Abstract
Objective:
Preplanned economic analysis of a pragmatic trial using electronic-medical-record–linked interactive voice recognition (IVR) reminders for enhancing adherence to cardiovascular medications (i.e., statins, angiotensin-converting enzyme inhibitors [ACEIs], and angiotensin receptor blockers [ARBs]).
Methods:
Three groups, usual care (UC), IVR, and IVR plus educational materials (IVR+), with 21,752 suboptimally adherent patients underwent follow-up for 9.6 months on average. Costs to implement and deliver the intervention (from a payer perspective) were tracked during the trial. Medical care costs and outcomes were ascertained using electronic medical records.
Results:
Per-patient intervention costs ranged from $9 to $17 for IVR and from $36 to $47 for IVR+. For ACEI/ARB, the incremental cost-effectiveness ratio for each percent adherence increase was about 3 times higher with IVR+ than with IVR ($6 and $16 for IVR and IVR+, respectively). For statins, the incremental cost-effectiveness ratio for each percent adherence increase was about 7 times higher with IVR+ than with IVR ($6 and $43 for IVR and IVR+, respectively). Considering potential cost offsets from reduced cardiovascular events, the probability of breakeven was the highest for UC, but the IVR-based interventions had a higher probability of breakeven for subgroups with a baseline low-density lipoprotein (LDL) level of more than 100 mg/dl and those with two or more calls.
Conclusions:
We found that the use of an automated voice messaging system to promote adherence to ACEIs/ARBs and statins may be cost-effective, depending on a decision maker’s willingness to pay for unit increase in adherence. When considering changes in LDL level and downstream medical care offsets, UC is the optimal strategy for the general population. However, IVR-based interventions may be the optimal choice for those with elevated LDL values at baseline.
Keywords: adherence, cardiovascular, diabetes, economics
Introduction
Adherence with cardiovascular medications is critical to successful treatment, but many patients are non-adherent, leading to poor outcomes and increased health care costs. A recent systematic review showed that patients with lower adherence to statins and other cardiovascular medications were more likely to experience excess hospital stays and costs, including incident coronary artery disease, cerebrovascular disease, heart failure, and mortality than were patients with better adherence [1]. In spite of this, reports [2] indicate that more than 26% of patients are non-adherent (i.e., <80% adherent) with statins and 22% are non-adherent with angiotensin converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB).
Successful interventions to enhance adherence with cardiovascular medications have included intensive, multimodal approaches that combine patient education with behavioral strategies (e.g., special pill packaging) and pharmacist follow-up [3]. Modest improvements in adherence to cardiovascular medications have been seen with less intensive efforts such as mailed educational materials [4] and for primary non-adherence with automated telephone reminders for patients taking statins [5].
Several studies have illustrated the relationship between better medication adherence and lower costs. For example, one recent study suggested that patients with congestive heart failure who were enrolled in health plans whose population had medication adherence in the lowest quartile had a 50% greater cost of care than similar patients in health plans with adherence in the highest quartile [6]. Relatively few studies, however, have examined the cost-effectiveness of interventions aimed at increasing adherence to cardiovascular medications. We conducted an economic analysis of a large pragmatic trial that used health system information-technology–enabled resources of varying intensities to enhance adherence to statins and ACEI/ARB.
Methods
Trial Design
Our economic analysis was conducted alongside PATIENT (Promoting Adherence to Improve Effectiveness of Cardiovascular Disease Therapies), a pragmatic trial that compared the effectiveness of two electronic medical record (EMR)-linked, health information-technology (HIT)–based interventions aimed at increasing adherence to statins and ACEI/ARB. The trial was conducted in three Kaiser Permanente regions (Northwest [Oregon and Southwest Washington], Southeast [Georgia], and Hawaii) and was approved by their respective institutional review boards. Participants were 40 years and older with diabetes mellitus (DM) and/or cardiovascular disease (CVD), with suboptimal adherence (<90%) to statins or ACEI/ARB during the previous 12 months, and were due or overdue for a refill at the time of randomization.
Full details of the parent study are available elsewhere [7]. Patients were randomized to receive usual care (UC), interactive voice recognition (IVR) calls, or IVR plus educational materials (IVR+). In both the IVR and IVR+ arms, patients received automated telephone calls when they were due or overdue for a statin or ACEI/ARB refill. At randomization, patients in the IVR and IVR+ arms were mailed pamphlets explaining that they would receive the calls. Calls lasted 2 to 3 minutes and used speech-recognition technology; content included education, the option of being transferred to a pharmacist, and assistance with prescription refills. The flow of the call was determined by specific patient responses and whether the patient was due and/or overdue for a refill. The initial IVR calls asked for permission to leave future detailed messages (electronically or with other household members). Calls for any given medication class were stopped on participants’ request, or if they had evidence of allergy/intolerance or medical contraindications; this happened in less than 1% of participants.
In addition to the IVR calls, patients in the IVR+ arm received a personalized reminder letter if they were 60 to 90 days overdue (about 0.6 per person) and a live outreach call (about 0.3 per person) if they were 90 days or more overdue, as well as EMR-based feedback to their primary care providers. They also received a personalized health report displaying their latest blood pressure and cholesterol levels, a pill organizer, and bimonthly mailings to answer common questions.
UC patients received a basic informational brochure; any adherence efforts occurred via routine medical care processes.
Clinical Trial Results
A total of 45,051 patients were identified who met the initial inclusion criteria, of which 6,158 were excluded because of medical contraindications (e.g., liver disease, pregnancy, stage 4 or worse chronic kidney disease, and allergy/intolerance) and 2,778 for administrative reasons (e.g., no valid telephone number). We randomly selected 25,323 patients for study inclusion and randomized them into the main study arms (n = 21,752) or to one of two ancillary treatment arms (see Vollmer et al. [7] for more information). The ancillary arms are not included in this analysis, but because they also received automated calls and other materials, we included them in the fixed cost calculations. There were no meaningful differences at baseline (Table 1); 16,380 ever qualified for statin calls; 13,036 ever qualified for the ACEI/ARB analyses. Electronic data systems identified patient outcomes over follow-up. Average follow-up was 9.6 months and did not vary by treatment arm [7]. Adherence was measured using pharmacy records of the proportion of days covered (PDC) based on the days supply [8,9]. For statins, adherence among IVR patients was 2.2 percentage points higher than UC (95% confidence interval [CI] 1.1, 3.4), and IVR+ adherence was 3.0 percentage points higher than UC (95% CI 1.9, 4.2), but IVR and IVR+ were not significantly different from each other. Results were similar for ACEI/ARB medications, but IVR+ was significantly more effective than IVR. Low-density lipoprotein (LDL) levels were captured from laboratory findings resulting from normal care processes. Among those with baseline LDL of more than 100 mg/dl, follow-up LDL measurements were lower for patients in both treatment arms, but blood pressure levels were not changed.
Table 1 –
Characteristics of the PATIENT study participants
| Characteristic | Usual care | IVR | IVR+ | Total |
|---|---|---|---|---|
| Number randomized | 7255 | 7247 | 7250 | 21752 |
| Age (y), mean ± SD | 63.6 ± 12.2 | 63.6 ± 12.1 | 63.5 ± 12.2 | 63.6 ± 12.2 |
| Sex: male (%) | 52.7 | 53.5 | 52.9 | 53.0 |
| Race (%) | ||||
| African American | 16.1 | 15.1 | 15.3 | 15.5 |
| American Indian/Alaskan Native | 0.7 | 0.6 | 0.6 | 0.6 |
| Asian | 17.5 | 17.8 | 17.3 | 17.5 |
| Native Hawaiian/Pacific Islander | 11.0 | 11.3 | 10.8 | 11.0 |
| White | 46.9 | 46.9 | 47.0 | 46.9 |
| Unknown | 7.8 | 8.3 | 9.0 | 8.4 |
| History of DM (%) | 78.1 | 78.7 | 77.6 | 78.1 |
| History of CVD (%) | 36.2 | 36.1 | 36.7 | 36.3 |
| LDL cholesterol within statin group (mg/dl), mean ± SD | 87.0 ± 42.0 | 85.5 ± 40.8 | 87.5 ± 41.1 | 86.7 ± 41.3 |
| No. of medications dispensed before randomization, mean ± SD | 7.5 ± 6.2 | 7.5 ± 6.3 | 7.6 ± 6.4 | 7.5 ± 6.3 |
| Statin adherence before randomization, mean ± SD | 0.51 ± 0.24 | 0.51 ± 0.24 | 0.51 ± 0.24 | 0.51 ± 0.24 |
| ACEI/ARB adherence before randomization, mean ± SD | 0.53 ± 0.23 | 0.53 ± 0.24 | 0.53 ± 0.23 | 0.53 ± 0.23 |
| Site (%) | ||||
| Kaiser Permanente Georgia | 30.9 | 30.9 | 31.0 | 30.9 |
| Kaiser Permanente Hawaii | 35.3 | 35.3 | 35.0 | 35.3 |
| Kaiser Permanente Northwest | 33.8 | 33.8 | 33.8 | 33.8 |
| Study medication use at baseline (N) | ||||
| Statin only | 2924 | 2873 | 2919 | 8716 |
| ACEI/ARB only | 1769 | 1787 | 1816 | 5372 |
| Statin and ACEI/ARB | 2562 | 2587 | 2515 | 7664 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; DM, diabetes mellitus; IVR, interactive voice recognition; IVR +, IVR plus educational materials; LDL, low-density lipoprotein; PATIENT, Promoting Adherence To Improve Effectiveness of Cardiovascular Disease Therapies.
Economic Outcomes
We reported three cost-effectiveness outcomes from the trial for the follow-up period: 1) cost per percent increase in ACEI/ARB adherence, 2) cost per percent increase in statin adherence, and 3) cost per mg/dL drop in LDL. We analyzed subgroups on baseline adherence (50% and under), baseline LDL (greater than 100 mg/dL), number of calls received/qualified for (i.e., IVR and IVR+, two or more calls when an individual was reached or a detailed message left; UC individuals qualifying for two or more calls) and individuals with CVD costs above the median at baseline.
Intervention Costs
Our analysis takes the payer (health plan) perspective. The cost of the intervention included both labor and non-labor inputs (Table 2). We estimated staff costs associated with the intervention using a weekly time tracking system, wherein staff reported the number of hours spent on tasks in support of the intervention. Recognizing that these costs include the cost of doing the research, we adjusted them to subtract our research protocol-driven costs. We also estimated the replication costs (i.e., the cost that might be incurred by a health plan adopting our intervention without major modification). After discussing principles of cost allocation, these adjustments were arrived at through consensus with study staff, investigators, and health plan employees whose positions currently required administering similar programs. We estimated cost per participant in two ways: 1) as-delivered (adjusted to exclude research-protocol–driven costs) and 2) replication cost (further adjusted to reflect adoption in other health plans). Wage rates came from study personnel salary; to fully allocate the labor costs, we added a fringe benefit of 30% and an overhead of 20%. All other intervention costs came from actual amounts paid to vendors in the trial. For subgroup analyses, per-patient fixed costs were assumed to be constant across all scenarios.
Table 2 –
Resource components and their sources and costs.
| Resource component | Description | Source | As-delivered cost (US $) |
Replication cost (US $) |
||||
|---|---|---|---|---|---|---|---|---|
| UC | IVR | IVR+ | UC | IVR | IVR+ | |||
| Labor | ||||||||
| Health plan coordination (IVR and IVR+) | Coordination with health plan leaders (travel, phone calls, e-mail, meeting preparation, etc.) | Clinical trial | - | 0.30 | 0.49 | - | 0.17 | 0.29 |
| Education materials development/updates (IVR+) | Personnel time to create educational materials (script writing, editing for clinical content, editing for tone of message, editing for appropriate language and literacy) graphics, includes customization for individual characteristics | Clinical trial | - | - | 0.05 | - | - | 0.02 |
| Focus groups/qualitative analysis (IVR and IVR+) | Time and supplies used in conducting focus groups to help refine phone call methodology (feedback on script, phone branching, mechanics of call) Review of educational materials | Clinical trial | - | 0.12 | 0.23 | - | 0.01 | 0.02 |
| Patient identification and outreach (IVR and IVR+) | Programming time for using, maintaining, and updating population selection algorithm | Clinical trial | - | 3.24 | 4.05 | - | 0.45 | 0.56 |
| Study design/outcome definition (IVR and IVR+) | Design and analysis planning and measurement of adherence variable | Clinical trial | - | 0.06 | 0.06 | - | 0.00 | 0.00 |
| Implementation and maintenance (IVR and IVR+) | Monitor intervention, manage mailing process, perform ongoing QA, etc. | Clinical trial | - | 4.75 | 7.64 | - | 1.65 | 2.82 |
| Administration (IVR and IVR+) | Administrative personnel, budget, reporting, monitoring workflow | Clinical trial | - | 0.27 | 0.66 | - | 0.07 | 0.18 |
| Meetings (IVR and IVR+) | Time spent in standing meetings | Clinical trial | - | 1.02 | 1.46 | - | 0.77 | 1.09 |
| Other (IVR and IVR+) | Clinical trial | - | 1.56 | 1.56 | - | 0.31 | 0.31 | |
| Pharmacy technician (IVR+) | Make live tardy calls, chart review for medication discontinuation before tardy letters and live calls, and documentation of contact with patient in chart, receive live transfer from automated calls, return calls from after-hours messages | Clinical trial | - | - | 3.36 | - | - | 3.36 |
| Pharmacy technician (IVR) | Receive live transfer from automated calls, return calls from after-hours messages | Clinical trial | - | 0.20 | - | - | 0.20 | - |
| Automated calls, printing, and postage | ||||||||
| Automated calls (IVR and IVR+) | Cost to make automated calls | Vendor charges | - | 5.02 | 5.02 | - | 5.02 | 5.02 |
| Introductory brochure (IVR) | Printing and postage of introductory brochure | Printing and mailing Vendor charges | - | 0.63 | - | - | 0.63 | - |
| Introductory brochure and educational mailings: printed materials (IVR+) | Printing and postage of educational materials, including Heart Health Report customization | Printing and mailing Vendor charges | - | - | 18.23 | - | - | 18.23 |
| Educational mailings: pill box (IVR+) | Pill organizer included with initial educational packet | Vendor charges | - | - | 4.11 | - | - | 4.11 |
| Total cost (US $) | - | 17 | 47 | - | 9 | 36 | ||
IVR, interactive voice recognition; IVR+, IVR plus educational materials; QA, Quality Assurance; UC, usual care.
Medical Care Utilization and Costs
Utilization data were extracted for all participants from electronic data warehouses containing comprehensive information from electronic medical records (EMRs), hospital discharge abstracts, claims, outpatient dispensing, and other health plan administrative data sources; data were standardized across plans [10]. Our analysis of health care utilization focused on the primary cost drivers of CVD: inpatient stays, outpatient clinic visits, and pharmacy dispensing.
We estimated the cost of inpatient and outpatient medical care encounters related to CVD using standardized costing methods [11] and International Classification of Diseases, Ninth Revision (ICD9) diagnostic codes wherein standard prices are applied to each visit, with adjustment for department, provider type, diagnosis related groups (DRG), and length of stay. These costs were based on Kaiser Permanente Northwest (KPNW) aggregate expenditures and applied to the other sites. Drug costs approximated those in the retail market. Costs for care delivered in non–Kaiser Permanente facilities came from paid amounts for visits, procedures, and related services. Costs were inflated to reflect 2012 US dollars; we did not discount costs or effects because of the short (1 year) time frame of the analysis.
Statistical Analysis
We calculated the incremental cost-effectiveness ratio (ICER) as the ratio of extra cost to the extra effect (i.e., ΔC/ΔE). We used net benefit regression methods [12–14] to estimate the intervention’s probability of being cost-effective for each outcome using replication costs. To assist translation of the results into practice, we reported the probability of the intervention being cost-effective across a wide range of thresholds of a health care plan’s willingness to pay (WTP) for an additional outcome by constructing cost-effectiveness acceptability curves and their frontier (plotting the probability that the optimal option was cost-effective across the range of WTP thresholds). Costs incorporated into the ICER calculations included intervention delivery and medication costs because these were clearly related to the adherence intervention but exclude LDL testing costs as its use was not influenced by the intervention. Additionally, as described above, we reported costs associated with inpatient and outpatient care for CVD. Using net benefit regression methods [12–14], all analyses were adjusted for age, sex, study site, number of unique medications at baseline (as proxy for disease severity and medication burden), presence of DM/CVD, and baseline level of the dependent variable.
We recognize that at present there is no guidance for WTP thresholds for a % increase in medication adherence. Thus, to aid in translating our findings for decision makers, we used net-benefit regression to estimate the probability that the costs of the intervention would (at least) be covered by expected offsets from reduced cardiovascular events due to LDL lowering. We performed this break-even analysis using estimates from the Cholesterol Treatment Trialists’ (CTT) Collaboration, who conducted extensive, patient-level meta-analyses of 26 randomized trials among 170,000 patients and showed that each 39 mg/dL decrease in LDL leads to annual reductions in major vascular events (e.g., coronary revascularization, stroke, myocardial infarction, and coronary death) of 21% (95% CI 19, 23), with no evidence of a threshold effect [15]. The estimated baseline rate of major vascular events from the CTT study was 4%, so each mg/dL reduction in LDL implies a reduction of approximately 0.00022 per person in major vascular events (i.e. 0.04×0.21/39 mg/dL). Because break-even is linked to cost of care, we applied that expected reduction in major vascular events to cost estimates [16] of caring for patients with those major vascular events. For example, if the cost of care for a major vascular event was $70,000, the above per person reduction estimate implies a break-even WTP per mg/dL LDL lowering of about $15 (0.00022 × $70,000).
As did the clinical trial reporting, [7] our analysis focuses on adherence effects for ACEI/ARB and statins separately. Thus our cost-effectiveness estimates are relevant to decision-makers who wish to influence adherence to those classes independently. Because there were some patients in the study sample who used both classes of medications (Table 1) we can estimate the effect of the economy of scope within the specific case-mix from our sample. We did this using assumptions of joint production after discussion with members of the study team; we report the associated ICER changes. Analyses on follow-up LDL included only patients on statins and controlled for baseline LDL levels. SAS [version 9.1, Cary, NC] and STATA [version 11, College Station, TX] were used for all the analyses.
Results
Intervention costs (Table 2) varied by arm and ranged from $9 to $17 (replication cost and as-delivered cost, respectively) for IVR, and from $36 to $47 for IVR+. Costs of statins and ACEI/ARB (data not shown) over follow-up for UC were $167 (95% CI $161–$172), $172 (95% CI $167–$177) for IVR, and $176 (95% CI $171–$181) for IVR+. CVD-related health care utilization costs over follow-up were $1566 (95% CI $1424, $1707) for UC, $1648 (95% CI $1506, $1790) for IVR, and $1564 (95% CI $1423, $1705) for IVR+.
The base case ICER on ACEI/ARB (Table 3) for IVR and IVR+ were $5.78 and $15.58, respectively, for a unit increase in adherence. At the lowest levels of WTP for a unit increase in adherence (e.g., <$5), UC had the highest probability of being cost-effective (i.e., ≥0.6; see Fig. 1); However, with higher WTP values, IVR and IVR+ were the optimal choices. ICER results for both IVR and IVR+ were slightly more favorable for the subgroup with baseline adherence of less than 50% and those with two or more calls, but were worse for patients with baseline costs above the median.
Table 3 –
Incremental cost-effectiveness ratios.
| Base case and subgroups | Replication cost ($) | Incremental cost ($) | Adherence (%)* | Incremental adherence (%) | ICER ($) |
|---|---|---|---|---|---|
| Cost-effectiveness for those taking ACEIs/ARBs | |||||
| Base case | |||||
| Control group (n = 4331) | 51.38 | 58 | |||
| Intervention IVR group (n = 4374) | 61.04 | 9.66 | 59 | 1.7 | 5.78 |
| Intervention IVR+ group (n = 4331) | 90.02 | 28.98 | 61 | 1.9 | 15.58 |
| Subgroup analysis: Baseline adherence <50% | |||||
| Control group (n = 2164) | 37.88 | 45 | |||
| Intervention IVR group (n = 2133) | 47.54 | 9.66 | 47 | 1.9 | 5.22 |
| Intervention IVR+ group (n = 2127) | 75.54 | 28.00 | 50 | 2.9 | 9.59 |
| Subgroup analysis: Individuals with baseline cost higher than the median | |||||
| Control group (n = 2195) | 49.01 | 57 | |||
| Intervention IVR group (n = 2277) | 60.52 | 11.51 | 58 | 1.7 | 6.65 |
| Intervention IVR+ group (n = 2236) | 88.68 | 28.16 | 60 | 1.5 | 18.41 |
| Subgroup analysis: Receiving/qualifying for two calls or more | |||||
| Control group (n = 4000) | 52.98 | 59 | |||
| Intervention IVR group (n = 2835) | 65.90 | 12.92 | 64 | 5.2 | 2.49 |
| Intervention IVR+ group (n = 2661) | 97.59 | 31.69 | 67 | 2.8 | 11.20 |
| Cost-effectiueness for those taking statins | |||||
| Base case | |||||
| Control group (n = 5486) | 120.93 | 55 | |||
| Intervention IVR group (n = 5460) | 135.74 | 14.81 | 57 | 2.4 | 6.24 |
| Intervention IVR+ group (n = 5434) | 163.76 | 28.02 | 58 | 0.7 | 42.64 |
| Subgroup analysis: Baseline adherence <50% | |||||
| Control group (n = 2852) | 92.24 | 52 | |||
| Intervention IVR group (n = 2856) | 110.25 | 18.01 | 55 | 3.0 | 6.08 |
| Intervention IVR+ group (n = 2815) | 128.84 | 18.59 | 56 | 0.8 | 23.83 |
| Subgroup analysis: Individuals with baseline cost higher than the median | |||||
| Control group (n = 2713) | 114.06 | 55 | |||
| Intervention IVR group (n = 2705) | 135.08 | 21.02 | 58 | 2.8 | 7.56 |
| Intervention IVR+ group (n = 2708) | 159.29 | 24.21 | 58 | 0.15 | 161.40 |
| Subgroup analysis: Receiving/qualifying for two calls or more | |||||
| Control group (n = 5031) | 123.21 | 56 | |||
| Intervention IVR group (n = 3525) | 148.56 | 25.35 | 62 | 6.3 | 4.00 |
| Intervention IVR+ group (n = 3320) | 177.36 | 28.80 | 63 | 1.4 | 20.00 |
| Replication cost ($) | Incrementalcost ($) | LDL level | Incremental change in LDL level | ICER ($) | |
|---|---|---|---|---|---|
| LDL cost-effectiueness for those taking statins | |||||
| Base case | |||||
| Control group (n = 4621) | 130.52 | 92.3 | |||
| Intervention IVR group (n = 4610) | 146.29 | * | 92.1 | * | * |
| Intervention IVR+ group (n = 4545) | 174.48 | 28.19 | 91.1 | −1.0 | 28.77 |
| Subgroup analysis: Baseline adherence <50% | |||||
| Control group (n = 2392) | 102.82 | 92.6 | |||
| Intervention IVR group (n = 2416) | 121.99 | * | 92.3 | * | * |
| Intervention IVR+ group (n = 2312) | 139.90 | 17.91 | 91.3 | −1.1 | 16.90 |
| Subgroup analysis: Baseline LDL >100 mg/dl | |||||
| Control group (n = 1352) | 120.26 | 114.5 | |||
| Intervention IVR group (n = 1329) | 122.12 | 1.86 | 114.0 | −0.5 | 3.74 |
| Intervention IVR+ group (n = 1346) | 152.86 | 30.74 | 111.5 | −2.5 | 12.13 |
| Subgroup analysis: Receiving/qualifying for two calls or more | |||||
| Control group (n = 4301) | 131.02 | 91.9 | |||
| Intervention IVR group (n = 3139) | 154.06 | 23.04 | 90.0 | −1.9 | 12.33 |
| Intervention IVR+ group (n = 2946) | 181.90 | 27.84 | 89.0 | −1.0 | 28.12 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ICER, incremental cost-effectiveness ratio; IVR, interactive voice recognition; IVR+, IVR plus educational materials; LDL, low-density lipoprotein.
Intervention is dominated.
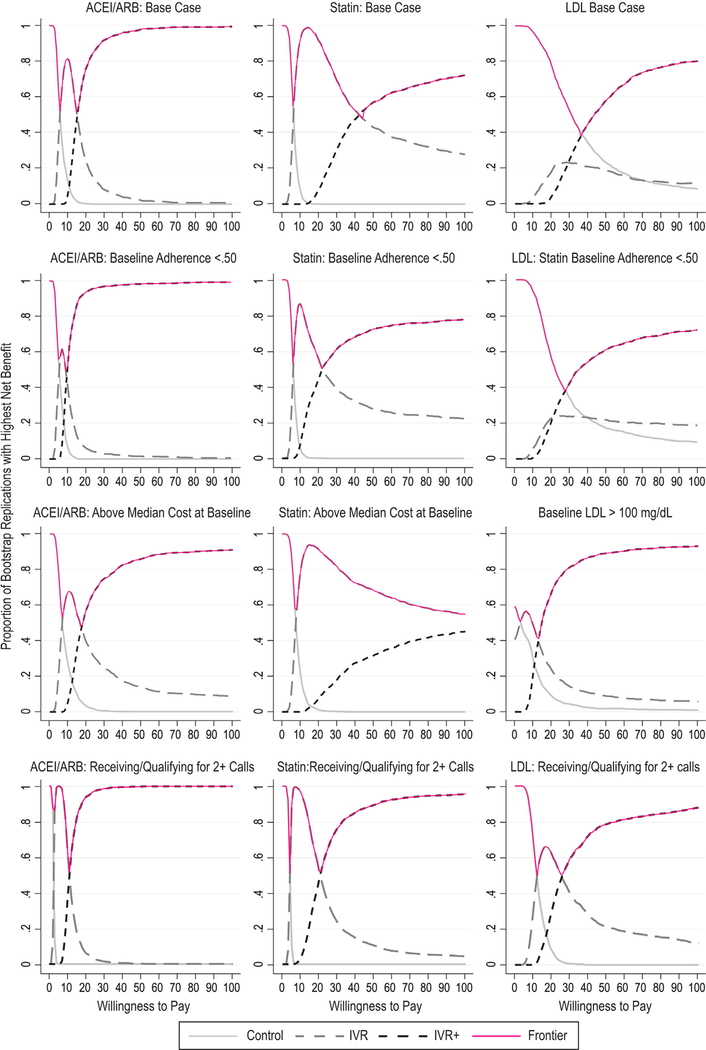
Fig. 1 –
Cost-effectiveness acceptability curves. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IVR, interactive voice recognition; IVR+, IVR plus educational materials; LDL, low-density lipoprotein.
In both IVR and IVR+, the ICER on adherence for patients using statins was always higher compared with ACEI/ARB patients; the base-case ICER was more than double for IVR+ ($15.58 and $42.64 for ACEI/ARB and statins, respectively). The probability of being cost-effective for IVR+ among statin users was much lower than among ACEI/ARB users over a broad range. For example, as demonstrated in Figure 1, in the base case, at a WTP of $50, the probability of IVR+ being cost-effective was nearly 1.0 for ACEI/ARB, but less than 0.6 for statins, reflecting the greater uncertainty in the statin adherence analysis. Subgroup analyses within statin users followed a similar pattern to that seen with ACEI/ARB, but were markedly worse for IVR+ patients, with costs above the median.
When considering the cost-effectiveness on mg/dL lowering of LDL for patients using statins (Table 3), IVR+ exhibited extended dominance [17] in the base case with an ICER of $28.77. While results improved for the subgroup with baseline adherence less than 50% (ICER $16.90), they became markedly more favorable for patients with a baseline LDL of more than 100 mg/dL (ICER $12.13). As seen in Figure 1, there was considerable uncertainty in the LDL analysis, particularly with a WTP between about $35 and $55 where the probability of IVR+ being optimal was less than 0.6. Among those with a baseline LDL of more than 100 mg/dL, however, the probability of IVR+ being cost-effective was more than 0.8 at a WTP of $55.
Table 4 presents the implementation and maintenance costs for various population sizes. Costs were divided into three components —fixed labor (e.g., materials development and patient identification), variable labor (e.g., time of pharmacy staff), and variable nonlabor costs (e.g., mailing and automated phone calls). Replication costs started at $14.52 and $44.10 for IVR and IVR+, respectively, for a population of 10,000 and were $8.41 and $34.69 for IVR and IVR+, respectively, for a population of 40,000.
Table 4 –
Implementation and maintenance costs for various population sizes: Resource categories and per person costs to implement and maintain the PATIENT intervention.
| Population size | As-delivered cost ($) |
Replication cost ($) |
||
|---|---|---|---|---|
| IVR | IVR+ | IVR | IVR+ | |
| 10,000 | ||||
| Fixed labor | 28.63 | 41.03 | 8.67 | 13.38 |
| Variable labor | 0.20 | 3.36 | 0.20 | 3.36 |
| Nonlabor (variable) | 5.65 | 27.36 | 5.65 | 27.36 |
| Total per person | 34.48 | 71.75 | 14.52 | 44.10 |
| 25,000 | ||||
| Fixed labor | 11.45 | 16.41 | 3.47 | 5.35 |
| Variable labor | 0.20 | 3.36 | 0.20 | 3.36 |
| Nonlabor (variable) | 5.65 | 27.36 | 5.65 | 27.36 |
| Total per person | 17.30 | 47.13 | 9.32 | 36.07 |
| 40,000 | ||||
| Fixed labor | 8.37 | 12.07 | 2.56 | 3.97 |
| Variable labor | 0.20 | 3.36 | 0.20 | 3.36 |
| Nonlabor (variable) | 5.65 | 27.36 | 5.65 | 27.36 |
| Total per person | 14.22 | 42.79 | 8.41 | 34.69 |
IVR, interactive voice recognition; IVR+, IVR plus educational materials; PATIENT, Promoting Adherence To Improve the Effectiveness of cardiovascular disease Therapies.
Table 5 focuses on break-even WTP for a 1mg/dL lowering of LDL. It illustrates that UC has the highest probability of being cost effective in the base case and for patients with lower baseline adherence; for patients receiving or qualifying for 2 calls or more IVR had the highest probability of being cost effective for all but the low cost of care estimate. Among patients with LDL>100 at baseline, IVR+ had the highest probability of being cost effective at the average and high cost of care estimates.
Table 5 –
Break-even analysis on inpatient care for major vascular events.
| Base case | Baseline adherence >50% | Baseline LDL > 100 mg/dl | Receiving/qualifying for two calls or more | ||
|---|---|---|---|---|---|
| Average cost per event | $71,000 | ||||
| Break even WTP per 1 mg/dl LDL lowering | $15 | ||||
| Probability of UC being cost-effective at WTP | 0.885 | 0.743 | 0.149 | 0.259 | |
| Probability of IVR being cost-effective at WTP | 0.112 | 0.168 | 0.334 | 0.64 | |
| Probability of IVR+ being cost-effective at WTP | 0.003 | 0.089 | 0.517 | 0.101 | |
| Low cost per event | $52,000 | ||||
| Break even WTP per 1 mg/dl LDL lowering | $11 | ||||
| Probability of UC being cost-effective at WTP | 0.949 | 0.903 | 0.251 | 0.583 | |
| Probability of IVR being cost-effective at WTP | 0.051 | 0.081 | 0.457 | 0.414 | |
| Probability of IVR+ being cost-effective at WTP | <0.001 | 0.016 | 0.292 | 0.003 | |
| High cost per event | $107,000 | ||||
| Break even WTP per 1 mg/dl LDL lowering | $23 | ||||
| Probability of UC being cost-effective at WTP | 0.687 | 0.461 | 0.067 | 0.033 | |
| Probability of IVR being cost-effective at WTP | 0.223 | 0.238 | 0.198 | 0.549 | |
| Probability of IVR+ being cost-effective at WTP | 0.09 | 0.301 | 0.735 | 0.418 |
IVR, interactive voice recognition; IVR+, IVR plus educational materials; LDL, low-density lipoprotein; UC, usual care; WTP, willingness to pay.
We found that accounting for joint production decreased the ICER in the base case for ACE/ARB to $4.84 from $5.78 (IVR) and to $11.89 from $15.58 (IVR+). For statins the decrease was to $5.72 from $6.24 (IVR) and to $34.27 from $42.64 (IVR+).
Discussion
We found that intervention costs varied by group from about $9 to $17 for IVR and from $36 to $47 for IVR+. In the case of ACEI/ARB adherence, the ICER for an additional unit of improvement was about 3 times higher with IVR+ ($5.78 and $15.58, IVR and IVR+, respectively). For statin adherence, the ICER for an additional unit improvement in adherence was about 7 times higher with IVR+ than IVR ($6.24 and $42.64 for IVR and IVR+, respectively). However, IVR+ dominated IVR when considering the ICER for an additional unit improvement in LDL level. These observations suggest that while more resource-intensive methods (i.e., IVR+) may appear to be a lower value-for-money option when considering medication adherence, at least in the case of statins, they are actually a better value for clinical (i.e., LDL reduction) outcomes. These inconsistent findings illustrate the difficulty decision makers face regarding the uptake of adherence promotion interventions. Although outside the scope and budget constraint of our analysis, using decision models where disparate sources of information are combined (including observational data on the impact of adherence on long-term costs and outcomes) may be a useful way forward. However, in the case of medication adherence, the remarkably strong “healthy adherer” bias [18] poses unique challenges to using observational data in this way. Pragmatic trials with longer-term, multi-year follow-up are necessary to fully gauge whether, and under what circumstances, the effects are durable and represent optimal use of resources.
During the planning of our study, we had discussions with health plan leaders regarding factors that would motivate them to adopt these adherence interventions if they were found effective. One consistent theme they described was a desire to fund interventions that had a positive return on investment, or were cost-neutral. There are several ways that medication adherence interventions can provide a positive return on investment, including their role in avoiding costly events. In addition, there are major incentive programs that payers have initiated to promote quality of care, including medication adherence. For example, the Centers for Medicare & Medicaid Services (CMS) in the United States reward Medicare providers for achieving medication adherence and other benchmarks. The interventions we evaluated are potentially an important part of a health plan’s overall strategy to increase such incentive payments. Our results can be used by health plans to assess the costs needed to achieve improvements in their medication adherence scores. However, it is difficult to provide guidance on the WTP for adherence that an individual decision maker should target. This is because, at least in the case of CMS, those quality targets and associated reward payments vary by many factors including geography, number of enrollees, and how the plan scores on other quality measures.
Our analysis focused on the cost per unit improvement in clinical parameters. Such analyses are consistent with standard cost-effectiveness analyses, but a recent systematic review [19] identified only one study reporting the cost per unit increase in adherence. The study was in a different clinical area (mental health) and the €149 per percentage point increase in adherence was not considered cost-effective by the authors. A recent study by Choudhry et al. [20] provided decision makers with some of the only WTP guidance for adherence changes that comes from a longer-term, randomized controlled trial following an intervention in cardiovascular medicine. That study randomized patients to free medication (or UC) after a myocardial infarction, and followed them for up to 3 years. Free medications included statins, ACEI/ARB, and beta blockers; adherence rates increased by 5.4 percentage points (95% CI, 3.6–7.2) across all three medication classes. Patients in the intervention group showed reduced cardiovascular expenditures of about $2000 over follow-up; this was 11% lower compared with UC (relative spending 0.89 [95% CI 0.77–1.02]). Delivering the IVR+ intervention over 3 years would cost about $108 ($36 × 3 years), a fraction of the potential savings demonstrated by Choudhry et al. However, patients in the Choudhry et al. study were likely sicker (recent myocardial infarction) than the patients in our study. It included beta blockers, and the adherence effect was nearly twice than what we found, so such a WTP threshold may not directly translate to patients in our study.
Among patients with an LDL level of more than 100 mg/dL, the IVR+ and IVR interventions resulted in LDL reductions compared to UC. In order to incorporate this clinical finding about the effect of LDL into our analysis, we examined the potential reduction in major vascular events. That analysis (Table 5) strongly suggested superiority for UC in the base case; the probability of UC being optimal ranged from 0.69 to 0.95, depending on the assumed cost of care for major vascular events. However, among patients with a starting LDL of more than 100 mg/dL, IVR had the highest probability of being optimal at the lowest cost of care (0.46), and IVR+ had the highest probability of being cost-effective at the average cost (0.46) and the highest cost (0.74) per event. Targeting the interventions at patients with high baseline LDL level may be a cost-effective option. However, this analysis must be viewed cautiously because it may not directly translate to our study population. For example, although the CTT analysis found no evidence of a threshold effect for lipid lowering, the decrease in LDL shown in our study was outside the range (lower) of the trials included in their analysis.
In the adherence analysis, we found that patients with a baseline adherence of less than 50% and those in the two-call subgroup had a somewhat more favorable ICER than patients in the base case. This general pattern of a more favorable ICER among such patients suggested that (depending on WTP) a less resource-intensive intervention such as IVR may be optimal for more motivated patients. These analyses were post hoc and should be viewed with caution as they may overstate the results, but they do illustrate that “lighter touch” interventions (e.g., IVR) may be optimal for those who are receptive to them. For example, IVR options might be offered and provided to patients who accept, while more intensive interventions would be reserved for less motivated patients. Such a targeted strategy would allow a more affordable set of interventions. The intervention was less cost-effective in those patients with higher baseline costs, suggesting that such patients may require other methods. This was particularly evident in the case of statins and IVR+ where the ICER nearly tripled from the base case.
The scalability of the intervention is an important component for decision makers. As the number of patients increases, the cost per patient decreases as fixed costs are being spread over more individuals, thus improving efficiency. However, the converse is also true; fewer patients leads to higher per-patient costs.
Strengths of our study include that it was conducted alongside a pragmatic, randomized trial, with careful assessment of costs. Commensurate with the pragmatic design, the nature of our main outcome assessment, pharmacy refill adherence, did not depend on patient contact, thus mitigating any such bias. The interventions tested had minimal impact on existing clinical work flows and, owing to the pragmatic design, allowed tailoring to site-specific requirements. Additionally, there were very few exclusion criteria, making our findings widely applicable to patients with the clinical profiles of those included. The main weakness of our analysis was the abbreviated follow-up (dictated by funding constraints), which did not allow for longer-term assessments of clinical outcomes. Follow-up averaged 9.6 months; this may have been too short of an induction period for us to observe the full intervention effects and costs. Even over this brief follow-up, however, we did observe clinically relevant changes in lipid parameters. We found that when CVD costs were included, mean health care costs were not meaningfully different (as evidenced by overlapping CIs) between the groups; we reported them for future analyses (e.g., modeling studies) that require care cost estimates. We also realize that our findings will be less relevant to those who prefer a societal perspective for economic evaluation; our findings would be a better fit for decision makers focused on a payer perspective.
Because the tested interventions rely heavily on information-technology–enabled resources (e.g., electronic pharmacy records and EMR) that can be costly to establish, intervention implementation may be favored by health systems that have existing functionality. However, existing data systems can potentially be linked to achieve similar results. Using EMR technology this way is consonant with “meaningful use” [21], and may provide an avenue to help providers meet those goals.
Conclusions
We found that the use of an automated voice messaging system to promote adherence to ACEI/ARB and statins may be cost-effective, depending on a decision maker’s WTP. When considering changes in LDL and downstream medical care offsets, UC is the optimal strategy for the general population. However, IVR-based interventions may be the optimal choice for those with elevated LDL values at baseline.
Acknowledgments
Source of financial support: This project was supported by the Agency for Healthcare Research and Quality (grant no. R01HS019341).
Footnotes
Conflicts of interest: The content of this article—which includes design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript—is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality by which this project was funded.
REFERENCES
- [1].Bitton A, Choudhry NK, Matlin OS, et al. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. Am J Med 2013;126:357. [DOI] [PubMed] [Google Scholar]
- [2].Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008;155:772–9. [DOI] [PubMed] [Google Scholar]
- [3].Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006;296:2563–71. [DOI] [PubMed] [Google Scholar]
- [4].Smith DH, Kramer JM, Perrin N, et al. A randomized trial of direct-to-patient communication to enhance adherence to {beta}-blocker therapy following myocardial infarction. Arch Intern Med 2008;168:477–83. [DOI] [PubMed] [Google Scholar]
- [5].Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med 2013;173:38–43. [DOI] [PubMed] [Google Scholar]
- [6].Seabury SA, Lakdawalla DN, Dougherty JS, et al. Medication adherence and measures of health plan quality. Am J Manag Care 2015;21:e379–89. [PubMed] [Google Scholar]
- [7].Vollmer WM, Owen-Smith A, Tom J, et al. Improving adherence to cardiovascular disease medications with information technology. Am J Manag Care 2014;20:SP502–10. [PMC free article] [PubMed] [Google Scholar]
- [8].Vollmer WM, Xu M, Feldstein A, et al. Comparison of pharmacy-based measures of medication adherence. BMC Health Serv Res 2012;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565–74. [DOI] [PubMed] [Google Scholar]
- [10].Hornbrook MC, Hart G, Ellis JL, et al. Building a virtual cancer research organization. J Natl Cancer Inst Monogr 2005;(35):12–25. [DOI] [PubMed] [Google Scholar]
- [11].Hornbrook M, Goodman MJ. Adjusting health benefit contributions to reflect risks In: Hornbrook M,ed., Risk Based Contributions to Private Health Insurance. Greenwich, CT: JAI Press Inc., 1991. [PubMed] [Google Scholar]
- [12].Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415–30. [DOI] [PubMed] [Google Scholar]
- [13].Hoch JS, Rockx MA, Krahn AD. Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of “community acquired” syncope. BMC. Health Serv Res 2006;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoch JS. Improving efficiency and value in palliative care with net benefit regression: an introduction to a simple method for cost-effectiveness analysis with person-level data. J Pain Symptom Manage 2009;38:54–61. [DOI] [PubMed] [Google Scholar]
- [15].Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Birkmeyer JD, Gust C, Baser O, et al. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res 2010;45:1783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gold M, Siegel J, Russel L, Weinstein M. Cost Effectiveness in Health and Medicine. New York, NY: Oxford University Press, 1996. [Google Scholar]
- [18].Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006;333:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Oberje EJ, de Kinderen RJ, Evers SM, et al. Cost effectiveness of medication adherence-enhancing interventions: a systematic review of trial-based economic evaluations. Pharmacoeconomics 2013;31:1155–68. [DOI] [PubMed] [Google Scholar]
- [20].Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med 2011;365:2088–97. [DOI] [PubMed] [Google Scholar]
- [21].US Department of Health and Human Services. EHR incentives and certification: meaningful use definition and objectives. 2014. Available from: http://www.healthit.gov/providers-professionals/meaningful-use-definition-objectives. [Accessed January 31, 2016].