Abstract
Steroid-resistant nephrotic syndrome (SRNS) is characterized by high-range proteinuria and most often focal and segmental glomerulosclerosis (FSGS). Identification of mutations in genes causing SRNS has improved our understanding of disease mechanisms and highlighted defects in the podocyte, a highly specialized glomerular epithelial cell, as major factors in disease pathogenesis. By exome sequencing, we identified missense mutations in TBC1D8B in two families with an X-linked early-onset SRNS with FSGS. TBC1D8B is an uncharacterized Rab-GTPase-activating protein likely involved in endocytic and recycling pathways. Immunofluorescence studies revealed TBC1D8B presence in human glomeruli, and affected individual podocytes displayed architectural changes associated with migration defects commonly found in FSGS. In zebrafish we demonstrated that both knockdown and knockout of the unique TBC1D8B ortholog-induced proteinuria and that this phenotype was rescued by human TBC1D8B mRNA injection, but not by either of the two mutated mRNAs. We also showed an interaction between TBC1D8B and Rab11b, a key protein in vesicular recycling in cells. Interestingly, both internalization and recycling processes were dramatically decreased in affected individuals’ podocytes and fibroblasts, confirming the crucial role of TBC1D8B in the cellular recycling processes, probably as a Rab11b GTPase-activating protein. Altogether, these results confirmed that pathogenic variations in TBC1D8B are involved in X-linked podocytopathy and points to alterations in recycling processes as a mechanism of SRNS.
Keywords: podocyte, nephrotic syndrome, endocytosis, recycling, inherited, rab11, child, trafficking, genetic
Main Text
Steroid-resistant nephrotic syndrome (SRNS) is a glomerular disease characterized by massive proteinuria, most often associated with focal and segmental glomerulosclerosis (FSGS).1 SRNS is responsible for chronic kidney disease and accounts for 15% of end-stage kidney disease (ESKD) cases in individuals under 25 years of age.2 In glomeruli, podocytes are terminally differentiated, highly specialized epithelial cells. Podocyte foot processes (FPs) wrap the outer surface of the glomerular capillaries and interdigitate with FP of neighboring podocytes forming specialized cell-cell junctions called slit-diaphragms (SD), a major constituent of the glomerular filtration barrier. Identification of monogenic causes of SRNS involving more than 40 genes has helped decipher podocyte physiology.3, 4 Many studies have reported a strong relationship between the SD and the actin cytoskeleton,4 and the role of vesicular trafficking has been well established in the maintenance of SD complexes.5, 6 Nevertheless, intracellular transport defects have rarely been related to monogenic SRNS.7
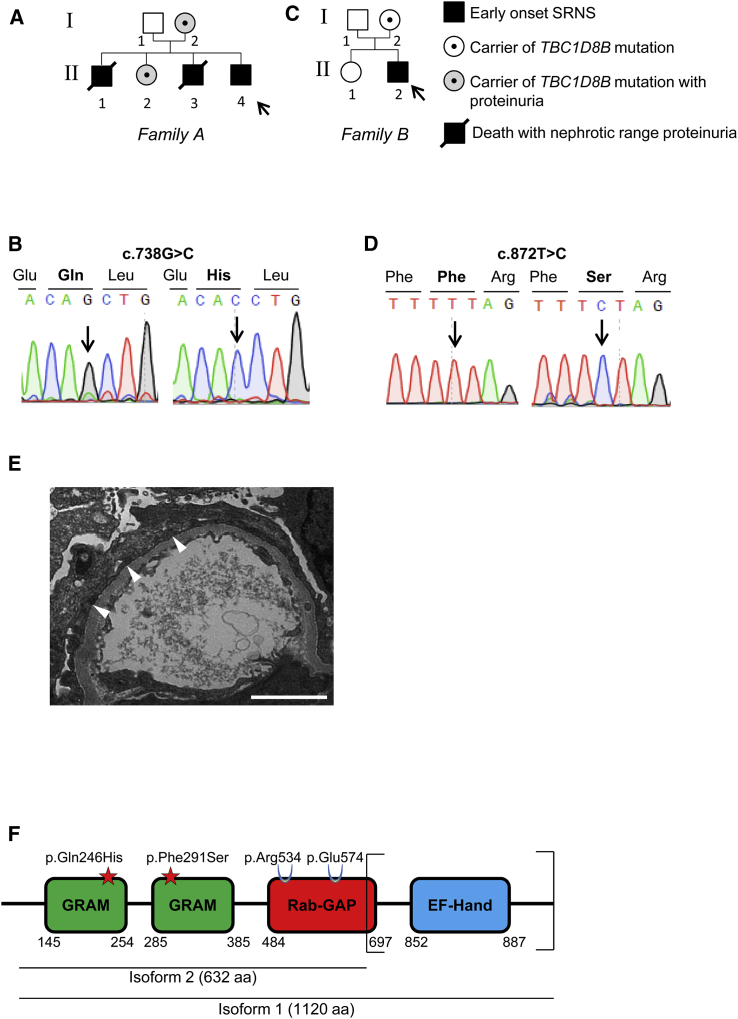
Now, through studies aiming at identifying new genes mutated in SRNS in two families, we identified a potential actor of endosomal trafficking. The first family (family A) was from Ecuador and presented with isolated congenital SRNS consistent with an X-linked inheritance. Pedigree and clinical features are described in Figure 1A and Table 1. Briefly, affected females (I-2 and II-2) exhibited non-nephrotic proteinuria, while affected boys (II-1, II-3, and II-4) developed congenital or early-onset NS. All pregnancies were marked by pre-eclampsia when carrying a male boy. In the proband (II-4) and her sister (II-2), kidney biopsy revealed FSGS lesions and electron microscopy (EM) showed FP effacement, a hallmark of NS.1 The proband (II-4) reached ESKD by the age of 2.8 years and did not exhibit recurrence after kidney transplantation. By exome sequencing (ES), we identified a c.738G>C variant in TBC1D8B (GenBank: NM_198881.1; HGNC:24715) localized on chromosome X (Figure 1B). This missense variant (p.Gln246His) was defined as probably damaging by pathogenicity prediction software. Through a European collaborative network (Eurenomics), we identified another sporadic SRNS-affected individual (II-2) in a family (family B) originated from the UK Renal Rare Disease Registry (RaDaR #425), with a missense c.872T>C (p.Phe291Ser) variant in the same gene (Figures 1C and 1D, Table 1). This variant also displayed highly pathogenic scores. The affected individual (II-2), a male from European ancestry presenting with early-onset SRNS at the age of 2 years, had no other systemic features. Kidney biopsy revealed global sclerosis on light microscopy and FP effacement on EM (Figure 1E). He reached ESKD by the age of 9 years and underwent kidney transplantation a year later. Both mutations segregated with the affected status in the respective families and were, respectively, present in 1/27,314 (with no hemizygous) and absent from gnomAD database in the population-matched control subjects. Both mutations involved residues conserved from C. elegans to H. sapiens (Figures S1A and S1B). For the UK NephroS study, ethical approval was obtained from North Somerset & South Bristol Research Ethics Committee (reference 09/H0106/72 for data collection and reference 09/H0106/80 for sample collection and data analysis). French approval for human subjects research was obtained from the Comité de Protection des Personnes Ile de France II.
Figure 1.
Clinical and Genetic Information for Families A and B
(A and C) Family pedigrees for families A (A) and B (C). In family A, the proband (II-4) and his brothers (II-1 and II-3) developed congenital nephrotic syndrome while the individual from family B (II-2) developed an early-onset nephrotic syndrome. Arrows indicate probands. TBC1D8B mutations c.738G>C and c.872T>C were detected in affected subjects and carriers of families A and B, respectively.
(B and D) Exome sequencing revealed two distinct missense variants in TBC1D8B (c.738G>C and c.872T>C). Segregation of variants was confirmed by Sanger sequencing in both families.
(E) Electron microscopy image from affected individual from family B (II-2) harboring the p.Phe291Ser mutation revealed foot processes effacement (white arrowheads) (scale bar, 2 μm).
(F) TBC1D8B protein exhibits two isoforms that include both mutations. Mutations localized in glucosyltransferases, Rab-like GTPase activators, and myotubularins (GRAM) domains at the N-terminal extremity. Catalytic residues on the Rab-GTPase activating (GAP) domain are maintained. C-terminal EF-hand domain is not conserved in isoform 2. EF-hand domain has been described in other proteins as a calcium-binding regulation site, which may downregulate the protein activity when bound to Ca2+.29
Table 1.
Mutations in TBC1D8B in Two Families with a Likely X-Linked FSGS
| Family | Individual | Ethnic Origin | Sex | Nucleotide Alteration | Exon | Satus | Protein Alteration | PolyPhen/Sift/MutationTaster | Amino Acid Conservation | Age at Proteinuria Onset | Immuno-sensitivity | Pathology | Age at ESRD | Age at Transplantation | Recurrence | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | NCR2230 (index) | Ecuador | M | c.738G>C | 5 | hemi | p.Gln246His | 0.996/0/DC | C. elegans | congenital | no | collapsing FSGS | 2.8 years | 4 years (thrombosis); 7 years | no | N/A |
| NCR1387 (mother) | Ecuador | F | hetero | adult | N/A | N/A | no | N/A | N/A | N/A | ||||||
| NCR1388 (sister) | Ecuador | F | hetero | 7 years | no | FSGS | no | N/A | N/A | N/A | ||||||
| brother 1 | Ecuador | M | N/A | N/A | N/A | N/A | N/A | N/A | congenital | no | N/A | N/A | N/A | N/A | 4 mo | |
| brother 2 | Ecuador | M | N/A | N/A | N/A | N/A | N/A | N/A | 5 months | no | N/A | N/A | N/A | N/A | 14 mo | |
| B | 104 | European | M | c.872T>C | 6 | hemi | p.Phe291Ser | 0.961/0/DC | C. elegans | 2 years | no | chronic progressive sclerosis | 9 years | 10 years | no | N/A |
Abbreviations: M, male; F, female; mo, months; FSGS, focal and segmental glomerulosclerosis; hemi, hemizygous; hetero, heterozygous; N/A, not applicable; ESRD, end stage renal disease
TBC1D8B is a member of the TBC domain protein family (Tre-2/Bub2/CDC16). Like other TBC proteins, TBC1D8B may function as a Rab-GTPase activating protein (Rab-GAP) by binding to specific Rab proteins and stimulating their GTPase activity.8 Interestingly, in addition to its Rab-GAP (TBC) domain, TBC1D8B contains one GRAM domain repeated two times, which allows binding to lipid rafts, critical elements of SD signaling in podocytes.9 The two mutations described above are localized within each GRAM domain (Figure 1F). TBC1D8B also possesses an EF-Hand domain localized at the C-terminal extremity. A second isoform of TBC1D8B results from alternative splicing events in intron 11 and leads to a shorter 632-amino acid protein, lacking the C-terminal extremity of the Rab-GAP domain and the entire EF-like domain. However, the catalytic residues (Arg534 and Glu574) are present in both protein isoforms.
The role of TBC1D8B has never been explored either in cell lines or animal models.
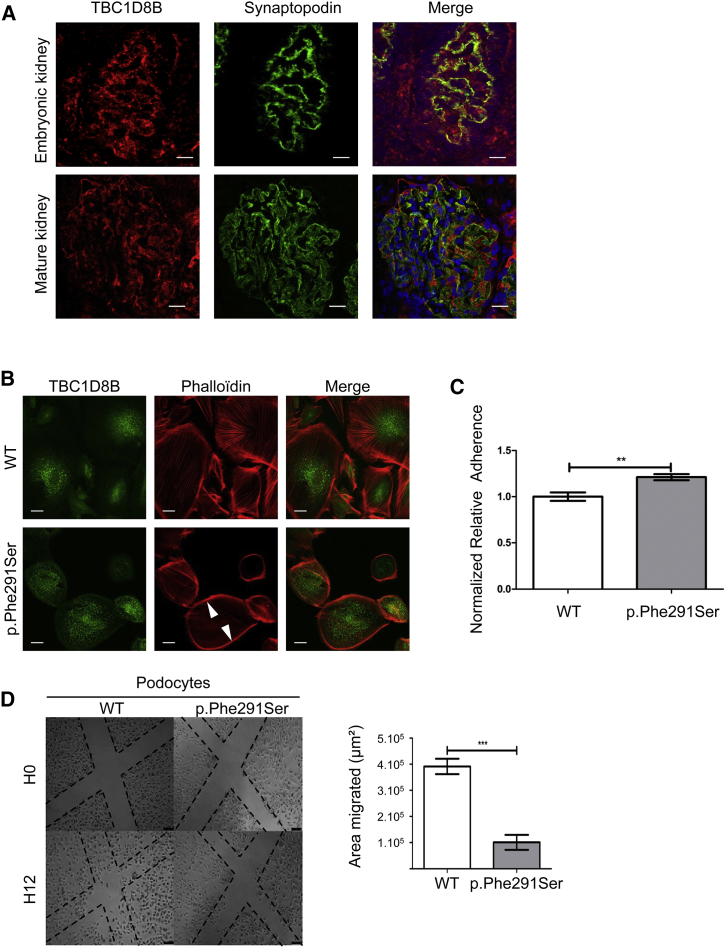
Immunofluorescence studies in human kidneys revealed the presence of TBC1D8B both in glomerular podocytes and tubules at early stages (25 weeks) and in mature kidney. In glomeruli, TBC1D8B colocalized with synaptopodin a specific cytosolic protein10 (Figure 2A). To further comprehend the role of TBC1D8B, we undertook functional studies in both zebrafish and human podocytes. Podocytes from individual B (harboring the p.Phe291Ser variant) were conditionally immortalized from the affected individual’s nephrectomy specimen using our published methodology.11 As previously described in some monogenic SRNS, podocytes often display alterations in actin cytoskeleton organization12, 13, 14, 15, 16 associated with changes in adhesion and migration.14, 17 Podocytes from affected individual B exhibited similar phenotypes, and TBC1D8B displayed an intracellular vesicular localization as shown in Figures 2B–2D. Podocytes from individual A were not available, since he underwent bilateral nephrectomy several years before.
Figure 2.
TBC1D8B Localization and Phenotype Observed in Mutated Podocytes
(A) Immunofluorescence studies were performed on human fetal kidney at 25 gestational weeks (top) and in mature kidney in a healthy 7-year-old male (bottom). TBC1D8B displayed a glomerular localization with partial colocalization with synaptopodin, a cytosolic podocyte protein (scale bar, 20 μm).
(B) In immunofluorescence experiments in immortalized podocytes, TBC1D8B displayed an intracellular vesicular expression. F-actin cytoskeleton was detected by immunofluorescence with phalloidin staining. p.Phe291Ser human mutant podocytes cortically (arrowhead) reorganized their F-actin cytoskeleton. Original magnification ×63.
(C) Cell adhesion was measured with a spectrophotometer at 570 nm optical density (n = 3, ∗∗p < 0.01, mean ± SEM).
(D) Human podocyte cell motility and migration were measured by scratch assays. Number of cells per unit was significantly higher in the WT group 12 hr after the scratch as shown on the graph on right (n = 3, ∗∗∗p < 0.0001, mean ± SEM).
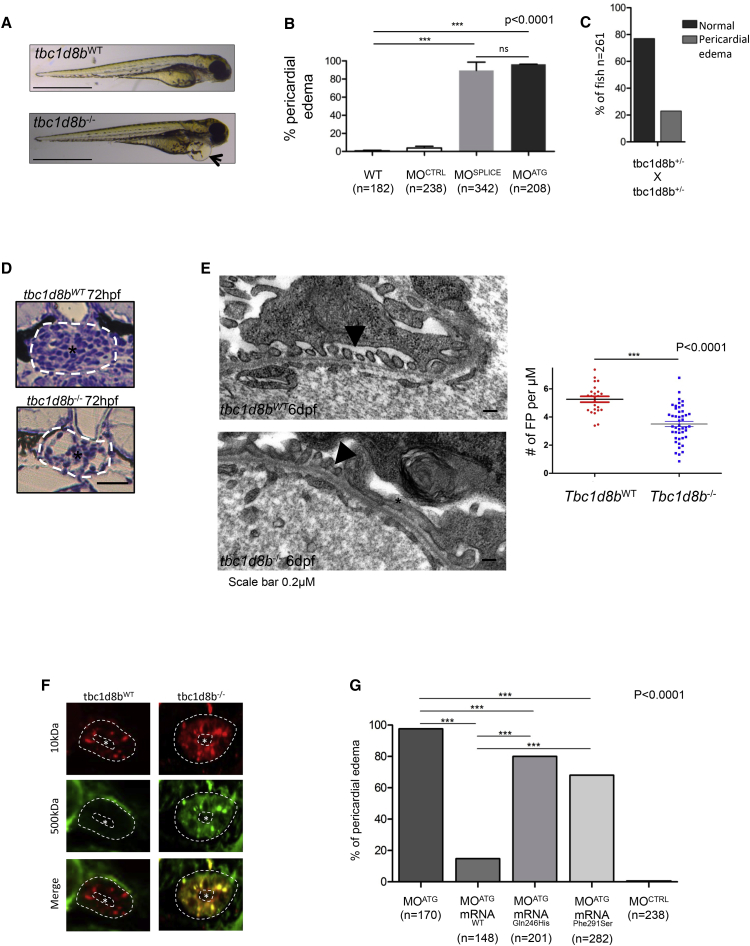
According to the Zebrafish Genome Reference Consortium (GRCz11), tbc1d8b is the only zebrafish ortholog and is located on autosome 5. Only one transcript has been described corresponding to the longer isoform in humans. By whole-mount in situ hybridization in 24 post-fertilization (hpf) zebrafish embryos, we demonstrated tbc1d8b expression in the neural tube, brain, pectoral fins, and the pronephric glomerulus (Figure S2). Functional analyses were then performed in both tbc1d8b knock-down (KD) morphants and knock-out (KO) fish. Morphants were obtained by specifically targeting tbc1d8b with either splice-blocking (MOSPLICE) or translation-blocking (MOATG) morpholino oligonucleotides. The efficacy of MOSPLICE was checked by RT-PCR (Figure S3A), and the efficacy of MOATG was demonstrated by the absence of fluorescence after co-injection of the MOATG with its tbc1d8b 5′ UTR mRNA target fused to the GFP as described in Figure S3B. The tbc1d8b−/− mutant fish line was obtained by ENU mutagenesis resulting in a nonsense mutation in exon 9 (European Zebrafish Resource Center [EZRC]). The final product is a truncated 468-amino acid protein missing the Rab-GAP domain (which starts at residue 466). In zebrafish, pericardial edema has been previously reported to be an indirect marker of a glomerular filtration barrier defect and proteinuria,16 although it is not specific and can be seen in other zebrafish abnormalities (i.e., heart defects, global developmental abnormalities). Such a phenotype was observed in >95% of both MOSPLICE and MOATG morphants at 96 hpf whereas it was found in fewer than 2% of control MO fish at 96 hpf (Figures 3A and 3B). An identical phenotype was obtained in the tbc1d8b−/− fish with the expected Mendelian ratio (n = 261; 22.9% phenotype in tbcd8b+/− offspring; Figure 3C).
Figure 3.
Phenotype Observed in tbc1d8b KO and KD Zebrafish
(A) KO fish displayed pericardial edema at 48 hpf on mutant fish (black arrow). Scale bar, 1 mm.
(B) Both MOATG and MOSPLICE also exhibited pericardial edema in more than 95% fish whereas MOCONTROL did not (n > 200, ∗∗∗p < 0.0001, mean ± SEM).
(C) tbc1d8b+/− offspring displayed pericardial edema with a Mendelian ratio.
(D) Regular optical microscopy revealed a retracted glomerulus (∗) in an enlarged Bowman’s capsule (white circle) in mutated tbc1d8b−/− fish compared to control (scale bar, 15 μm).
(E) Electron microscopy findings showed a mixture of foot processes (FP) effacement (∗) with regular FP (black arrowheads). Graph on the right shows a significantly lower rate of FP per uM in tbc1d8b−/− fish compared to control (n = 3 fish per condition, ∗∗∗p < 0.001, mean ± SEM).
(F) Dye filtration assay was performed in KO and control fish. While Texas-Red 10 kDa fluorescence uptake was physiologically found in both tbc1d8b−/− and +/−, FITC 500 kDa fluorescence was only found in KO fish (n = 3). Asterisk represents tubular lumen (scale bar, 5 μm).
(G) In rescue experiments, only human WT mRNA was able to significantly decrease pericardial edema whereas both mutated mRNA were able to only partially reduce phenotype in fish (n > 100/condition).
To characterize the effect of tbc1d8b loss on kidney anatomy, histology was performed and showed a retracted glomerulus in an enlarged Bowman’s capsule in tbc1d8b−/− fish compared to controls (Figure 3D). Similar to affected individuals, EM revealed significant FP effacement and disappearance of SD in tbc1d8b−/− KO fish compared to controls (number of FP per μm 3.5 ± 0.2 and 5.3 ± 0.2, respectively - p < 0.0001) as indicated in Figures 3E and S4.
To confirm the glomerular permeability defect, we set up a dye-filtration assay. A high-molecular-weight FITC-labeled dextran (500 kDa) was injected in the cardinal vein of MOSPLICE and MOCONTROL and tbc1d8b−/− or tbc1d8bWT zebrafish at 96 hpf. We then screened the filtration defects in two different ways. We first measured fluorescence in the retinal vein and showed that fluorescence in the vasculature of MOSPLICE fish was significantly decreased compared to MOCONTROL (Figure S5). We then showed specific glomerular leakage by imaging dye uptake in tubular cells 6 h post-injection (6 hpi). Fluorescence was observed in tubular cells in KO tbc1d8b−/− fish, but not in control fish, revealing a glomerular protein leakage consistent with pericardial edema and EM findings (Figure 3F).
To investigate the functional consequences of both mutations, we performed rescue experiments in zebrafish using the human short isoform of WT and mutant mRNAs, since in wild-type human cultured podocytes, the 632-amino acid short isoform seems to be predominantly expressed (Figure S6). As mentioned previously, edema was described in 97.6% of morphants. However, when mRNAWT (100 pg) was co-injected with MO, this ratio fell to 16% (n = 148) (Figure 3G) with a dose-response effect (Figure S7). Conversely, the mutated mRNAp.Gln246His and mRNAp.Phe291Ser were only able to very partially rescue the phenotype with 80% (n = 201) and 68% (n = 282) of fish exhibiting pericardial edema, respectively (Figure 3G). These results indicate that although both mutations are clearly damaging in both individuals, a modest protein residual activity is likely.
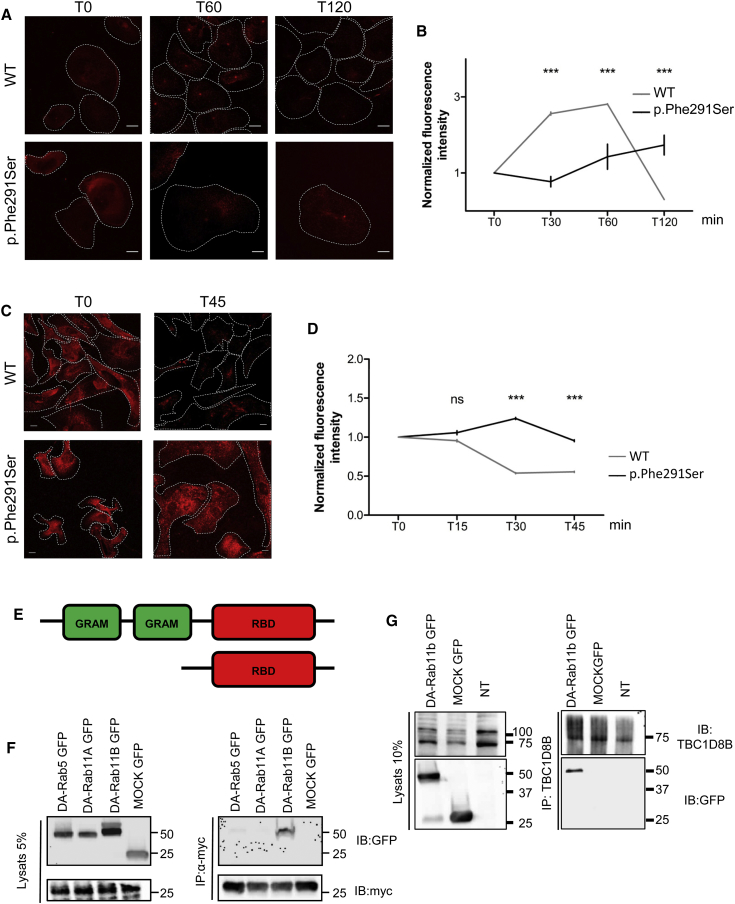
As mentioned above, TBC1D8B may act as Rab-GAP, promoting GTP hydrolysis in specific Rab proteins.18 In mammalian cells including podocytes, Rab-GTPases are mostly involved in vesicular trafficking.19, 20 To further analyze the role of TBC1D8B in this process, we performed transferrin endocytosis and recycling assays in affected individuals’ podocytes and fibroblasts harboring p.Phe291Ser and p.Gln246His variants, respectively. Transferrin is a widely used marker for both clathrin-mediated internalization and recycling pathways in most cell types. After internalization from the plasma membrane and before being recycled back to cell surface, transferrin and its receptor accumulate in the perinuclear recycling compartment (PNRC).21, 22 We detected a delay in transferrin uptake in affected individuals’ cells compared to control subjects. After 15 and 60 min of incubation (in fibroblasts and podocytes, respectively), most of the fluorescence was found in the PNRC of WT cells. However, endocytosis was significantly slower in mutant fibroblasts and podocytes (Figures 4A, 4B, S8A, and S8B). In addition, analysis of transferrin recycling revealed a significant decrease of normalized fluorescence intensity 45 min after transferrin loading in WT podocytes as well as in fibroblasts, whereas it was maintained in the PNRC in affected individuals’ cells (Figures 4C, 4D, S8C, and S8D). Altogether, these results strongly suggest that both endocytosis and vesicular recycling are altered in affected podocytes.
Figure 4.
TBC1D8B Interaction and Effect of Mutations on Vesicular Trafficking
(A and B) Transferrin uptake is evaluated by incubation with fluorescently labeled transferrin. Fluorescence intensity progressively increased in control podocytes whereas it increased much more slowly in mutated podocytes harboring the p.Phe291Ser mutation suggesting an endocytosis defect. Graph B represents the quantification of normalized fluorescence in both mutated and control cells (∗∗∗p < 0.001, mean ± SEM; scale bar, 25 μm).
(C and D) Transferrin chase evaluates the recycling process in podocytes. Measuring recycling of fluorescently labeled transferrin in WT and mutant podocytes revealed persistence of fluorescence 45 min after internalization in mutant cells while it reached 50% of T0 intensity in control podocytes, indicating a delay in recycling processes in mutant cells (C). Graph D represents the quantification of normalized fluorescence in both mutated and control cells (∗∗∗p < 0.001 at 45 min, mean ± SEM; scale bar, 25 μm).
(E) Construct used for to perform co-immunoprecipitation with a specific plasmid harboring only the TBC1D8B Rab-binding domain (RBD) tagged with the myc epitope, and under the CMV promoter.
(F) In co-transfected HEK293T, we showed that the (myc-)RBD of TBC1D8B was able to interact with Dominant Active (DA)-Rab11b but not DA-Rab11A nor DA-Rab5.
(G) The endogenous TBC1D8B protein interacts with transfected DA-Rab11b-GFP in HEK cells.
In cells, PNRC dynamics and recycling are mostly regulated by Rab11. We then hypothesized that TBC1D8B might be a crucial GAP for Rab11 that displays at least two isoforms (Rab11a and Rab11b)22 both present in mouse podocytes.23 By co-immunoprecipitation experiments in HEK293T cells, we showed that the Rab-binding domain (RBD) of TBC1D8B was able to interact with a dominant active, GTP-bound, mutant of Rab11b (DA-Rab11b), but not with DA-Rab11a (Figures 4E and 4F). We also observed a specific interaction between endogenous TBC1D8B and DA-Rab11b (Figure 4G). In addition, we showed that DA-Rab11b and TBC1D8B Rab-binding domain colocalized at the PNRC when co-expressed in podocytes (Figure S9), but interestingly, the widespread Rab11b signal in WT cells shifted to a very restricted localization to the PNRC in mutant podocytes, which suggests that GTP-bound Rab11b is trapped in the PNRC in cells from affected individuals (Figures S10A and S10B). Altogether, these data strongly suggest that TBC1D8B is a specific Rab11b-GTPase activating protein.
Numerous proteins are subject to Rab11-dependent recycling in various cell types, especially in neurons.24 However, although it has been shown that some proteins are recycled in a Rab11b-dependent manner,25 only a few studies explored the role of Rab11b on vesicular trafficking. In 2016, Grimsey et al. reported the crucial role of Rab11b GTP hydrolysis for the initiation of transferrin recycling from the PNRC to the plasma membrane.26 In podocytes SD integrity is of utmost importance and several SD proteins are subjected to endocytic and recycling events to maintain their regulation.27 Very recently, mutations in GAPVD1, a Rab5 effector showed to be involved in nephrin regulation at the SD, were reported as disease causing.7 In our study, we did not find any interaction between TBC1D8B and Rab5 (Figure 4F), suggesting that Rab5 is not a target of TBC1D8B. Thus, our results highly suggest that some podocyte-specific proteins could specifically use the Rab11b-dependent recycling pathway, as it has been shown for some SD proteins and the use of the Rab5-dependent pathway.7 By this means, alterations in Rab11b activation would lead to SD dysregulation, implying that not only endocytosis but also recycling are of fundamental importance for SD integrity. Indeed, it is likely that mutations in TBC1D8B lead to strong defect in recycling processes in podocytes, which could also induce a defect in transferrin uptake linked to decrease number of receptors at the plasma membrane at steady state.
Altogether, our results confirmed that mutations in TBC1D8B are involved in the development of early SRNS in rare affected individuals. We show herein that vesicular trafficking plays a fundamental role in podocyte disease, especially in SRNS, through a Rab11-dependent recycling process. Recent discoveries in monogenic SRNS and especially in SD regulation during disease through endocytic and recycling pathways would certainly help to develop targeted therapy for affected individuals.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We express gratitude to the affected individuals and their families for contributing to improve medical knowledge in nephrotic syndrome. We thank Imagine’s platform of cell imaging for help in images acquisition, and the Bioinformatics Platform of Paris Descartes University and the Imagine Genomics Platform for exome sequencing. We also thank the MRC in addition to the Wolfson Foundation for establishing the Wolfson Bioimaging Facility, University of Bristol. We thank Prof. Iain Drummond, Dr. Marion Delous, and Dr. MC. Gubler for helpful discussion and Elizabeth Angus (Southampton) for EM images.
This research was supported by the Investments for the Future Program (grant ANR-10-IAHY-01 to C.A.), the European Union’s Seventh Framework Programme (FP7/2007–2013) grant 305608 (EURenOmics) (to C.A.), and the Fondation Recherche Medicale (project DEQ2015031682) (to C.A.). This research was also supported by grants from Kidney Research UK, NIHR-TRC, Nephrotic Syndrome Trust, and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. G.D. was supported by the “Programme Santé-Science” (MD-PhD) of Imagine Institute and Fondation Bettencourt Schueller, France. V.K. was supported by a Kidney Research UK PhD studentship.
The UK affected individuals were recruited via NephroS, the National Nephrotic Syndrome Study based within RaDaR, the UK renal Rare Disease Registry.
Published: January 17, 2019
Footnotes
Supplemental Data include 11 figures, 1 table, and Supplemental Material and Methods and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.12.016.
Contributor Information
Moin A. Saleem, Email: m.saleem@bristol.ac.uk.
Corinne Antignac, Email: corinne.antignac@inserm.fr.
Web Resources
Eurenomics, https://eurenomics.eu/
MutationTaster, http://www.mutationtaster.org/
OMIM, http://www.omim.org/
PolyPhen, http://genetics.bwh.harvard.edu
SIFT, http://sift.jcvi.org
Supplemental Data
References
- 1.D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin. Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 2.Smith J.M., Stablein D.M., Munoz R., Hebert D., McDonald R.A. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatr. Transplant. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Lovric S., Ashraf S., Tan W., Hildebrandt F. Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol. Dial. Transplant. 2016;31:1802–1813. doi: 10.1093/ndt/gfv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierzynska A., McCarthy H.J., Soderquest K., Sen E.S., Colby E., Ding W.Y., Nabhan M.M., Kerecuk L., Hegde S., Hughes D. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int. 2017;91:937–947. doi: 10.1016/j.kint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Faul C., Asanuma K., Yanagida-Asanuma E., Kim K., Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Tossidou I., Teng B., Menne J., Shushakova N., Park J.K., Becker J.U., Modde F., Leitges M., Haller H., Schiffer M. Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PLoS ONE. 2010;5:e10185. doi: 10.1371/journal.pone.0010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermle T., Schneider R., Schapiro D., Braun D.A., van der Ven A.T., Warejko J.K., Daga A., Widmeier E., Nakayama M., Jobst-Schwan T. GAPVD1 and ANKFY1 Mutations Implicate RAB5 Regulation in Nephrotic Syndrome. J. Am. Soc. Nephrol. 2018;29:2123–2138. doi: 10.1681/ASN.2017121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda M. TBC proteins: GAPs for mammalian small GTPase Rab? Biosci. Rep. 2011;31:159–168. doi: 10.1042/BSR20100112. [DOI] [PubMed] [Google Scholar]
- 9.Huber T.B., Schermer B., Müller R.U., Höhne M., Bartram M., Calixto A., Hagmann H., Reinhardt C., Koos F., Kunzelmann K. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc. Natl. Acad. Sci. USA. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsch T., Endlich N., Gökce G., Doroshenko E., Simpson J.C., Kriz W., Shaw A.S., Endlich K. Association of CD2AP with dynamic actin on vesicles in podocytes. Am. J. Physiol. Renal Physiol. 2005;289:F1134–F1143. doi: 10.1152/ajprenal.00178.2005. [DOI] [PubMed] [Google Scholar]
- 11.Saleem M.A., O’Hare M.J., Reiser J., Coward R.J., Inward C.D., Farren T., Xing C.Y., Ni L., Mathieson P.W., Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma K., Kim K., Oh J., Giardino L., Chabanis S., Faul C., Reiser J., Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J. Clin. Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akilesh S., Suleiman H., Yu H., Stander M.C., Lavin P., Gbadegesin R., Antignac C., Pollak M., Kopp J.B., Winn M.P., Shaw A.S. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 2011;121:4127–4137. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashraf S., Gee H.Y., Woerner S., Xie L.X., Vega-Warner V., Lovric S., Fang H., Song X., Cattran D.C., Avila-Casado C. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gee H.Y., Saisawat P., Ashraf S., Hurd T.W., Vega-Warner V., Fang H., Beck B.B., Gribouval O., Zhou W., Diaz K.A. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Invest. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gee H.Y., Zhang F., Ashraf S., Kohl S., Sadowski C.E., Vega-Warner V., Zhou W., Lovric S., Fang H., Nettleton M. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J. Clin. Invest. 2015;125:2375–2384. doi: 10.1172/JCI79504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura H., Nakazato H., Kuraoka S., Yoneda K., Takahashi W., Endo F. Reduced INF2 expression in nephrotic syndrome is possibly related to clinical severity of steroid resistance in children. Nephrology (Carlton) 2016;21:467–475. doi: 10.1111/nep.12627. [DOI] [PubMed] [Google Scholar]
- 18.Kramer-Zucker A.G., Wiessner S., Jensen A.M., Drummond I.A. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev. Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan X., Eathiraj S., Munson M., Lambright D.G. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature. 2006;442:303–306. doi: 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer S.R. Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell. 2017;28:712–715. doi: 10.1091/mbc.E16-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant B.D., Donaldson J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S., Ghosh R.N., Maxfield F.R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 23.Kann M., Ettou S., Jung Y.L., Lenz M.O., Taglienti M.E., Park P.J., Schermer B., Benzing T., Kreidberg J.A. Genome-Wide Analysis of Wilms’ Tumor 1-Controlled Gene Expression in Podocytes Reveals Key Regulatory Mechanisms. J. Am. Soc. Nephrol. 2015;26:2097–2104. doi: 10.1681/ASN.2014090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapierre L.A., Dorn M.C., Zimmerman C.F., Navarre J., Burnette J.O., Goldenring J.R. Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp. Cell Res. 2003;290:322–331. doi: 10.1016/s0014-4827(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 25.Li X., DiFiglia M. The recycling endosome and its role in neurological disorders. Prog. Neurobiol. 2012;97:127–141. doi: 10.1016/j.pneurobio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Grimsey N.J., Coronel L.J., Cordova I.C., Trejo J. Recycling and Endosomal Sorting of Protease-activated Receptor-1 Is Distinctly Regulated by Rab11A and Rab11B Proteins. J. Biol. Chem. 2016;291:2223–2236. doi: 10.1074/jbc.M115.702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue K., Ishibe S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 2015;309:F398–F405. doi: 10.1152/ajprenal.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo L.I., Liao Y., Ruiz W.G., Clayton D.R., Li M., Liu Y.J., Jiang Y., Fukuda M., Apodaca G., Yin X.M. TBC1D9B functions as a GTPase-activating protein for Rab11a in polarized MDCK cells. Mol. Biol. Cell. 2014;25:3779–3797. doi: 10.1091/mbc.E13-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.