomy (n=17/19; 89%), a three-drug chemotherapy regimen (n=9/10; 90%), and radiation (n=7/10; 70%). There were 3 (30%) patients with chemotherapy-induced nausea and vomiting (severity not specified); no other significant toxicities were reported as a result of the maintenance chemotherapy, including transaminitis/hepatoxicity, peripheral neuropathy, febrile neutropenia, or other toxicities requiring admission. The patient with Beckwith-Wiedemann syndrome relapsed at 11 months after initiation of maintenance chemotherapy (Figure 1). All patients are currently alive and free of disease with a median follow-up of 40 (range: 15–87) months.
Table 1.
Patient, tumor, and treatment characteristics of Wilms tumor patients with bilateral nephroblastomatosis treated with maintenance chemotherapy
| No. | % | Median (Range) | |
|---|---|---|---|
| Overall | 10 | ||
| Duration of follow-up, months | 10 | 40 (15–87) | |
| Age at diagnosis, months | 10 | 31 (11–51) | |
| Sex | |||
| Male | 1 | 10 | |
| Female | 9 | 90 | |
| Tumor laterality | |||
| Bilateral | 9 | 90 | |
| Unilateral | 1 | 10 | |
| Local Stage | |||
| 1 | 15 | 79 | |
| 2 | 1 | 5 | |
| 3 | 3 | 18 | |
| Metastases | |||
| Pulmonary | 3 | 30 | |
| Non-Pulmonary | 0 | 0 | |
| None | 7 | 70 | |
| Histology | |||
| Favorable | 18 | 95 | |
| Focal Anaplasia | 1 | 5 | |
| Diffuse Anaplasia | 0 | 0 | |
| Nephrogenic Rests | |||
| Interlobar | 2 | 20 | |
| Perilobar | 5 | 50 | |
| Insufficient Information | 3 | 30 | |
| Cancer Predisposition | |||
| Beckwith-Wiedemann Syndrome | 1 | 10 | |
| Isolated Hemihypertrophy | 2 | 20 | |
| None | 7 | 70 | |
| Surgery | |||
| Partial nephrectomy | 17 | 89 | |
| Total nephrectomy | 2 | 11 | |
| Radiation | |||
| None | 4 | 40 | |
| Flank | 4 | 40 | |
| Abdomen | 2 | 20 | |
| Lung | 2 | 20 | |
| Chemotherapy Regimen | |||
| VINC, DACT, DOX | 9 | 90 | |
| VINC, DACT, DOX, CPM, ETOP | 1 | 10 | |
| Complications during Maintenance | |||
| Vomiting | 3 | 30 | |
| Peripheral Neuropathy | 0 | 0 | |
| Transaminitis or Hepatotoxicity | 0 | 0 | |
| Febrile Neutropenia | 0 | 0 | |
| Admissions | 0 | 0 | |
| Relapse | |||
| No | 9 | 90 | |
| Yes | 1 | 10 | |
| Alive with no evidence of disease | |||
| No | 0 | 0 | |
| Yes | 10 | 100 |
VINC = Vincristine, DACT = Dactinomycin, DOX = Doxorubicin, CPM = Cyclophosphamide, ETOP = Etoposide
Figure 1.
Event‐free survival of patients with Wilms tumor with bilateral nephroblastomatosis treated with maintenance chemotherapy
Discussion
Children at our institution with Wilms tumor and bilateral nephroblastomatosis receive a 1-year maintenance regimen consisting of vincristine and dactinomycin after standard of care treatment, which includes surgery, chemotherapy, and often radiation. In our series of 10 patients, this maintenance regimen was well tolerated without significant toxicities. Although one patient (10%) developed a second Wilms tumor, she was retreated and remains alive with no evidence of disease. Given our small number of patients, we were unable to determine if this maintenance regimen prevented or reduced the incidence of second de novo Wilms tumors or primary recurrence. Notably, AREN0534, the first prospective multi-institutional treatment study in children with bilateral Wilms tumors, reported a 4-year event-free survival of 82%.8
Estimating any individual child’s risk of developing a new Wilms tumor is challenging. Approximately 1% of children with unilateral Wilms tumor will develop disease in the contralateral tumor.9 Patients with Wilms tumor who are younger than 1 year with nephrogenic rests, particularly perilobar rests, have a nearly 10% chance of developing a second Wilms tumor.9 Greater numbers of proliferative rests, as seen in nephroblastomatosis, is associated with an increased risk of developing a Wilms tumor. For example, Wilms tumors develop in approximately 50% of patients with numerous proliferative perilobar nephrogenic rests, termed hyperplastic perilobar nephroblastomatosis (HPLN).7 Cancer predisposition syndromes, such as Beckwith-Wiedemann, increase the risk of developing a Wilms tumor, but the precise risk of a second primary Wilms tumor is less well established.5
Both the index case and the one patient in our study cohort who developed recurrence had Beckwith-Wiedemann syndrome. The index patient developed multiple prior metachronous Wilms tumors before initiating maintenance therapy; the other patient who developed recurrence was younger than 12 months at diagnosis. Although our limited study size precluded a rigorous statistical analysis, these clinical differences suggest that there are likely biological factors that drove the development of a second Wilms tumor in these patients in spite of the maintenance chemotherapy.
Although maintenance chemotherapy has not been previously studied as a means of preventing a second de novo Wilms tumor, prophylactic chemotherapy has been shown to reduce the incidence of developing a Wilms tumor in patients with diffuse HPLN. However, pre-treatment of nephroblastomatosis is an independent risk factor for worse outcome and may predispose to anaplastic transformation.6,7 Notably, there was no anaplastic transformation identified in our study.
While our small, single-institution study cannot provide a definitive conclusion regarding efficacy of maintenance chemotherapy, it does highlight a novel approach to the challenging problem of preventing the development of a second primary Wilms tumor. An alternative or potentially complementary approach to our maintenance chemotherapy could be treatment with a retinoic acid-based differentiating agent. Emerging evidence suggests that the retinoic acid pathway may play a role in nephroblastomagenesis.10,11 One infant with bilateral diffuse nephroblastomatosis, who progressed on vincristine and dactinomycin, responded when 13-cis retinoic acid was added to her regimen.12 Future studies are warranted to determine the optimal duration and interventions needed to prevent development of Wilms tumors in patients with predisposing lesions.
Acknowledgements
The authors thank nurse practitioners Maura Byrnes-Casey, Lauren Kushner, Kateri McGuire, Joseph Reyes, and Marjorie Weis for their invaluable help in caring for these patients. Dr. Ortiz receives support from the Family and Friends of Caroline Bhatt as well as Met Life Foundation. Research reported in this publication was supported by grants from the National Cancer Institute of the National Institutes of Health (#K12CA184746 and #P30CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
None of the authors reported a conflict of interest for this manuscript.
References
- 1.Beckwith JB. Precursor lesions of Wilms tumor: clinical and biological implications. Medical and Pediatric Oncology. 1993;21(3):158–168. [DOI] [PubMed] [Google Scholar]
- 2.Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms’ tumor. Pediatric Pathology. 1990;10(1–2):1–36. [DOI] [PubMed] [Google Scholar]
- 3.Vujanic GM, Apps JR, Moroz V, Ceroni F, Williams RD, Sebire NJ, Pritchard-Jones K. Nephrogenic rests in Wilms tumors treated with preoperative chemotherapy: The UK SIOP Wilms Tumor 2001 Trial experience. Pediatric Blood & Cancer. 2017;64(11). [DOI] [PubMed] [Google Scholar]
- 4.Breslow NE, Beckwith JB, Perlman EJ, Reeve AE. Age distributions, birth weights, nephrogenic rests, and heterogeneity in the pathogenesis of Wilms tumor. Pediatric Blood & Cancer. 2006;47(3):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalish JM, Doros L, Helman LJ, Hennekam RC, Kuiper RP, Maas SM, Maher ER, Nichols KE, Plon SE, Porter CC, Rednam S, Schultz KAP, States LJ, Tomlinson GE, Zelley K, Druley TE. Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clinical Cancer Research. 2017;23(13):e115–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furtwangler R, Schmolze M, Graber S, Leuschner I, Amann G, Schenk JP, Niggli F, Kager L, von Schweinitz D, Graf N. Pretreatment for bilateral nephroblastomatosis is an independent risk factor for progressive disease in patients with stage V nephroblastoma. Klinische Padiatrie. 2014; 226(3):175–181. [DOI] [PubMed] [Google Scholar]
- 7.Perlman EJ, Faria P, Soares A, Hoffer F, Sredni S, Ritchey M, Shamberger RC, Green D, Beckwith JB. Hyperplastic perilobar nephroblastomatosis: long-term survival of 52 patients. Pediatric Blood & Cancer. 2006;46(2):203–221. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich P, Chi YY, Chintagumpala MM, Hoffer FA, Perlman EJ, Kalapurakal JA, Warwick A, Shamberger RC, Khanna G, Hamilton TE, Gow KW, Paulino AC, Gratias EJ, Mullen EA, Geller JI, Grundy PE, Fernandez CV, Ritchey ML, Dome JS. Results of the First Prospective Multi-institutional Treatment Study in Children With Bilateral Wilms Tumor (AREN0534): A Report From the Children’s Oncology Group. Annals of Surgery. 2017;266(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppes MJ, Arnold M, Beckwith JB, Ritchey ML, D’Angio GJ, Green DM, Breslow NE. Factors affecting the risk of contralateral Wilms tumor development: a report from the National Wilms Tumor Study Group. Cancer. 1999;85(7):1616–1625. [DOI] [PubMed] [Google Scholar]
- 10.Percicote AP, Mardegan GL, Gugelmim ES, Ioshii SO, Kuczynski AP, Nagashima S, de Noronha L. Tissue expression of retinoic acid receptor alpha and CRABP2 in metastatic nephroblastomas. Diagnostic Pathology. 2018;13(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegert J, Bausenwein S, Kneitz S, Roth S, Graf N, Geissinger E, Gessler M. Retinoic acid pathway activity in wilms tumors and characterization of biological responses in vitro. Molecular Cancer. 2011;10(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witt O, Hammerling S, Stockklausner C, Schenk JP, Gunther P, Behnisch W, Hamad B, Al Mulla NA, Kulozik A. 13-cis retinoic acid treatment of a patient with chemotherapy refractory nephroblastomatosis. Journal of Pediatric Hematology/Oncology. 2009;31(4):296–299. [DOI] [PubMed] [Google Scholar]