Abstract
Aim:
CaCO3 nanoparticles (nano-CaCO3) can neutralize the acidic pHe of solid tumors, but the lack of intrinsic imaging signal precludes noninvasive monitoring of pH-perturbation in tumor microenvironment. We aim to develop a theranostic version of nano-CaCO3 to noninvasively monitor pH modulation and subsequent tumor response.
Materials & methods:
We synthesized ferromagnetic core coated with CaCO3 (magnetite CaCO3). Magnetic resonance imaging (MRI) was used to determine the biodistribution and pH modulation using murine fibrosarcoma and breast cancer models.
Results:
Magnetite CaCO3-MRI imaging showed that nano-CaCO3 rapidly raised tumor pHe, followed by excessive tumor-associated acid production after its clearance. Continuous nano-CaCO3 infusion could inhibit metastasis.
Conclusion:
Nano-CaCO3 exposure induces tumor metabolic reprogramming that could account for the failure of previous intermittent pH-modulation strategies to achieve sustainable therapeutic effect.
Keywords: : magnetite, MRI, nano-CaCO3, pH modulation, theranostic
Effective cancer therapy remains difficult because of significant side effects and off-target toxicity associated with common approaches such as chemotherapy [1] and radiotherapy [2]. Further complications arise from the development of resistance as tumor cells undergo mutations and adaptive reprogramming to escape therapy. Recent studies have shown that the tumor microenvironment plays important roles in cancer growth and metastasis. As a result, interest in developing therapies that can modulate the extracellular milieu and avoid development of therapeutic resistance has intensified. For example, one such target is the relatively acidic extracellular pH of many solid tumors. Cancer cells utilize aerobic glycolysis to fuel their growth (Warburg metabolism), synthesizing lactic acid from glucose in the presence or absence of oxygen. Efflux of the excess lactic acid and protons via various mechanisms decreases the extracellular pH (pHe = 6.8) of solid tumors relative to the physiologic pHe in tissues (pHe 7.2–7.4) [3,4] and this process has been noted as a hallmark of cancer [5,6]. Low pHe activates matrix degradation enzymes to facilitate metastasis and inactivates some chemotherapeutics. Thus, targeting or modulating the pHe of tumors can improve drug-delivery via pH-responsive nanoparticles [7,8] or cell-penetrating peptides [9]. Neutralizing tumor acidity can also directly inhibit growth and metastasis via selective proton pump inhibitors [10], bicarbonate infusions [11] or nanoparticle-based buffering [12]. Recent studies have also found that changing tumor extracellular pH synergizes with other therapies, including chemotherapy [13,14], anti-angiogenesis therapy [15], radiation therapy [16] and immunotherapy [10].
Of these methods used to change pHe, CaCO3 nanoparticles (nano-CaCO3) have been particularly effective due to their high payload and buffering capacity, allowing them to deliver drugs [7,17] and directly modulate the local pHe [12] in acidic environments, respectively. Recent studies have shown that formation of albumin coronal shell stablizes most forms of CaCO3 crystalline forms in vivo. Changing their crystalline properties can confer different degrees of dissolution [18]. Biocompatiblity of diverse calcium-based nanoparticles has favored their use in the diagnosis and treatment of diseases [19]. In particular, the strong buffering capacity of nano-CaCO3 compared with microparticle versions makes them particularly attractive for in vivo applications [18–20]. These materials have a low potential for side effects, as they degrade into calcium (regulated by the kidney and deposited into the bone) and CO2 (exhaled by the lungs), and only increases the pHe to a maximum of 7.4 [12], the normal physiologic pH of most healthy tissues. As a result, nano-CaCO3 provides buffering when needed but does not induce alkalosis, even at high concentrations. We previously reported tumor inhibition in a fibroasarcoma xenograft murine model with daily administration of nano-CaCO3, associated with a pH increase that reversed upon ceasing administration of the nano-CaCO3 [12]. The effect of nano-CaCO3 is not dependent on inhibiting any specific acid secretion pathway, avoiding traditional drug resistance via genetic mutation. However, we have not yet assessed pHe changes after clearance of nano-CaCO3, or its effect, if any, on tumor glycolytic metabolism.
Despite recent progress in the therapeutic applications of nano-CaCO3, it has been difficult to determine the biodistribution of nano-CaCO3 and noninvasively map subsequent pH changes in vivo. This handicap reduces our ability to optimize its therapeutic potential. Unlike some nanoparticles, such as gold and silver nanoparticles, nano-CaCO3 does not have an intrinsic signaling moiety to track in vivo distribution. Nanoparticle-derived calcium and carbonate are difficult to differentiate from endogenous ions. The conventional approach to directly incorporate imaging moieties such as fluorescent dyes or radionuclides into the nanoparticle's surface is less applicable to nano-CaCO3 because the imaging component will dissociate from the bulk nanoparticles as they dissolve in vivo. Coating the surface of the nano-CaCO3 with polymers or other materials that provide linkage groups for conjugation of imaging agents alters the highly pH-controlled CaCO3 release that sets these particles apart from other pH modulators. Although radioactive calcium could be used, this approach presents challenges on several levels, including the use of dedicated synthesis room and apparatus, as well as the costs associated with the disposal of long-lived radioisotopes. For these reasons, there are no reports of imaging nano-CaCO3 distribution in vivo.
In this study, we synthesized magnetite encapsulated by nano-CaCO3, (magnetite CaCO3 [m-CaCO3]) for detection by MRI. MRI has been used to image magnetite nanoparticle distributions in vitro and in vivo [21,22]. We correlated biodistribution with changes in pHe and glucose uptake measured through acido-chemical exchange saturation transfer (acidoCEST)-MRI and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). Additionally, we developed a proof-of-concept nano-CaCO3 therapy for metastasis inhibition. Thus, m-CaCO3 serves as a theranostic, with both diagnostic and imaging uses as well as therapeutic potential. In the process, we discovered that nano-CaCO3 induces tumor metabolic reprogramming, unique among nanoparticles.
Materials & methods
Synthesis of nano-CaCO3
This method has been previously published [12]. CaCl2 hexahydrate (100 mg; 0.46 mmol; Sigma–Aldrich, Saint Louis, MO, USA) was dissolved in anhydrous ethanol (50 ml) in a 400 ml beaker in a desiccator containing with drierite. The beaker was surrounded by 16 glass vials (20 ml) filled with ammonium bicarbonate (typically 20 g per vial) and placed under vacuum for 1–3 days with completion determined by a change in the turbidity of the solution with a visual change to a light blue, cloudy solution. The volume was reduced by approximately 10 ml ethanol during the reaction. The solid nanoparticles were then isolated from the ethanol solution by centrifuging at 6800 × g for 10 min in 1 ml Eppendorf tubes, then air dried. The resultant solid was recombined in fresh ethanol and resuspended by sonication, followed by storage at room temperature. Yield was determined by sampling 10 ml of the resultant solution, and weighing the dry mass.
Synthesis of m-CaCO3
Monodisperse iron oxide nanoparticles (IONPs; magnetite) were synthesized by thermal decomposition of iron carboxylate Fe(CO)5 at high temperature. In a typical synthesis of 15 nm iron oxide nanoparticles, a mixture of 2 mmol FeO(OH) fine powder and 2 mmol oleic acid in 5.0 g of 1-octadecene was heated and stirred under argon atmosphere at 320°C. The reaction proceeded for 2 h before cooling to room temperature. The suspension was purified with a standard washing procedure using hexane and acetone, then dispersed in hexanes. The purified particles were transferred from hexane to water by ligand addition of sodium monododecyl phosphate using sonication. During this process, 0.5 ml of IONPs in hexane solution and 1 mM sodium monododecyl phosphate were added to 10 ml of ultrapure water in a glass vial. The mixture of organic and aqueous phase was then ultra-sonicated for 20 min. After sonication, the brown suspension was heated to 70°C for 1 h to evaporate residual hexane. The IONPs were further purified via ultracentrifugation (Sorvall WX Ultra 80, Thermo Scientific, MA, USA), filtration through a syringe filter (pore size of 0.2 um, Millipore) and redispersion by sonication [23]. A premixed solution of CaCl2•2H2O (0.1 M) and NaHCO3 (0.1 M) in water and ethylene glycol (1:5 v/v; Sigma–Aldrich) were dispersed in the aqueous dispersion of monodisperse IONPs and mixed at room temperature. The synthesized m-CaCO3 particles were collected by sequential wash with ethanol, methanol and acetone, as reported previously, and stored as a dry powder at room temperature [12,20].
Transmission electron microscope
Transmission electron microscope (TEM) micrographs were obtained using an FEI Spirit TEM (OR, USA) operated at 120 kV. A 400-mesh Formvar carbon-coated copper grid was glow-discharged in a vacuum evaporator (Denton, NJ, USA) for 30 s. The sample was prepared by placing 2 μl of sonicated CaCO3 nanoparticles solution onto the grid and wicking off the excess sample with filter paper after 30 s. The grid left to dry out at room temperature before TEM analysis.
x-ray diffraction
x-ray diffraction (XRD) patterns were obtained by using the Bruker d8 Advance x-ray diffractometer (Bruker, MA, USA) configured with a Cu x-ray tube with 1.5418 Å for analysis of powder samples using LYNXEYE_XE detector. For the analysis, fine ground CaCO3 nanoparticles were kept on a Zero diffraction plate (MTI Corporation, CA, USA). XRD data were scanned from 20–60 degrees, with a 0.04 degree step size, a 0.5 s per step count time, with sample rotation turned on (15 r.p.m.), and with a coupled two-theta/theta scan. The Bruker Diffrac. Eva program was used for the evaluation and processing of XRD scan data. Search-match operations included search by diffraction index list, by name, using chemistry filters and via an International Centre for Diffraction Data (ICCDD PDF) database filter to confirm crystal type.
Hydrodynamic diameter & electro-kinetic zeta potential
To study agglomeration kinetics of CaCO3 nanoparticles, the hydrodynamic diameter was measured using time-resolved dynamic light scattering (Malvern Instruments, MA, USA). The zeta potential was measured using a Malvern zetasizer nano ZS instrument. An applied voltage of 100 V was used for the analyses. A minimum of three measurements was made per sample.
Animal studies & tumor models
All animal studies were conducted in compliance with Washington University and Arizona University Animal Welfare Committees’ requirements for the care and use of laboratory animals in research. BALB/c and athymic nu/nu mice were purchased from Frederick Cancer Research and Development Center. HT1080 tumors were generated by subcutaneous injection of 4 × 106 cells in 100 μl of Dulbecco's phosphate-buffered saline (PBS) into the dorsal flanks of athymic nu/nu mice. Likewise, MDA-MB-231 tumors were generated by injecting MDA-MB-221 cells into the dorsal flanks of BALB/c mice or by tail vein injection. 4T1 tumors were generated by injection of 4 × 105 cells in 100 μl of PBS into the mammary fat pads of BALB/c mice or 1 × 106 cells intravenously via tail vein.
Phantom imaging of m-CaCO3 nanocomposite
Eighty-one milligram of m-CaCO3 particles were suspended in 1 ml of 2% (v/v) bovine serum albumin (BSA) in Dulbecco's PBS supplemented with MgCl2 and CaCl2, and then serially diluted five times, each time by a factor of 10 in 2% BSA–PBS. The resulting 1 ml solutions (81, 8, 0.8, 0.08 and 0.008 mg/ml) were placed in microcentrifuge tubes and imaged by a 7T Biospec MRI scanner (Bruker Biospin, Inc., MA, USA). The transverse relaxation rate constant (R2) was measured for each dilution using a Carr-Purcell-Meiboom-Gill type spin-echo pulse sequence by employing 12 echo times ranging from 13 to 300 ms and a repetition time of 2 s.
In vivo imaging of m-CaCO3
Mice bearing tumors between 1.0 and 1.5 cm in diameter were injected with 1 mg w/v m-CaCO3 via tail vein. Each mouse was anesthetized, secured to a customized cradle, inserted into a 7T Biospec MRI scanner (Bruker Biospin, Inc.) and measured using the Carr-Purcell-Meiboom-Gill sequence described above. The mouse's breathing rate was monitored and the core body temperature was regulated at 37.0 ± 0.5°C. Depending on the tumor model, measurements were taken pre-injection, immediately postinjection and after 1, 2, 4–6, and 24 h postinjection. The transverse relaxivity of the particle (r2) was calculated based on the linear relation (assuming low concentrations of particles) between R2 and particle/iron concentration:
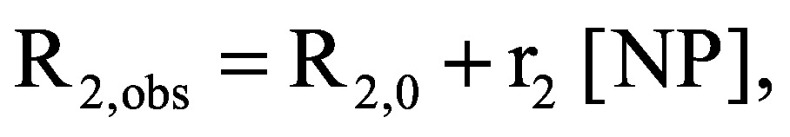 |
where R2,obs is a measured transverse relaxation rate constant; R2,0 is the calculated transverse relaxation rate constant in the absence of the nanoparticle, also referred as the ‘background’ R2; and [NP] is the nanoparticle concentration. Images were analyzed in MATLAB (Mathworks, Inc., MA, USA).
18F-Fluorodeoxyglucose positron emission tomography
BALB/c mice bearing HT1080 tumors were shaved and injected intravenously with FDG at 5 mCi/kg dose. FDG activity was measured by PET over a 60 min tumor uptake study after overnight fasting. Mice were then injected with 1 mg of nano-CaCO3 in 100 μl of 2% murine serum albumin in PBS and allowed to feed again before an overnight fast. At approximately 24 h post-nano-CaCO3 administration, mice were again injected with FDG and then measured for PET activity over 60 min.
AcidoCEST-MRI pH measurements in vivo
AcidoCEST protocol has been previously described [24–26]. Briefly, each mouse was anesthetized, catheterized and secured to a customized cradle, and inserted into the MRI machine. A bolus of 200 μl of iopromide was injected via the catheter, followed by infusion of iopromide at 400 μl/h for the duration of imaging. A respiration-gated CEST-FISP (fast imaging with steady state precession) MRI protocol acquired images with 54 saturation frequencies using 2.8 μT saturation power and a saturation period of 5 s. This CEST MRI protocol was repeated four times before injection and six times after injection, for a total time of approximately 55 min. The acidoCEST magnetic resonance imaging (MRI) images were processed with MATLAB R2012B (Mathworks, Inc.), as previously described [24–26].
Continuous infusion of nano-CaCO3
In BALB/c mice bearing mammary fat pad 4T1luc-GFP tumors, bioluminescence imaging (BLI) was performed using the IVIS Lumina System (Perkin Elmer, MA, USA) to estimate tumor size on day 1 after implantation. Nano-CaCO3 (3 mg) was suspended in EtOH (200 μl) along with cypate (100 μM in ethanol 100 μl). The mixture was placed in a Lynch coil connected to an Alzet osmotic pump (Model 2004, DURECT Corporation, CA, USA) with a 28-day infusion time (0.15 μl/h). The pump was implanted on day 2 post-tumor implantation and connected via a catheter into the jugular vein. Animals rested for 2–3 days after surgery. On day 6, nano-CaCO3 (1 mg) in 100 ul 2% BSA–PBS was injected intravenously, and the animals were imaged by BLI the same day as well as approximately every 4 days up to day 30. To detect lung metastasis, the primary tumor was covered with a black material and a long exposure was taken of the chest region. Control mice were implanted with tumors but not osmotic pumps.
For extravasation experiments requiring intravenous (iv.) injection of tumor cells, pumps were similarly implanted. Three days after pump implantation, nano-CaCO3 (1 mg) was injected via tail vein, followed 3–5 min later by 4T1luc-GFP cells. The mice were imaged by BLI at 2 h and then days 2, 6, 9 and 13 days after iv. tumor injection before being euthanized.
Determination of toxicity in cells
HT1080 fibroadenoma cells were grown to near-confluency in a 96 well plate. The cells were plated in a 96-well plate and incubated with m-CaCO3 nanoparticles at various concentrations from 0 to 1.2 mg/ml for 24 h, under Iscove's Modified dulbecco's medium media (pH 7.4). The sulforhodamine B assay [27] was used to determine the cytotoxicity, which was expressed as a percentage of viable cells relative to the untreated control. Each condition was plated with four technical replicates.
Determination of toxicity in rats
Three month old Sprague Dawley rats (n = 6; 2 females and 4 males) were used to determine the in vivo toxicity of m-CaCO3 nanoparticles. The nanoparticles were dispersed in 2% w/v rat serum albumin in PBS supplemented with CaCl2 and MgCl2. The 2% (v/v) m-CaCO3 nanoparticles were injected with an allometrically dosed 25.4 mg/kg of a stock solution (30 mg/ml), approximately equivalent to a 40 mg/kg mouse injection reported previously [12]. As a control, six rats (n = 6; 3 females and 3 males) were injected with a vector of 2% rat serum albumin–PBS using an equivalent volume (1.333 ml/kg) as in the treated group. Toxicity was then analyzed by histology, complete blood count, white blood cell differential and a basic metabolic panel.
Statistical methods
Statistics were calculated using Prism (GraphPad, CA, USA) for Kaplan–Meier log rank tests, and Microsoft Excel (Microsoft, WA, USA) for two tailed t-tests.
Data availability
Underlying raw data are available on request.
Results
Synthesis & characterization of m-CaCO3
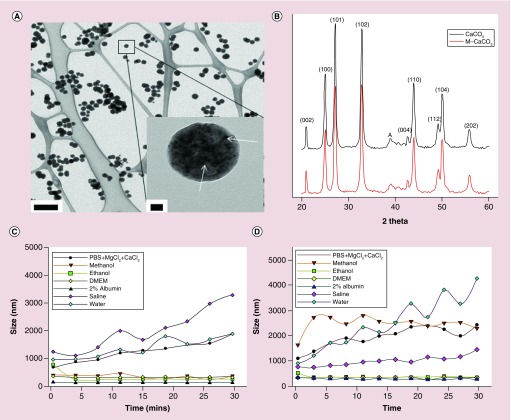
Magnetite (iron oxide) nanoparticles were synthesized by thermal decomposition of iron carboxylate purified, and then mixed with CaCl2 hexahydrate and sodium bicarbonate in water and ethylene glycol to form m-CaCO3 nanoparticles. TEM micrographs of m-CaCO3 demonstrated a characteristic spherical shape and size range (100 ± 8.5 nm) of the particles dispersed in methanol (Figure 1A). The images show the integration of the magnetite particles into the nano-CaCO3 as dark spots of the appropriate size (inset of Figure 1A). XRD patterns clearly showed that both nano-CaCO3 and m-CaCO3 were vaterite crystals (Figure 1B). The images clearly show the composite of nano-CaCO3 alone (Supplementary Figure 1) and magnetite (Supplementary Figure 2). The known ratio of presynthesized magnetite nanoparticles added to the final product of m-CaCO3 matched well with the final concentration of iron measured by Inductively coupled plasma mass spectrometry (ICP-MS) analysis.
Figure 1. . Characterization of magnetite CaCO3.
(A) Transmission electron microscope images of m-CaCO3 particles when dispersed in methanol (scale bar 500 nm). Inset in (A): higher magnification of the m-CaCO3 particles (scale bar of 20 nm). The black dots indicated by arrows within the bulk of the CaCO3 particle are the magnetite nanoparticles. (B) x-ray diffraction characterization of nano-CaCO3 (black) and m-CaCO3 (red) composite shows vaterite composition for both m-CaCO3 and nano-CaCO3. (C) Stability test of nano-CaCO3 in various solvents measured by time-resolved dynamic light scattering over 30 min. (D) Stability test of m-CaCO3 in various solvents measured by time-resolved dynamic light scattering. For both particle types, the order of stability is 2% albumin > ethanol = DMEM > saline > PBS + MgCl2 > methanol > water.
m-CaCO3: Magnetite CaCO3; Nano-CaCO3: CaCO3 nanoparticles; PBS: Phosphate-buffered saline.
Stability of m-CaCO3 in aqueous medium is achieved with an albumin solution
Using time-resolved dynamic light scattering, the stability of both nano-CaCO3 and m-CaCO3 were determined in a variety of solvents including water, saline, BSA, DMEM, ethanol, methanol and PBS. The m-CaCO3 shared similar stability as nano-CaCO3 in 2% albumin/PBS or DMEM, with both solutions providing the greatest stability and least aggregation over time (Figure 1C & D). In contrast, water and saline destablized the particles, probably due to rapid equilibration or ion exchange.
Imaging of nano-CaCO3 distribution ex vivo & in vivo using m-CaCO3 in a fibrosarcoma xenograft murine model
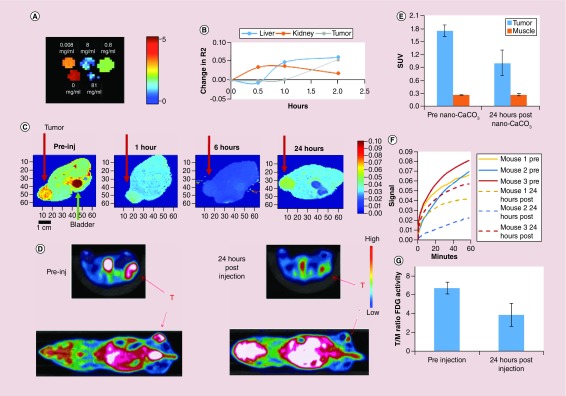
Incorporation of magnetite allowed the use of MRI to image CaCO3 nanoparticles in vitro and in vivo. Phantom imaging of in vitro solutions demonstrated the ability to detect a sample of m-CaCO3 at 0.1 mg/ml, a 100-fold dilution from normal injection doses of 1 mg in 100 μl for nano-CaCO3 (Figure 2A). Accumulation of m-CaCO3 in fibrosarcoma HT1080 tumors in vivo caused a decrease in T2 relaxation time (increase in R2 or 1/T2), which appeared first in the kidney, then liver and finally tumor (Figure 2B). Images of liver and kidney obtained during the experiment are shown in Supplementary Figure 3.
Figure 2. . Analysis of in vivo CaCO3 nanoparticles penetration in fibrosarcoma HT1080 xenografts and subsequent metabolic changes.
(A) Ex vivo phantom imaging using magnetic resonance imaging of magnetite-CaCO3 (m-CaCO3) at different concentrations (0.008–81 mg/ml range). Increase in m-CaCO3 concentration leads to a reduction in T2. Scale is T2. For (B & C), m-CaCO3 was administered to HT1080 tumor-bearing mice intravenous injection. (B) Change in R2 (1/T2) over time in the liver, kidney and HT1080 tumor after injection of m-CaCO3, representative profile. (C) Visualization of T2 changes with m-CaCO3 injection in a cross section of a mouse (representative images of n = 3 mice). Red arrow shows tumor, green arrow shows the mouse's bladder. Note the drop in T2 throughout, with recovery by 24 h. Heat map shows relative T2, with red and blue indicating high and low activity, respectively. For (D–G), HT1080 tumor-bearing mice were given a tail vein injection of CaCO3 nanoparticles (nano-CaCO3) and imaged using FDG-PET (n = 3 mice). (D) Representative PET activity heat maps in cross section of a mouse at 0 and 24 h after nano-CaCO3 injection. White is beyond threshold, red arrow (T) indicates tumor. (E) Tumor versus muscle uptake of FDG, area under the curve over 1 h. (F) Difference in FDG uptake pre- and post-nano-CaCO3 administration over the course of glucose uptake. Pretreatment (solid lines); 24 h post-treatment (dashed lines). (G) Comparison of FDG tumor/muscle ratio.
FDG: 18F-Fluorodeoxyglucose positron; m-CaCO3: Magnetite CaCO3; Nano-CaCO3: CaCO3 nanoparticles; PET: Positron emission tomography.
The T2 signal throughout the animal decreased rapidly with a trough at around 6 h, followed by the return of signal approaching the original T2 profile by 24 h (Figure 2C). The significant reduction of T2 in the bladder suggests a substantial component of renal clearance, whereas T2 recovery in the tumor indicates that m-CaCO3 is dissolving in the tumor's acidic pH as designed and the free magnetite is clearing from the tissue (Figure 2C). Subsequent studies were conducted with nano-CaCO3, except where m-CaCO3 was used for imaging the particles’ distribution in vivo.
Measuring metabolic reprogramming in the fibrosarcoma model
HT1080 tumor-bearing mice were treated with nano-CaCO3, and glucose metabolism was measured by uptake of the glucose analog FDG using PET. FDG signal in tumors 24 h after nano-CaCO3 introduction showed a statistically significant reduction in glucose uptake in the tumor (p = 0.046, 2-tailed paired t-test), but with no change in the muscle (p = 0.69; Figure 2D). This finding was true for total FDG uptake by the tumor (area under the curve, Figure 2E) and at each time point post-FDG administration (Figure 2F). This reduction was localized primarily to the tumor and not the muscle (Figure 2G).
MRI-derived pH measurement of tumors with CaCO3 nanoparticles in breast cancer murine models
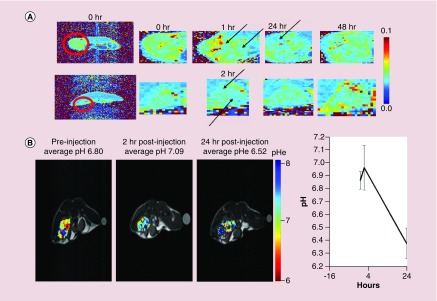
For imaging the biodistribution of particles, we investigated m-CaCO3 in a subcutaneous xenograft model of human breast cancer (MDA-MB-231). Analysis of m-CaCO3 penetration into the breast tumor showed a limited reduction in T2, suggesting less uptake than observed previously in the HT1080 fibrosarcoma model (Figure 2C vs Figure 3A). Differential penetration and kinetics were observed depending on the size of the tumor. To determine the tumor pH, we used the acidoCEST-MRI method. Pioneered by the Pagel group, acidoCEST detects the differential rate of proton transfer of the amine group of iopamidol under different pH conditions, creating two separate peaks on MRI [28]. By measuring the ratio of the intensity of the two peaks, we can estimate the pH in vivo [29]. We used nano-CaCO3 instead of m-CaCO3 to prevent confounding signals from the magnetite and iopamidol imaging agents. We found that the tumors exhibited an average extracellular pH (pHe) of 6.8 before nano-CaCO3 injection, which increased to 7.04 before dropping to 6.54 at 24 h postinjection, (p < 0.054; Figure 3B). This result indicates that the clearance of nano-CaCO3 from the tumor region at 24 h decreased the pH to a level lower than baseline.
Figure 3. . Magnetite CaCO3 measurement in breast cancer model and subsequent effect on pH.
(A) Measurement of magnetite CaCO3 distribution in the cross-section of mice with an MDA-MB-231 xenograft. Heat map shows T2 signal. Red circle indicates tumor, with enlarged cutouts to the right. Black arrows show drops in T2 attributed to the magnetite core, with differential penetration in large and small tumors, and distribution peaking somewhere between 1 and 24 h dependent on size. Representative images (n = 3 mice, one experiment). (B) MRI-based pH measurement in a breast cancer MDA MB-231 model treated with CaCO3 nanoparticle. Left, representative images, heat map shows pH. Right, average tumor pH (n = 3 mice, one experiment).
Continuous infusion of nano-CaCO3 decreases lung metastasis
Rebound of the acidic environment of tumors after clearance of nano-CaCO3 suggests that continuous infusion of particles could maintain the pH above 7 and potentially induce a sustained inhibition in tumor metastasis and growth. As current osmotic pumps for infusion in murine models only last 30 days, we opted to use the aggressively metastasizing 4T1luc-GFP orthotopic breast cancer model. Mice were implanted with an osmotic pump containing nano-CaCO3 and cypate, a near-infrared (NIR) dye [30], followed by mammary fat pad implantation of the tumor cells. Metastasis to the lung was measured by transcutaneous bioluminescence. Planar NIR fluorescence imaging suggested that most of the materials were released by day 19 (Figure 4A). The residual NIR fluorescence on day 19 is from the pump implanted in the dorsum of the mice with a catheter into their internal jugular vein. Although nano-CaCO3 treatment nearly doubled the time to metastasis, the long-term survival benefit was not statistically significant (Figure 4B; p = 0.19, Kaplan–Meier log rank test), indicating that nano-CaCO3 may act by inhibiting extravasation. Next, we explored the feasibility of using nano-CaCO3 to reduce metastatic tumor growth in the lungs due to extravasation of circulating tumor cells. The nano-CaCO3 infusion inhibited metastatic growth of 4T1luc-GFP cells in the lungs of mice after intravenous injection (Figure 4C & D). There was a strong trend in metastasis inhibition in the iv. infusion model (p = 0.0576, two-way Analysis of variance [ANOVA], repeated measures). There was a statistically significant difference in growth at day 2 postinjection (p = 0.02, 2-tailed unpaired t-test), but no statistically significant differences were found on other days (p = 0.16, 0.46, 0.22, 0.16, at day 0, 6, 9, 13 postinjection, respectively, 2-tailed unpaired t-test). The control experiment ran in parallel and had to be terminated early due to weight loss in the control animals. Thus, continuous infusion of nano-CaCO3 limited extravasation and metastasis of tumor cells.
Figure 4. . Proof-of-concept continuous infusion of nano-CaCO3 may inhibit metastasis.
Mice were implanted with osmotic infusion pumps containing nano-CaCO3 nanoparticle and near-infrared dye cypate (treatment) or left untreated (control). (A) Near-infrared fluorescence of cypate as evidence of nano-CaCO3 release over time, heat map shows near-infrared signal. (B) Mice were implanted with orthotopic 4T1luc tumors and metastasis from orthotopic site to the lungs was tracked by bioluminescence. Time to metastasis is graphed, with control in black (n = 8, two experiments) and treatment in red (n = 3, one experiment). In (C–D), tumor cells were introduced via – tail vein injection, and tumor seeding and growth was tracked by bioluminescence over time (n = 3/group, one experiment). (C) Representative images of control and nano-CaCO3 treated mice. Scale is radiance (photons/sec/cm2/steradian). Note, bioluminescence outside of the lung region in the treated group is attributed to the infusion pump itself as described by the manufacturer. (D) Quantification of lung bioluminescence in photons/s. Error bars are standard error of the mean [31].
Nano-CaCO3: CaCO3 nanoparticles.
Evaluating toxicity of CaCO3 nanoparticles in vitro & in vivo
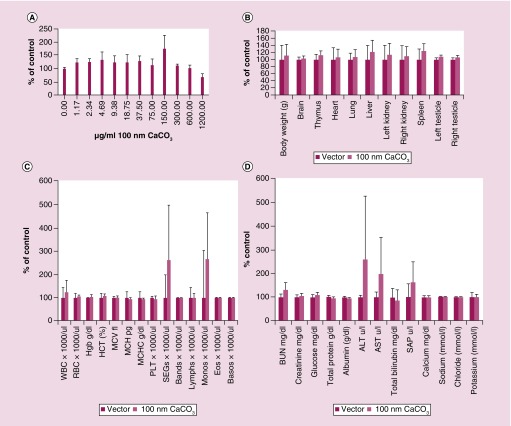
Toxicity of nanoparticles can arise from several factors, including size, charge, constituents and metabolites. As such, we characterized the toxicity of nano-CaCO3. Data analysis showed that the particles did not exhibit specific cytotoxicity on HT1080 cells treated with up to 1.20 mg/ml in vitro (Figure 5A). For in vivo studies, rats were used in order to obtain sufficient blood samples for analysis at multiple time points. Nano-CaCO3-treated rats did not exhibit significant changes in organ weights, blood chemistries and blood counts up to 24 h after 25 mg/kg nano-CaCO3 injection (Figure 5B–D). A slight increase in blood urea nitrogen was observed, which is not likely a toxic effect because there was no concurrent increase in the creatinine or liver enzymes. A single animal exhibited an increase in neutrophil counts as well as liver enzyme levels (ALT and AST) that were confounded by the presence of pneumonia in the animal (Figure 5C–D).
Figure 5. . CaCO3 nanoparticle does not show toxicity in vitro or in vivo.
(A) Cytotoxicity of CaCO3 nanoparticles as measured by the percentage of HT1080 cell surviving when incubated with 100 nm CaO3 nanoparticles in standard pH 7.4 media (n = 4 technical replicates, one experiment). For (B–D), rats were injected with 30 mg/ml at 25 mg/kg CaO3 nanoparticles or vector alone (n = 3/group, one experiment). (B) Organ weights with solvent versus 100 nm CaO3 nanoparticles. (C) Complete blood count. (D) Serum biomarker values.
MCH: Mean cell hemoglobin; MCV: Mean corpuscular volume; RBC: Red blood cell; WBC: White blood cell.
Discussion
The ability to visualize CaCO3 by impregnating magnetite nanoparticles into the existing nanoparticle formulations allows for the noninvasive assessment of the in vivo assessment of the biodistribution of this traceless material in animal models of cancer. The magnetite is uniformly distributed across the particle and remains in the particle even during dissolution, allowing for a better approximation of the location of the entire particle. Despite the different synthesis methods used to prepare both m-CaCO3 and nano-CaCO3, the nanoparticles exhibited similar stability in different solvents. Because m-CaCO3 maintains the same vaterite-like crystal properties as shown by XRD, it may have exhibited the same surface properties as nano-CaCO3. By maintaining similar surface properties, m-CaCO3 can be used to predict the behavior of the non-signaling nano-CaCO3 under similar in vivo conditions.
MRI of m-CaCO3 clearly shows differing distributions between the fibrosarcoma and breast cancer models, a diversity suspected to also exist in vivo clinically. These differences point to the importance of determining the biodistribution of nanoparticles in different tumor types for therapeutic optimization. The noninvasiveness of MRI-based tracking of the particles biodistribution makes it ideal for both preclinical optimization and eventual in vivo human studies.
Additionally, the combination of MRI for m-CaCO3 distribution and acidoCEST-MRI or FDG-PET for metabolic changes permits fully noninvasive and clinically translatable assessment of nano-CaCO3 efficacy in vivo. Previous studies on nano-CaCO3 used invasive methods to measure pH changes, which detected pH increase but failed over time due to acidity induced by pressure necrosis. Noninvasive pH measurements remain difficult because of limited pH sensitivity above 7 for many probes. However, noninvasive techniques such as acidoCEST imaging are useful to measure any potential drops in pH in vivo, including a return to pretreatment acidic pHe value. To map the metabolic reprogramming of the tumor, FDG-PET can be used to monitor glucose uptake, which can be overlaid with MRI data showing m-CaCO3 biodistribution from the same individual. With these multiple imaging modalities, we could perform parallel experiments to correlate biodistribution of m-CaCO3 and changes in pHe and glucose uptake in vivo and provide complementary information on how these particles modulate the pHe of tumors in multiple tumor models.
Biodistribution data using m-CaCO3 was able to shed light on previous findings using nano-CaCO3. We had previously demonstrated a tumor-static effect of nano-CaCO3 administration and pHe increase in an HT1080 fibrosarcoma model [12]. We found that daily nano-CaCO3 injections were required for therapeutic efficacy, and tumor growth resumed after nano-CaCO3 injections were stopped. These effects may be explained by MRI imaging showing the majority of the nanoparticles were cleared within in 24 h. Thus, continuous retention of nano-CaCO3 is likely required to prevent tumor growth, which matches with the results we found here using continuous infusion to prevent metastasis. Understanding the dissolution of these particles will require determination of the elemental composition of these nanoparticles over time. This information could further elucidate the release kinetics of calcium and iron from the m-CaCO3.
Our results from the FDG-PET and acidoCEST studies appear to demonstrate tumor metabolic reprogramming. In the HT1080 fibrosarcoma model, we found reduced FDG uptake in the tumor at 24 h, indicating nano-CaCO3 exposure may have affected the tumor's reliance on Warburg metabolism. Conversely, nano-CaCO3 produced a drop in tumor pH after nanoparticle clearance in the MDA-MB-231 model. A previous study using a pH electrode showed that nano-CaCO3 can increase the pHe of tumors from 6.8 to 7.4 over the course of several hours [12]. However, in this study, noninvasive imaging using acidoCEST-MRI detected a drop in pHe to 6.54 at 24 h post-treatment, which is below the average pH before injection. This finding suggests that CaCO3 induces a biological response from tumor cells that overcompensates for the initial alkalization, resulting in a lower pHe after the buffering nanoparticles are cleared from the tumor. This overcompensation suggests that tumors tightly and actively maintain acidic pHe, indicating that this process is not simply a spontaneous byproduct of lactic acid accumulation. This overcompensation confirms the need to sustain local nano-CaCO3 concentration in tumor microenvironment for effective inhibition of cancer progression and metastasis.
To combat this overcompensation, we explored the use of continuous infusion of nano-CaCO3 in a metastasis model. Increasing pH has been demonstrated to inhibit both metastasis and cancer cell extravasation in tumor models [11]. In our study, we found that the release of the majority of nanoparticles over 19 days correlated with lower levels of metastasis to the lung. A previous report showed a similar trend of metastasis inhibition when systemic pH was raised with sodium carbonate [11]. In contrast, our nanoparticle approach inhibited extravasation at approximately 30,000-fold lower dose than the previously reported required oral dosing of sodium bicarbonate. More study, however, is required to confirm that nano-CaCO3 can indeed limit metastasis, ideally utilizing a pump with longer duration in larger animals, and with concurrent long term placebo controls.
Alteration of systemic acid/base status outside physiological normal pH (7.2–7.4) is known to induce pathological effects [12]. Therefore, we evaluated the potential toxicity of nano-CaCO3 in rats, which were used to obtain sufficient blood samples for analysis. The results from the rat studies suggest minimal acute toxicity, except one rat that appeared to suffer from pneumonitis of unknown etiology, which showed increased liver enzymes and neutrophil counts. Nevertheless, further studies on the potential for long-term toxicity from multiple doses or continuous infusion with larger cohort will be required before human application.
Although the results of this study are promising, there are limitations to m-CaCO3 and the available methods for detecting its effects. Although magnetite allows for MRI-based noninvasive imaging, measurements are not quantitative and have low sensitivity. We were not able to obtain a longitudinal quantification of nanoparticle distribution because of technical constraints that include hypothermia caused by prolonged anesthesia. Future studies will explore alternative ways to determine quantitatively the pharmacokinetics of m-CaCO3 in the kidney and liver for longer durations than presented here. As such, the inability to estimate what concentration of nano-CaCO3 accumulates in tissues or at what time after injection it is eliminated as opposed to when the amount drops below signal visibility, prevents accurate estimation of the total nano-CaCO3 effect on pHe. In addition, acidoCEST allows noninvasive monitoring of the effect of CaCO3 on pHe, but has limited ability to detect pH above 7, confining its use to pretreatment or postclearance than during nano-CaCO3 therapy. A limitation of acidoCEST-MRI is that it does not allow, at the time of experimentation, the capability for continuous monitoring or extended daily time point imaging to avoid deleterious effects on mouse welfare, which limited the exploration of pH acidification. Future studies will continue to explore this question of acidification at longer time points as new and user-friendly pH measurement methods become available.
Conclusion
We developed m-CaCO3 for MRI and used it to determine nano-CaCO3 biodistribution noninvasively in two separate solid tumor types. A combination of acidoCEST-MRI and FDG-PET allowed us to assess the effects of nano-CaCO3 treatment. We discovered that nano-CaCO3-induced pH changes can cause tumors to reprogram their metabolic function, as evidenced by changes in pH and 18-FDG uptake in MDA-MB-231 and HT1080 tumors, respectively. Based on these results, several directions are currently under investigation with the particles, including methods to increase targeting and retention of the nano-CaCO3 at the tumor region and understanding how these particles stimulate cellular reprogramming. Given the substantial literature surrounding chemotherapy sensitivity to pH, the ability of nano-CaCO3 to increase and decrease tumor pHe may be used to optimize the retention and potentiation of a wide range of existing therapies [32]. Rat toxicity data suggest minimal side effects of treatment, enabling future translation to larger animal studies. The combination of this novel material synthesis and biological analysis offers the first evidence of tumor reprogramming by extracellular environment change by a nanoparticle with multiple possible applications in cancer therapy.
Summary points.
Incorporation of magnetite into CaCO3 nanoparticle (nano-CaCO3) produces an MRI imaging agent that is capable of assessing the nanoparticles biodistribution and pH modulation noninvasively.
Magnetite CaCO3 has similar surface and solubility properties as nano-CaCO3 in in vivo.
Nano-CaCO3 induces a transient pH increase in the tumor microenvironment and downregulation of radiolabeled glucose uptake in tumors.
After clearance, nano-CaCO3 also leads to an overcompensation of acidity by the tumor, demonstrating a metabolic reprogramming response to extracellular pH change.
Continuous infusion of nano-CaCO3 could prevent rapid clearance and inhibit tumor metastasis.
Nano-CaCO3 is biocompatible, exhibiting minimal toxic effects in vitro and in vivo.
Differences in tumor response to nano-CaCO3 suggest a need to optimize dosage, administration protocol and molecularly targeted delivery for different cancer types.
Incorporation of magnetite into carbonates-based nanoparticles provides an opportunity to intelligently design diverse nanometal carbonates and noninvasively determine their biodistribution, therapeutic effects and pH modulation of tissues.
Supplementary Material
Acknowledgments
The authors would like to acknowledge D Hathi for help with the MR analysis, as well as the help by the staff of the Mallinckrodt Institute of Radiology microPET Facility and SC Greco of the Washington University in St Louis Division of Comparative Medicine for aid in toxicity studies.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/full/10.2217/nnm-2018-0302
Financial & competing interests disclosure
A Som was supported by the NCI F30CA189435-03 and NIH training Grant Number T32 GM07200. R Raliya, L Habimana-Griffin and N Reed were supported by NIH-NCI U54CA199092. AY Mah-Som was supported by NIAID 1F30AI129110 and NIH training Grant Number T32 GM07200. WJ Akers was supported by NCI R50 CA211481. JL Prior was supported by NIH P50 CA094056 (Molecular Imaging Center) and NCI P30 CA091842 (Siteman Cancer Center Small Animal Cancer Imaging shared resource). The study was funded in part by grants from the National Institutes of Health (U54 CA199092, R01 EB021048, R01 CA171651, P50 CA094056, P30 CA091842, S10 OD016237, S10 RR031625 and S10 OD020129), Department of Defense Breast Cancer Research Program (W81XWH-16-1-0286), and the Alvin J Siteman Cancer Research Fund (11-FY16-01). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All animal studies were conducted in compliance with Washington University and Arizona University Animal Welfare Committees’ requirements for the care and use of laboratory animals in research. The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology. 2003;61(9):1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- 2.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat. Rev. Cancer. 2009;9(2):134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojugo AS, McSheehy PM, McIntyre DJ, et al. Measurement of extracellular pH of solid tumors in mice by MR Spectroscopy. Comparison of exogenous 19F and 31P probes. NMR in Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J. Nucl. Med. 2010;51(8):1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Ueno Y, Futagawa H, Takagi Y, Ueno A, Mizushima Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J. Control. Release. 2005;103(1):93–98. doi: 10.1016/j.jconrel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Lee ES, Shin HJ, Na K, Bae YH. Poly(l-histidine)–PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003;90(3):363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90(5):604–610. doi: 10.1002/bip.20989. [DOI] [PubMed] [Google Scholar]
- 10.Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology. 2013;2(1):e22058. doi: 10.4161/onci.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Robey et. al.'s work on bicarbonate inhibiting metastasis was one of the first to demonstrate that pH in the extracellular environment is more than a byproduct of dysregulated glycolysis and serves the reader as a base on which much pH modulation work was done.
- 12.Som A, Raliya R, Tian L, et al. Monodispersed calcium carbonate nanoparticles modulate local extracellular pH and inhibit tumor growth in vivo . Nanoscale. 2015;8(25):12639–12647. doi: 10.1039/c5nr06162h. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Our work here first showed how to synthesize, stabilize and use therapeutically nano-CaCO3 and is a good connected article.
- 13.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J. Control. Rel. 2008;132(3):164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006;8(2):143–152. doi: 10.1593/neo.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntyre A, Patiar S, Wigfield S, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin. Cancer Res. 2012;18(11):3100–3111. doi: 10.1158/1078-0432.CCR-11-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004;14(3):198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Peng H, Li K, Wang T, et al. Preparation of hierarchical mesoporous CaCO3 by a facile binary solvent approach as anticancer drug carrier for etoposide. Nano Express. 2013;(8):321. doi: 10.1186/1556-276X-8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauth V, Maas M, Rezwan K. An evaluation of colloidal and crystalline properties of CaCO3 nanoparticles for biological applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;78:305–314. doi: 10.1016/j.msec.2017.04.037. [DOI] [PubMed] [Google Scholar]; • The work by Lauth shows a detailed understanding of CaCO3 crystalline properties and interaction in vivo.
- 19.Qi C, Lin J, Fu LH, Huang P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018;47(2):357–403. doi: 10.1039/c6cs00746e. [DOI] [PubMed] [Google Scholar]
- 20.Raliya R, Som A, Shetty N, Reed N, Achilefu S, Biswas P. Nano-antacids enhance pH neutralization beyond their bulk counterparts: synthesis and characterization. RSC Adv. 2016;6(59):54331–54335. doi: 10.1039/c6ra12856d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenblum LT, Kosaka N, Mitsunaga M, Choyke PL, Kobayashi H. In vivo molecular imaging using nanomaterials: general in vivo characteristics of nano-sized reagents and applications for cancer diagnosis. Mol. Membr. Biol. 2010;27(7):274–285. doi: 10.3109/09687688.2010.481640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin TH, Choi Y, Kim S, Cheon J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rrv. 2015;44(14):4501–4516. doi: 10.1039/c4cs00345d. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Liu D, Wu J, Kim C, Fortner JD. Aqueous aggregation and surface deposition processes of engineered superparamagnetic iron oxide nanoparticles for environmental applications. Environ. Sci. Technol. 2014;48(20):11892–11900. doi: 10.1021/es502174p. [DOI] [PubMed] [Google Scholar]
- 24.Chen LQ, Howison CM, Spier C, et al. Assessment of carbonic anhydrase IX expression and extracellular pH in B-cell lymphoma cell line models. Leuk. Lymphoma. 2015;56(5):1432–1439. doi: 10.3109/10428194.2014.933218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LQ, Randtke EA, Jones KM, Moon BF, Howison CM, Pagel MD. Evaluations of tumor acidosis within in vivo tumor models using parametric maps generated with acido-CEST MRI. Mol. Imaging Biol. 2015;17(4):488–496. doi: 10.1007/s11307-014-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This work goes through how acido-CEST MRI can be used to measure pH noninvasively and is a critical novel technique for analysis for noninvasive extracellular pH measurement.
- 26.Akhenblit PJ, Hanke NT, Gill A, et al. Assessing metabolic changes in response to mTOR inhibition in a mantle cell lymphoma xenograft model using Acido-CEST MRI. Mol. Imaging. 2016;15 doi: 10.1177/1536012116645439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 28.Sheth VR, Li Y, Chen LQ, Howison CM, Flask CA, Pagel MD. Measuring in vivo tumor pHe with CEST-FISP MRI. Magn. Reson. Med. 2012;67(3):760–768. doi: 10.1002/mrm.23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acido-CEST MRI. Magn. Reson. Med. 2014;72(5):1408–1417. doi: 10.1002/mrm.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achilefu S, Dorshow RB, Bugaj JE, Rajagopalan R. Novel receptor-targeted fluorescent contrast agents for in vivo tumor imaging. Invest. Radiol. 2000;35(8):479–485. doi: 10.1097/00004424-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Alzet. Alzet Technical Sheet 1.1, in vivo bioluminescence imaging using alzet osmotic pumps. www.alzet.com/resources/documents/BLITechSheet_applications.pdf
- 32.Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Underlying raw data are available on request.