Abstract
In a recent clinical trial, a patient exhibited regression of several pancreatic cancer metastases following the administration of the immune modulator Ipilimumab (anti-CTLA-4 antibody). We sought to characterize the immune cells responsible for this regression. Tumor infiltrating lymphocytes (TIL-2742) and an autologous tumor line (TC-2742) were expanded from a regressing metastatic lesion excised from this patient. Natural killer (NK) cells predominated in the TIL (92% CD56+) with few T cells (12% CD3+). A majority (88%) of the NK cells were CD56brightCD16−. TIL-2742 secreted IFN-γ and GM-CSF following co-culture with TC-2742 and major histocompatibility complex mismatched pancreatic tumor lines. After sorting TIL-2742, the purified CD56+CD16−CD3− subset showed reactivity similar to TIL-2742 while the CD56−CD16−CD3+ cells exhibited no tumor recognition. In co-culture assays, TIL-2742 and the NK subset expressed high reactivity to several pancreatic and prostate cancer cell lines and could lyse the autologous tumor as well as pancreas and prostate cancer lines. Reactivity was partially abrogated by blockade of TRAIL. We thus identified a unique subset of NK cells (CD56brightCD16dim) isolated from a regressing metastatic pancreatic cancer in a patient responding to Ipilimumab. This represents the first report of CD56+CD16− NK cells with apparent specificity for pancreatic and prostate cancer cell lines and associated with tumor regression following the treatment with an immune modulating agent.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0897-y) contains supplementary material, which is available to authorized users.
Keywords: Natural killer cells, Pancreatic cancer, Immunotherapy, Anti-CTLA-4
Introduction
Metastatic pancreatic cancer remains to be one of the most lethal malignancies with an estimated median survival of <6 months. Although patients with metastases from other solid organ malignancies, such as colon and breast cancer have seen improvements in outcomes with the advent of new chemotherapeutic agents; there has been not much increase in survival for patients with pancreatic cancer. New strategies are needed to combat this aggressive disease.
Immunotherapy is being actively studied as a cancer treatment. Non-specific stimulators, such as interleukin 2 (IL-2) can produce durable tumor regressions in patients with metastatic renal cell cancer and melanoma [1, 2]. Other strategies involve manipulation of the adaptive immune system by activating antigen-specific T lymphocytes in vivo with vaccines [3], or expanding cells ex vivo as in adoptive cell transfer (ACT) [4]. Although more recent trials have used T lymphocytes as the antitumor immune effector, past research focused on activated natural killer (NK) cells.
NK cells are immune cells that exhibit cytotoxicity without the need for prior sensitisation. They compose 5–15% of human peripheral blood lymphocytes, and are defined by their abundant granular cytoplasm and surface expression of the neural cell adhesion molecule like marker CD56. Culture of resting NK cells with IL-2 results in activation and allows lysis of tumor lines resistant to resting NK cells [5, 6]. Recent studies have identified two unique subsets of NK cells distinguishable by the density of CD56 on the cell surface as well as the presence or absence of the cell surface marker CD16 [7, 8]. Although a majority of NK (~90%) cells have relatively low density CD56 and abundant CD16 (CD56+CD16+), a smaller subset (~10%) is CD56brightCD16dim/negative. Along with differences in surface markers, these two subsets function differently. CD56+CD16+ behave like traditional NK cells efficiently lysing a wide variety of tumor lines with negligible cytokine production. CD56+CD16− cells are less cytolytic, but are capable of secreting cytokines, such as interferon-γ (IFN-γ), IL-2, IL-10, IL-12 and granulocyte macrophage colony stimulating factor (GM-CSF) which may indicate that they function as intermediaries between the innate and adaptive immune systems [8]. Although the recognition and lysis of tumor targets by NK cells depends on a delicate balance between activating and inhibitory receptors, the induction of cytokine secretion appears less specific and dependent primarily on the presence of cytokines.
In this study, we report the identification and characterization of a unique immune cell grown from a regressing metastatic lesion of a patient with pancreatic adenocarcinoma following the treatment with the immune modulatory antibody anti-CTLA-4. Analysis of cell surface markers identified these cells as CD56brightCD16− NK cells which possess very few inhibitory receptors and overexpress the natural cytotoxicity receptor (NCR) p46. When cultured in high dose IL-2, these cells were capable of lysing all tested tumor targets and secreted IFN-γ to an autologous tumor line. Following co-culture with a panel of pancreatic and other solid organ neoplastic cell lines, cytokine secretion appeared specific for pancreatic and prostate cancers. Although a source of the apparent specificity could not be definitively identified, the reactivity was partially blocked by an antibody to tumor necrosis factor-related apoptosis inducing ligand (TRAIL). This represents the first report of CD56+CD16− NK cells with apparent specificity for pancreatic and prostate cancer cell lines and associated with tumor regression following treatment with an immune modulating agent.
Materials and methods
TIL-2742 and TC-2742
TIL-2742 was established from the resected gastrohepatic ligament lymph node (Fig. 1a supplemental) of a patient with stage IV pancreatic cancer following treatment with Ipilimumab, a monoclonal antibody against CTLA-4, as previously described [9]. In brief, the surgical specimen was taken from the operating room to a dedicated laboratory, where the lesion was dissected free of surrounding fat and necrosis and divided into 16 fragments. After mechanical disruption with surgical blades, a single cell suspension was achieved by overnight enzymatic digestion. Fragments composed of tumor cells and lymphocytes were placed separately into culture with AIM-V (Invitrogen, Inc. Rockville, MD) media supplemented with 5% human ab serum (Valley Biomedical, Winchester, VA) and 6,000 international units (IU) of IL-2 (Chiron Corp., Emmerville, CA) for 14 days and then evaluated for lymphocyte growth. Growth positive wells were frozen for later analysis.
TC-2742 was established from the same lesion by serial passage of adherent cells in RPMI media (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT) and 7.2 μg/ml of recombinant human insulin (Invitrogen). Cytopathologic analysis was performed to confirm that the established line was pancreatic adenocarcinoma (Fig. 1b supplemental).
Cell lines
Pancreatic cancer (Capan-1, MIA Paca-2, Panc 08.13, HPAF-II, SU86.86), prostate cancer (DU-145, PC3, LNCaP), colon cancer (WiColo, SN486), ovarian cancer (SK-OV3, NIH-OVCAR-3), glioma (SNB-75, U571), breast cancer (SKBR3, MDA-MB-468), lung cancer (NCI-H1299, Calu-6) and melanoma (526mel, 624mel, 888mel, 938mel, SK-23, 568mel, 1359mel, 836mel, 1300mel) tumor lines were established at the National Cancer Institute (NCI), Bethesda, MD, or acquired from ATCC (Rockville, MD) and maintained in RPMI with 10% FBS. Renal cell cancer lines were established in the NCI Surgery Branch as previously described and cultured in DMEM (Invitrogen) with 10% FBS. Peripheral blood mononuclear cells (PBMC) and PBL were acquired via leukapheresis of patients on trials at the NCI and maintained in AIM-V with 300 IU/ml IL-2.
Peripheral NK (pNK) cells
Whole PBMC were acquired via leukapheresis and CD3+ cells removed using CD3+-conjugated magnetic bead separation per the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). The remaining cells were expanded in AIM-V with 10% human AB serum, 600 IU IL-2 and 30 ng/ml OKT-3 (Ortho-McNeil, Titusville, NJ). Feeder cells consisting of irradiated (4,000 gy) PBMC were added at a 10:1 ratio. After 14–21 days, the cells were harvested and FACS analysis performed to confirm NK phenotype. Cells were rested for at least 2 days in AIM-V with 300 IU IL-2 prior to use.
FACS analysis
Protein cell-surface expression was assessed using fluorochrome-conjugated antibodies against HLA-A2, CD3, CD8, CD56, CD16, NKG2D, CD94, p46, CD158a, CD158b, NKB1 and TRAIL (Becton–Dickinson). Isotype controls included PE, FITC and APC conjugated anti-human IgG (Becton–Dickinson). Cells were incubated for 40 min at 4° with the staining antibodies and immunofluorescence was measured as relative log fluorescence of live cells using a FACScan flow cytometer (Becton–Dickinson).
In vitro stimulation assays
To assess for the reactivity of various TIL-2742 fragments, an overnight co-culture with autologous tumor line was performed. Because both the primary tumor as well as the established line had lost HLA-A2 expression, TC-2742 was transduced with a retroviral vector encoding this protein using a previously described transduction protocol (TC-2742-A2) [10]. After trypsinization, 1 × 105 tumor targets were co-cultured with 1 × 105 TIL cells. After 20 h, supernatants were harvested and tested for IFN-γ or GM-CSF levels using a colorimetric ELISA kit per the manufacturer’s protocol (Endogen, Cambridge, MA).
Cell lysis assay
Target cell lysis was assessed by standard 4-h 51Cr release assay as previously described [11]. In brief, tumor targets were cultured with 200 μCi of 51Cr for 2 h at 37°. After thorough washing, the labeled cells were plated at a concentration of 2.5 × 103 cells per well in a 96-well round bottom plate (Becton–Dickinson biosciences, San Jose, CA). Effector cells were prepared and added to the wells at various E:T ratios. After 4 h in culture, the supernatants were harvested and counted using a Wallace 1470 Wizard automatic gamma counter (Perkin Elmer, Waltham, MA). Minimum and maximum release was determined by culture of labeled cells with media and 2% SDS, respectively. Values were recorded as percent lysis using the formula (experimental cpm − spontaneous cpm)/(maximum cpm − minimum cpm)× 100.
Intracellular IFN-γ staining
To evaluate IFN-γ production by immune subsets, TIL-2742 cells were cultured with various targets in the presence of protein transport inhibitor (GolgiStop, Becton–Dickinson) for 5 h. After washing, cell surface markers were stained as above. Cells were then fixed and permeabilized using BD Cytofix/Cytoperm kit. The presence of IFN-γ within the cells was determined using a FITC-conjugated anti-IFN-γ antibody (Becton–Dickinson).
Results
Phenotypic characterization of TIL-2742
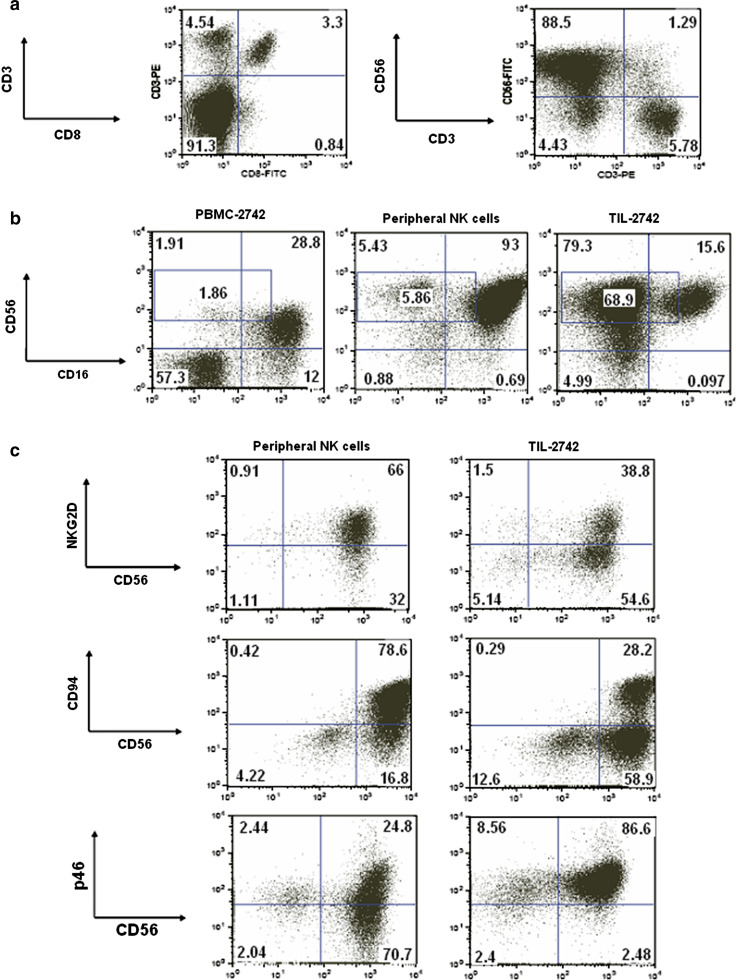
Immune cells were grown from fragments of a metastatic tumor which regressed following systemic treatment with anti-CTLA-4 (Fig. 1a supplemental). Immunohistochemical staining of this metastasis showed a predominant CD3+ infiltrate with even distribution of CD4 and CD8 lymphocyte subsets. CD56 cells were a minority in the tumor infiltrate. Of the 16 fragments put into culture, 13 resulted in successful growth and were available for further analysis. After 14 days in lymphocyte growth media, there were no visible tumor cells present. FACS analysis was used to further characterize the phenotype of TIL-2742. A majority of cells in TIL-2742 (92%) were CD3− with approximately 50% of CD3+ cells also expressing CD8. Staining for NK cell markers revealed 90% of cells to be CD56+, with 1.3% CD56+CD3+ (Fig. 1a). CD16 staining revealed 84% of the CD56+ cells from TIL-2742 to be CD56+CD16−. This was quite different from CD3 negatively gated PBMC from the same patient as well as enriched ex vivo activated NK cells in which CD56+CD16− cells comprise only 6 and 5.5% respectively (Fig. 1b). The density of the CD56 on the cell surface appeared higher on the activated NK cells and TIL-2742 as compared to resting NK cells from PBMC-2742.
Fig. 1.
TIL-2742 is composed of a CD56brightCD16− NK cell subset with high expression of the natural cytotoxicity receptor p46. a FACS analysis using fluorochrome-conjugated antibodies to CD3, CD8 and CD56 revealed TIL 2742 to be composed of CD56+CD3− NK cells. b Further characterization using FITC-labeled anti-CD16 identified 68.9% of these cells as CD56brightCD16−. Included for comparison was CD3−-gated PBMC from the same patient as well as peripheral NK (pNK) cells which were stimulated and grown under identical conditions as TIL-2742. c The expression of NKG2D and CD94 appeared lower on TIL-2742 than activated pNK cells. p46, however, appeared to be more abundant
TIL-2742 recognizes the autologous pancreatic cell line TC-2742 with and without HLA-A2
To test for autologous reactivity, a tumor cell line was created using a fragment from the same lesion. After serial passage in insulin containing media, TC-2742 tumor line was established and cells were sent for cytopathologic analysis of the pancreatic cancer markers CA 19-9, CEA and CK-7. Staining was also performed to assess major histocompatibility complex (MHC) class I and HLA-A2 expression (Fig. 1b supplemental). Comparison of the cell line with the original tumor pathology determined the staining pattern [CA 19-9(+), CEA(+) and CK7(+) and MHC class I(+), HLA-A2(−)] to be identical.
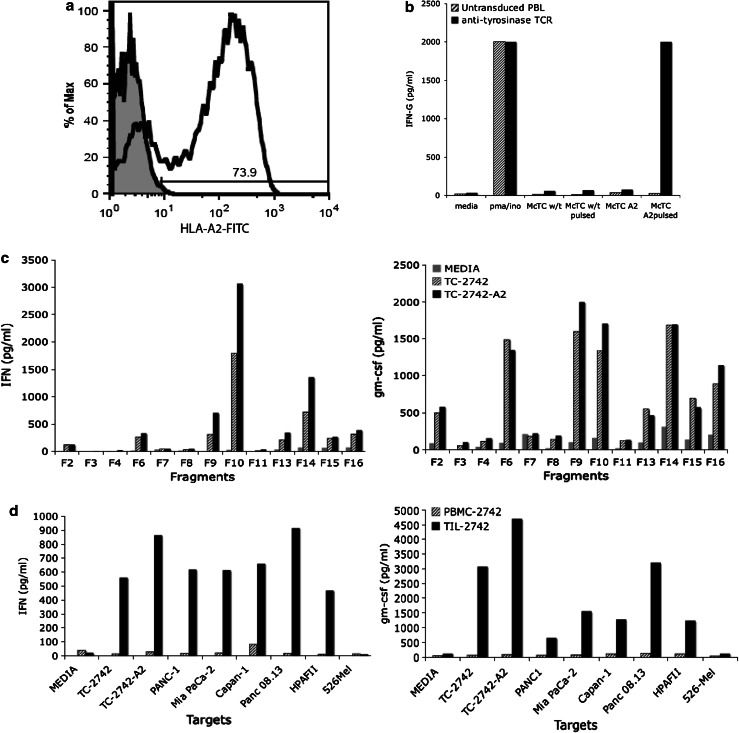
Although blood analysis revealed the treated patient was HLA-A2-positive, cytopathologic analysis revealed the tumor had lost expression. TC-2742 was retrovirally transduced with a plasmid encoding the HLA-A2 protein. Surface expression was confirmed by FACS analysis with 73.9% of cells staining positive (Fig. 2a). To assess the ability of the newly introduced HLA-A2 to present peptide, TC-2742-A2 and TC-2742 were pulsed with 1 μg of tyrosinase:368-76 peptide for 2 h. Following co-culture with effector cells bearing TCRs specific for this HLA-A2 presented peptide, reactivity was seen against TC-2742-A2, but not TC-2742 (Fig. 2b).
Fig. 2.
TIL-2742 is reactive against the autologous tumor line TC-2742 and a panel of pancreatic tumor lines irrespective of HLA type. a To ensure HLA-A2 reactivity was not missed, TC-2742 was retrovirally transduced with a plasmid encoding the HLA-A2 protein. The expression was confirmed using FITC-labeled antibody for the protein. b The ability of T cells to recognize the introduced HLA-A2 was confirmed by pulsing the transduced line with tyrosinase peptide followed by co-culture with T cells bearing an anti-tyrosinase TCR. Reactivity was seen against the transduced line, but not the wild-type TC-2742. c Immune cells grown from various fragments of the resected tumor were co-cultured with both TC-2742 and TC-2742-A2. After 20 h, supernatants were harvested and assayed for both IFN-γ and GM-CSF revealing reactivity from 8/13 fragments. d A co-culture of TIL-2742 with a panel of pancreatic tumor lines representing various HLA types and revealed reactivity against all tested pancreatic cancer lines, but not the melanoma line 526mel
Reactivity of TIL-2742 fragments was assessed by overnight co-culture with TC-2742 and TC-2742-A2. Abundant IFN-γ and GM-CSF release was identified from fragments 2, 6, 9, 10, 13, 14, 15, 16 (Fig. 2c). These fragments were used for further analysis.
TIL-2742 recognizes multiple pancreatic cancer lines irrespective of HLA type
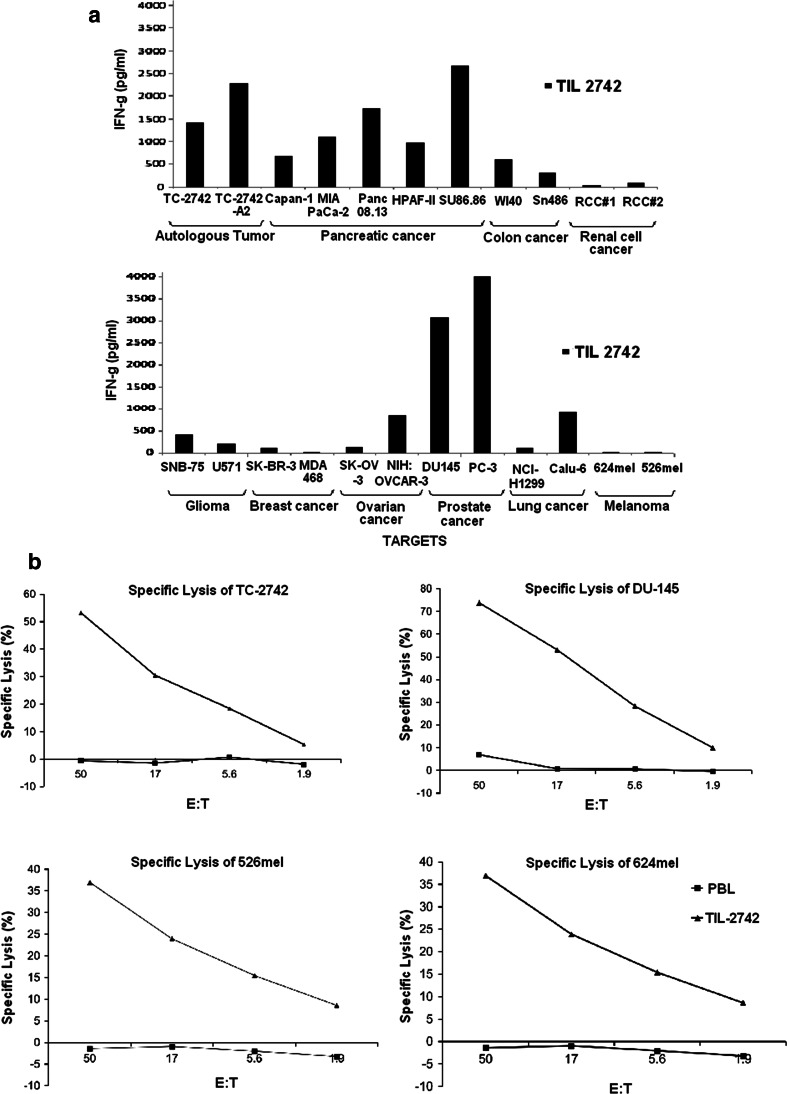
A panel of pancreatic cell lines were established which represented the various HLA-A, B, C subtypes of TIL-2742. Co-culture revealed reactivity against the five tested pancreatic cell lines irrespective of HLA type, but not against a melanoma line (526mel) (Fig. 2d). To further test the reactivity of TIL-2742, co-culture was performed with tumor lines from other solid organ malignancies, including colon, renal cell, glioma, breast, ovarian, prostate, lung and melanoma (Fig. 3a). In addition, strong reactivity was seen against the pancreatic lines (TC-2742, Capan-1, MIA Paca-2, Panc 08.13, HPAF-II and SU.86.86). No reactivity was seen against the renal cell cancer lines (RCC#1 and RCC#2), breast cancer lines (SK-BR-3 and MDA-MB-468) and melanoma lines (624mel and 526mel). Minimal reactivity was seen against the glioma lines (SNB-75 and U571) and the ovarian cancer line NIH-OVCAR-3 and the lung cancer line Calu-6. Strong reactivity similar to that seen against the pancreatic lines was seen against the prostate cancer lines DU-145 and PC-3. A broader panel of negative lines was tested to confirm the apparent specificity of TIL-2742 for pancreatic and prostate cancers (Table 1 supplemental) including 9 melanoma and 4 renal cell cancer lines.
Fig. 3.
TIL-2742 demonstrated reactivity against pancreatic and prostate cancer lines, but not others. a Overnight co-culture of pancreatic, colon, renal cell, glioma, breast, ovarian, prostate, lung cancer and melanoma cell lines with TIL-2742 revealed reactivity against the pancreatic and prostate lines, but not melanoma, renal cell or breast cancer. Marginal reactivity was seen against colon, glioma, ovarian and lung cancer lines. b 51Cr release assay demonstrated that unlike the cytokine release, the cytolytic ability of TIL-2742 was not specific and all assayed tumor lines were lysed
To evaluate the cytolytic ability of TIL-2742, 51Cr release assay was performed using tumor lines that both induced (TC-2742 and DU-145) and failed to induce cytokine production (526mel and 624mel). TIL-2742 was able to lyse all evaluated cell lines although the average percent specific lysis was slightly higher in the lines that elicited cytokine release (62 vs. 37%) (Fig. 3b). Donor PBL was used a negative control and no lysis was seen.
CD56+CD16− cells from TIL-2742 are responsible for the specific cytokine release
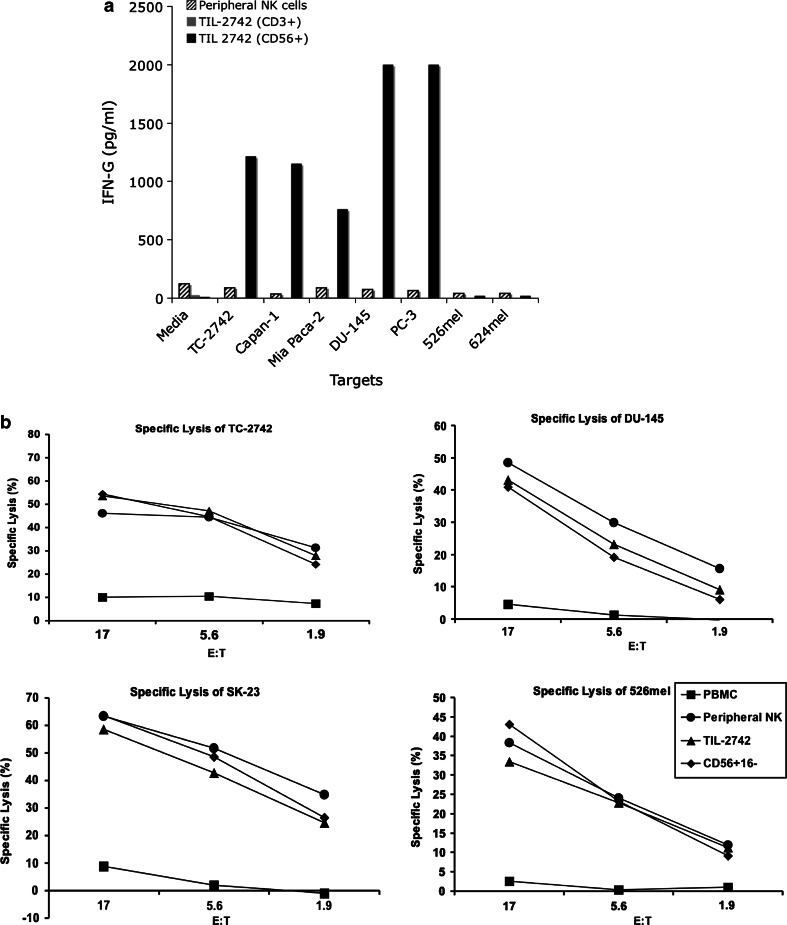
To confirm the cell type responsible for the specific release of cytokines, CD56+CD16− cells from TIL-2742 were separated by negative selection using magnetic beads (CD3+ and CD16+). Co-culture with cell lines which were (TC-2742, Capan-1, MIA Paca-2, DU-145 and PC-3) and were not (526mel, 624mel) able to elicit cytokine release confirmed both the reactivity and specificity of this subset (Fig. 4a). pNK cells which were grown in a similar fashion to TIL-2742 showed no cytokine secretion. When lytic ability was compared, TIL-2742 lysed all evaluated cell lines similar to activated pNK cells (Fig. 4b). To assess the contribution of CD16+ versus CD16− cells to the observed cytokine release, intracellular staining for IFN-γ was used. Following 5-h co-culture with TC-2742, 3% of CD56+ cells stained positive for IFN-γ as compared to 0.6% after stimulation with 526mel. There was only background staining of CD56− cells following the same co-culture. Cells were then gated on CD56+ and stained for CD16. After stimulation with TC-2742, 3.73% of CD56+CD16− cells were positive for IFN-γ as compared to 0.3% of CD56+CD16+ cells (Fig. 5b).
Fig. 4.
Although both activated pNK cells and TIL-2742 can lyse tumor cell lines, cytokine release is restricted to the later. a pNK cells and TIL-2742 sorted into the CD3+/CD56− or CD3−/CD56+ fractions were co-cultured with pancreatic cancer and melanoma cell lines. IFN-γ release measured in the supernatant 20 h later revealed abundant cytokine production by TIL-2742 to pancreatic, but not melanoma cell lines, but none from activated pNK cells. b 4-h 51Cr release assay found near identical lytic ability of TIL-2742 and pNK cells against pancreatic (TC-2742), prostate (DU-145) and melanoma lines (SK-23 and 526mel). To confirm the cytolysis was not due to contamination by CD56+CD16+, TIL-2742 was purified using CD16 conjugated beads. There was no change in cytolysis following removal of these cells
Fig. 5.
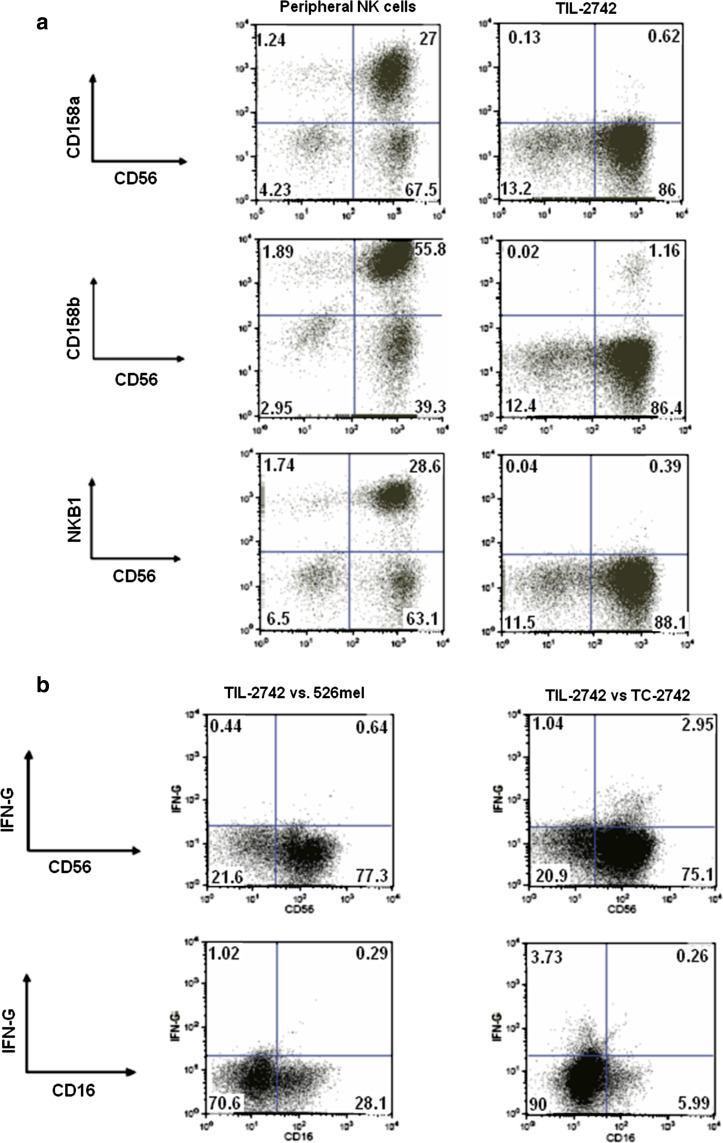
There is minimal KIR expression on TIL-2742. a FACS analysis found little expression of CD158a, CD158b and NKB1 on TIL-2742, as has been previously demonstrated. b TIL-2742 were co-cultured with TC-2742 and 526mel for 5 h and them fixed and permeabilized. Staining for NK cell markers and intracellular IFN-γ identified the reactive cells as the CD56brightCD16− NK subset
Activating and inhibitory NK cell receptor expression
Further FACS analysis was performed to quantify the activating and inhibitory receptors on CD56+ cells from TIL-2742. For comparison, peripheral NK (pNK) cells which were stimulated in a similar fashion were used. NKG2D was positive on 67% of pNK cells as compared to 41% on TIL-2742. Similarly, there was greater CD94 expression on pNK cells (82%) as compared to TIL-2742 (32%). p46 staining was nearly ubiquitous on TIL-2742 with 97% of cells staining positive (Fig. 1c). There were very few inhibitory receptors present on TIL-2742. Antibodies to CD158a, CD158b and NKB1 stained 0.7, 1.3 and 0.4, respectively. pNK cells had a higher proportion of cells that stained positive for these receptors (29, 59 and 31%, respectively) (Fig. 5a).
TIL-2742 activity is dependent on TRAIL
It has been suggested that TRAIL plays a role in NK cell function and tumor suppression [12, 13]. To evaluate the role of TRAIL in TIL-2742, CD56+ was analyzed for surface expression of TRAIL and identified 74.7% of cells staining positive (Fig. 6a). Co-culture of reactive tumor lines with TIL-2742 in the presence of anti-TRAIL antibody demonstrated a slight decrease in reactivity against TC-2742, but a dramatic (60%) drop in cytokine release against DU-145 (Fig. 6b). Similar results were seen with the blockade of the TRAIL dependent T cell clone 2G1 [14]. 51Cr release assay performed in the presence of anti-TRAIL antibody identified a similar decrease in cytolysis of both TIL-2742 and 2G1 (Fig. 6c). T cells transduced with a chimeric antigen receptor against erb2 [15] showed no abrogation of reactivity or lysis in the presence of anti-TRAIL antibody.
Fig. 6.
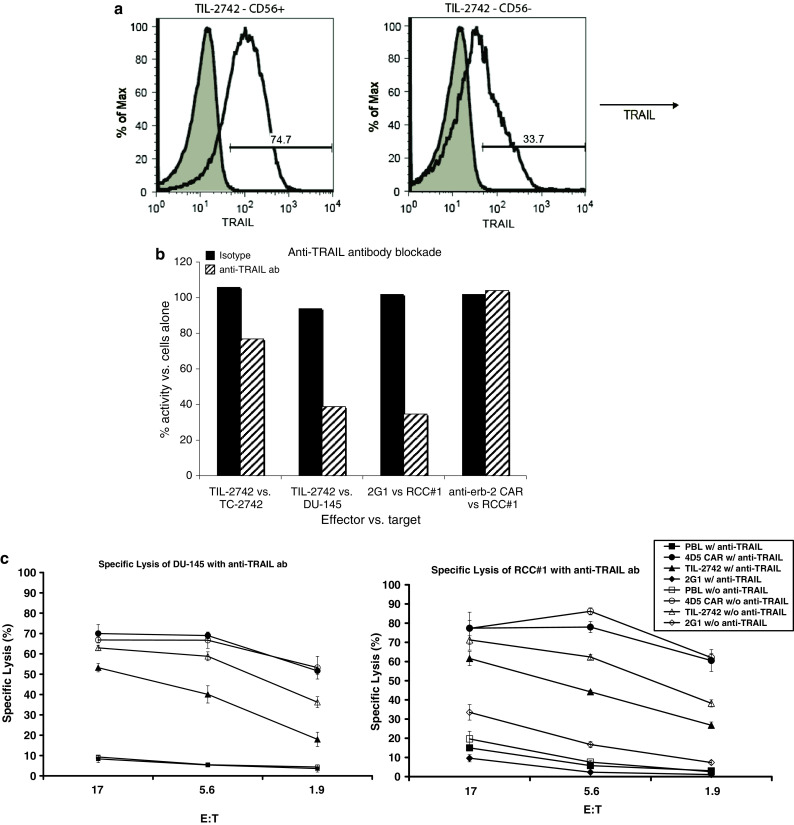
Function of TIL-2742 is modulated by TRAIL. a TRAIL expression on TIL-2742 was assessed using PE conjugated anti-TRAIL antibody. Displayed are the FACS analyses for the CD56+ and CD56− fractions. b TIL-2742 was incubated with TRAIL blocking antibody at 20 μg/ml for 1 h followed by co-culture with TC-2742 and DU-145. Experimental controls included clone 2G1, a T cell clone reactive against surface bound TRAIL [14] and PBL transduced with an HER-2 chimeric antigen receptor (CAR) which is TRAIL independent [15]. While there was no decrease in reactivity following TRAIL blockade of anti-erb2 CAR, there was significant abrogation of TIL-2742 activity against DU-145 and 2G1 against RCC#1. There was only a slight decrease in reactivity against TC-2742
Discussion
Pancreatic cancer remains the fourth leading cause of cancer mortality in the United States with an estimated 35,000 deaths in 2008. Even with rapid diagnosis, patients with early stage disease have a 5-year survival of only 20% following surgical resection. Unfortunately, most patients present with late stage, inoperable disease for which, few survive 1 year. Despite advances in chemotherapy and radiation, not much progress has been made in improving the survival of patients with metastatic pancreatic cancer.
Immunotherapy is based on recruiting the immune system to target and destroy cancer cells. This is done by either non-specific stimulation or targeting of specific populations through signaling molecules or antigen-specific T cell receptors. Clinical trials using IL-2 have shown durable regression of metastatic renal cell carcinoma and melanoma [1, 2]. Lymphodepletion followed by ACT of tumor infiltrating lymphocytes can mediate objective response rates of up to 70% in melanoma patients [16]. Because of its ability to cause dramatic and durable regression of aggressive malignancies, immunotherapy is potentially an attractive strategy for treating metastatic pancreatic cancer.
Several trials have attempted to harness the immune system to target pancreatic cancer. Jaffe et al. [17] reported on the adjuvant administration of irradiated tumor cells genetically modified to secrete GM-CSF. Although tumor regression could not be evaluated, delayed type hypersensitivity (DTH) to re-challenge with tumor cells as well as the development of mesothelin specific T cells was seen. In a study by Hirooka et al. [18], gemcitabine was administered to patients with locally advanced pancreatic cancer followed by intratumoral injection of dendritic cells pulsed with OK-432, a non-specific immune stimulator. Although only five patients were treated, one patient experienced a partial response.
A phase II trial performed at the National Cancer Institute, evaluated the use of Ipilimumab, a monoclonal antibody against the T cell inhibitory molecule CTLA-4, as a single agent for patients with locally advanced or metastatic pancreatic cancer. The goal of the study was to disrupt the immune suppressive signals on cells in the tumor microenvironment [19, 20]. Of the 27 patients treated, there were no objective responders by RECIST criteria although one patient showed a dramatic regression of multiple lesions. This subject was a 67-year-old woman who had been diagnosed with metastatic pancreatic cancer 8 months prior to enrollment. She was symptomatic with a low performance status and a 5.5 cm pancreatic primary and 16 hepatic metastases. After two courses of Ipilimumab, she had a 90% reduction in her tumor volume with complete resolution of multiple hepatic metastases. These radiographic findings coincided with a dramatic fall in her serum tumor markers (CA-19.9, CEA and β-HCG) and a return to baseline performance status. Following the removal of metastatic lesions, an effort was undertaken to identify the immune cells associated with her dramatic regression to anti-CTLA4 antibody administration.
A 2-cm lymph node, which had decreased in size from six centimeters after treatment with anti-CTLA-4, was removed from the gastrohepatic ligament (Fig. 1a supplemental). Tumor infiltrating lymphocytes were grown from the lesion, as well as a tumor cell line. Analysis of the tumor cell line revealed that although MHC class I expression was present, there had been selective loss of HLA-A2, a phenomena which has been previously described [20, 21]. A new line was created by retrovirally transducing the parent line with HLA-A2 to establish TC-2742-A2 (Fig. 2a, b).
FACS analysis using antibodies against surface markers specific for different immune cells revealed TIL-2742 was composed of NK cells. Further staining identified these NK cells as a unique subset with high surface density of CD56 and low or absent CD16. When the tumor infiltrating immune cells were subsequently co-cultured with the autologous lines, reactivity was demonstrated by the release of the cytokines IFN-γ and GM-CSF. Further investigation revealed that these cells were also reactive against a broad panel of pancreatic tumor lines irrespective of HLA antigen expression. Co-culture with a broader panel of tumor lines representing multiple solid organ malignancies revealed this reactivity to be specific for pancreatic and prostate cancers.
First described by Lanier et al. [22], CD56brightCD16− cells compose 10% of circulating NK cells and 1% of peripheral blood lymphocytes. They are generally felt to be less cytolytic than the more common CD56+CD16+ NK cells, but upon activation with IL-2 can lyse even traditionally NK-resistant tumor lines [23]. The most widely studied functional difference between NK cell subsets is the unique ability of CD56brightCD16− NK cells to secrete cytokines upon stimulation. Cooper et al. [8] demonstrated robust release of cytokines IFN-γ, TNF-β and GM-CSF following culture of various monokines with CD56brightCD16−, but not CD56+CD16+ NK cells. Although other studies [24, 25] have shown similar results using non-specific stimulators, there have been no reports of specific inducers of cytokine release.
While the CD56brightCD16− NK cells of TIL-2742 were able to produce cytokines following non-specific activation with PMA-ionomycin (data not shown), they also released abundant IFN-γ and GM-CSF following co-culture with pancreatic and prostate cell lines. Intracellular IFN-γ staining confirmed the cytokine release from TIL-2742 was from the CD56brightCD16− NK cells. This specificity may imply the TIL-2742 reactivity was not random, but relied on the interaction between cell surface molecules that were present only on certain tumor types. Although the ability to induce cytokine secretion appears specific, TIL-2742 was able to lyse all evaluated cell lines in a pattern similar to traditional CD56+CD16+ NK cells.
To further investigate molecules responsible for specific cytokine release, we first looked at cell surface expression of known activating and inhibitory NK cell receptors. The c-type lectin receptor family is composed of various NKG2 molecules covalently bound to a common CD94 subunit. FACS analysis of CD94 expression on TIL-2742 demonstrated lower surface expression of the NKG2 when compared with peripheral NK cells stimulated under similar conditions (Fig. 1c). This along with confirmed expression of MHC class I on the tumor cells implied this class of receptors was likely not involved in the observed immune response.
NKG2D is the best described activating c-type lectin receptor and has been implicated as an important mediator of anti-tumor immunity [26]. Its principle ligands MHC class I chain-related (MIC) peptides and UL16-binding proteins (ULBPs) are rarely present on normal cells, but often found on malignant lesions and tumor cell lines [26–28]. As with the other c-type lectin receptors, there was less cell surface expression of NKG2D on TIL-2742 and expression of MICa/b and ULBPs on tumor targets did not correlate with cytokine release (data not shown).
A second family of receptors, the killer immunoglobulin-like receptors (KIR), is the primary mechanism by which NK cells identify cells that have lost MHC class I expression. It has been previously reported that CD56brightCD16− NK cells differ in their KIR expression as compared to traditional NK cells [29]. Our findings echo this report with little to no expression of the KIRs 158a, 158b and NKB1 on TIL-2742 (Fig. 5a). Whether this lack of inhibition may contribute to the activity of these cells warrants further investigation.
The NCRs are activating receptors and have been implicated in recognition of tumor cells by NK cells [30, 31]. Although their ligands on tumor cells are unknown, they can bind to influenza hemagglutinin and activate NK cells [32]. One receptor from this family, p46, was of particular interest to us as Sivori et al. [33] demonstrated strong production of IFN-γ and TNF-α following incubation with a p46-triggering antibody. The analysis of p46 expression on TIL-2742 revealed higher expression as compared to activated pNK cells. However, it should be noted that the level of expression on the pNK cells was lower than has been previously reported [33]. Unfortunately, because the ligand for p46 is unknown, tumor lines could not be evaluated for potential correlation with reactivity.
Another molecule which is reportedly involved in NK cell antitumor function is TRAIL [34]. This type-II transmembrane protein is a member of the TNF family and induces apoptotic cell death upon ligation with the death receptors (DR) 4 and DR5. Takeda et al. [13] demonstrated that TRAIL was directly involved in NK cell-mediated suppression of tumor growth and metastases in mice. The analysis of TRAIL on TIL-2742 found high expression on NK cells with 75% staining positive. Antibody blockade of TRAIL resulted in decreased cytokine release and cytolysis following co-culture with tumor targets (Fig. 6). FACS analysis of DR4 and 5 expression on tumor lines failed to reveal any correlation with cytokine release (data not shown). Although TRAIL appears to partially modulate the function of TIL-2742, the mechanism of tumor recognition by TIL-2742 is unknown and warrants further investigation.
In conclusion, we have identified a unique NK cell subset extracted from a metastatic pancreatic cancer deposit which regressed following treatment with the immune modulator anti-CTLA-4. These CD56brightCD16− NK cells were capable of lysing cells from a broad panel of malignant histologies, but released IFN-γ only when co-cultured with pancreatic and prostate lines. Although it is unknown whether these cells were directly responsible for the impressive tumor reduction in the patient who provided these cells, they were able to lyse and release cytokine ex vivo following stimulation with an autologous line. It is important to note that these cells were extracted from a single patient and further investigation into other samples is needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig 1 (supplemental) Establishment of an autologous pancreatic cancer line from metastasis which regressed after anti-CTLA-4 treatment. A. Computed tomography (CT) scan of a patient with metastatic pancreatic cancer shows dramatic resolution of hepatic and intra-abdominal lymph node disease following treatment with Ipilimumab, an anti-CTLA-4 monoclonal antibody. After surgical resection, tumor infiltrating immune cells and an autologous tumor cell line were derived from a gastrohepatic ligament lymph node (white arrow). B. Cytopathologic characterization of the established tumor line identified positive staining for CA 19-9, CEA, CK- 7 and MHC class I, but not HLA-A2, similar to the resected lesion (DOC 1620 kb)
References
- 1.Parkinson DR, Abrams JS, Wiernik PH, Rayner AA, Margolin KA, Van Echo DA, Sznol M, Dutcher JP, Aronson FR, Doroshow JH, et al. Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J Clin Oncol. 1990;8:1650–1656. doi: 10.1200/JCO.1990.8.10.1650. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. doi: 10.1001/jama.271.12.907. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimm EA, Robb RJ, Roth JA, Neckers LM, Lachman LB, Wilson DJ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983;158:1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 9.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA (2010) Phase 2 trial of single agent ipilimumab (anti-CTLA4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother (in press) [DOI] [PMC free article] [PubMed]
- 10.Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, Wunderlich J, Hawley RG, Moayeri M, Rosenberg SA, Morgan RA. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol. 1989;142:3714–3725. [PubMed] [Google Scholar]
- 12.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 14.Wang QJ, Hanada K, Yang JC. Characterization of a novel nonclassical T cell clone with broad reactivity against human renal cell carcinomas. J Immunol. 2008;181:3769–3776. doi: 10.4049/jimmunol.181.6.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, Sadelain M, Eshhar Z, Rosenberg SA, Morgan RA. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O’Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Yamamoto K, Kaneko T, Nieda M, Yokokawa K, Goto H. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69–e74. doi: 10.1097/MPA.0b013e318197a9e3. [DOI] [PubMed] [Google Scholar]
- 19.Liyanage UK, Goedegebuure PS, Moore TT, Viehl CT, Moo-Young TA, Larson JW, Frey DM, Ehlers JP, Eberlein TJ, Linehan DC. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother. 2006;29:416–424. doi: 10.1097/01.cji.0000205644.43735.4e. [DOI] [PubMed] [Google Scholar]
- 20.Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, Altermann W, Handke D, Atkins D, Seliger B, Kiessling R. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–6394. doi: 10.1158/0008-5472.CAN-06-0029. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J Exp Med. 1999;190:205–215. doi: 10.1084/jem.190.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–4486. [PubMed] [Google Scholar]
- 23.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 24.Perussia B, Chen Y, Loza MJ. Peripheral NK cell phenotypes: multiple changing of faces of an adapting, developing cell. Mol Immunol. 2005;42:385–395. doi: 10.1016/j.molimm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Terunuma H, Deng X, Dewan Z, Fujimoto S, Yamamoto N. Potential role of NK cells in the induction of immune responses: implications for NK cell-based immunotherapy for cancers and viral infections. Int Rev Immunol. 2008;27:93–110. doi: 10.1080/08830180801911743. [DOI] [PubMed] [Google Scholar]
- 26.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 27.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Kimura R, Miyagi T, Mochizuki K, Sasaki Y, Hayashi N. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- 28.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 29.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–3127. doi: 10.1002/1521-4141(2001010)31:10<3121::AID-IMMU3121>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 33.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Yamaguchi N, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol. 2001;214:194–200. doi: 10.1006/cimm.2001.1896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig 1 (supplemental) Establishment of an autologous pancreatic cancer line from metastasis which regressed after anti-CTLA-4 treatment. A. Computed tomography (CT) scan of a patient with metastatic pancreatic cancer shows dramatic resolution of hepatic and intra-abdominal lymph node disease following treatment with Ipilimumab, an anti-CTLA-4 monoclonal antibody. After surgical resection, tumor infiltrating immune cells and an autologous tumor cell line were derived from a gastrohepatic ligament lymph node (white arrow). B. Cytopathologic characterization of the established tumor line identified positive staining for CA 19-9, CEA, CK- 7 and MHC class I, but not HLA-A2, similar to the resected lesion (DOC 1620 kb)