Abstract
Lung protective mechanical ventilation (LPV) even in patients with healthy lungs is associated with a lower incidence of postoperative pulmonary complications (PPC). The pathophysiology of ventilator-induced lung injury and the risk factors of PPCs have been widely identified, and a perioperative lung protective concept has been elaborated. Despite the well-known advantages, results of recent studies indicated that intraoperative LPV is still not widely implemented in current anaesthesia practice.
No nationwide surveys regarding perioperative pulmonary protective management have been carried out previously in Hungary. This study aimed to evaluate the routine anaesthetic care and adherence to the LPV concept of Hungarian anaesthesiologists during major abdominal surgery.
A questionnaire of 36 questions was prepared, and anaesthesiologists were invited by an e-mail and a newsletter to participate in an online survey between January 1st to March 31st, 2018.
A total of one hundred and eleven anaesthesiologists participated in the survey; 61 (54.9%), applied low tidal volumes, 30 (27%) applied the entire LPV concept, and only 6 (5.4%) regularly applied alveolar recruitment manoeuvres (ARM). Application of low plateau and driving pressures were 40.5%. Authoritatively written protocols were not available resulting in markedly different perioperative pulmonary management. According to respondents, the most critical risk factors of PPCs are chronic obstructive pulmonary diseases (103; 92.8%); in contrast malnutrition, anaemia or prolonged use of nasogastric tube were considered negligible risk factors. Positive end-expiratory pressure (PEEP) and regular ARM are usually ignored. Based on the survey, more attention should be given to the use of LPV.
Keywords: lung protective ventilation, low tidal volumes, positive end-expiratory pressure, alveolar recruitment manoeuvres, postoperative pulmonary complications, perioperative respiratory protocols
Introduction
Lung protective mechanical ventilation (LPV) even in patients with healthy lungs is associated with a lower incidence of postoperative pulmonary complications (PPC), resulting in better outcomes, shorter length of hospital stay, and lower healthcare-associated costs [1,2]. The multifactorial pathophysiology of ventilator-induced lung injury (VILI), the surgery, the anaesthesia and the patient-related risk factors of PPCs have been widely reported in the literature [3, 4, 5, 6, 7]. Based on this, the concept of perioperative lung protective management emerged, including preoperative breathing physiotherapy, positive pressure respiratory support, prophylactic perioperative positive pressure ventilation (POP-ventilation), continuous positive airway pressure (CPAP), non-invasive ventilation (NIV), intraoperative LPV, applying low tidal volumes, moderate levels of positive end-expiratory pressure (PEEP) and regular ARM has been elaborated [8,9,10]. Despite the well-known advantages, Schultz MJ et al. (2017) concluded that intraoperative LPV is still not widely implemented in everyday anaesthesia practice even in high-risk surgical patients and it has been suggested that much more attention should be given to the use of lung protective strategies during general anaesthesia [11,12].
Several differences are known to exist between Eastern and Western Europe health care systems and patient management[13]. As no data exists from Eastern Europe, including Hungary, a decision was made to survey members of the Hungarian Society of Anaesthesiology and Intensive Therapy (HSAIT) regarding the routine anaesthetic care, awareness and adherence to the LPV concept during major abdominal surgery.
Materials and Methods
A questionnaire of thirty-six “mandatory-to-answer” multiple-choice questions divided into five sections had been prepared and tested on a pilot sample of three expert anaesthesiologists to check the clarity and validity of the questions and to estimate the completion time of the survey. Agreement of any ethics committee was not necessary as the questionnaire was about the professional practice of anaesthesiologists, and participation was voluntary and anonymous. There were no exclusion criteria and the study complied with the survey-reporting list.
After the questionnaire was considered appropriate, Hungarian anaesthesiologists were invited by email and by a newsletter, to participate in an online survey between January 1st to March 31st, 2018, using the public e-mail database of the Hungarian Hospital Federation (Magyar Kórházszövetség). A cover letter containing the investigators’ names and contact details, the objectives, aims and methodology of the study was attached. The online questionnaire was published using Google Forms (Google Inc., Mountain View, CA).
Demographic data of respondents, routine preoperative, intraoperative and postoperative pulmonary management and opinions of participants about the risk factors of PPCs were evaluated in different sections. The primary endpoint was the frequency of consistent application of the three basic elements of LPV: low tidal volume (TV) ≤ 6 ml/kg ideal body weight (IBW), PEEP of 6 cmH2O at least and regular ARMs. Secondary endpoints were the respiratory rate, application of permissive hypercapnia [end tidal carbon dioxide tension (EtCO2) 35-40 mmHg], low plateau pressure (Pplat < 25 cmH2O) and low driving pressure (ᐃPaw < 20 cmH2O), use of neuromuscular blocking agent antagonists (NMBA-A) and prevalence of perioperative pulmonary management protocols. The tertiary endpoint was the opinion of respondents about the risk factors of PPCs.
The difference, if any, in the way trainees and specialists practised and the difference in the standard of care between university hospitals and other hospitals was assessed.
Statistical analysis
Data are expressed as the number and percentage of survey respondents with associated 95% confidence interval (CI). Odds ratios (OR) were calculated and the level of significance set at α =0.05.
MedCalc Statistical Software v14.8.1 (MedCalc Software bvba, Ostend, Belgium) was used for statistical analysis.
Results
Demographic Data
Ten institutions from the 117 hospitals stated that they do not perform major abdominal surgery. In total, 111 anaesthesiologists completed the survey, 25 (22.5%) after the first e-mail and 86 (77.5%) after the newsletter published on the website.
The survey population’s professional details and demographic characteristics are summarised in Table 1. Most of the anaesthesiologists worked in hospitals with significant patient turnover [> 300 major abdominal surgeries annually, 72 (64.9%)]. 24 (21.6%) of the respondents worked in university medical centres of which 89 (80.2%) were specialists. 70 (63.1%) of these had more ten years of surgical experience.
Table 1.
Demographic data and respondents’ professional details
| n (=111) | % | ||
|---|---|---|---|
| Type of institution | |||
| University medical centre | 24 | 21.6 | |
| Hospital in capital | 30 | 27.1 | |
| County hospital | 44 | 39.6 | |
| Other hospitals | 13 | 11.7 | |
| Respondents’ post | |||
| Specialist candidate (trainees) | 22 | 19.8 | |
| Specialist | 58 | 52.3 | |
| Chief medical officer | 31 | 27.9 | |
| Length of practice in anaesthesia | |||
| < 5 yrs | 20 | 18.0 | |
| 5 – 10 yrs | 21 | 18.9 | |
| > 10 yrs | 70 | 63.1 | |
| The annual number of major abdominal surgery per centre | |||
| < 100 | 6 | 5.4 | |
| 100 – 200 | 11 | 9.9 | |
| 200 – 300 | 22 | 19.8 | |
| 300 – 400 | 12 | 10.8 | |
| > 400 | 60 | 54.1 | |
Data are expressed as the number and percentage of respondents
Primary Endpoint
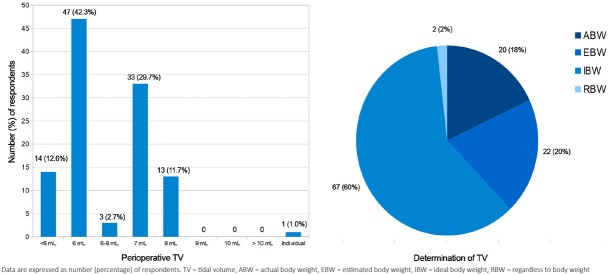
61 (54.9%) (95% CI 48.7 – 78.4) of the anaesthesiologists applied low tidal volume (TV) of less than 6 ml/kg) and 67 (60.4%) [95% CI 51.9 – 85.1] used ideal body weight (IBW) to determine the appropriate TV (Figure 1).
Fig. 1.
Use of low tidal volume (TV) and ideal body weight (IBW) to determine the appropriate TV are common: 54.9% of respondents apply a low TV of 6 ml/kg or less and 60% of them use IBW. However, applying a TV of 7 ml/kg is also frequent and 38% of respondents use actual or estimated body weight to determine the appropriate TV and 2% of them do not take the patient’s weight into account (RBW).
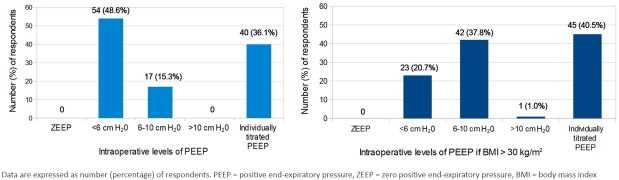
None of the respondents used zero PEEP, 54 [48.6% (95% CI 40.6 – 70.5)] always used lower levels of PEEP and 58(52.3%) [95% CI 44.0 – 74.9] never performed a PEEP titration procedure to determine the optimal levels of PEEP. Higher (6-10 cmH2O) or individually titrated levels of PEEP were more common during anaesthesia in obese patients with a BMI greater than 30 kg/m2 (Figure 2).
Fig. 2.
None of the respondents apply zero positive end-expiratory pressure (PEEP) during mechanical ventilation. Half of the respondents commonly use lower levels of PEEP (48.6%), and only 36.1% apply an individually optimal level of PEEP determined during a PEEP titration procedure. In contrast to these results, presumably based on pathophysiological rationality, both moderate (6-10 cmH2O, 37.8%) and individually titrated levels of PEEP (40.5%) are commonly considered appropriate for obese patients (body mass index greater than 30 kg/m2).
The most commonly used PEEP titration procedure, used by 32 (28.8%) of respondents, was the “pressure-volume curve determined method” and the “fraction of inspired oxygen” (FiO2) adapted PEEP was by 20 (18%). Neither Electrical Impedance Tomography (EIT) nor oesophageal pressure monitoring were available during anaesthetic care according to respondents.
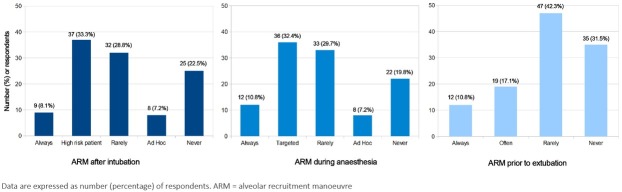
The use of ARMs after induction of anaesthesia and endotracheal intubation during general anaesthesia and before the removal of the endotracheal tube is summarised in Figure 3.
Fig. 3.
Routine and regular use of alveolar recruitment manoeuvres (ARM) is rare after endotracheal intubation (8.1%), during general anaesthesia (10.8%) and prior to extubation procedure (10.8%). Based on our data ARM is a procedure for high-risk patients (33.3%) and usually used during anaesthesia when a decreasing oxygen saturation is detected (32.4%). Approximately 20-30% of respondents never use ARM during any phase of general anaesthesia.
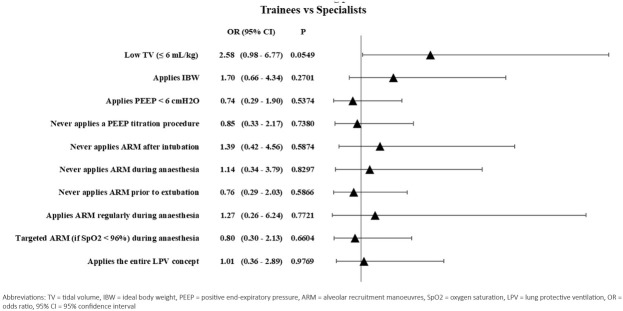
30 (27%) [95% CI 20.2 – 42.8)] of all the anaesthesiologists applied the three basic elements of LPV, but only 6(5.4%) [95% CI 2.2 – 13.1] applied ARMs regularly every 30 or 60 minutes. Although there were obvious practice variations between doctors and institutes, there were no statistically significant differences neither in the intraoperative pulmonary management practice of trainees and specialists nor in the practice of university centres and other hospitals. Results are summarised in Table 2 and Figure 4.
Table 2.
Use of the basic elements of lung protective ventilation
| Trainees | Specialists | ||||||
|---|---|---|---|---|---|---|---|
| n (=22) | % | n (=89) | % | OR (95% CI) | p | ||
| Low TV (≤ 6 mL/kg) | 8 | 36.4 | 53 | 59.6 | 2.58 | (0.98 – 6.77) | 0.0549 |
| Applies IBW | 11 | 50.0 | 56 | 62.9 | 1.70 | (0.66 – 4.34) | 0.2701 |
| PEEP < 6 cmH2O | 12 | 54.5 | 42 | 47.2 | 0.74 | (0.29 – 1.90) | 0.5374 |
| Never applies a PEEP titration procedure | 12 | 54.5 | 45 | 50.6 | 0.85 | (0.33 – 2.17) | 0.7380 |
| Never applies ARM after intubation | 4 | 18.2 | 21 | 23.6 | 1.39 | (0.42 – 4.56) | 0.5874 |
| Never applies ARM during anaesthesia | 4 | 18.2 | 18 | 20.2 | 1.14 | (0.34 – 3.79) | 0.8297 |
| Never applies ARM before extubation | 8 | 36.4 | 27 | 30.3 | 0.76 | (0.29 – 2.03) | 0.5866 |
| Applies ARM regularly during anaesthesia | 2 | 9.1 | 10 | 11.2 | 1.27 | (0.26 – 6.24) | 0.7721 |
| Targeted ARM (if SpO2 < 96%) during anaesthesia | 8 | 36.4 | 28 | 31.5 | 0.80 | (0.30 – 2.13) | 0.6604 |
| Applies the entire LPV concept | 6 | 27.3 | 24 | 26.9 | 1.01 | (0.36 – 2.89) | 0.9769 |
TV = tidal volume, IBW = ideal body weight, PEEP = positive end-expiratory pressure, ARM = alveolar recruitment manoeuvres, SpO2 = oxygen saturation, LPV = lung protective ventilation, OR = odds ratio, 95% CI = 95% confidence intervals
Fig. 4.
Forest plot for the application of the basic elements of lung-protective ventilation. Differences between groups with P values less than 0.05 were considered significant. Despite obvious practice variations were evaluated between trainees and specialist, these differences were not significant statistically.
Secondary Endpoints
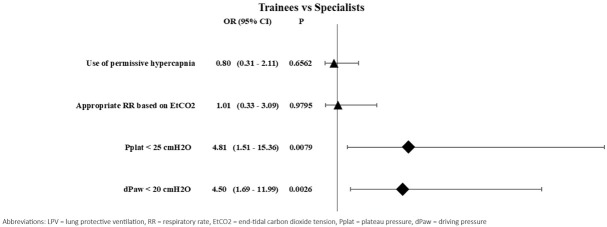
More than half of respondents. 66(59.5%) [95% CI 51.0 – 83.9] applied permissive hypercapnia (EtCO2 = 35-40 mmHg) during surgery and the great majority, 86 (77.5%) [95% CI 68.8 – 106.2] determined the appropriate respiratory rate based on capnography. Application of low plateau pressure (Pplat) and low ᐃPaw were 40.5% [45 (95% CI 32.8 – 60.2)] and the difference in the application of these two parameters between trainees and specialists was statistically significant [OR: 4.81 (95% CI 1.51 – 15.36) p=0.0079; OR: 4.50 (95% CI 1.69 – 11.99) p=0.0026] (Table 3 and Figure 5). Most patients, 93.7% [95% CI 84.9 – 126.0] were extubated in the operating theatre. The use of nondepolarizing neuromuscular blocking agents (NMBA-As) nondepolarizing neuromuscular blocking agents
Table 3.
Use of other elements of lung protective ventilation
| Trainees | Specialists | ||||||
|---|---|---|---|---|---|---|---|
| n (=22) | % | n (=89) | % | OR (95% CI) | p | ||
| Use of permissive hypercapnia | 14 | 63.6 | 52 | 58.4 | 0.80 | (0.31 – 2.11) | 0.6562 |
| Appropriate RR based on EtCO2 | 17 | 77.3 | 69 | 77.5 | 1.01 | (0.33 – 3.09) | 0.9795 |
| Pplat < 25 cmH2O | 4 | 18.2 | 46 | 51.7 | 4,81 | (1.51 – 15.36) | 0.0079 |
| dPaw < 20 cmH2O | 4 | 18.2 | 25 | 28.1 | 4,50 | (1.69 – 11.99) | 0.0026 |
RR = respiratory rate, EtCO2 = end-tidal carbon dioxide tension, Pplat = plateau pressure, dPaw = driving pressure, OR = odds ratio, 95% CI = 95% confidence intervals
Fig. 5.
Forest plot for the application of the other elements of lung-protective ventilation. Differences between groups with P values less than 0.05 were considered significant. Differences in the application of low Pplat and low dPaw between trainees and specialists was statistically significant. Application of these two target parameters are more common among specialists.
(NMBA-As) was common, but only 19 [17.1% (95% CI 11.4 – 29.7)] respondents considered the necessity of these agents based on neuromuscular transmission monitoring (NMT). Also, 8.1% of respondents considered “head lifting test” to be appropriate.
On the one hand, during the preoperative assessment, a large number of examinations such as chest X-ray, spirometry and arterial blood gas analysis (ABGA), were carried out, mainly in high-risk patients. On the other hand, substantive interventions such as breathing physiotherapy and positive pressure ventilatory support (CPAP) and non-invasive ventilation (NIV) were not reported in the survey. (Table 4). The same holds for postoperative care.
Table 4.
Preoperative assessment: examinations and prescribed interventions
| Physiotherapy | Chest X-ray | Spirometry | ABGA | PPPVS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Always | 3 | (2.7) | 46 | (41.1) | 0 | (0) | 7 | (6.3) | 0 | (0) |
| In patients with COPD | 49 | (43.8) | 44 | (39.3) | 101 | (90.2) | 63 | (56.3) | 8 | (7.1) |
| In patients with bronchial asthma | 25 | (22.3) | 30 | (26.8) | 84 | (75.0) | 22 | (19.6) | 3 | (2.7) |
| Inactive smokers | 18 | (16.1) | 22 | (19.6) | 18 | (16.1) | 10 | (8.9) | 0 | (0) |
| In case of actual intermittent respiratory disease | 11 | (9.8) | 38 | (33.9) | 30 | (26.8) | 25 | (22.3) | 5 | (4.5) |
| In patients with abnormal chest X-ray or lung CT scan | 17 | (15.2) | n/a | 47 | (42.0) | 24 | (21.4) | 2 | (1.8) | |
| If low SpO2 (< 96%) is observed during an assessment | 20 | (17.9) | 41 | (36.6) | 46 | (41.1) | 63 | (56.3) | 7 | (6.3) |
| Prior to acute or vital surgery | n/a | 16 | (14.3) | n/a | 45 | (40.2) | 7 | (6.3) | ||
| Never prescribed | 56 | (50) | 9 | (8) | 6 | (5.4) | 9 | (8.0) | 96 | (85.7) |
Data are expressed as the number (and percentage) of answers. COPD = chronic obstructive pulmonary disease, CT = computer tomography, SpO2 = oxygen saturation, ABGA = arterial blood gas analysis, PPPVS = perioperative positive pressure ventilatory support
Written institutional perioperative pulmonary management protocols general were unavailable, regardless of the type of institution (Table 5). Neither CPAP nor NIV were available 24 hours a day in several hospitals, resulting in 45 (40.5%) [95% CI 32.8 – 60.2] of respondents never use POP.
Table 5.
Availability of perioperative breathing and intraoperative LPV protocols
| Other hospitals | University Medical Centres | ||||||
|---|---|---|---|---|---|---|---|
| n (=87) | % | n (=24) | % | OR (95% CI) | p | ||
| Availability of perioperative breathing protocols | 10 | 11.5 | 8 | 33.3 | 0.39 | (0.14 – 1.10) | 0.0747 |
| The absence of perioperative breathing protocols | 79 | 90.8 | 18 | 75.0 | 0.42 | (0.14 – 1.28) | 0.1262 |
| Availability of intraoperative LPV protocols | 6 | 6.9 | 2 | 8.3 | 0.82 | (0.15 – 4.32) | 0.8099 |
| The absence of intraoperative LPV protocols | 81 | 93.1 | 22 | 91.7 | 1.22 | (0.25 – 6.07) | 0.8062 |
LPV = lung protective ventilation, OR = odds ratio, 95% CI = 95% confidence intervals
Tertiary Endpoints
Regarding knowledge about the surgical factors, anaesthetic issues and patient-related risk factors of PPCs, respondents considered that the most critical risk factors are: thoracic and major abdominal surgery, COPD, obesity and residual neuromuscular blockade after surgery. In contrast transplant and intracranial surgery, chronic malnutrition, anaemia and prolonged use of nasogastric tube after surgery were considered negligible risk factors (Table 6). These last three results indicated the lack of early recovery after surgery (ERAS) approach.
Table 6.
Opinions about the risk factors of postoperative pulmonary complications
| Risk factors of PPC | Considered as important RF | ||
|---|---|---|---|
| n (=111) | % | 95% CI | |
| Thoracic surgery | 103 | 92.8 | 84.1 – 124.9 |
| Major abdominal surgery | 100 | 90.1 | 81.4 – 121.6 |
| COPD | 109 | 98.9 | 90.4 – 132.6 |
| Obesity | 97 | 87.4 | 78.7 – 118.3 |
| Residual neuromuscular blockade after surgery | 106 | 95.5 | 86.8 – 128.2 |
| Transplant surgery | 42 | 37.8 | 30.3 – 56.8 |
| Intracranial surgery | 38 | 33.3 | 26.1 – 51.0 |
| Chronic malnutrition | 39 | 35.8 | 28.6 – 54.5 |
| Anaemia | 37 | 33.7 | 23.5 – 47.5 |
| Prolonged use of NGT after surgery | 28 | 25.3 | 17.8 – 39.3 |
PPC = postoperative pulmonary complications, RF = risk factor, COPD = chronic obstructive pulmonary disease, NGT = nasogastric tube, 95% CI = 95% confidence intervals
Discussion
The questionnaire was designed to evaluate the routine perioperative pulmonary management practice during major abdominal surgery in Hungary. The reporting list described by Story et al., (2017) was used to obtain consistency, clarity, reproducibility and validity of the survey report [14].
Major abdominal surgery is considered a high-risk intervention associated with the risk of development of PPCs [6,15]. Furthermore, it is often an urgent or vital procedure performed in high-risk patients with serious comorbidities such as cardiovascular and chronic pulmonary diseases, life-threatening intraabdominal
infections or malignancies leading to chronic malnutrition. Applying LPV during major abdominal surgery is considered rational or even appropriate.
Advantages of LPV in patients with acute respiratory distress syndrome (ARDS) were described in the early ‘90s leading to intensive research [16, 17, 18]. Amato et al. (1998) found significantly better survival rates in the LPV group than in the conventional ventilatory group, and this finding was strengthened by the investigators of the Acute Respiratory Distress Network (2000) [19,20].
Results of the study by Futier et al. (2013) emphasised that LPV during abdominal surgery, even in patients with healthy lungs, is associated with a lower incidence of PPCs, resulted in improved outcomes, shorter length of stay in a hospital and reduced health care utilisation.[1] These findings were confirmed and the multifactorial pathophysiology of VILI and the risk factors of PPCs had been thoroughly evaluated. [2,3,4, 9,10]. Based on this knowledge and the pathophysiological rationale, Futier et al. (2014) established a new integrated approach called “perioperative positive pressure ventilation” (POP concept) to improve pulmonary care [8]. Despite existing evidence, the work of Fischer et al. (2016) indicated that ventilatory management practice in cardiac surgery varied markedly between anaesthesiologists [21]. Colinet et al. (2017) were of the opinion that the use of protective ventilation during anaesthetic care is still not used frequently enough. This may be due to lack of knowledge and therefore indicates an urgent need for education and regular training [22]. Schultz et al. (2017) opined that intraoperative LPV is still not widely implemented in everyday anaesthesia practice even in high-risk surgical patients, further suggesting that attention should be given to the use of lung protective strategies during general anaesthesia [11].
The present results indicate that applying low TV based on IBW is common and it is implemented in everyday anaesthesia practice, although the use of moderate levels of PEEP and even more regular ARMs are usually ignored, not to mention that individually titrated levels of PEEP are seldom employed. In patients with a BMI greater than 30 kg/m2, slightly higher levels of PEEP are accepted, and PEEP titration procedures seem to be employed more commonly in this patient group. Based on this survey, ARM is a procedure used when a decreasing oxygen saturation (SpO2) is detected. Application of permissive hypercapnia and determination of appropriate respiratory rate based on capnography are common during general anaesthesia, but somewhat more sophisticated elements such as low Pplat and ᐃPaw are used only by experts, which may be due to the low availability rate of written intraoperative ventilatory protocols or the shortcomings of regular education and training sessions. A significant number of examinations such as chest X-ray, spirometry and ABGA are carried out during the preoperative assessment, especially in the high-risk patient groups with chronic obstructive pulmonary disease (COPD), patients with actual respiratory diseases or patients with decreased SpO2.
However, perioperative pulmonary care, the socalled POP concept, is not generally used according to the survey findings. It is also important to note that constant access to CPAP or NIV devices is limited in several institutions. These findings altogether explain that consistent and entire application of LPV and POP concepts are rare, resulting markedly, but insignificant differences between anaesthesiologists and institutions.
The main risk factors of PPCs are well-known, but some issues such as chronic malnutrition or prolonged use of nasogastric tube after surgery as negligible factors indicate the absence of an ERAS approach, maybe due to reasons such as the absence of written protocols or the shortcomings of regular education, described earlier.
The survey suffers from some limitations. First, the survey was declarative, and the response rate was relatively low with only approximately 15% of all anaesthesiologists responding. Secondly, to maintain anonymity, sensitive personal or institutional data were not collected; therefore, neither the exact number of participating institutions nor regional distribution were evaluated. Thirdly, the anchoring effect may have influenced the answers to the subsequent questions. Randomising the order of questions could have eliminated this problem, however, this approach could have affected the coherence of the survey significantly.
Conclusions
The results of a nationwide survey are very similar to that of earlier international surveys and reports, indicating that variations in practice of perioperative respiratory management occur nationally and worldwide. More attention should be given to the use of lung protective strategies during general anaesthesia. Implementation of recent guidelines, developing local institutional protocols and continuous, high-quality education and regular training sessions are essential to improve postoperative outcomes in high-risk patients undergoing major abdominal surgery.
Acknowledgements
The authors extend thanks to everyone who participated in the survey and especially to Professor Ákos Csomós, former President of HSAIT, for editing a newsletter for members of HSAIT.
Footnotes
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest The authors declare that they did not get any funding source supporting the manuscript and the submitted work. The authors disclose any commercial and noncommercial affiliations that are or may be perceived to be a conflict of interest with the work. The authors declare that they did not use or demand any other consultancies.
References
- 1.Futier E, Constantin J-M, Paugam-Burtz C. A Trial of Intraoperative Low-Tidal-Volume Ventilation in Abdominal Surgery. N Engl J Med. 2013;369:428–37. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 2.Hemmes SN, Gama De Abreu M, Pelosi P, Schulz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): A multicentre randomised controlled trial. The Lancet. 2018;384:495–503. doi: 10.1016/S0140-6736(14)60416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slutsky AS, Ranieri VM. Ventilator-Induced Lung Injury. N Eng J Med. 2013;369:2126–36. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 4.Ricard J-D, Dreyfuss D, Saumon G.. Ventilator-induced Lung Injury. Eur Respir J. 2003;22(Suppl 42):2–9. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 5.Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care. 2014;18:211. doi: 10.1186/cc13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res. 2015;198(2):441–49. doi: 10.1016/j.jss.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Davies OJ, Husain T, Stephen R CM. Postoperative pulmonary complications following non-cardiothoracic surgery. BJA Education. 2017;17:295–300. [Google Scholar]
- 8.Futier E, Marret E, Jaber S. Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology. 2014;121:400–8. doi: 10.1097/ALN.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 9.Hartland BL, Newell TJ, Damico N. Alveolar recruitment manoeuvres under general anaesthesia: a systematic review of the literature. Respir Care. 2015;60:609–20. doi: 10.4187/respcare.03488. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, Grant MC, Stone A, Wu Cl, Wick EC. A Meta-Analysis of Intraoperative Ventilation Strategies to Prevent Pulmonary Complications: Is Low Tidal Volume Alone Sufficient to Protect Healthy Lungs? Ann Surg. 2016;263:881–7. doi: 10.1097/SLA.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 11.Schultz MJ. Epidemiology, the practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS - an observational study in 29 countries. Eur J Anaesth. 2017;34:492–507. doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haller G, Walder B. Postoperative pulmonary complications - Still room for improvement. Eur J Anaest. 2017;34:489–91. doi: 10.1097/EJA.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 13.Molnar Z. SepsEast: Bridging between East and West. J Crit Care. 2017;40:323. doi: 10.1016/j.jcrc.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Story DA, Gin V, na Ranong V, Stephanie BN P, Daryl J. Inconsistent Survey Reporting in Anaesthesia Journals. Anesth Analg. 2011;113:591–5. doi: 10.1213/ANE.0b013e3182264aaf. [DOI] [PubMed] [Google Scholar]
- 15.Patel K, Hadian F, Ali A. Postoperative pulmonary complications following major elective abdominal surgery: a cohort study. Perioper Med (Lond) 2016;5:10. doi: 10.1186/s13741-016-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee PC, Helsmoortel CM, Cohn SM, Fink MP. Are Low Tidal Volumes Safe? Chest. 1990;97:430–4. doi: 10.1378/chest.97.2.430. [DOI] [PubMed] [Google Scholar]
- 17.Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: A prospective study. Crit Care Med. 1994;22:1568–78. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Pelosi P, Croti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1807–14. doi: 10.1164/ajrccm.151.6.7767524. [DOI] [PubMed] [Google Scholar]
- 19.Amato MBP, Barbas CSV, Medeiros DM. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N Engl J Med. 1998;338:347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 20.The Acute Respiratory Distress Syndrome Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Fischer MO, Courteille B, Guinot PG. Perioperative Ventilatory Management in Cardiac Surgery: A French Nationwide Survey. Medicine (Baltimore) 2016;95:e2655. doi: 10.1097/MD.0000000000002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colinet B, Van der Linden P, Bissot M, Sottiaux N, Sotiaux T. Mechanical Ventilation Practices in the Operating Room. Survey of the Anesthesiology Society of Charleroi “VENTISAC”. Acta Anaesth Belg. 2017;68:81–6. [Google Scholar]