Abstract
Prostate cancer is the second most prevalent cancer in men worldwide and its incidence is expected to double by 2030. Multi-parametric magnetic resonance imaging (MRI) incorporating anatomical and functional imaging has now been validated as a means of detecting and characterising prostate tumours and can aid in risk stratification and treatment selection. The European Society of Urogenital Radiology (ESUR) in 2012 established the Prostate Imaging—Reporting and Data System (PI-RADS) guidelines aimed at standardising the acquisition, interpretation and reporting of prostate MRI. Subsequent experience and technical developments have highlighted some limitations, and a joint steering committee formed by the American College of Radiology, ESUR, and the AdMeTech Foundation have recently announced an updated version of the proposals. We summarise the main proposals of PI-RADS version 2, explore the evidence behind the recommendations, and highlight key differences for the benefit of those already familiar with the original.
Introduction
Prostate cancer is the second leading cause of cancer death in men and its incidence is expected to double by 2030 due to the aging global population.1 The traditional diagnostic tests employed for detection of prostate cancer, namely prostate-specific antigen (PSA) and transrectal ultrasound (TRUS)-guided biopsy lack sensitivity and specificity. The former is specific to the prostate gland but not to prostate cancer and the latter can miss approximately 30% of tumours and underestimate tumour aggressiveness in around a third of cases.2,3 Imaging was initially employed for loco-regional staging with magnetic resonance imaging (MRI) and distant staging with computed tomography (CT) or bone scintigraphy in patients with biopsy-proven cancer; however, more recent advances in MRI technology and the incorporation of functional sequences alongside anatomical imaging, termed multiparametric (mp) MRI, have improved our ability to both detect and characterise prostate tumours. MRI for biopsy guidance has been shown to improve accuracy of tumour detection and grading,4,5 and MRI is now seen as an essential step prior to enrolment on active surveillance programmes.6 As a result, some authors even suggest that MRI should replace TRUS biopsy as the initial diagnostic test for prostate cancer to enable guidance of subsequent biopsy.7
This change in practice is reflected in the updated 2014 National Institute for Health and Care Excellence (NICE) guidelines, which recommend more widespread use of MRI in the work-up of prostate cancer.6 A recently published report from the National Prostate Cancer Audit revealed that although 99% of trusts in England that provide prostate cancer services have access to onsite MRI, only 75% provide mpMRI.8 In addition, interpretation of prostate MRI entails a steep learning curve9,10 and requires regular reporting and audit in order to maintain reporting standards.11
The European Society of Urogenital Radiology (ESUR) in 2012 established clinical guidelines for the acquisition, interpretation, and reporting of mpMRI of the prostate in order to facilitate a greater level of standardisation and consistency.12 These recommendations, popularly referred to as Prostate Imaging—Reporting and Data System (PI- RADS), were based on literature evidence and consensus expert opinion and were modelled on similar efforts in mammography (BI-RADS) that led to the transformation of breast cancer care. A number of studies have subsequently validated PI-RADS in certain research and clinical settings; however, experience has highlighted some limitations, in part due to technical improvements and also changes in clinical practice. A joint steering committee formed by the American College of Radiology (ACR), ESUR, and the nonprofit organisation AdMeTech Foundation, have recently attempted to update and improve on the original proposals. PI-RADS version 2 (v2) was officially launched at RSNA 2014, and is now available online.13
Herein, we summarise the main features of PI-RADS v2, explore the background evidence that form the basis of the recommendations, and highlight the key differences with PI-RADS v1 for the benefit of those already familiar with the original.
Format and scope
The incorporation of the ACR into the consortium results in the subtle change to US English, from the UK English of the original European-based ESUR guidelines. The format is also fundamentally different, with the original being a 12-page article published in a peer-reviewed journal, whereas PI-RADS v2 is a 55-page PDF (portable document format) document available online. This is necessary to meet the intended aims of being a “living” document that has scope to evolve with the accrual of clinical experience and scientific data; indeed, some sections, such as report templates and sample protocols, remain listed as “under construction” on the earliest iteration.
The mention of PI-RADS tends to invoke thoughts of the proposed scoring system, but, in truth, the original document was far more than this, with information on risk stratification in prostate cancer, enrolment criteria for active surveillance programmes, recommendations on MRI protocol parameters, and how to report findings. The format of v2 allows it to be even more comprehensive, with detailed background information including an overview of normal anatomy and benign findings, and a lexicon of terminology with relevant definitions.
PI-RADS v2 is more explicit in its scope and aims. It is designed to promote global standardisation and diminish variation in the acquisition, interpretation, and reporting of prostate mpMRI examinations. Unlike the original document, which struck a compromise by suggesting both “minimal” and “optimal” requirements, v2 only proposes to establish minimal technical parameters that should result in an acceptable mpMRI examination. A further aim that differs from v1 is to facilitate the use of MRI data for targeted biopsy, a reflection of the recent evolution of clinical practice.14 Common to both, although more explicitly stated in v2, the guidelines are not intended for MRI application in the setting of suspected post-therapy recurrent prostate cancer, nor progression during surveillance, although they could be easily adopted for the latter. Unlike the original, separate protocols for “detection”, “staging”, and “node and bone” are not suggested, rather a common protocol is presented, and MRI for evaluation of the skeletal system is explicitly not covered.
A combined MRI and biopsy definition of “significant” prostate cancer is proposed based on recent clinical experience15,16: Gleason score ≥7 (including 3+4 with prominent but not predominant Gleason 4 component), and/or volume ≥0.5 cm3, and/or extra-prostatic extension (EPE). This is relevant to the stated aims of improving detection of significant cancer, whilst increasing confidence in calls of benign or insignificant disease, in order to reduce unnecessary biopsies and treatment.
MRI acquisition
PI-RADS v1 recommended the combination of high-resolution T2-weighted imaging (T2WI), and at least two functional MRI techniques, which could be diffusion- weighted imaging (DWI), dynamic contrast-enhanced (DCE), or MR spectroscopy imaging (MRSI). In v2, MRSI is no longer recommended for PI-RADS assessment, with mpMRI consisting of anatomical imaging, DWI, and DCE alone. It is further noted that if functional imaging with both DWI and DCE is absent or inadequate, the MRI assessment should be limited to staging.
Pre-procedure
Minimum expected requirements are explicitly stated now for providing a relevant clinical history, namely recent PSA level and PSA history, family history, previous treatment, digital rectal examination findings, and biopsy status and result. PI-RADS v1 recommended waiting at least 4–6 weeks between biopsy and MRI. The new version recommends waiting at least 6 weeks when the purpose is for staging, but acknowledges that there may be no need to delay post-biopsy imaging if the primary purpose of the examination is to detect and characterise clinically significant cancer within the gland, based on the superior specificity of DWI utilising high b-values.17
Patient preparation
It is acknowledged that, at present, there is a lack of consensus regarding issues of patient preparation. Anti- spasmodic agents are considered beneficial but not essential and use can be decided locally, based on considerations of cost, drug availability, and potential for adverse drug reactions. An endorectal coil (ERC) is not routinely recommended, although with older generation 1.5 T MRI systems, an ERC may be indispensable for achieving diagnostic quality imaging.18 The patient should evacuate the rectum just prior to the MRI examination to minimise the presence of stool and rectal air, which can lead to susceptibility artefact and distortion on DWI. If significant air is noted on initial imaging, consideration should be given to performing the remainder of the examination in a prone position or attempts made to decompress the rectum. Although in some centres abstention from ejaculation 72 hours prior to scanning is employed to maintain seminal vesicle distention, a benefit for this is not established and is, therefore, not recommended.
MRI protocols
In contradistinction to the original guidance, v2 proposes just one protocol for MRI of the prostate, with no separate parameters offered for 1.5 T protocols. Three tesla is considered superior to 1.5 T; however, both 1.5 and 3 T can provide adequate and reliable diagnostic examinations when acquisition parameters are optimised and appropriate contemporary technology is employed. 1.5 T should be used for patients with implants that are MR conditional at 1.5 T but not at 3 T, or where artefact from implants, such as hip replacements will compromise imaging at 3 T.
There is also a strong recommendation that the imaging plane angle, location, and section thickness are identical for all sequences. If image quality of any sequence is suboptimal, measures should be taken to repeat the sequence. The technical parameters recommended for T2, DWI, and DCE sequences are summarised in Table 1, a brief overview of the key points for each sequence and changes is given below.
Table 1.
Acquisition protocols: technical parameters.
| • T2-weighted imaging (axial, sagittal, coronal planes) |
| − Field-of-view: 12–20 cm to encompass the entire prostate gland and seminal vesicles |
| − 3 mm section thickness, 0 mm gap |
| − In-plane resolution: ≤0.7 mm (phase) × ≤0.4 mm (frequency) |
| • Diffusion-weighted imaging (axial plane) |
| − Free-breathing spin echo EPI sequence combined with spectral fat saturation is recommended |
| − Section thickness to match T2WI |
| − TE: ≤90 ms; TR: >3000 msI |
| − Field-of-view: 16–22 cm |
| − In plane dimension: ≤2.5 mm phase and frequency |
| − At least two b-values should be acquired in three orthogonal directions. If only two b-values are utilised, these should be 50 −100 s/mm2 (low) and 800–1000 s/mm2 (high) |
| − “High b-value” acquisition with b=1400–2000 s/mm2, depending on achievable quality of SNR |
| − ADC map calculation: low b-value should be ≥50 s/mm2, high b-value should be >800 s/mm2, up to a maximum of 1000 s/mm2 |
| • Dynamic contrast-enhanced MRI (axial plane) |
| − Fat suppression and/or subtraction is recommended |
| − 2D or 3D T1 gradient echo (GRE) sequence; 3D is preferred |
| − Section thickness to match T2WI |
| − Injection rate: 2–3 ml/s |
| − TR/TE: <100 ms/<5 ms |
| − In-plane dimension: ≤2 mm × ≤2 mm |
| − Temporal resolution: ≤10s(<7s is preferred) |
| − Pharmacodynamic analysis does not have to be performed |
EPI, echo planar imaging; ADC, apparent diffusion coefficient; 2D, two-dimensional; 3D, three-dimensional; TR, repetition time, TE, echo time; T2WI, T2-weighted imaging; GRE, gradient echo.
Anatomical imaging
Axial T1WI remains essential to assess for biopsy-related haemorrhage. This can be with or without fat suppression and should match the other axial sequences, although lower spatial resolution may be employed. Multiplanar T2WI should incorporate the axial, sagittal, and coronal planes; the “detection” protocol in v1 only recommended axial and sagittal imaging. Three-dimensional (3D) axial acquisitions may be used; however, in-plane resolution may be lower than the equivalent two-dimensional (2D) acquisition, and therefore, these should be used as an adjunct to, rather than a replacement for, 2D imaging.
DWI
DWI should incorporate a minimum of two b-values and a mono-exponential model of signal decay, which is in contrast to the original PI-RADS where at least three b-values were recommended, with prescribed values of 0,100, and 800–1000 s/mm2. This is set as the minimum requirement, and if only two b-values are acquired, the lowest should be 50–100 s/mm2 (not b=0) and the highest should be 800–1000 s/mm2. In addition, the acquisition of “high b-value” images is recommended, utilising a b-value of at least 1400 s/mm2, or up to 2000 s/mm2, if the signal-to-noise (SNR) remains adequate. The original v1 recommended using the ≥800 s/mm2 as the “high b-value” series for interpretation purposes; however, despite these changes, the recommendations for apparent diffusion coefficient (ADC) maps essentially remain the same with exclusion of any b=0 data to avoid pseudo-perfusion effects,19 and utilising a high b-value of no greater than 1000 s/mm2 for calculation purposes.
DCE MRI
PI-RADS v2 now recommends a fat-suppressed sequence to improve lesion conspicuity; subtraction can be used as a replacement or as a post-processing adjunct in cases of biopsy-related haemorrhage. The most widely used method of assessing DCE is visual assessment of each section by cine loop or manually scrolling, and there is currently insufficient evidence to recommend the routine use of pharmacodynamic (PD) analysis. Parametric maps can be calculated depending on local preference, but any suspicious findings should always be confirmed on the source images. An injection rate of 2–3 ml/s should be employed, with a temporal resolution of <10 seconds and preferably <7 seconds in order to depict focal early enhancement. This differs from the original guidelines of a 15 seconds temporal resolution, with a higher temporal resolution of <10 seconds suggested when PD analysis is to be performed. Whilst v1 stated the minimum length of acquisition post-injection as being 5 minutes in order to detect washout, v2 simply states a total observation time of ≥2 minutes, this relates to the deemphasis of PD analysis that requires longer acquisition periods for DCE.
MRI interpretation
It is emphasised that the assigned PI-RADS v2 score is based solely on mpMRI findings and does not take into account clinical factors that may be of key relevance, such as PSA, clinical history, or previous biopsy results. As such, reporters may choose to use a Likert scale of probability to reflect these factors (Fig.1).Typically, an overall PI-RADS score of 4 or 5 means a biopsy is recommended. Subtle changes have been made to the five-point scoring systems for T2WI findings in the peripheral zone (PZ) and transition zone (TZ) and for DWI findings (Table 2). DCE-MRI has changed from a five-point scale to simply being either “positive” or “negative”. Additionally, at the end of the document, example images are provided for all PI-RADS categories for each of the three sequences in both the PZ and TZ.
Figure 1.

Importance of clinical context. A 60-year-old man referred for raised PSA. (a) Axial T2WI demonstrates a focal lesion in the left mid PZ (arrows), with matching restricted diffusion (b); PI-RADS score 4. Subsequent transperineal fused TRUS-MRI targeted-biopsy is planned (c), with sagittal US image confirming needle placement in the lesion (d). Histology confirms high-grade PIN. MRI repeated at 18 months shows no change in the previously sampled lesion (e, f). Although the PI-RADS score is based solely on mpMRI findings and remains 4, when taking account of clinical context, the report conclusion would more appropriately state that clinically significant cancer is unlikely to be present.
Table 2.
PI-RADS v2 scoring systems.
| Score criteria |
|---|
| T2WI for peripheral zone |
| 1. Uniform high signal intensity |
| 2. Linear, wedge-shaped, or geographic areas of lower SI, usually not well demarcated |
| 3. Heterogeneous signal intensity or non-circumscribed, rounded, moderate hypointensity. Or other not in categories 1/2 or 4/5 [Intermediate, not 1/2 or 4/5] |
| 4. Circumscribed, homogeneous moderate hypointense focus/mass confined to prostate and <1.5 cm in greatest dimension |
| [No size stated] |
| 5. Same as 4 but ≥1.5 cm in greatest dimension or definite ECE/invasive behaviour |
| [Broad >1.5 cm capsule contact] |
| T2WI for transition zone |
| 1. Heterogeneous intermediate SI |
| [Used terms well-defined margins and “organised chaos”] |
| 2. Circumscribed hypointense or heterogeneous encapsulated nodule(s) (BPH) |
| [Used well-marginated] |
| 3. Heterogeneous signal intensity with obscured margins. Or other not in categories 1/2 or 4/5 |
| [Intermediate, not 1/2 or 4/5] |
| 4. Lenticular or non-circumscribed, homogeneous, moderately hypointense, and <1.5 cm in greatest dimension |
| [No size stated. Used term “erased charcoal sign”] |
| 5. Same as 4, but ≥ 1.5 cm in greatest dimension or definite ECE /invasive behaviour |
| [Involving the anterior fibromuscular stroma or the anterior horn of the PZ] |
| DWI |
| 1. No abnormality (i.e. normal) on ADC and high b-value DWI |
| 2. Indistinct hypointense on ADC |
| [Also: diffuse hyper SI on ≥b=800 image; no focal features] |
| 3. Focal mildly/moderately hypointense on ADC and isointense/mildly hyperintense on high b-value DWI |
| [Intermediate, not 1/2 or 4/5] |
| 4. Focal markedly hypointense on ADC and markedly hyperintense on high b-value DWI; <1.5 cm in greatest dimension |
| [Iso-intense on high b-value. No size stated] |
| 5. Same as 4 but ≥1.5 cm in greatest dimension or definite ECE/invasive behaviour |
| [Focal, hyper SI on the high b-value images with reduced ADC] |
| Dynamic contrast-enhanced MRI |
| −No early enhancement OR |
| Diffuse enhancement not corresponding to a focal finding on T2 and/or DWI OR |
| Focal enhancement corresponding to a lesion demonstrating features of BPH on T2WI |
| + Focal AND |
| Earlier than or contemporaneously with enhancement of adjacent normal prostatic tissues AND
Corresponds to suspicious finding on T2 and/or DWI |
Differences from version 1 stated in parentheses.
PI-RADS v2, Prostate Imaging—Reporting and Data System version 2; T2W1, T2-weighted imaging; SI, signal intensity; ECE, extra-capsular extension; BPH, benign prostatic hypertrophy; ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging.
The concept of a “dominant sequence” is formally introduced. DWI is the key sequence for the PZ, and T2WI predominates in the TZ. Simplified guidance tables help to emphasise this interpretation and how to assign the final PI-RADS v2 score (Table 3), essentially the dominant sequence score is the final score for 1, 2, 4, or 5 and for scores of 3, the secondary sequence (DCE in PZ and DWI in TZ) may change the score from 3 to 4. The system proposed helps provide some clarity, as previous authors have used PI-RADS v1 in a variety of ways: using a Likert system based on PI-RADS descriptors,20 employing a summed PI-RADS score from all sequences with a cut-off,21–23 or using an average score.24 Early studies have already been performed assessing the PI-RADS v2 system,25 and it will be interesting to test interobserver variation and outcomes compared to final pathology.
Table 3.
Guidance for assignment of overall PI-RADS v2 score.
| Peripheral zone | |||
| DWI score | DCE score | T2W1 | Overall |
| (dominant | (secondary | score | PI-RADS |
| sequence) | sequence) | v2 score | |
| 1 | Any | Any | 1 |
| 2 | Any | Any | 2 |
| 3 | − | Any | 3 |
| 3 | + | Any | 4 |
| 4 | Any | Any | 4 |
| 5 | Any | Any | 5 |
| Transition zone | |||
| T2W1 score | DW1 score | DCE score | Overall |
| (dominant | (secondary | P1-RADS | |
| sequence) | sequence) | v2 score | |
| 1 | Any | Any | 1 |
| 2 | Any | Any | 2 |
| 3 | ≤4 | Any | 3 |
| 3 | 5 | Any | 4 |
| 4 | Any | Any | 4 |
| 5 | Any | Any | 5 |
PI-RADS v2, Prostate Imaging—Reporting and Data System version 2; T2W1, T2-weighted imaging; DW1, diffusion-weighted imaging; DCE dynamic contrast-enhanced.
T2-weighted scoring
Anatomical imaging was previously the mainstay of prostate MRI and axial T2WI remains the key sequence for local staging, including extracapsular extension and seminal vesicle invasion (SVI), and for the assessment of the TZ. If the T2WI PI-RADS score in the TZ is 3, this can be upgraded to a score of 4 if DWI, the secondary sequence, shows strong restricted diffusion (category 4, 5); however, it is noted that diffusion is often reduced in benign prostatic hypertrophy (BPH) nodules, so caution should be exercised in this setting. T2WI findings in the PZ lack specificity: low signal can be due to acute or chronic prostatitis, post-inflammatory scars and atrophy, haemorrhage, and previous treatment, as well as tumour.26 As a result, DWI is the dominant sequence used in the PZ. It should be emphasised that in the normal PZ, it is unusual for diffusion to be very restricted in conditions other than cancer.
The main change in the T2WI scoring system relates to category 4 and 5. Previously lesions needed to show either a suspicion for early (capsular bulging or broad contact) or established extra-capsular extension (ECE) to reach a score of 5. In the updated system, the criteria retain the latter, but replace the former with a size cut-off of 1.5 cm (Fig. 2). Of note, the use of the terms “erased charcoal” to described TZ tumours and “organised chaos” to describe the normal TZ appearance due to BPH on T2 have been dropped from the summary scoring system. These somewhat evocative terms are, however, retained within the text of the article, and it is sensible for the reader to keep them in mind when interpreting the T2 sequences. The description of TZ tumours having a lenticular or “tear-drop” shape27 is retained. A formal definition of what constitutes category 3 findings is provided for PZ and TZ alongside the original article’s slightly ambiguous statement of not otherwise being in categories 1, 2, 4, or 5.
Figure 2.
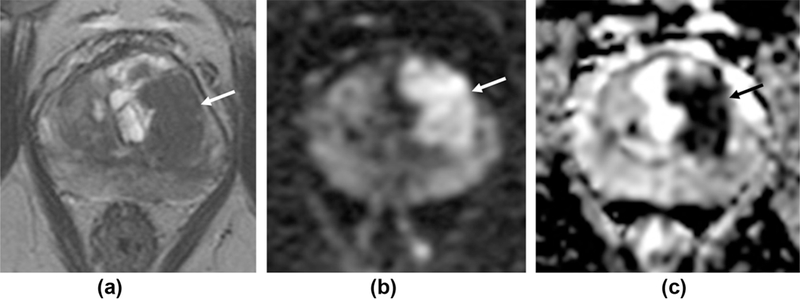
Differences inT2 scoring for high probability targets. A 77-year-old patient with a PSA of 17 ng/ml. (a)T2W1 shows a large (2.5 × 1.5 cm) lesion centred in the left TZ at the level of the mid-gland (arrow), with matching restricted diffusion (b-c). P1-RADS v1 scores: 4 for T2 as no features of ECE and no broad capsular contact, 5 for DW1. P1-RADS v2 overall score 5: T2 is the dominant sequence and the lesion is >1.5 cm, despite no features of ECE. Subsequent targeted biopsy confirms Gleason 4+5 disease (90% core involvement) in the left medial mid-gland and Gleason 5+5 (60% involvement) in the left posterior lateral gland.
DWI scoring
Non-cancerous nodules of BPH can contain stroma or have a high cellular density and can, therefore, demonstrate restricted diffusion,28 thus DWI is the secondary sequence to T2 for TZ interpretation. However, DWI is the main sequence for interpretation of the PZ, with DCE the secondary sequence, thus a DWI score of 3 in the PZ can be upgraded to category 4 if the matching DCE is positive. The scoring system for DWI in categories 2–5 has changed considerably compared to v1 (Table 2). As with the T2WI scoring, the distinction between categories 4 and 5 is based on a size cut-off of 1.5 cm or features of ECE, this offers consistency between the two dominant sequences.
It is emphasised that absolute ADC values should be used with caution as these can vary substantially depending on the value and number of b-values selected, the magnet strength, the vendor, and inter-patient variability.29–31 P1-RADS v2 does suggest that an ADC threshold of 750–900 mm2/s can be used for differentiation between benign and malignant prostate tissues in the PZ. It is also recommended that ADC maps are consistently viewed with the same contrast windowing, set to portray clinically significant prostate cancers adequately as markedly hypointense on ADC maps. Clearly, these will need to be tailored to the particular scanner and advice from radiologists who have experience with a similar magnet may be helpful. In our experience, using a window width of 1400 μm2/s and window level of 1400 μm2/s sets the grey-scale range from 700–2100 μm2/s, with potentially significant ADC values of <700 μm2/sec therefore appearing as “black”.
DCE MRI scoring
The interpretation of DCE and the application of its results have changed significantly from v1. Although the role of DCE-MR1 appears downplayed, PI-RADS v2 states explicitly that DCE should be included in all prostate mpMRl examinations to avoid missing some small but significant tumours.32 Additionally, in situations when one of the other sequences is non-diagnostic, DCE can be helpful in assessing risk.
Considerable effort was previously expended in v1 looking at “curvology” of contrast wash-in and wash-out from the prostate and prostatic lesions. Three curve patterns are described: Type I, progressive enhancement, Type II, plateau of enhancement, and Type III, contrast washout. Curve typing works well in breast imaging where Type I is considered benign (only 8.9% of tumours demonstrate this pattern), Type II is intermediate probability, and a Type III curve is considered malignant (only 5.5% of benign lesions show this pattern).33 P1-RADS v1 endorsed the visual assessment of specific curve types along with focality and asymmetry of enhancement in order to create a five-point DCE score; however, the enhancement characteristics of prostate show great heterogeneity: BPH nodules are often hypervascular (Fig. 3), the absence of early enhancement usually adds little information, and diffuse enhancement can be seen in the setting of prostatitis. Furthermore, there is little evidence in the literature to support the qualitative use of curve types for identifying prostate cancer.34 1ndeed, Hansford et al.35 recently showed that despite good interobserver agreement on curve-type assignment, curvology cannot reliably differentiate prostate cancer from benign prostate tissue (Fig. 4), with the majority of tumours demonstrating Type 2 enhancement curves.
Figure 3.
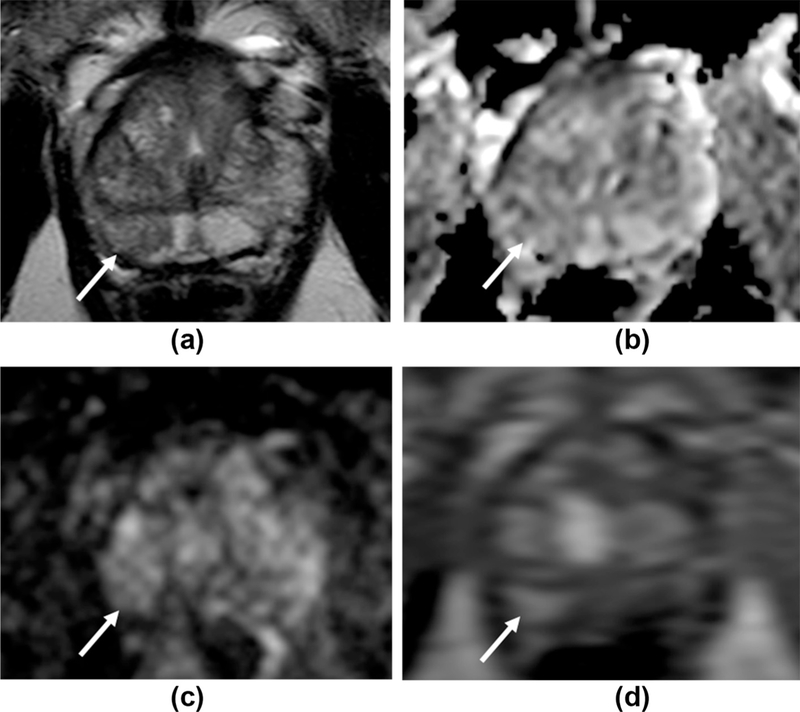
False-positive DCE result, correctly classed as low probability for tumour with PI-RADS v2 criteria. 63 year-old man, serum PSA=2.31 ng/ml. a: T2W1 imaging shows an area of intermediate signal with geographical features in the right mid/apex PZ (arrows) with no matching restricted diffusion (b,c). d: The region shows focal early enhancement on DCE-MRI (arrow), with a Type III curve. PI-RADS v1 scores: 2 for T2, 2 for DWI, 5 for DCE, summed score=9. PI-RADS v2 overall score=2 (DWI is the dominant PZ sequence) despite being “positive” with DCE. Targeted biopsy of this region was benign.
Figure 4.
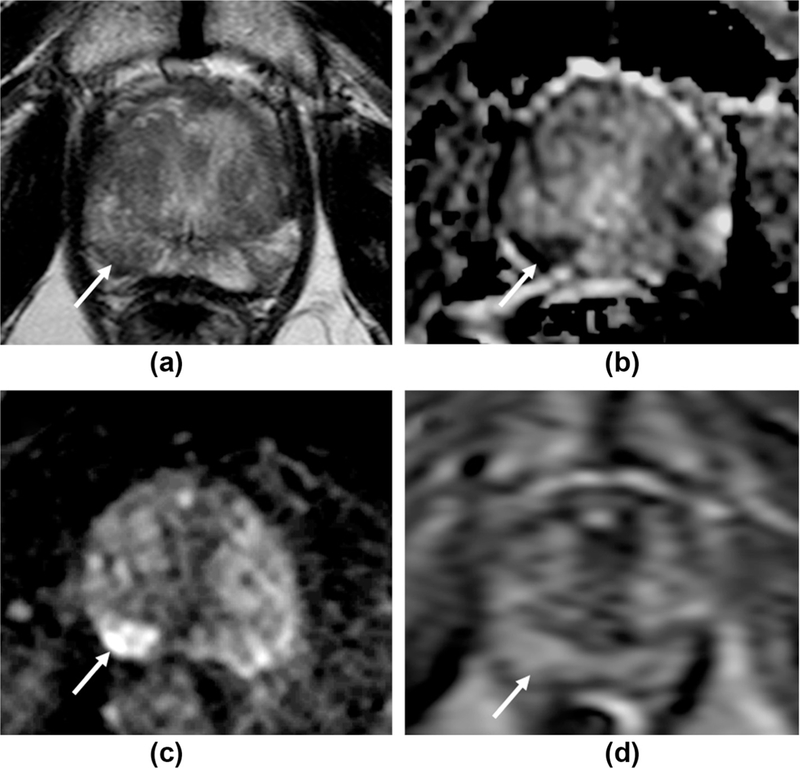
False-negative DCE, correctly evaluated as likely tumour using PI-RADS v2 criteria. 60 year-old patient with a PSA of 4.48 ng/ml. a: T2W1 imaging shows a focal area of intermediate/low signal the right mid/apex PZ (arrows), with marked matching restricted diffusion (b, c). d: The region shows diffuse, but no focal or early enhancement on DCE-MRI (arrow), with a Type I curve. PI-RADS v1 scores: 3 for T2, 5 for DWI, 1 for DCE, summed score=9. PI-RADS v2 overall score=5 (DWI is the dominant PZ sequence) despite being “negative” with DCE. Targeted biopsy demonstrated a Gleason 3+3 tumour in 10% of the cores.
As a result, PI-RADS v2 does not consider the type of curve important, with DCE only classed as “positive” in the presence of focal enhancement that corresponds to a suspicious finding on T2 and/or DWI. DCE is considered the secondary sequence for the PZ, and lesions scoring 3 on DWI can be upgraded to category 4 overall if DCE is positive. DCE may also be helpful in prioritisation when multiple lesions are present in the same patient, or for identification of the index lesion. Although DCE-MRI has no role to play in the TZ in the presence of good-quality T2WI and DWI, it can be utilised as a secondary sequence if DWI evaluation in part or all of the gland is technically compromised.
Although DCE MRI is often the key sequence for identifying recurrent disease after previous focal or whole-gland treatment,36–38 PI-RADS v2 is specifically aimed at assessment of treatment naive patients.
Staging
T2WI is the key sequence for determining the “T-stage” of tumours. Distinction between T1 and T2 disease and their subsets is less important than determining organ-confined (T1–2 disease) versus extra-prostatic extension (T3a disease), seminal vesicle involvement (T3b), or invasion of the pelvic side wall or other organs (T4 disease). Although some work suggests DWI may help in the detection of ECE,39 this benefit may partially relate to more confident tumour localisation; clearly initial identification of the tumour using anatomical and functional sequences is an aid to accurate staging. In v1, extra-prostatic involvement (T3a/ b) was also scored on a five-point scale and, although a formal scale is no longer employed, the features to look for are retained.
Imaging features suggesting extraprostatic extension include overt ECE, capsular bulging or irregularity, broad capsular contact (>1 cm), filling in of the retroprostatic angle, and asymmetry or invasion of the neurovascular bundles. Recent work has demonstrated that the degree of tumour contact length with the capsule is a better predictor than both pathological tumour volume and clinical nomograms: capsular contact >2 cm demonstrated an accuracy of 82% for predicting ECE, whereas with contact <1 cm the chance of ECE was <5%.40
The MRI features of SVI are low T2W signal, restricted diffusion, abnormal contrast enhancement, obliteration of the angle between the seminal vesicle and prostatic base, and demonstration of contiguous tumour extension. Three patterns of SVI have been described: Type I, direct spread along the ejaculatory ducts, Type II, direct invasion from tumour through the capsule into the seminal vesicles, and Type III, metastasis from a remote non-contiguous primary in the prostate. In 1993, Ohori et al.41 demonstrated SVI as being Type I in 26%, Type II in 33%, combined Types I and II in 28%, and Type III in 13% of cases.41 A more recent study showed an almost equal split between Types I and II, but with no Type III disease.42 Although the type of SVI may not be clinically relevant and is not routinely reported by pathologists,43 it is worth noting the relative rarity of Type III involvement if the tumour is confidently identified in a location remote from the base or the confluence with the ejaculatory ducts at the verumontanum.
Unlike v1, there is no dedicated “nodes and bones” protocol, as the imaged population will be expected to have either organ-confined or locally advanced disease. At least one sequence should employ a field-of-view (FOV) that permits evaluation of lymph nodes up to the level of the aortic bifurcation. MRI assessment of lymph nodes remains limited to morphological features and size, with nodes >8 mm in short axis regarded as suspicious. DWI is not considered a reliable discriminator between benignity and metastatic involvement; normal nodes can have an impeded diffusion due to their high cellularity, and lymph nodes have a relatively long T2 relaxation time, and therefore, appear as high signal intensity structures on b-value DWI.44 This latter property can be exploited by using the high b-value sequence as a means of identifying and mapping out nodes,45 which can then be evaluated on the anatomical sequences. The imaged bones should be reviewed for metastases, and again the high b-value imaging may aid conspicuity (Fig. 5).
Figure 5.
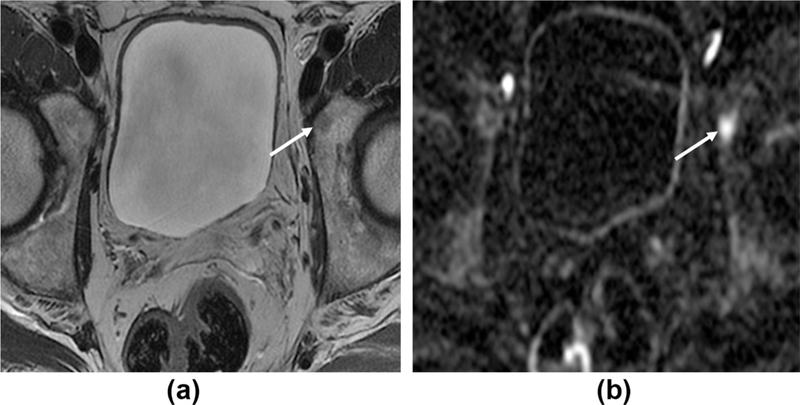
High b-value images aid detection of bone metastases. A 68-year-old man with rising PSA, 2 years post-prostatectomy. (a) Axial T2W1 shows a subtle low intensity area in the left acetabulum (arrow). (b) Axial b=1400 DWI sequence; the lesion demonstrates restricted diffusion and appears more conspicuous on these high b-value images.
MRI reporting
The original PI-RADS article stated the use of a structured reporting scheme was preferred. The benefits of proforma-type reporting have been championed,46 and this is consistent with the RSNA radiology reporting initiative for improving reporting practices. PI-RADS v2 has provision for example template reports in Appendix I, but these remain “under construction”.
Lesions with a PI-RADS score of 3, 4, or 5 should be reported, up to a maximum of four. The index (dominant) lesion should be identified. This is defined as the lesion with the highest PI-RADS score, if this is assigned to two or more lesions, the index lesion should be the largest lesion, or the one that shows EPE. The minimum requirement is to report the largest dimension of a suspicious finding on an axial image, if the largest dimension is derived from the sagittal and/or coronal images, this measurement and plane should also be reported. Consistent with the predominant sequences previously described, PZ lesions should be measured on ADC maps and TZ lesions measured on T2WI. Unless direct lesion volumetry is available, overall gland volume should be recorded using the ellipse formula (maximum anteroposterior diameter) × (maximum transverse diameter) × (maximum longitudinal diameter) × 0.52.
Lesion location should be reported according to the sector map to enable localisation of findings and as a visual aid for subsequent biopsy and/or treatment planning. Either a paper copy or scanned electronic version of the map should be used for recording. PI-RADS v1 suggested a minimum requirement of dividing the prostate into 16 regions, with an optimal requirement of 27 regions. The updated recommendations are 36 sectors for the prostate, with an additional two for the seminal vesicles and one for the external urethral sphincter.
Although there was no diagrammatic representation in the original paper, the recommendations were based on the scheme proposed by Dickinson et al.47 and developed from earlier work by Villers et al.48 and Haffner et al.49 Herein, the gland is separated into left and right and medial and lateral at the level of the apex, mid, and base. There is further division into anterior and posterior by the “17-mm line”, which is based on the likely reach of a 20 mm transrectal biopsy needle. An interim update by Barentsz et al.50 proposed the 36-sector partition, with additional division of the anterior stroma levels into left and right and further splitting the TZ into anterior and posterior above and below the 17-mm line. For PI-RADS v2, a new diagram has been drawn based on the earlier work, which now incorporates the central zone (Fig. 6), but includes only alphabetical abbreviations in contradistinction to the alphanumeric labels of the earlier schemes.
Figure 6.

Sector maps for division of prostatic regions. A PI-RADS v2 schema incorporating the central zone and utilizing alphabetic abbreviations; PZ, peripheral zone; TZ, transition zone; a, anterior; p, posterior; pm, posteromedial; pl, postero-lateral; AS, anterior stroma. Used with Permission David Rini ©, Johns Hopkins University.
Conclusions
PI-RADS v2 offers a comprehensive overview of the role of mpMRI for the initial assessment of prostate cancer (Table 4). The diagnostic work-up and treatment of prostate cancer continues to progress rapidly, and the nature of PI-RADS v2 as a “living” online document will allow it to adapt and match this clinical evolution. It is now the task of the imaging community to test the validity of PI-RADS v2, including its sensitivity and specificity for clinically significant disease, its reproducibility among readers with different experience levels and between centres, and its role in active surveillance decisions. As a living document, there is no doubt that v3 is already in its earliest stages.
Table 4.
Summary of key differences proposed in PIRADS-v2.
| • Format and scope |
| − Intended as a “living” web-based document that will evolve with clinical practice |
| − Explicitly only for initial MRI examination, not for follow-up in active surveillance or assessment post-treatment |
| − Only proposes minimal technical parameters for an acceptable mpMRI examination, with the previous “optimal” parameters omitted |
| • MRI acquisition |
| − Spectroscopy is no longer used for PI-RADS assessment |
| − No delay in MRI post-biopsy if the primary purpose is to detect and characterise “clinically significant” cancer |
| − Anti-peristaltic medications recommended but no longer considered essential |
| − Single MRI protocol recommended, in contrast to original “detection”, “staging” and “node and bone” protocols |
| • Interpretation |
| − PI-RADS v2 score is based solely on mpMRI findings and does not take into account clinical factors |
| − Typically an overall PI-RADS score of 4 or 5 means a biopsy should be considered in this region, but the score does not explicitly state recommendations for management |
| − DWI is the dominant sequence for interpretation in the PZ, T2WI predominates for assessment of the TZ |
| − The secondary sequences are DCE in the PZ and DWI in the TZ. If the primary sequence has a PI-RADS score of 3, the secondary sequence may change the category to 4. |
| − For category 5 assessment in both the transition zone and peripheral zone, lesions must be >1.5 cm in size or demonstrate extraprostatic or invasive changes |
| − ADC maps should be consistently viewed with the same contrast windowing. Absolute ADC values should be interpreted with caution. |
| − For DCE-MRI interpretation, the presence of focal enhancement is important rather than the type of curve |
| − T2WI remains key for the purposes of staging |
| • Reporting |
| − Structured reports are recommended. Examples are yet to be provided |
| − Lesions with an overall PI-RADS score ≥3 should be reported, up to a maximum of 4 lesions, with the index lesion identified |
| − The maximal axial dimensions of a lesion should be reported using the ADC map as reference for PZ lesions and T2WI for TZ lesions |
| − Sector maps consist of 36 prostatic regions, compared to 16 (minimal) and 27 (optimal) in v1. The gland is again divided into anterior and posterior by the “17-mm line”, based on the likely reach of a 20 mm transrectal biopsy needle. |
| − The new schema incorporates the central zone and includes only alphabetic rather than alphanumeric abbreviations |
PI-RADS v2, Prostate Imaging—Reporting and Data System version 2; MRI, magnetic resonance imaging; mp, multiparametric; PZ, peripheral zone; TZ, transition zone; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; DCE dynamic contrast-enhanced; ADC, apparent diffusion coefficient.
Acknowledgments
☆ Author TB is supported by the Cancer Research UK and Engineering and Physical Sciences Research Council Imaging Centre in Cambridge and Manchester and the NIHR Biomedical Research Centre.
References
- 1.Maddams J, Utley M, Moller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer 2012;107:1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine MA, Ittman M, Melamed J, et al. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol 1998;159:471–5. [DOI] [PubMed] [Google Scholar]
- 3.Kvåle R, Møller B, Wahlqvist R, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int 2009;103:1647–54. [DOI] [PubMed] [Google Scholar]
- 4.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multi- parametric magnetic resonance imaging. J Urol 2011;186:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013;63:125–40. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Prostate cancer: diagnosis and treatment. Clinical guideline 175 (Update of clinical guideline 58). 2014. Available at: http://www.nice.org.uk/guidance/cg175/evidence/cg175-prostate-cancer-full-guideline3 [accessed 14.04.15].
- 7.Ahmed HU, Kirkham A, Arya M, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol 2009;6:197–206. [DOI] [PubMed] [Google Scholar]
- 8.National Prostate Cancer Audit First Year Annual Report — Organisation of Services and Analysis of Existing Clinical Data. London: The Royal College of Surgeons of England; 2014. Available at: http://www.npca.org.uk/wp-content/uploads/2014/11/NPCA-Annual-Report-FINAL-10_11_14.pdf [accessed 14.04.15].
- 9.Gaziev G, Wadhwa K, Barrett T, et al. Defining the learning curve for multi-parametric MRI of the prostate using MRI-TRUS fusion guided transperineal prostate biopsies as a validation tool. BJU Int 2014. August 7 10.1111/bju.12892 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Akin O, Riedl CC, Ishill NM, et al. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol 2010;20:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkham AP, Haslam P, Keanie JY, et al. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin Radiol 2013;68:1016–23. [DOI] [PubMed] [Google Scholar]
- 12.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACR, ESUR, AdMeTech Foundation. Prostate Imaging and Reporting and Data System: Version 2. 2014. Available at: 14/4/2015, http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf [accessed 14.04.15].
- 14.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkbey B, Mani H, Aras O, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 2013;268:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bott SR, Ahmed HU, Hindley RG, et al. The index lesion and focal therapy: an analysis of the pathological characteristics of prostate cancer. BJU Int 2010;106:1607–11. [DOI] [PubMed] [Google Scholar]
- 17.Barrett T, Vargas HA, Akin O, et al. Value of the hemorrhage exclusion sign on T1-weighted prostate MR images for the detection of prostate cancer. Radiology 2012;263:751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turkbey B, Merino MJ, Gallardo EC, et al. Comparison of endorectal coil and nonendorectal coil T2W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging 2014;39:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence EM, Tang SY, Barrett T, et al. Prostate cancer: performance characteristics of combined TZW and DW-MRI scoring in the setting of template transperineal re-biopsy using MR-TRUS fusion. Eur Radiol 2014;24:1497–505. [DOI] [PubMed] [Google Scholar]
- 21.Renard-Penna R, Mozer P, Cornud F, et al. Prostate Imaging Reporting And Data System and Likert scoring system: multiparametric MR imaging validation study to screen patients for initial biopsy. Radiology 2015;275:458–68. [DOI] [PubMed] [Google Scholar]
- 22.Vaché T, Bratan F, Mege-Lechevallier F, et al. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology 2014;272:446–55. [DOI] [PubMed] [Google Scholar]
- 23.Renard-Penna R, Mozer P, Cornud F, et al. Prostate Imaging Reporting and Data System and Likert scoring system: multiparametric MR imaging validation study to screen patients for initial biopsy. Radiology 2015;275:458–68. [DOI] [PubMed] [Google Scholar]
- 24.Kuru TH, Roethke MC, Rieker P, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int 2013;112:1080–7. [DOI] [PubMed] [Google Scholar]
- 25.Muller BG, Shih JH, Sankineni S, et al. Inter-observer agreement and accuracy of the revised Prostate Imaging — Reporting and Data System (PI-RADS) for interpreting multiparametric MRI of the prostate. Radiology 2015. June 18:142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenkrantz AB, Taneja SS. Radiologist, be aware: ten pitfalls that confound the interpretation of multiparametric prostate MRI. AJR Am J Roentgenol 2014;202:109–20. [DOI] [PubMed] [Google Scholar]
- 27.Akin O, Sala E, Moskowitz CS, et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology 2006;239:784–92. [DOI] [PubMed] [Google Scholar]
- 28.Oto A, Kayhan A, Jiang Y, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion- weighted and dynamic contrast-enhanced MR imaging. Radiology 2010;257:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thörmer G, Otto J, Reiss-Zimmermann M, et al. Diagnostic value of ADC in patients with prostate cancer: influence of the choice of b values. Eur Radiol 2012;22:1820–8. [DOI] [PubMed] [Google Scholar]
- 30.Litjens GJ, Hambrock T, Hulsbergen-van de Kaa C, et al. Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology 2012;265:260–6. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki M, Yamada K, Watanabe Y, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology 2008;249:624–30. [DOI] [PubMed] [Google Scholar]
- 32.Iwazawa J, Mitani T, Sassa S, et al. Prostate cancer detection with MRI: is dynamic contrast-enhanced imaging necessary in addition to diffusion- weighted imaging? Diagn Interv Radiol 2011;17:243–8. [DOI] [PubMed] [Google Scholar]
- 33.Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 1999;211:101–10. [DOI] [PubMed] [Google Scholar]
- 34.Ren J, Huan Y, Wang H, et al. Dynamic contrast-enhanced MRI of benign prostatic hyperplasia and prostatic carcinoma: correlation with angio-genesis. Clin Radiol 2008;63:153–9. [DOI] [PubMed] [Google Scholar]
- 35.Hansford BG, Peng Y, Jiang Y, et al. Dynamic contrast-enhanced MR imaging curve-type analysis: is it helpful in the differentiation of prostate cancer from healthy peripheral zone? Radiology 2015;275:448–57. [DOI] [PubMed] [Google Scholar]
- 36.Haider MA, Chung P, Sweet J, et al. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:425–30. [DOI] [PubMed] [Google Scholar]
- 37.Barrett T, Davidson SR, Wilson BC, et al. Dynamic contrast enhanced MRI as a predictor of vascular-targeted photodynamic focal ablation therapy outcome in prostate cancer post-failed external beam radiation therapy. Can Urol Assoc J 2014;8:E708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouviere O, Girouin N, Glas L, et al. Prostate cancer transrectal HIFU ablation: detection of local recurrences using T2-weighted and dynamic contrast-enhanced MRI. Eur Radiol 2010;20:48–55. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence EM, Gallagher FA, Barrett T, et al. Preoperative 3-T diffusion-weighted MRI for the qualitative and quantitative assessment of extracapsular extension in patients with intermediate- or high-risk prostate cancer. AJR Am J Roentgenol 2014;203:W280–6. [DOI] [PubMed] [Google Scholar]
- 40.Vlatkovic L, Svindland A, Eggesb0 HB, et al. Predictive value of magnetic resonance imaging determined tumor contact length for extracapsular extension of prostate cancer. J Urol 2015;193:466–72. [DOI] [PubMed] [Google Scholar]
- 41.Ohori M, Scardino PT, Lapin SL, et al. The mechanisms and prognostic significance of seminal vesicle involvement by prostate cancer. Am J Surg Pathol 1993;17:1252–61. [DOI] [PubMed] [Google Scholar]
- 42.Roethke M, Kaufmann S, Kniess M, et al. Seminal vesicle invasion: accuracy and analysis of infiltration patterns with high-spatial resolution T2-weighted sequences on endorectal magnetic resonance imaging. Urol Int 2014;92:294–9. [DOI] [PubMed] [Google Scholar]
- 43.Berney DM, Wheeler TM, Grignon DJ, et al. , ISUP Prostate Cancer Group. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 4: seminal vesicles and lymph nodes. Mod Pathol 2011;24:39–47. [DOI] [PubMed] [Google Scholar]
- 44.Glazer GM, Orringer MB, Chenevert TL, et al. Mediastinal lymph nodes: relaxation time/pathologic correlation and implications in staging of lung cancer with MR imaging. Radiology 1988;168:429–31. [DOI] [PubMed] [Google Scholar]
- 45.Qayyum A Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. RadioGraphics 2009;29:1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahn CE Jr, Langlotz CP, Burnside ES, et al. Toward best practices in radiology reporting. Radiology 2009;252:852–6. [DOI] [PubMed] [Google Scholar]
- 47.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European Consensus Meeting. Eur Urol 2011;59:477–94. [DOI] [PubMed] [Google Scholar]
- 48.Villers A, Lemaitre L, Haffner J, et al. Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: implications for focal therapy and active surveillance. Curr Opin Urol 2009;19:274–82. [DOI] [PubMed] [Google Scholar]
- 49.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int 2011. ;108:E171–8. [DOI] [PubMed] [Google Scholar]
- 50.Barentsz J, Villers A, Schouten M. ESUR prostate MR guidelines. Author reply. Eur Radiol 2013;23:2322–3. [DOI] [PubMed] [Google Scholar]


