Abstract
Transforming growth factor β (TGF-β), signaling induced by cigarette smoke (CS), plays an important role in the progression of airway diseases, like chronic bronchitis associated with chronic obstructive pulmonary disease (COPD), and in smokers. Chronic bronchitis is characterized by reduced mucociliary clearance (MCC). Cystic fibrosis transmembrane conductance regulator (CFTR) plays an important role in normal MCC. TGF-β and CS (via TGF-β) promote acquired CFTR dysfunction by suppressing CFTR biogenesis and function. Understanding the mechanism by which CS promotes CFTR dysfunction can identify therapeutic leads to reverse CFTR suppression and rescue MCC. TGF-β alters the microRNAome of primary human bronchial epithelium. TGF-β and CS upregulate miR-145-5p expression to suppress CFTR and the CFTR modifier, SLC26A9. miR-145-5p upregulation with a concomitant CFTR and SLC26A9 suppression was validated in CS-exposed mouse models. While miR-145-5p antagonism rescued the effects of TGF-β in bronchial epithelial cells following transfection, an aptamer to block TGF-β signaling rescues CS- and TGF-β-mediated suppression of CFTR biogenesis and function in the absence of any transfection reagent. These results demonstrate that miR-145-5p plays a significant role in acquired CFTR dysfunction by CS, and they validate a clinically feasible strategy for delivery by inhalation to locally modulate TGF-β signaling in the airway and rescue CFTR biogenesis and function.
Keywords: TGFBR2 aptamer, microRNAome, CFTR, miR-145-5p, SLC26A9, TGFBR2, TGF-β signaling, cigarette smoke, COPD
The authors demonstrate that TGF-β and cigarette smoke upregulate miR-145-5p to suppress CFTR biogenesis. This is also the first report of microRNA-mediated regulation of SLC26A9, an important CFTR-modifying gene, and the authors demonstrate that miR-145-5p is also involved in TGF-β- and cigarette smoke-mediated suppression of SLC26A9.
Introduction
Mucociliary clearance (MCC) is a primary innate defense mechanism of mammalian airways that works in concert with other antimicrobial substances, like lactoperoxidase, lysozyme, and lactoferrin, to protect the host from the noxious effects of airborne pathogens, pollutants, and allergens.1, 2 The mucociliary apparatus consists of cilia, a protective mucus layer, and a periciliary airway surface liquid (ASL) layer to optimize ciliary beating.3 Abnormalities in any compartment of the mucociliary system can compromise mucus clearance, leading to mucus impaction. The accumulated mucus entraps bacteria and promotes chronic bacterial infection.4, 5, 6 The ASL layer lining the airway surfaces is crucial for mediating MCC rates,7 and it is tightly regulated under normal conditions.8 Cystic fibrosis transmembrane conductance regulator (CFTR) plays a pivotal role in MCC and other mechanisms of airway innate immunity, like the lactoperoxidase thiocyanate defense mechanism of the airways.9, 10, 11 CFTR regulates ASL due to Cl− efflux and reciprocal inhibition of Na+ absorption.12 Water follows through the transcellular or paracellular pathway maintaining ASL height leading to efficient MCC.13, 14 We have demonstrated that CFTR also regulates paracellular space and, consequently, water transport by increasing paracellular permeability.15 Hence, CFTR plays a critical role in regulating the ASL depth. CFTR dysfunction leads to decreased ASL depth,13 ASL acidification,16, 17 and increased mucus viscoelasticity,18 which in turn impairs MCC and cough clearance mechanisms.
An extensive body of evidence has established that smoking also induces an acquired state of CFTR dysfunction in patients with normal copies of the CFTR gene.19, 20 We have shown that acquired CFTR dysfunction due to smoke exposure rapidly and severely inhibits the MCC apparatus of the airways.21 In our earlier report, we demonstrated that transforming growth factor β (TGF-β) signaling and cigarette smoke (CS) (via TGF-β signaling) suppress CFTR mRNA, which translates to a concomitant and proportional suppression of CFTR function.15 TGF-β1 is ubiquitously expressed and secreted by several cell types, including airway epithelial, smooth muscle, fibroblast, and most immune cells. Airway epithelia of smokers, as well as patients with chronic bronchitis or chronic obstructive pulmonary disease (COPD), show increased TGF-β1 expression.22, 23, 24, 25
In this report, we determine the mechanism by which CS and TGF-β suppress CFTR. We show that miR-145-5p plays an important role in CFTR suppression in primary bronchial epithelial cells redifferentiated at the ALI treated with TGF-β1 and in small animal models exposed to CS. We also demonstrate that miR-145-5p modulates another important chloride channel, SLC26A9, which physically interacts with CFTR and plays an important role in CFTR biogenesis and activation.26, 27, 28 We demonstrate that antagonizing miR-145-5p rescues CFTR and SLC26A9. Finally, we show that a neutralizing aptamer to TGFBR2 can rescue CFTR mRNA and function in primary bronchial epithelial cells exposed to CS.
Results
TGF-β1-Mediated CFTR mRNA Suppression Is Due to miRNA-Mediated Post-transcriptional Gene Silencing
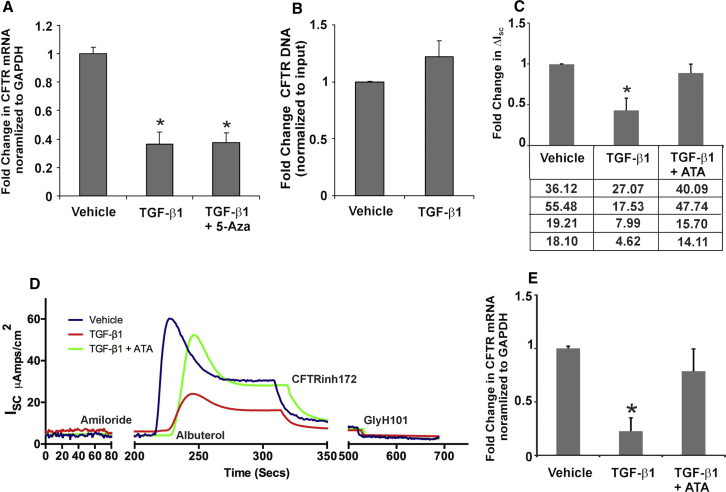
Given reports that TGF-β can regulate transcription of a number of genes, we tried to determine if any effects of TGF-β-mediated CFTR suppression were due to a suppression of transcription from the CFTR promoter. To determine if CFTR mRNA suppression is a result of transcriptional suppression, we first tested whether TGF-β induces CFTR promoter hypermethylation, given reports that CFTR promoter is silenced by hypermethylation in several cancers.29 Normal human bronchial epithelial (NHBE) air-liquid interface (ALI) cultures were pretreated with 5-aza-2′-deoxycytidine (5-aza-CdR) for 24 hr, and they were retained for the remainder of the experiment. 5-aza-CdR can reactivate epigenetic silencing established due to histone methyl transferases.29 Recombinant TGF-β1 (10 ng/mL) or vehicle (as control) was added apically and basolaterally. At 16 hr post-treatment, total RNA was isolated, and CFTR mRNA levels (normalized to GAPDH) were determined. Figure 1A demonstrates that pretreatment with 5-aza-CdR does not rescue TGF-β-mediated CFTR mRNA suppression.
Figure 1.
TGF-β1 Signaling Does Not Suppress Transcription from the CFTR Promoter
(A) 5-aza-CdR did not block TGF-β1-mediated CFTR mRNA suppression, suggesting that non-coding RNA-mediated transcriptional gene silencing is not involved. n = 3 experiments from different lungs. (B) NHBE ALI cultures were treated with recombinant TGF-β1 (10 ng/mL) or vehicle (as control). Transcriptional initiation from the CFTR promoter was determined in real time by ChIP. The amount of immunoprecipitated DNA was quantified by qPCR using primers designed to hybridize 150 bp downstream of the CFTR promoter. TGF-β1 treatment does not affect transcription initiation from the CFTR promoter. n = 3 experiments from different lungs. (C) CFTR function was determined in Ussing chambers (ΔISC), and CFTR mRNA from TGF-β1- or TGF-β1 plus ATA-treated cells was analyzed by qRT-PCR. ATA completely rescued TGF-β-mediated CFTR function. The table below the graph shows actual ΔISC values for the experiment. (D) Ussing chamber trace for TGF-β1 treatment and its rescue by ATA. (E) Total RNA from these cells shows the concomitant rescue of CFTR RNA (D). n = 4 experiments from different lungs. *Significant (p < 0.05) from control.
To further confirm or rule out transcriptional suppression, we performed a chromatin immunoprecipitation (ChIP) experiment on NHBE ALI cultures treated with TGF-β1 or vehicle. NHBE ALI cultures were treated with recombinant TGF-β1 (10 ng/mL) or vehicle (as control). At 16 hr post-treatment, the effect of TGF-β1 on transcriptional initiation from the CFTR promoter was determined in real time by ChIP, using a strategy that was used by Sandoval et al.30 Chromatin was immunoprecipitated using an antibody against the large subunit of RNA polymerase II, and it was probed with primers designed to hybridize 150 bp downstream of the transcription start site. Figure 1B demonstrates that TGF-β1 treatment does not affect transcription initiation from the CFTR promoter.
To determine if TGF-β-mediated CFTR mRNA suppression is due to post-transcriptional gene silencing, NHBE ALI cultures grown on snapwells were treated with TGF-β1. Separately, another subset of cells was treated with aurintricarboxylic acid (ATA) 3 hr prior to TGF-β1 treatment. ATA is a small molecule inhibitor of DROSHA,31 the enzyme involved in pri-miRNA processing. Hence, inhibiting DROSHA will block the entire microRNA (miRNA) pathway. Snapwells were mounted in Ussing chambers, and CFTR activity was determined with albuterol, as reported earlier by us.15 Figure 1C shows that blocking the miRNA pathway rescues TGF-β-mediated CFTR functional suppression, as measured by Ussing chamber experiments. Figure 1D shows an Ussing chamber trace of the rescue of CFTR function by ATA. Upon addition of the β2-agonist albuterol, a peak CFTR response was observed, which subsides possibly due to internalization of fraction of β2-adrenergic receptors. These data show that rescue of CFTR function by ATA is a consequence of rescue of CFTR mRNA levels in NHBE ALI cultures treated with TGF-β1. The addition of CFTR inhibitors CFTRinh172 and GlyH101 completely inhibited the change in short-circuit current. Total RNA was isolated from these cells and analyzed for CFTR mRNA levels. Figure 1E shows that a rescue of CFTR function by ATA correlates with a rescue of CFTR mRNA as well. Together these data demonstrate that TGF-β mediates CFTR suppression by miRNA-mediated post-transcriptional gene silencing.
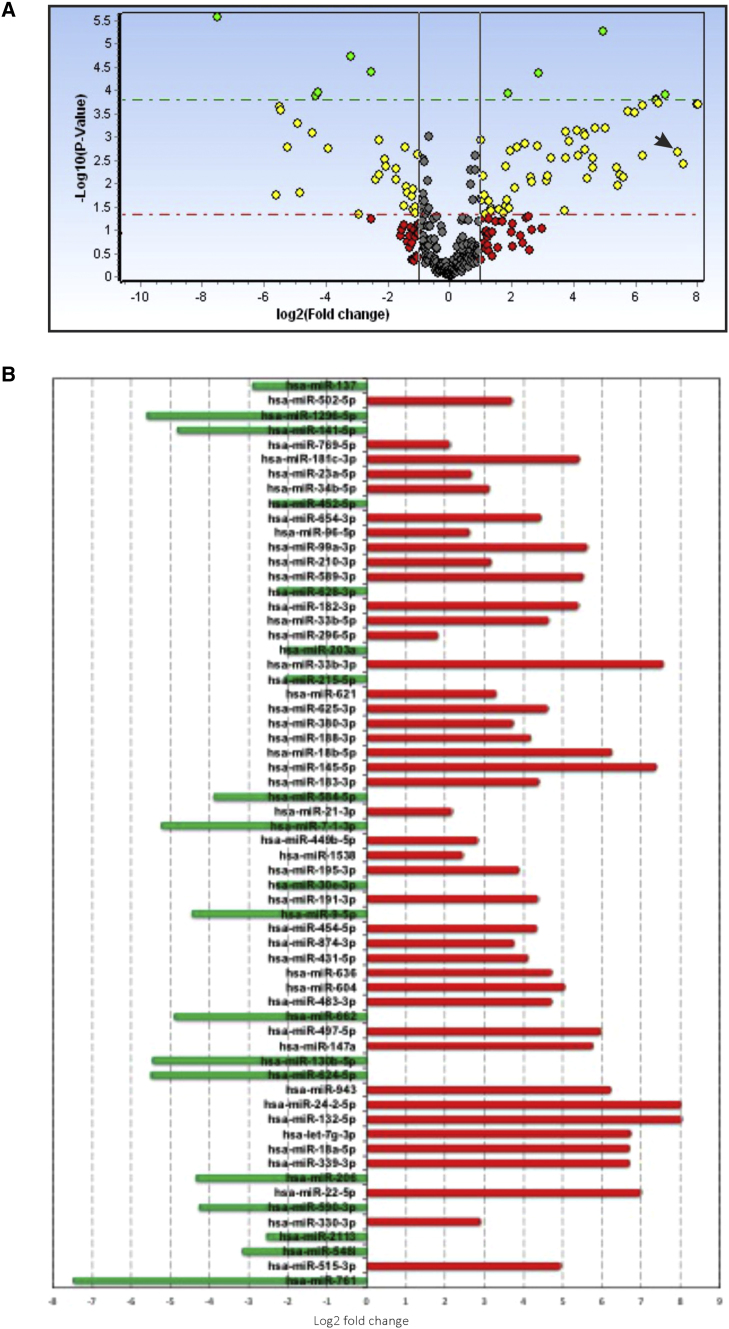
TGF-β Alters the Bronchial Epithelial MicroRNAome, Affecting Diverse Cellular Pathways
NHBE ALI cultures were treated with TGF-β1 as reported by us earlier.15 Change in miRNA expression profile was determined using the Exiqon microRNA human panel I and II, version (v.)4. This allowed us to assay 754 mature human miRNAs. Data were analyzed using the GenEx software. Figure 2A shows a volcano plot of the TGF-β1-altered microRNAome in NHBE ALI cultures. TGF-β alters the microRNAome of NHBE ALI cultures with a statistically significant Log2-fold change (p < 0.05) in the expression of 62 different miRNAs, including our previously reported miR-141-5p.32 Analysis of Log2-fold or higher changes showed that TGF-β upregulates the expression of 43 different miRNAs and downregulates the expression of 19 miRNAs (Figure 2B).
Figure 2.
TGF-β1 Alters the Bronchial Epithelial MicroRNAome
(A) Volcano plot of the altered miRNA expression profile of primary NHBE ALI cultures treated with TGF-β1. Primary bronchial epithelial cultures redifferentiated at the ALI were treated with TGF-β1 (10 ng/mL) or vehicle (as control) as described by us earlier.15 TGF-β-mediated changes in miRNA expression profile in NHBE ALI cultures were determined using Exiqon microRNA Human panel I and II, v.4 (Exiqon, Woburn, MA, USA). Data were analyzed using the GenEX software. (B) TGF-β1 shows statistically significant (p < 0.05) Log2-fold change in the expression of 62 miRNAs. Green, downregulated miRNAs; red, upregulated miRNAs. n = 3 different lungs. Arrow indicates the position of miR-145-5p.
TGF-β Suppresses CFTR via miR-145-5p-Mediated Silencing of CFTR
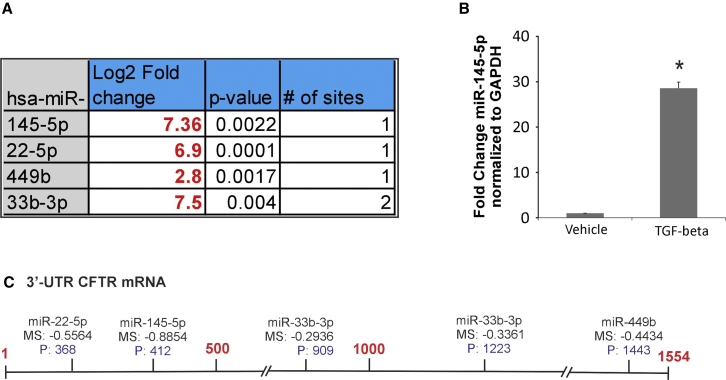
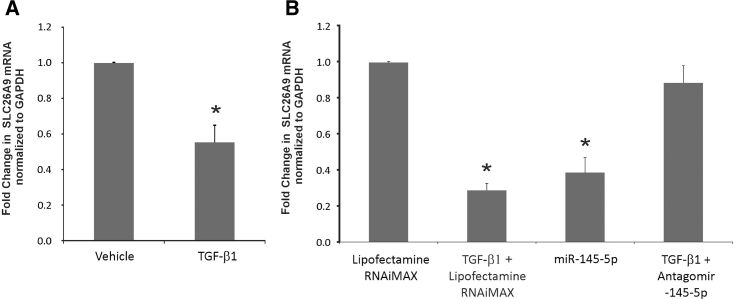
In airway epithelial cells, CFTR can be regulated by multiple different miRNAs.33, 34 miR-145-5p has been experimentally validated to suppress CFTR function, and its upregulation by TGF-β signaling has been demonstrated in lung myofibroblasts.35 More recently, Lutful Kabir et al.36 demonstrated that miR-145-5p antagonism reverses TGF-β-mediated inhibition of F508del CFTR correction in airway epithelia. Using a combination of target site prediction algorithm miRanda, miRSVR scores (<−0.5), and miRTARbase (a database of experimentally validated miRNAs), we identified 4 different miRNAs predicted to suppress CFTR function (Figure 3A). We first validated our array data to determine upregulation of miR-145-5p. NHBE ALI cultures were treated with TGF-β1, and the expression of miR-145-5p was validated using specific probes (TaqMan). Figure 3B shows that TGF-β1 treatment upregulates miR-145-5p expression. Figure 3C shows the schematic of the putative target sites of the identified miRNAs on the 3′ UTR of CFTR mRNA.
Figure 3.
Identification of Potential CFTR-Targeting miRNAs Induced by TGF-β1
(A) miRNAs targeting CFTR were identified by a combination of target site prediction algorithm miRanda (miRSVR scores <−0.5) and miRTARbase (database of experimentally validated miRNAs). miRNAs are listed in order of their scores starting with the best. (B) Array validation for miR-145-5p. NHBE ALI cultures were treated with TGF-β1 (10 ng/mL) or vehicle (as control). Total RNA was analyzed for miR-145-5p expression using qRT-PCR and normalized to GAPDH. TGF-β1 treatment upregulates miR-145-5p in NHBE ALI cultures. n = 3 different lungs. *Significant (p < 0.05) from control. (C) Schematic of the putative target sites of the identified miRNAs on the 3′ UTR of CFTR mRNA.
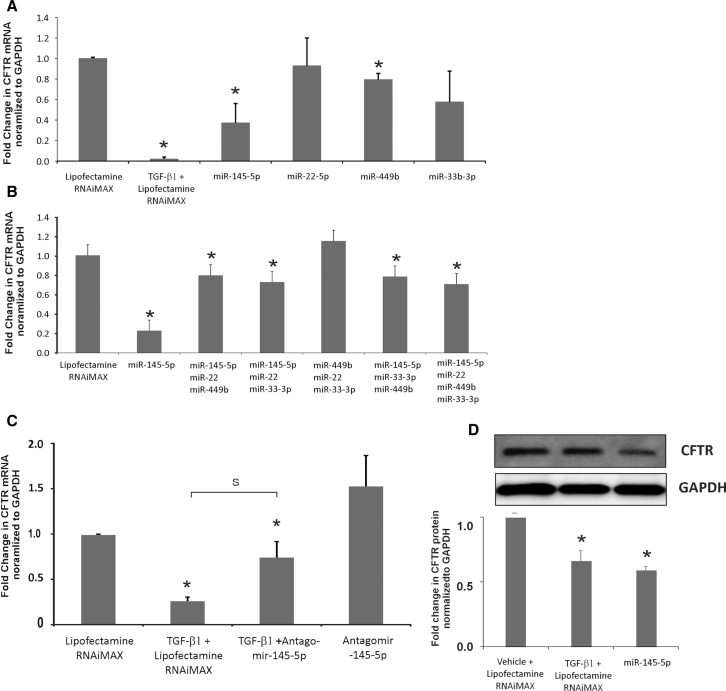
To determine if the miRNAs identified by us in Figure 3A suppress CFTR mRNA, we transfected miRNA mimics in BEAS-2B airway epithelial cells, and we looked for CFTR mRNA suppression. We preferred BEAS-2B cells for transfection experiments because BEAS-2B cells are easier to transfect and are airway epithelial in origin while also demonstrating CFTR expression. Using BEAS-2B cell lines for miRNA target validation experiments will not affect our observations, as mimic and antagomir are added exogenously. BEAS-2B cells were transfected with the respective miRNA mimics (20 nM) of the miRNAs identified in Figure 3B. Lipofectamine RNAiMAX alone was used as the control. TGF-β1 treatment was used for comparison. As seen in Figure 4A, only miR-145-5p mimic suppressed CFTR mRNA.
Figure 4.
miRNA Validation
(A) miRNA mimics for the 4 miRNAs identified in Figure 3B were tested for their ability to suppress CFTR mRNA by transient transfection in the airway epithelial cell line BEAS-2B. Lipofectamine RNAiMAX plus vehicle was used as the control and lipofectamine RNAiMAX plus TGF-β1 treatment was used for comparison. Only miR-145-5p mimic suppressed CFTR mRNA. A minimal suppression (∼10%) was observed with miR-449b. Some suppression was also observed with miR-33b-3p, but this was not found to be statistically significant. n = 5 different experiments. (B) To check for cooperative effects of miRNA, the miRNA mimics were pooled into 4 pools of 3 miRNAs each (Figure 3B). BEAS-2B cells were transfected with each of the pools. Lipofectamine RNAiMAX alone was used as the control, and lipofectamine RNAiMAX and TGF-β1-treated cells and miR-145-5p-transfected cells were used for comparison. Only the pools containing miR-145-5p showed some suppression of CFTR mRNA, and the suppression was not comparable to that observed for miR-145-5p, suggesting that the suppression by the pool was due to the presence of miR-145-5p and not any cooperative effects. n = 5 different experiments. (C) BEAS-2B cells were transfected with antagomir to miR-145-5p. Lipofectamine RNAiMAX plus vehicle was used as the control, and lipofectamine RNAiMAX plus TGF-β1 was used for comparison. At 24 hr post-transfection, cells were treated with TGF-β1 (lipofectamine RNAiMAX and TGF-β alone with lipofectamine RNAiMAX for comparison). Antagomir-145-5p rescues TGF-β1-mediated CFTR suppression, confirming its role in TGF-β-mediated CFTR mRNA suppression. n = 5 different experiments. (D) BEAS-2B cells were transfected with miR-145-5p as described. TGF-β1 and lipofectamine RNAiMAX-treated cells were used for comparison. miR-145-5p transfection suppresses CFTR protein levels comparable to that observed with TGF-β1-treated cells. *Significant (p < 0.05) from control. S, significant from each other (p < 0.05).
Since no suppression was observed by other miRNAs, we tried to determine if any miRNAs work in a cooperative manner with miR-145-5p. We made four pools of three miRNAs each. BEAS-2B cells were transfected with these miRNA pools to determine if increased or synergistic suppression was observed by the miRNA pools. miR-145-5p mimic alone was transfected for comparison. The total miRNA mimic concentration for each pool was 20 nM. As seen in Figure 4B, only the pool that had miR-145-5p showed a small suppression of CFTR, suggesting that the suppression by the pool was because of miR-145-5p and not any other miRNA. The pool in which miR-145-5p was excluded did not show any CFTR suppression.
We further tried to confirm the role of miR-145-5p in TGF-β-mediated CFTR suppression using miR-145-5p antagonism. BEAS-2B cells were transfected with an antagomir to miR-145-5p. Separately, another set of antagomir-transfected BEAS-2B cells was treated with TGF-β1. Lipofectamine RNAiMAX alone- and TGF-β plus lipofectamine RNAiMAX alone-treated cells were used for comparison. As seen in Figure 4C, antagomir to miR-145-5p rescued TGF-β-mediated CFTR mRNA suppression, confirming the role of miR-145-5p in TGF-β-mediated CFTR suppression. BEAS-2B cells were transfected with miR-145-5p mimic using lipofectamine RNAiMAX (TGF-β1 treatment was used for comparison). After 48 hr, total protein was isolated and analyzed for CFTR expression by western blot analyses. Figure 4D shows that miR-145-5p mimic suppresses CFTR protein levels as well, comparable to that observed with TGF-β1-treated BEAS-2B cells.
TGF-β Signaling Suppresses SLC26A9 via miR-145-5p-Mediated Gene Silencing
miR-145-5p is an extensively studied miRNA and plays an important role as a tumor suppressor. Its expression is regulated by multiple signaling pathways.37 We tried to determine if miR-145-5p can alter the expression of other genes involved in CFTR expression and function. Using miRanda and miRSVR algorithms for target identification, we identified SLC26A9, a constitutively active Cl− channel,38 as a potential target of miR-145-5p (miRSVR score of −0.99; Figure S1). SLC26A9 plays an important role in CFTR expression and function,26 and it also modulates airway response to CFTR-directed therapeutics.28 Likewise, defects in CFTR protein inhibit the function of SLC26A9.39 The mechanism of SLC26A9 regulation is poorly understood, with one report suggesting that lysine-deficient protein kinases (WNKs) inhibit the expression of SLC26A9.40 However, none of the reports has demonstrated TGF-β-mediated suppression or the role of miRNA in the regulation of SLC26A9.
Since TGF-β1 upregulates miR-145-5p, we tried to determine if TGF-β signaling also suppresses SLC26A9. NHBE ALI cultures were treated with TGF-β1, and total RNA was analyzed for SLC26A9 expression by qRT-PCR. As seen in Figure 5A, TGF-β1 suppresses the expression of SLC26A9. Next, we tried to determine if SLC26A9 is a target of miR-145-5p. BEAS-2B cells were transfected with mimic or an antagomir to miR-145-5p. At 24 hr post-transfection with antagomir, transfected cells were treated with TGF-β1. Lipofectamine RNAiMAX alone- and TGF-β1 plus lipofectamine RNAiMAX-treated cells were used for comparison. Experiments were terminated after a further 16 hr, and total RNA was analyzed for SLC26A9 expression using specific TaqMan probes. As seen in Figure 5B, transfection with miR-145-5p mimic suppressed SLC26A9, comparable to that observed with TGF-β1 treatment, and miR-145-5p antagonism rescued SLC26A9 in TGF-β1-treated BEAS-2B cells, confirming the TGF-β signaling ⇒ miR-145-5p ⇒ SLC26A9 pathway.
Figure 5.
TGF-β Suppresses the Expression of SLC26A9 by miR-145-5p-Mediated Silencing
(A) NHBE cultures redifferentiated at the ALI were treated with TGF-β (10 ng/mL) apically and basolaterally. 16 hr post-treatment, total RNA was isolated and SLC26A9 mRNA levels were determined. TGF-β signaling suppresses the expression of SLC26A9. n = 3 different lungs. (B) To determine the role of miR-145-5p in this suppression, BEAS-2B cells were transfected with miR-145-5p mimic. Lipofectamine RNAiMAX alone was used as the control. Another subset of cells was transfected with antagomir to miR-145-5p. 24 hr post-transfection, cells were treated with TGF-β1 (lipofectamine RNAiMAX and TGF-β alone for comparison). miR-145-5p mimic suppresses SLC26A9 expression while antagomir-145-5p rescues TGF-β-mediated SLC26A9 suppression, confirming the role of miR-145-5p in TGF-β-mediated SLC26A9 mRNA suppression. n = 5 experiments. *Significant (p < 0.05) from control.
Non-CF A/J Mice Exposed to CS Suppress CFTR and SLC26A9 mRNA with a Concomitant Increase in miR-145-5p Expression
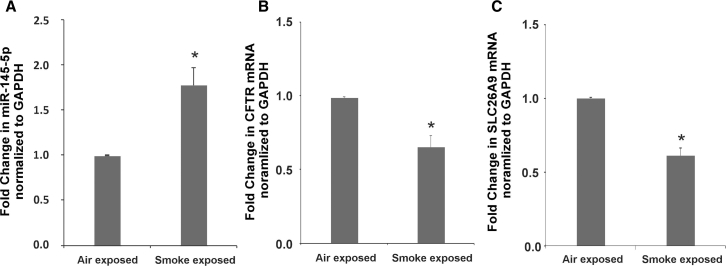
CS exposure leads to an acquired state of CFTR dysfunction in smokers with normal copies of the CFTR gene.19, 20 A number of reports have demonstrated that CS upregulates TGF-β signaling in airway epithelial cells.15, 41, 42 We have demonstrated that CS suppresses CFTR expression and function via TGF-β signaling.15 We have also shown that chronic smoke exposure leads to CFTR suppression in non-CF A/J mice.43 We tried to determine if CS also upregulates miR-145-5p to suppress CFTR and SLC26A9. Non-CF A/J mice were exposed to air or CS. Following 5 weeks of smoke exposure, mice were sacrificed, and total RNA was isolated from lungs and analyzed for CFTR, SLC26A9, and miR-145-5p expressions. As seen in Figure 6, CS upregulates miR-145-5p (Figure 6A) with a concomitant suppression of CFTR (Figure 6B). Upregulation of miR-145-5p also translates to a suppression of SLC26A9 in smoke-exposed mice (Figure 6C).
Figure 6.
Cigarette Smoke Upregulates miR-145-5p with Concomitant Suppression of CFTR and SLC26A9 mRNA in Mouse Models of Acquired CFTR Dysfunction
(A) Non-CF A/J mice were exposed to cigarette smoke for 5 weeks. Mice exposed to room air were used as controls. Mice were sacrificed and lungs were obtained. Total RNA was isolated from lungs and analyzed for miR-145-5p. Smoke exposure upregulates miR-145-5p in mouse lung samples. (B andC) Total RNA was also analyzed for CFTR and SLC26A9 expression using specific TaqMan probes designed to amplify mouse genes. Cigarette smoke exposure decreases mRNA levels of CFTR (B) and SLC26A9 (C). Values were plotted as fold change normalized to GAPDH. n = 8 mice per group. *Significant (p < 0.05) from air-exposed mice.
Neutralizing Aptamer to Transforming Growth Factor Beta Receptor-2 Rescues CFTR Suppression in NHBE ALI Cultures Exposed to Cigarette Smoke or Treated with TGF-β1
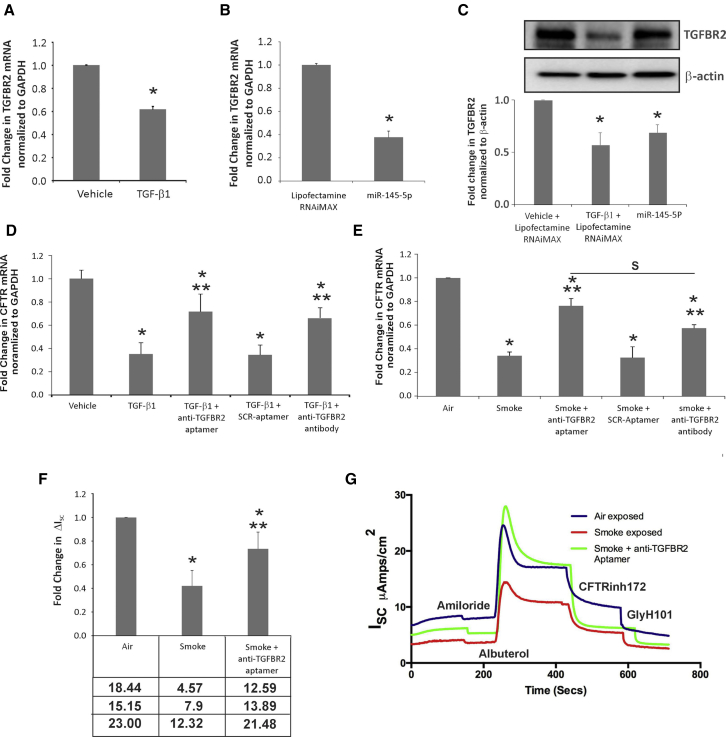
Transforming growth factor beta receptor-2 (TGFBR2) is one of the experimentally validated targets of miR-145-5p.44, 45 Hence, the upregulation of miR-145-5p should suppress TGFBR2. To determine if this is the case, NHBE cultures redifferentiated at the ALI were treated with TGF-β1 (10 ng/mL) apically and basolaterally. At 16 hr post-treatment, total RNA was isolated and TGFBR2 mRNA levels determined by qRT-PCR. As seen in Figure 7A, TGF-β1 suppresses TGFBR2 levels in NHBE ALI cultures.
Figure 7.
Anti-TGFBR2 Aptamer Rescues TGF-β- and Cigarette Smoke-Mediated CFTR Suppression
(A) TGF-β signaling suppresses TGFBR2 expression in NHBE ALI cultures. 16 hr post-TGF-β1 treatment, total RNA was isolated and TGFBR2 mRNA levels were determined. TGF-β1 signaling suppresses the expression of TGFBR2. (B) BEAS-2B cells were transfected with miR-145-5p mimic (lipofectamine RNAiMAX as control). BEAS-2B cells transfected with miR-145-5p mimic show suppression of TGFBR2 mRNA. (C) BEAS-2B cells were transfected with miR-145-5p mimic (lipofectamine RNAiMAX as control; TGF-β1 plus lipofectamine RNAiMAX for comparison). miR-145-5p suppresses TGFBR2 protein levels as observed by western blot analyses. (D) TGFBR2 aptamer rescues TGF-β-mediated suppression of CFTR function. NHBE ALI cultures were treated with TGF-β1 or vehicle. Separately, a subset of cells treated with TGF-β1 was pretreated with anti-TGFBR2 neutralizing aptamer (or scrambled aptamer [SCR] as the control), reported by Zhu et al.46 TGFBR2-neutralizing antibody was used for comparison. Anti-TGFBR2 aptamer rescues TGF-β signaling-mediated CFTR mRNA suppression possibly by blocking the receptor and preventing TGF-β signaling. (E) NHBE ALI cultures were exposed to cigarette smoke (or air as the control) using a smoke regimen described by us.15 Separately, a subset of cigarette smoke-exposed cells was treated with anti-TGFBR2 aptamer (or scrambled aptamer [SCR] as the control). TGFBR2-neutralizing antibody was used for comparison. TGFBR2 aptamer rescued cigarette smoke effects on CFTR mRNA comparable to that observed with the TGFBR2-neutralizing antibody. (F and G) NHBE ALI cultures grown on snapwells were exposed to cigarette smoke (or air as the control) using the smoke regimen described by us.15 Separately, a subset of cigarette smoke-exposed cells was treated with anti-TGFBR2 aptamer. (F) CFTR function was determined in Ussing chambers (ΔISC). TGFBR2 aptamer rescues smoke-induced suppression of CFTR function, possibly by rescuing CFTR mRNA suppression. The table below the graph shows actual ΔISC values for the experiment. (G) An Ussing chamber trace for anti-TGFBR2 aptamer-mediated rescue of CFTR function. n = 3 lungs *Significant from vehicle; **significant from TGF-β1 and/or CS exposed.
To determine if TGFBR2 is a target of miR-145-5p mimic, BEAS-2B airway epithelial cell lines were transfected with miR-145-5p mimic (lipofectamine RNAiMAX as the control). Transfection with miR-145-5p mimic likewise suppressed TGFBR2 mRNA in BEAS-2B cells (Figure 7B). However, western blot analyses showed that significant levels of TGFBR2 protein were still available to mediate TGF-β signaling (Figure 7C). This could explain continued CFTR suppression even when TGFBR2 levels were downregulated in the context of increased miR-145-5p. Hence, neutralizing TGFBR2 at the surface can be considered a good strategy in airway diseases where TGF-β signaling (and miR-145-5p) is upregulated, as this can synergize with miR-145-5p-mediated suppression of TGFBR2 to maximize CFTR rescue.
In our previous work, we demonstrated that a TGFBR2-neutralizing antibody rescues CFTR function in NHBE ALI cultures.15 We tried to rescue CFTR mRNA suppression in TGF-β-treated and smoke-exposed cells using a neutralizing aptamer reported by Zhu et al.46 Aptamers are high-affinity single-stranded nucleic acid ligands that exhibit specificity and avidity comparable to or exceeding that of antibodies, and they can be generated against most targets.47, 48 NHBE ALI cultures were pretreated with recombinant TGF-β1 or vehicle as described by us.15 Separately, another subset of cells was pretreated with TGFBR2-neutralizing aptamer reported by Zhu et al.46 (scrambled aptamer [SCR] as the control). TGFBR2-neutralizing antibody was used for comparison. At 16 hr following TGF-β1 treatment, total RNA was analyzed for CFTR mRNA levels by qRT-PCR. As seen in Figure 7D, anti-TGFBR2 aptamer rescued TGF-β-mediated CFTR mRNA suppression comparable to that observed by TGFBR2-neutralizing antibody.
To determine if the aptamer can rescue the effects of CS on CFTR expression, NHBE ALI cultures were exposed to CS as described by us earlier.15 Separately, another set was pre-treated with anti-TGFBR2-neutralizing aptamer (or scrambled aptamer [SCR] as the control) 3 hr prior to smoke exposure, and it was retained for the remainder of the experiment. At 16 hr following smoke exposure, total RNA was analyzed for CFTR mRNA levels by qRT-PCR. As seen in Figure 7E, TGFBR2-neutralizing aptamer rescued CS-mediated suppression on CFTR mRNA comparable to that observed with the TGFBR2-neutralizing antibody.
Finally, we tried to determine if CFTR mRNA rescue by the TGFBR2-neutralizing aptamer translates to a functional rescue of CFTR. NHBE ALI cultures grown on snapwells were similarly exposed to CS in the presence or absence of anti-TGFBR2 aptamer. Cells were mounted in an Ussing chamber, and CFTR function was determined by the addition of albuterol, as reported by us earlier.15, 49 As seen in Figure 7F, anti-TGFBR2 aptamer significantly rescued the effects of CS on CFTR function. Figure 7G shows an Ussing chamber trace of the rescue of CFTR function by anti-TGFBR2 aptamer in smoke-exposed NHBE ALI cultures grown on snapwells.
Discussion
Lung infections are a significant co-morbidity in smokers and COPD patients.50, 51 COPD and recurrent lung infections also increase the risk of lung cancer.52 CS and COPD lead to impaired MCC, thereby promoting microbial colonization and lung infections. The etiology of pneumonias associated with COPD and tobacco smoking is similar to that seen in cystic fibrosis.53, 54 CFTR dysfunction (and MCC dysfunction) plays an important role in the early pathogenesis of these chronic airway diseases.55, 56 CFTR tightly regulates the ASL height, which is crucial for mediating ciliary beating and MCC rates,7, 8 and its dysfunction can compromise ASL and ciliary beat frequency (CBF) decreasing MCC rates.57 CFTR-mediated HCO3− secretion maintains ASL pH16, 17 and mucus viscosity18 while also regulating airway antimicrobial host defenses.58, 59 CFTR plays an important antibacterial role in the innate immune response mediated by the lactoperoxidase-thiocyanate-H2O2 antibacterial system by secreting thiocyanate (SCN(−)9, 10). Decreased SCN(−) secretion in cystic fibrosis patients has been implicated in lung infections.60, 61, 62 CFTR also secretes reduced glutathione, a key redox buffer to regulate H2O2 levels in the ASL.63 Hence, CFTR suppression can have global effects on airway innate immunity by suppressing mucus clearance and antimicrobial responses, setting up a vicious cycle of infection, inflammation, and injury.64, 65, 66
TGF-β signaling is increased in chronic airway diseases like chronic bronchitis, asthma, and COPD,22, 23, 24, 25 and TGF-β levels correlate with the severity of obstruction.25, 67 We have shown that TGF-β signaling is also induced by HIV Tat. TGF-β1 increases HIV infection of bronchial epithelial cells and also leads to CFTR suppression.32, 49 TGF-β isoforms are expressed and secreted by several cell types in the airway, including epithelia.68 We have already demonstrated that TGF-β and CS (via TGF-β) signaling suppresses CFTR mRNA expression, which translates to a functional suppression.
In this study, we tried to determine the mechanism by which TGF-β signaling suppresses CFTR mRNA and if targeting TGF-β signaling or the intermediates can rescue CFTR function in the context of CS or TGF-β signaling. We first tried to determine if CFTR mRNA suppression is due to a decreased transcription from the CFTR promoter (transcriptional suppression) and/or a contribution of epigenetic silencing if any. Our data demonstrated that TGF-β does not affect transcriptional initiation from the CFTR promoter, suggesting that the effect was not due to transcriptional suppression, further positing the role of post-transcriptional silencing mechanisms. TGF-β signaling is known to alter miRNA homeostasis by directly acting on components of the miRNA-processing pathway.69, 70 CFTR also has a longer (∼1.5 kb) than average 3′ UTR, which strongly correlates with miRNA regulation.71 Hence, we determined if TGF-β signaling suppresses CFTR mRNA by miRNA-mediated silencing. Our data demonstrate that ATA, a small molecule inhibitor of the miRNA-processing enzyme DROSHA, completely reverses the effects of TGF-β on CFTR mRNA with a concomitant rescue of CFTR function. We used the β2-agonist albuterol to study the effects on CFTR activation, as β2-agonists are routinely prescribed during exacerbations or used as maintenance medications in chronic airway diseases. β2-adrenergic receptor physically interacts with CFTR,72 and it has been shown to activate mucociliary clearance73 by activating CFTR as well as ciliary beat frequency,15, 74 suggesting that β2-agonsits can also serve in a dual role of increasing CFTR activity and mucociliary clearance.
We next performed an miRNA array to determine mature miRNAs dysregulated by TGF-β signaling. An miRNA array of NHBE ALI cultures treated with TGF-β1 demonstrates that TGF-β signaling significantly alters the microRNAome by dysregulating the expression of 62 different miRNAs, which can impact the signaling of several different pathways involved in lung diseases. Specifically, we observed altered expression of four different miRNAs that can potentially regulate CFTR, including miR-145-5p reported by Gillen et al.75 and others.36 Using multiple experiments involving mimics, individually and in pools, we confirmed that, of the four miRNAs, only miR-145-5p suppresses CFTR. We further confirmed the role of miR-145-5p using antagomir to miR-145-5p to rescue TGF-β-mediated CFTR mRNA suppression. Using miRNA-target algorithms, we predicted and then confirmed that miR-145-5p can also suppress SLC26A9.
None of the reports to date has identified miRNA-mediated suppression of this very important CFTR-modifying chloride channel. The deleterious effects of miR-145-5p on SLC26A9 can be 2-fold, considering that miR-145-5p directly regulates SLC26A9 while CFTR suppression by miR-145-5p could affect its localization to the surface.39 Our experiments validate the role of miR-145-5p, and they also suggest a potential therapeutic approach to rescue CFTR and SLC26A9 suppression. Not surprisingly, our data confirm observations of other groups that miR-145-5p mimic (and TGF-β1 treatment in NHBE ALI cultures) suppresses TGFBR2 mRNA and protein levels. However, we were not able to detect this suppression in smoke-exposed mice (data not shown). This could be due to other signaling mechanisms induced by CS that may counter this suppression. However, most COPD patients have already quit smoking, but the chronic inflammation in the airways and the resultant TGF-β signaling would manifest as decreased TGFBR2 levels in airway epithelial cells.
Lutful Kabir et al.36 have suggested that antagomir to miR-145-5p rescues TGF-β signaling-mediated inhibition of F508del CFTR correction in airway epithelia. However, this approach can have several limitations in clinical application, as miR-145-5p is a potent tumor suppressor in multiple cancers (for a review, see Cui) and the inhibition of miR-145-5p can have adverse effects. Moreover, any therapeutic effects of miRNA antagonism will require uptake of antagomir by airway bronchial cells, which is difficult with current delivery mechanisms. A neutralizing antibody or an aptamer on the other hand that acts on the cell surface to modulate TGF-β signaling in the airway is more clinically feasible. We have already demonstrated that anti-TGFBR2 antibody reverses the effects of CS on CFTR in NHBE ALI cultures. Our western blot data demonstrate that significant levels of TGFBR2 remain even after TGF-β1 treatment or miR-145-5p transfection.
We tested a nucleic acid-based aptamer to determine if this can have effects similar to that of the antibody. Aptamers are high-affinity single-stranded nucleic acid ligands, which exhibit specificity and avidity comparable to or exceeding that of antibodies, and they can be generated against most targets.47, 48 Unlike antibodies, aptamers can be synthesized chemically, and, hence, they offer significant advantages in terms of production cost, simpler regulatory approval, and lower immunogenicity even when administered in preclinical doses 1,000-fold greater than those necessary for animal and human therapeutic application.76, 77 TGFBR2-neutralizing aptamer can cooperate with miR-145-5p-mediated suppression of TGFBR2 expression, by neutralizing the diminished receptor levels at the surface, and synergistically rescue CFTR and SLC26A9 in smokers and chronic airway diseases. Our data show that the anti-TGFBR2 aptamer rescues the effects of TGF-β signaling- and CS-mediated decreases in CFTR mRNA and this rescues CFTR function in NHBE ALI cultures exposed to CS. While we did not get a complete rescue of CFTR function, this could be due to transient effects of CS on surface CFTR, independent of TGF-β signaling.56 Aptamer-based therapeutics have several advantages over small molecule TGF-β inhibitors, as the size and cation selectivity of airway epithelial tight junctions78, 79 will prevent transmigration across the epithelium and restrict TGF-β inhibition to the airway.
In conclusion, this study demonstrates that miR-145-5p plays a crucial role in TGF-β-mediated suppression of CFTR and SLC26A9 mRNA. A neutralizing aptamer to TGFBR2 can reverse these effects to rescue CFTR function. Rescue of CFTR function can restore CFTR functions in airway innate immunity and redox balance, thereby decreasing the incidence of recurrent lung infections and disrupting the cycle of infection, inflammation, and MCC dysfunction.
Materials and Methods
Chemical and Reagents
5-aza-CdR and ATA were purchased from Sigma-Aldrich (St. Louis, MO, USA). TGF-β1 (Recombinant Human Protein) was from Life Technologies. The high-capacity cDNA reverse transcription kit was from Applied Biosystems. Taqman Fast Advanced Master Mix was from Life Technologies. Antibody against the large subunit of RNA polymerase, RPB1, was from Santa Cruz Biotechnology (sc-899). Lipofectamine RNAiMAX Transfection Reagent and Opti-MEM Reduced-Serum Medium were purchased from Thermo Fisher Scientific (13778150 and 31985062). Mimics of hsa-miR-145-5p, -22-5p, -449b, and -33b-3p, were purchased from Sigma-Aldrich (Mission miRNA mimics; St. Louis, MO, USA). Antagomir to miR-145-5p was purchased from Integrated DNA Technologies (IDT, Coralville, IA, USA). Likewise, the TGFBR2 aptamer was custom synthesized by IDT. Amiloride (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.1% DMSO (Sigma-Aldrich) to make a concentration of 10 mM. Albuterol hemisulfate (also known as salbutamol; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sterile water to make a concentration of 10 mM. CFTR(inh)-172 (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in DMSO to make a concentration of 20 nM.
NHBE ALI Cultures and Cell Lines
Primary human bronchial epithelial cells were isolated and redifferentiated at the ALI cultures, as described by Fulcher et al.80, 81 and adapted by us.15, 82 Cells were obtained from properly consented donors, whose lungs were not suitable for transplantation for causes unrelated to airway complications, and supplied by the University of Miami Life Alliance Organ Recovery Agency. Since the material was obtained from deceased individuals with minor, de-identified information, its use does not constitute human subject research, as defined by CFR 46.102. A signed and well-documented consent of each individual or legal healthcare proxy for the donation of lungs for research purpose is on file with the Life Alliance Organ Recovery Organization and allows research purpose of this material.
Unless otherwise mentioned, experiments used cells from non-smokers to not confound the findings in unknown ways. These primary cultures undergo mucociliary differentiation at the ALI, reproducing both the in vivo morphology and key physiologic processes to recapitulate the native bronchial epithelium ex vivo.80, 81 All experiments with NHBE used cultures redifferentiated at the ALI. With the exception of Ussing chamber experiments, NHBE cultures were redifferentiated at the ALI in 10-mm transwell filers (Corning 3460). For Ussing chamber experiments, NHBE cells were redifferentiated on snapwell filters (Corning 3801). The immortalized NHBE cell line BEAS-2B (ATCC CRL-9609; immortalized with the T SV40 antigen) was purchased from the American Type Culture Collection (Manassas, VA, USA). BEAS-2B cells were cultured in BioLite 75-cm2 flasks (130190, Thermo Scientific) containing bronchial epithelial cell growth medium (BEGM) and were not differentiated. BEGM was supplemented with 0.1% (v/v) human recombinant epidermal growth factor, 0.1% (v/v) insulin, 0.1% (v/v) hydrocortisone, 0.1% (v/v) ethanolamine, 0.1% phosphoryl ethanolamine, 0.1% (v/v) retinoic acid, 0.1% (v/v) epinephrine, 0.24% (v/v) transferrin, 1% (v/v) penicillin-streptomycin, and 0.1% (v/v) bovine pituitary extract, as reported by Fulcher et al.80 The cells were cultured in 95% air and 5% CO2 at 37°C and maintained free of mycoplasma contamination.
Animal Experiments
All animal protocols were reviewed and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. A/J mice aged 6–8 weeks old and expressing wild-type CFTR (+/+) were evenly divided along gender for all studies. As previously reported,83 mice were exposed in whole-body chambers (28 × 19 × 15 in) to diluted mainstream CS (up to 200 μg/L of total particulate matter, 35 mL puffs of 2-s duration at a rate of 3 L/s each minute for 40 min) from 3R4F reference cigarettes (University of Kentucky, Lexington, KY) for 2 sessions/day for 5 weeks, using a computer-controlled CS generator (SCIREQ, InExpose model, Montreal, QC, Canada). Control mice were exposed to room air in same-sized chambers. Characterizations of whole CS exposures (e.g., volumetric flow rate calibration, aerosol concentration, and particle size distribution) were previously reported.43 Animals were humanely euthanized, and freshly isolated lung sections were flash frozen in liquid nitrogen for expression analyses.
TGF-β1 Treatment of NHBE ALI Cultures
Recombinant TGF-β1 (R&D Systems, 240-B-002) was dissolved according to the manufacturer’s instructions at a stock concentration of 10 μg/μL. A working dilution was prepared at 10 ng/mL in ALI media. ALI media containing TGF-β1 or vehicle were added basolaterally and apically (50 μL added apically) to mimic physiological conditions. While one report demonstrated expression of TGFBR1 on the basolateral side,84 a number of reports have demonstrated TGF-β1 on the mucosal (apical) side under physiological conditions.85 Likewise, TGF-β has been demonstrated in the bronchoalveolar lavage (BAL) fluid in both healthy and CF subjects, suggesting that at least a fraction of TGF-β signaling also occurs from the apical side.86 The concentration of TGF-β1 used was within the mean physiological range (2–20 ng/mL) suggested by Sun et al.87
Treatment with 5-aza-CdR
NHBE ALI cultures were pretreated with 5-aza-CdR for 24 hr and retained for the remainder of the experiment. Recombinant TGF-β1 (10 ng/mL) was added apically and basolaterally. At 16 hr post-treatment of TGF-β1, total RNA was isolated and CFTR mRNA levels were determined by qRT-PCR.
ATA Treatment in NHBE ALI Cultures
NHBE ALI cultures grown on snapwells were treated with TGF-β1, and, separately, cells were treated with ATA (25 μL/mL) 3 hr prior to TGF-β1 treatment and retained for the remainder of the experiment. At 16 hr post-treatment, cells were mounted in Ussing chambers, Cl− efflux in response to albuterol addition was determined as an index of CFTR function, and CFTR mRNA from these cells was analyzed by qRT-PCR.
CS Exposure
NHBE ALI cultures were exposed to air or CS using a SCIREQ smoke robot (Montreal, QC, Canada). Four research-grade cigarettes (University of Kentucky) were smoked, with a puff volume of 35 mL for 2 s every 60 s, and blown over cell culture filter at rate of 5 mL/min, according to ISO 3308. Control cells were similarly treated without smoke (air control). We have used this regimen before, and the regimen was not found to affect the viability or trans-epithelial electrical resistance of NHBE ALI cultures (data not shown).15 Cells were allowed to recover for 16 hr before initiating experiments.
ChIP Assay
ChIP was performed using a commercially available ChIP kit (Chroma Flash high sensitivity ChIP kit, Epigentek), where NHBE ALI cultures were treated with recombinant TGF-β1 (10 ng/mL) or vehicle. At 16 hr post-treatment of TGF-β1, chromatin from vehicle and TGF-β1-treated NHBE cells was precipitated using an antibody against the large subunit of RNA polymerase, RPB1 (Santa Cruz Biotechnology, sc-899), according to the manufacturer’s instructions. The amount of immunoprecipitated DNA was quantified by qPCR using primers designed to hybridize 150 bp downstream of the transcription start site (5′ primer: 5′-GAGGCTGGGAGTCAGAATCGG-3′ and 3′ primer: 5′-CATGGTCTCTCGGGCGCTGGGGT-3′).
Electrophysiology Experiments
Ussing chambers were used to determine CFTR activation as reported by our lab previously.49 Briefly, NHBE cultures redifferentiated at the ALI on snapwells were mounted in EasyMount Chambers (Physiologic Instruments, San Diego, CA) with Krebs Henseliet (KH) in apical and basolateral chambers. KH consisted of 118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 1.2 mM CaCl2, and 5.5 mM glucose (pH 7.35) when gassed with 95% O2/5% CO2. Solutions were maintained at 37°C by heated water jackets, and they were continuously bubbled with a 95% O2/5% CO2 mixture.
To monitor the short-circuit current (ISC), the transepithelial membrane potential was clamped at 0 mV with a single-channel voltage-current clamp (VCC600, Physiologic Instruments), using Ag-AgCl electrodes in agar bridges. Signals were digitized and recorded with DAQplot software (Acquire and Analyze v. 2.3.300, Physiologic Instruments). Amiloride (10 μM) was added apically to inhibit epithelial sodium channel influences. CFTR activation was measured by the addition of albuterol (10 μM) as described by us before.49 Albuterol was used for CFTR activation to maintain conformity with our earlier report15 and for potential therapeutic use in CFTR dysfunction, given that β2-agonists are already prescribed in these diseases. Change in short circuit current (ΔISC) in response to albuterol was determined as an indicator of CFTR activity. CFTR currents were confirmed by the addition of CFTRinh172. GlyH101, another inhibitor of CFTR and SLC26A9, was added to inhibit residual CFTR currents as well as SLC26A9.39 All additions were prepared at 1,000× stock solution. Albuterol hemisulfate was dissolved in sterile water. Amiloride, CFTR inhibitor CFTRinh172, and GlyH101 were dissolved in DMSO to make a 1,000× stock. The total DMSO concentration at the end of experiments was 0.003%.
Microarray Screening of Cellular miRNAs
Exiqon miRCURY Ready-to-Use PCR Human panel I and II, v.4 (Exiqon, Woburn, MA, USA) was used to detect different expression profiles of miRNA between vehicle as control and TGF-β1 as treatment. 5 ng/μL of each template RNA from NHBE cells was reverse transcribed using the miRCURY LNA Universal RT cDNA Synthesis Kit (Exiqon, 203301). The cDNA template was then amplified using the miRNA Ready-to-Use PCR, Human Panel I + II (Exiqon, 203615) in 384-well plates, according to the manufacturer’s instructions. The qPCR reactions were run on a CFX384 (Bio-Rad) using the thermal-cycling parameters recommended by Exiqon (denaturation at 95°C 10 min and 40 amplification cycles at 95°C for 10 s and 60°C for 1 min). The relative quantification of miRNA expression levels was performed using the delta Cq method. Ct value <37 and assays detected with 5 Cts less than the negative control (no template control [NTC]) were allowed for analysis. The amplification curves were analyzed by GenEX software (Exiqon). From the 768 wells, 754 miRNA primer sets were used for miRNA expression profiling; the remaining wells contained interplate calibrator oligonucleotides, spike-in control oligonucleotides for quality controls. Targeted miRNAs that were identified from the initial screen phase were subjected to validation through RT and qRT-PCR according to the manufacturer’s protocol (Taqman MicroRNA Reverse Transcription Kit, PN 4366596 and PN 4366597).
Transfection of miRNA Mimics and Antagomir in BEAS-2B Cells
BEAS-2B cells were transfected with miRNA mimics or antagomir to miR-145-5p using lipofectamine RNAiMAX, according to the manufacturer’s instructions. 20 nM of each mimic individually or as pools was transfected in BEAS-2B cells. Cells were harvested 48 hr post-transfection for RNA isolation and qRT-PCR. In the case of TGF-β1 treatment for comparison, cells were treated with lipofectamine RNAiMAX at the same time as mimic or antagomir transfection. At 24 hr following treatment, 10 ng/mL TGF-β1 (or vehicle) was added. Experiments were terminated after an additional 24 hr for qRT-PCR analysis.
mRNA Extraction and qRT-PCR
Total RNA was extracted from NHBE cells or BEAS-2B cells following the termination of experiments using an RNeasy mini kit (QIAGEN, Valencia, CA). The concentration and integrity of the extracted RNA were analyzed by measurement of the OD260/280 (Synergy HTX Multi-Mode Microplate Reader, Winooski, VT, USA). cDNA was reverse transcribed using the Applied Biosystems High performance kit (Carlsbad, CA). Reverse transcription of 2 μg total cellular RNA was performed in a final volume of 20 μL containing 10 μL RNA, 2 μL 10× RT buffer, 0.8 μL deoxyribonucleotide triphosphate (dNTP) mix (100 mM), 2.0 μL 10× RT random hexamer primers, 1.0 μL MultiScribe reverse transcriptase, 1 μL RNase inhibitor, and 3.2 μL nuclease-free water. The reverse transcription reaction conditions were 25°C for 10 min, 37°C for 120 min, and 85°C for 5 s. cDNA samples were stored at −20°C. qPCR for reverse-transcribed cDNA was performed on the Bio-Rad CFX96 real-time system (Bio-Rad, Hercules, CA, USA), using validated TaqMan probes (GAPDH, Hs02758991_g1; CFTR, HS00357011-m1; and hsa-miR-145-5P, PM11480) and cycling conditions specified by the manufacturer. Gene-specific or miRNA expression was normalized to GAPDH. The results were determined using the delta-delta method and expressed as fold change.
Statistical Analysis
Unless otherwise mentioned, data were expressed as mean ± SEM from NHBE ALI cultures from at least three lungs. The data were subjected to statistical analysis using unpaired t tests for two groups or ANOVA followed by Tukey Kramer honestly significant difference test for multiple comparisons as appropriate. The significance was considered at the level of p < 0.05.
Author Contributions
Drafting Manuscript, R.K.D. and H.J.U.; Performing Experiments, R.K.D., L.R., and S.C.; Analysis and Interpretation, R.K.D., S.V.R., and H.J.U.; Designing Experiments, S.V.R. and H.J.U.; Conception and overall Design, H.J.U.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
We would like to acknowledge Dr. Barbara R. Gould (Qiagen) for help with analysis of the microRNA qPCR array data. This work was supported by funding from the Flight Attendant Medical Research Institute (FAMRI) grant CIA150086 to H.U. S.V.R. was supported by American Lung Association grant RG-305752 and FAMRI grant YFA130008.
Footnotes
Supplemental Information includes one figure and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.11.017.
Supplemental Information
References
- 1.Wanner A., Salathé M., O’Riordan T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T. Antimicrobial polypeptides in host defense of the respiratory tract. J. Clin. Invest. 2002;109:693–697. doi: 10.1172/JCI15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanner A. Mucociliary clearance in the trachea. Clin. Chest Med. 1986;7:247–258. [PubMed] [Google Scholar]
- 4.Gibson R.L., Burns J.L., Ramsey B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 5.Boucher R.C. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur. Respir. J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 6.Wilson R., Dowling R.B., Jackson A.D. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur. Respir. J. 1996;9:1523–1530. doi: 10.1183/09031936.96.09071523. [DOI] [PubMed] [Google Scholar]
- 7.Boucher R.C. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliv. Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 8.Boucher R.C. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 9.Dawson D.C., Smith S.S., Mansoura M.K. CFTR: mechanism of anion conduction. Physiol. Rev. 1999;79(1, Suppl):S47–S75. doi: 10.1152/physrev.1999.79.1.S47. [DOI] [PubMed] [Google Scholar]
- 10.Conner G.E., Wijkstrom-Frei C., Randell S.H., Fernandez V.E., Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581:271–278. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson M., Bye P.T. Mucociliary clearance in cystic fibrosis. Pediatr. Pulmonol. 2002;33:293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 12.Smith J.J., Karp P.H., Welsh M.J. Defective fluid transport by cystic fibrosis airway epithelia. J. Clin. Invest. 1994;93:1307–1311. doi: 10.1172/JCI117087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarran R., Grubb B.R., Gatzy J.T., Davis C.W., Boucher R.C. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J. Gen. Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinton P.M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990;4:2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- 15.Unwalla H.J., Ivonnet P., Dennis J.S., Conner G.E., Salathe M. Transforming growth factor-β1 and cigarette smoke inhibit the ability of β2-agonists to enhance epithelial permeability. Am. J. Respir. Cell Mol. Biol. 2015;52:65–74. doi: 10.1165/rcmb.2013-0538OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr. Pulmonol. 2015;50(Suppl 40):S24–S30. doi: 10.1002/ppul.23247. [DOI] [PubMed] [Google Scholar]
- 17.Kim D., Steward M.C. The role of CFTR in bicarbonate secretion by pancreatic duct and airway epithelia. J. Med. Invest. 2009;56(Suppl):336–342. doi: 10.2152/jmi.56.336. [DOI] [PubMed] [Google Scholar]
- 18.Tang X.X., Ostedgaard L.S., Hoegger M.J., Moninger T.O., Karp P.H., McMenimen J.D., Choudhury B., Varki A., Stoltz D.A., Welsh M.J. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dransfield M.T., Wilhelm A.M., Flanagan B., Courville C., Tidwell S.L., Raju S.V., Gaggar A., Steele C., Tang L.P., Liu B., Rowe S.M. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloane P.A., Shastry S., Wilhelm A., Courville C., Tang L.P., Backer K., Levin E., Raju S.V., Li Y., Mazur M. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju S.V., Lin V.Y., Liu L., McNicholas C.M., Karki S., Sloane P.A., Tang L., Jackson P.L., Wang W., Wilson L. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am. J. Respir. Cell Mol. Biol. 2017;56:99–108. doi: 10.1165/rcmb.2016-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubert J.D., Dalal B.I., Bai T.R., Roberts C.R., Hayashi S., Hogg J.C. Transforming growth factor beta 1 gene expression in human airways. Thorax. 1994;49:225–232. doi: 10.1136/thx.49.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignola A.M., Chanez P., Chiappara G., Merendino A., Zinnanti E., Bousquet J., Bellia V., Bonsignore G. Release of transforming growth factor-beta (TGF-beta) and fibronectin by alveolar macrophages in airway diseases. Clin. Exp. Immunol. 1996;106:114–119. doi: 10.1046/j.1365-2249.1996.d01-811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignola A.M., Chanez P., Chiappara G., Merendino A., Pace E., Rizzo A., la Rocca A.M., Bellia V., Bonsignore G., Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 25.Takizawa H., Tanaka M., Takami K., Ohtoshi T., Ito K., Satoh M., Okada Y., Yamasawa F., Nakahara K., Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am. J. Respir. Crit. Care Med. 2001;163:1476–1483. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 26.Avella M., Loriol C., Boulukos K., Borgese F., Ehrenfeld J. SLC26A9 stimulates CFTR expression and function in human bronchial cell lines. J. Cell. Physiol. 2011;226:212–223. doi: 10.1002/jcp.22328. [DOI] [PubMed] [Google Scholar]
- 27.Ousingsawat J., Schreiber R., Kunzelmann K. Differential contribution of SLC26A9 to Cl(-) conductance in polarized and non-polarized epithelial cells. J. Cell. Physiol. 2012;227:2323–2329. doi: 10.1002/jcp.22967. [DOI] [PubMed] [Google Scholar]
- 28.Strug L.J., Gonska T., He G., Keenan K., Ip W., Boëlle P.Y., Lin F., Panjwani N., Gong J., Li W. Cystic fibrosis gene modifier SLC26A9 modulates airway response to CFTR-directed therapeutics. Hum. Mol. Genet. 2016;25:4590–4600. doi: 10.1093/hmg/ddw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son J.W., Kim Y.J., Cho H.M., Lee S.Y., Lee S.M., Kang J.K., Lee J.U., Lee Y.M., Kwon S.J., Choi E. Promoter hypermethylation of the CFTR gene and clinical/pathological features associated with non-small cell lung cancer. Respirology. 2011;16:1203–1209. doi: 10.1111/j.1440-1843.2011.01994.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandoval J., Rodriguez J.L., Tur G., Serviddio G., Pereda J., Boukaba A., Sastre J., Torres L., Franco L., Lopez-Rodas G. RNAPol-ChIP: a novel application of chromatin immunoprecipitation to the analysis of real-time gene transcription. Nucleic Acids Res. 2004;32:e88. doi: 10.1093/nar/gnh091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan G.S., Chiu C.H., Garchow B.G., Metzler D., Diamond S.L., Kiriakidou M. Small molecule inhibition of RISC loading. ACS Chem. Biol. 2012;7:403–410. doi: 10.1021/cb200253h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinnapaiyan S., Dutta R., Bala J., Parira T., Agudelo M., Nair M., Unwalla H.J. Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function. Sci. Rep. 2018;8:7984. doi: 10.1038/s41598-018-26095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillen A.E., Gosalia N., Leir S.H., Harris A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem. J. 2011;438:25–32. doi: 10.1042/BJ20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan F., Nuovo G.J., Crawford M., Boyaka P.N., Kirkby S., Nana-Sinkam S.P., Cormet-Boyaka E. MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. PLoS ONE. 2012;7:e50837. doi: 10.1371/journal.pone.0050837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S., Cui H., Xie N., Icyuz M., Banerjee S., Antony V.B., Abraham E., Thannickal V.J., Liu G. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–2391. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutful Kabir F., Ambalavanan N., Liu G., Li P., Solomon G.M., Lal C.V., Mazur M., Halloran B., Szul T., Gerthoffer W.T. MicroRNA-145 Antagonism Reverses TGF-β Inhibition of F508del CFTR Correction in Airway Epithelia. Am. J. Respir. Crit. Care Med. 2018;197:632–643. doi: 10.1164/rccm.201704-0732OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui S.Y., Wang R., Chen L.B. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J. Cell. Mol. Med. 2014;18:1913–1926. doi: 10.1111/jcmm.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand C.A., Zhang R., Pilewski J.M., Frizzell R.A. SLC26A9 is a constitutively active, CFTR-regulated anion conductance in human bronchial epithelia. J. Gen. Physiol. 2009;133:421–438. doi: 10.1085/jgp.200810097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertrand C.A., Mitra S., Mishra S.K., Wang X., Zhao Y., Pilewski J.M., Madden D.R., Frizzell R.A. The CFTR trafficking mutation F508del inhibits the constitutive activity of SLC26A9. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L912–L925. doi: 10.1152/ajplung.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorwart M.R., Shcheynikov N., Wang Y., Stippec S., Muallem S. SLC26A9 is a Cl(-) channel regulated by the WNK kinases. J. Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R.D., Wright J.L., Churg A. Transforming growth factor-beta1 drives airway remodeling in cigarette smoke-exposed tracheal explants. Am. J. Respir. Cell Mol. Biol. 2005;33:387–393. doi: 10.1165/rcmb.2005-0203OC. [DOI] [PubMed] [Google Scholar]
- 42.Milara J., Peiró T., Serrano A., Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68:410–420. doi: 10.1136/thoraxjnl-2012-201761. [DOI] [PubMed] [Google Scholar]
- 43.Raju S.V., Jackson P.L., Courville C.A., McNicholas C.M., Sloane P.A., Sabbatini G., Tidwell S., Tang L.P., Liu B., Fortenberry J.A. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am. J. Respir. Crit. Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao N., Koenig S.N., Trask A.J., Lin C.H., Hans C.P., Garg V., Lilly B. MicroRNA miR145 regulates TGFBR2 expression and matrix synthesis in vascular smooth muscle cells. Circ. Res. 2015;116:23–34. doi: 10.1161/CIRCRESAHA.115.303970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang S., Wang S., Luo A., Ding T., Lai Z., Shen W., Ma X., Cao C., Shi L., Jiang J. Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary. Biol. Reprod. 2013;89:126. doi: 10.1095/biolreprod.113.107730. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X., Li L., Zou L., Zhu X., Xian G., Li H., Tan Y., Xie L. A novel aptamer targeting TGF-β receptor II inhibits transdifferentiation of human tenon’s fibroblasts into myofibroblast. Invest. Ophthalmol. Vis. Sci. 2012;53:6897–6903. doi: 10.1167/iovs.12-10198. [DOI] [PubMed] [Google Scholar]
- 47.Nimjee S.M., Rusconi C.P., Sullenger B.A. Aptamers: an emerging class of therapeutics. Annu. Rev. Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 48.Que-Gewirth N.S., Sullenger B.A. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007;14:283–291. doi: 10.1038/sj.gt.3302900. [DOI] [PubMed] [Google Scholar]
- 49.Chinnapaiyan S., Parira T., Dutta R., Agudelo M., Morris A., Nair M., Unwalla H.J. HIV Infects Bronchial Epithelium and Suppresses Components of the Mucociliary Clearance Apparatus. PLoS ONE. 2017;12:e0169161. doi: 10.1371/journal.pone.0169161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xavier R.F., Ramos D., Ito J.T., Rodrigues F.M., Bertolini G.N., Macchione M., de Toledo A.C., Ramos E.M. Effects of cigarette smoking intensity on the mucociliary clearance of active smokers. Respiration. 2013;86:479–485. doi: 10.1159/000348398. [DOI] [PubMed] [Google Scholar]
- 51.Sethi S. Infection as a comorbidity of COPD. Eur. Respir. J. 2010;35:1209–1215. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 52.Shepshelovich D., Goldvaser H., Edel Y., Shochat T., Lahav M. High Lung Cancer Incidence in Heavy Smokers Following Hospitalization due to Pneumonia. Am. J. Med. 2016;129:332–338. doi: 10.1016/j.amjmed.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 53.Lyczak J.B., Cannon C.L., Pier G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sethi S., Murphy T.F. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 2001;14:336–363. doi: 10.1128/CMR.14.2.336-363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantin A.M., Hanrahan J.W., Bilodeau G., Ellis L., Dupuis A., Liao J., Zielenski J., Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am. J. Respir. Crit. Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 56.Clunes L.A., Davies C.M., Coakley R.D., Aleksandrov A.A., Henderson A.G., Zeman K.L., Worthington E.N., Gentzsch M., Kreda S.M., Cholon D. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarran R., Button B., Picher M., Paradiso A.M., Ribeiro C.M., Lazarowski E.R., Zhang L., Collins P.L., Pickles R.J., Fredberg J.J., Boucher R.C. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J. Biol. Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abou Alaiwa M.H., Reznikov L.R., Gansemer N.D., Sheets K.A., Horswill A.R., Stoltz D.A., Zabner J., Welsh M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA. 2014;111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah V.S., Meyerholz D.K., Tang X.X., Reznikov L., Abou Alaiwa M., Ernst S.E., Karp P.H., Wohlford-Lenane C.L., Heilmann K.P., Leidinger M.R. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerson C., Sabater J., Scuri M., Torbati A., Coffey R., Abraham J.W., Lauredo I., Forteza R., Wanner A., Salathe M. The lactoperoxidase system functions in bacterial clearance of airways. Am. J. Respir. Cell Mol. Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- 61.Wijkstrom-Frei C., El-Chemaly S., Ali-Rachedi R., Gerson C., Cobas M.A., Forteza R., Salathe M., Conner G.E. Lactoperoxidase and human airway host defense. Am. J. Respir. Cell Mol. Biol. 2003;29:206–212. doi: 10.1165/rcmb.2002-0152OC. [DOI] [PubMed] [Google Scholar]
- 62.Lorentzen D., Durairaj L., Pezzulo A.A., Nakano Y., Launspach J., Stoltz D.A., Zamba G., McCray P.B., Jr., Zabner J., Welsh M.J. Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions. Free Radic. Biol. Med. 2011;50:1144–1150. doi: 10.1016/j.freeradbiomed.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linsdell P., Hanrahan J.W. Glutathione permeability of CFTR. Am. J. Physiol. 1998;275:C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- 64.Boucher R.C. Pathogenesis of cystic fibrosis airways disease. Trans. Am. Clin. Climatol. Assoc. 2001;112:99–107. [PMC free article] [PubMed] [Google Scholar]
- 65.Hogg J.C., Chu F., Utokaparch S., Woods R., Elliott W.M., Buzatu L., Cherniack R.M., Rogers R.M., Sciurba F.C., Coxson H.O., Paré P.D. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 66.Noone P.G., Leigh M.W., Sannuti A., Minnix S.L., Carson J.L., Hazucha M., Zariwala M.A., Knowles M.R. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am. J. Respir. Crit. Care Med. 2004;169:459–467. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 67.de Boer W.I., van Schadewijk A., Sont J.K., Sharma H.S., Stolk J., Hiemstra P.S., van Krieken J.H. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998;158:1951–1957. doi: 10.1164/ajrccm.158.6.9803053. [DOI] [PubMed] [Google Scholar]
- 68.Annes J.P., Munger J.S., Rifkin D.B. Making sense of latent TGFbeta activation. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 69.Butz H., Rácz K., Hunyady L., Patócs A. Crosstalk between TGF-β signaling and the microRNA machinery. Trends Pharmacol. Sci. 2012;33:382–393. doi: 10.1016/j.tips.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 70.Davis B.N., Hilyard A.C., Lagna G., Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stark A., Brennecke J., Bushati N., Russell R.B., Cohen S.M. Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution. Cell. 2005;123:1133–1146. doi: 10.1016/j.cell.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 72.Naren A.P., Cobb B., Li C., Roy K., Nelson D., Heda G.D., Liao J., Kirk K.L., Sorscher E.J., Hanrahan J., Clancy J.P. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc. Natl. Acad. Sci. USA. 2003;100:342–346. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lafortuna C.L., Fazio F. Acute effect of inhaled salbutamol on mucociliary clearance in health and chronic bronchitis. Respiration. 1984;45:111–123. doi: 10.1159/000194607. [DOI] [PubMed] [Google Scholar]
- 74.Salathe M. Effects of beta-agonists on airway epithelial cells. J. Allergy Clin. Immunol. 2002;110:S275–S281. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- 75.Gillen A.E., Gosalia N., Leir S.H., Harris A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem. J. 2011;438:25–32. doi: 10.1042/BJ20110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eyetech Study Group Preclinical and phase 1A clinical evaluation of an anti-VEGF pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina. 2002;22:143–152. doi: 10.1097/00006982-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Eyetech Study Group Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology. 2003;110:979–986. doi: 10.1016/S0161-6420(03)00085-X. [DOI] [PubMed] [Google Scholar]
- 78.Coyne C.B., Gambling T.M., Boucher R.C., Carson J.L., Johnson L.G. Role of claudin interactions in airway tight junctional permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 79.Flynn A.N., Itani O.A., Moninger T.O., Welsh M.J. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc. Natl. Acad. Sci. USA. 2009;106:3591–3596. doi: 10.1073/pnas.0813393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fulcher M.L., Gabriel S., Burns K.A., Yankaskas J.R., Randell S.H. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 81.Fulcher M.L., Randell S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 82.Unwalla H.J., Horvath G., Roth F.D., Conner G.E., Salathe M. Albuterol modulates its own transepithelial flux via changes in paracellular permeability. Am. J. Respir. Cell Mol. Biol. 2012;46:551–558. doi: 10.1165/rcmb.2011-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raju S.V., Rasmussen L., Sloane P.A., Tang L.P., Libby E.F., Rowe S.M. Roflumilast reverses CFTR-mediated ion transport dysfunction in cigarette smoke-exposed mice. Respir. Res. 2017;18:173. doi: 10.1186/s12931-017-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snodgrass S.M., Cihil K.M., Cornuet P.K., Myerburg M.M., Swiatecka-Urban A. Tgf-β1 inhibits Cftr biogenesis and prevents functional rescue of ΔF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS ONE. 2013;8:e63167. doi: 10.1371/journal.pone.0063167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magnan A., Frachon I., Rain B., Peuchmaur M., Monti G., Lenot B., Fattal M., Simonneau G., Galanaud P., Emilie D. Transforming growth factor beta in normal human lung: preferential location in bronchial epithelial cells. Thorax. 1994;49:789–792. doi: 10.1136/thx.49.8.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harris W.T., Muhlebach M.S., Oster R.A., Knowles M.R., Noah T.L. Transforming growth factor-beta(1) in bronchoalveolar lavage fluid from children with cystic fibrosis. Pediatr. Pulmonol. 2009;44:1057–1064. doi: 10.1002/ppul.21079. [DOI] [PubMed] [Google Scholar]
- 87.Sun H., Harris W.T., Kortyka S., Kotha K., Ostmann A.J., Rezayat A., Sridharan A., Sanders Y., Naren A.P., Clancy J.P. Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS ONE. 2014;9:e106842. doi: 10.1371/journal.pone.0106842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.