Abstract
Radiotherapy has been a central part in curing non-small cell lung cancer (NSCLC). However, it is possible that not all of the tumor cells are destroyed by radiation; therefore, it is important to effectively control residual tumor cells that could become aggressive and resistant to radiotherapy. In this study, we aimed to investigate the molecular mechanism of decreased NSCLC radioresistance by low-dose radiation (LDR) pretreatment. The results indicated that miR-30a and miR-30b, which effectively inhibited plasminogen activator inhibitor-1 (PAI-1), were overexpressed by treatment of LDR to NSCLC cells. Phosphorylation of Akt and ERK, the downstream survival signals of PAI-1, was decreased by PAI-1 inhibition. Reduced cell survival and epithelial-mesenchymal transition by PAI-1 inhibition were confirmed in NSCLC cells. Moreover, in vivo orthotopic xenograft mouse models with 7C1 nanoparticles to deliver miRNAs showed that tumor growth and aggressiveness were efficiently decreased by LDR treatment followed by radiotherapy. Taken together, the present study suggested that PAI-1, whose expression is regulated by LDR, was critical for controlling surviving tumor cells after radiotherapy.
Keywords: non-small cell lung cancer, radiotherapy, plasminogen activator inhibitor-1, low-dose radiation, therapeutic resistance
The common pattern of failure among non-small cell lung cancer patients treated with radiotherapy is development of therapeutic resistance. Youn et al. reveals that low-dose radiation-pretreating radiotherapy strategy leads to PAI-1 suppression, which is important for controlling side effects of radiotherapy.
Introduction
Lung cancer is the most lethal form of cancer worldwide. Despite efforts to treat lung cancer, the 5-year survival rate remains only 15%.1 Non-small cell lung cancer (NSCLC), which accounts for over 75% of lung cancer, is one of the two main types of lung cancer, the other being small-cell lung cancer. The response rate to NSCLC therapeutic agents is significantly low, with possible therapies in clinical trials showing limited success.2 Radiotherapy is an important mode of treatment for NSCLC, and it is administered to approximately 64%–75% of NSCLC patients overall.3 However, clinical outcomes of radiotherapy are not always satisfactory due to the development of radioresistance in NSCLC.4, 5 Recent reports have suggested that NSCLC radioresistance could be caused by the intercellular communication of heterogeneous tumor cells, but this mechanism is largely unknown.6, 7 Thus, elucidating how molecules interact between tumor cells having different characteristics could provide novel radiotherapeutic targets for NSCLC. Moreover, radiotherapy used in cancer treatment is also known to modulate microRNA (miRNA) expression to increase therapeutic efficacy.8 The different roles of miRNAs in tumor development have been investigated in previous studies.9 In breast cancer, miR-520 has tumor-suppressive effects and inhibits the expression of transforming growth factor-β (TGF-β) receptor 2, which can promote metastasis.10 On the other hand, miR-155 is an important tumor-promoting miRNA that downregulates the expression of WEE1 G2 checkpoint kinase, which is critical for DNA-damage responses.11 Therefore, it is important to understand radiation-induced regulatory mechanisms of miRNAs and their targets to overcome the therapeutic resistance during NSCLC radiotherapy.
Recent reports have suggested that increased expression of plasminogen activator inhibitor-1 (PAI-1) indicates poor prognosis of many cancer types, including NSCLC.12, 13 PAI-1 is an inhibitor of fibrinolysis. It is a physiological process that degrades fibrin and the extracellular matrix (ECM) proteins by inhibiting the urokinase-type plasminogen activator (uPA), which cleaves plasminogen to form plasmin. PAI-1, which is associated with the remodeling of the surrounding tissues, is a key factor in cancer proliferation, invasion, dissemination, and release of tumor growth factors and cytokines.14 PAI-1 secreted from mesenchymal stem cells also increased proliferation and migration of colon cancer cells.15 Moreover, PAI-1 is known to regulate several intracellular signaling pathways, such as phosphoinositide 3-kinase (PI3K)/Akt, focal adhesion kinase, and extracellular signal-regulated kinase (ERK) signaling, which induce tumor cell survival and aggressiveness.16, 17 In addition to the well-known functions of PAI-1 in tumor progression, some reports have demonstrated that PAI-1 could promote the epithelial-mesenchymal transition (EMT) process by regulating EMT-related proteins such as E-cadherin, Snail, and vimentin.18 These previous reports suggested that PAI-1, as a secreted protein, might significantly promote tumor metastasis and therapeutic resistance via paracrine signaling.
Low-dose radiation (LDR), which is not known to cause serious DNA damage, is defined as a dose of 100 mGy or less. The human body can be exposed to LDR in a variety of ways, most of which occur via medical examinations such as X-rays, computed tomography (CT), and positron emission tomography-CT scans.19 The biological effects of LDR are still controversial. The linear non-threshold (LNT) model suggests that there is no threshold for LDR risk, and even extremely low doses of radiation may exert a bad influence on human health. On the other hand, the hormesis model proposes that LDR is safe and beneficial to human health.20 A recent report indicated that LDR could inhibit the inflammation of articular chondrocytes induced by interleukin-1β and suggested LDR as a therapeutic tool for patients with cartilage disorders.21 Adaptive response is another mechanism postulating the positive effect of LDR.22 It suggests that LDR activates multiple cell-defense signaling pathways such as DNA repair systems and antioxidant production.23 Moreover, a previous study demonstrated that normal human dermal fibroblasts exposed to LDR showed alterations in some miRNA expressions.24 In this study, the expression of 168 miRNAs was altered 6 hr post-LDR, and several of their targets, such as collagen type I alpha 1 and matrix metalloproteinase 1, were suggested. However, the regulatory mechanisms of miRNAs and their targets by LDR remain obscure.
The current study was conducted to investigate the mechanisms by which LDR treatment increased the efficacy of lung cancer radiotherapy. Here, we suggested that LDR-induced miR-30a and miR-30b inhibited the expression and secretion of PAI-1, which was responsible for inducing NSCLC aggressiveness. Moreover, ERK1/2 and Akt phosphorylation was downregulated by PAI-1 inhibition. Consequently, our findings indicated that LDR treatment or LDR-induced miRNAs can be a strategy to improve lung cancer radiotherapy.
Results
LDR Sensitized NSCLC Cells to High-Dose Radiation by Downregulating PAI-1
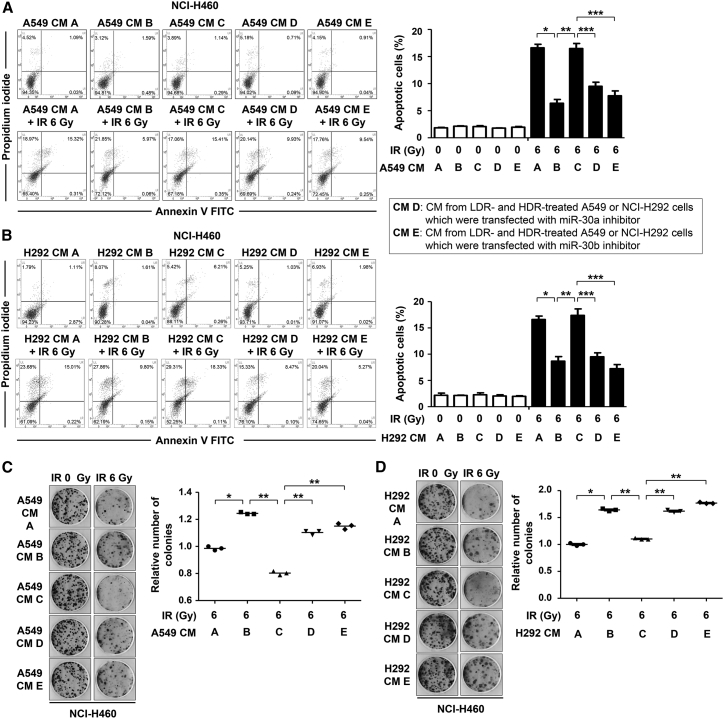
Our previous study revealed that increased expression and secretion of PAI-1 enhanced the radioresistance and aggressiveness of NSCLC.7 To identify a strategy to control NSCLC radioresistance using LDR, we examined the effects of LDR (50 mGy) prior to high-dose radiation (HDR, 6 Gy) on relatively radioresistant A549 and NCI-H292 cells. A previous study suggested that some NSCLC cell lines have relatively radioresistant or radiosensitive properties,25 and A549 and NCI-H292 cells showed higher cell survival upon HDR than NCI-H460 cells (Figure S1A). The dose of LDR that elicited a biological impact was selected by our screening of various LDR doses (Figure S1B). The conditioned media (CM) from the A549 and NCI-H292 cells were added to cultures of relatively radiosensitive NCI-H460 cells to determine the role of secreted factors. NCI-H460 cells were then irradiated, and their sensitivity to HDR was analyzed using the Annexin V/propidium iodide (PI)-staining assay. As shown in Figures 1A and 1B, the percentage of Annexin V-positive NCI-H460 cells that were unirradiated was less than 5% regardless of the CM treatments. On the contrary, the proportion of the apoptotic radiosensitive cells that received HDR varied with the types of CM that were used. The proportion of apoptosis was lower in NCI-H460 cells incubated with the CM from the HDR-treated radioresistant cells (CM B) than in the cells incubated with the CM from the unirradiated radioresistant cells (CM A) (Figures 1A and 1B). Remarkably, a higher proportion of apoptotic cells was observed in NCI-H460 cells incubated with the CM from radioresistant cells subjected to LDR followed by HDR (CM C) than in NCI-H460 cells incubated with CM B. From these results, we hypothesized that the higher rate of apoptosis in the CM C-treated NCI-H460 cells could be attributed to the LDR-regulated secreted factors from the radioresistant cells. Additionally, a colony-forming assay was performed to determine the long-term cell proliferation rate in NCI-H460 cells in response to treatment with different types of CM. The formation of colonies was upregulated in CM B-treated NCI-H460 cells, and downregulated in CM C-treated NCI-H460 cells (Figures 1C and 1D). We further observed that PAI-1 expression increased after HDR-only treatments but not after treatments with LDR or LDR followed by HDR in A549 and NCI-H292 cells (Figures 1E and 1F). Therefore, these results suggested that LDR pretreatment-induced PAI-1 downregulation might be involved in increased apoptosis of radioresistant cells.
Figure 1.
LDR Pretreatments Induced Apoptosis and Reduced Cell Proliferation and PAI-1 Levels in HDR-Treated NSCLC Cells
(A and B) Effects of CM derived from A549 (A) or NCI-H292 (B) cells on the HDR-induced apoptosis of NCI-H460 cells were analyzed by Annexin V/PI staining assays. The percentage of Annexin V-positive apoptotic NCI-H460 cells are indicated in the graph. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells. (C and D) The long-term effects of CM derived from A549 (C) or NCI-H292 (D) cells on the proliferation of NCI-H460 cells in response to HDR were measured by colony-forming assays. The number of NCI-H460 cell colonies relative to the control is indicated in the graph. The number of cell colonies of the control group was set as 1. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells. (E and F) The PAI-1 levels influenced by LDR and/or HDR in A549 (E) or NCI-H292 (F) cells were confirmed by western blotting. The number below the western blot bands indicates normalized expression (divided by α-tubulin expression) relative to control. IR, ionizing radiation. CM A, CM from the unirradiated A549 or NCI-H292 cells; CM B, CM from the HDR-treated A549 or NCI-H292 cells; CM C, CM from A549 or NCI-H292 cells subjected to LDR followed by HDR.
LDR-Induced miR-30a and miR-30b Were Selected as PAI-1 Post-transcriptional Repressors
Several previous reports have suggested that miRNAs regulated the radiation responses of NSCLC and that the miR-143/-145 cluster reduced the expression of PAI-1.26, 27, 28 Therefore, we screened for miRNAs that were complementary to the PAI-1 mRNA sequences using the TargetScan (http://www.targetscan.org), miRBase (http://www.mirbase.org), and microRNA.org (http://www.microrna.org/microrna/home.do) databases and selected 10 miRNAs (Figure 2A). We then confirmed whether the expressions of these miRNAs were altered by LDR and HDR. As miR-30a and miR-30b levels increased after LDR and decreased after HDR, they were selected as possible negative regulators of PAI-1 that functioned by binding to the SERPINE1 mRNA (which encodes PAI-1) in irradiated NSCLC cells (Figure 2B). The binding sites for miR-30a or miR-30b were present in the 3′ UTR of SERPINE1 (Figure 2C). To confirm the direct regulation of SERPINE1 by miR-30a or miR-30b, luciferase reporter vectors containing SERPINE1 3′ UTR with the miR-30a or miR-30b target site in its wild-type or mutated form (Figure 2C) were transfected with miR-30a or miR-30b mimic and the luciferase activity was measured (Figure 2D). In the presence of miR-30a or miR-30b mimic, the luciferase activity of the wild-type reporter in the coexpressed A549 or NCI-H292 cells was inhibited, but inhibitory effects by miRNA mimics were not observed in the mutant reporter-transfected cells (Figure 2D). Next, we measured the effect of miR-30a and miR-30b overexpression on PAI-1 mRNA levels using miR-30a and miR-30b mimics. The miR-30a or miR-30b level was significantly increased by treatment of miR-30a or miR-30b mimic treatment, respectively (Figure S2). PAI-1 mRNA and protein levels were lower in HDR-treated radioresistant cells transfected with the miR-30a and miR-30b mimics (Figures 2E and 2F). Therefore, we confirmed that miR-30a and miR-30b acted as post-transcriptional repressors of PAI-1. Collectively, the results suggest that LDR increased miR-30a and miR-30b levels, which then decreased PAI-1 mRNA and protein levels by inhibiting PAI-1 transcription.
Figure 2.
The Expressions of miR-30a and miR-30b, Which Target PAI-1, Were Affected by LDR
(A) Ten miRNAs were selected from several predicted PAI-1-binding miRNAs. The TargetScan, miRbase, and miRNA.org databases were used to predict the miRNAs whose sequences were complementary to the PAI-1 mRNA sequences. (B) Levels of the 10 miRNAs in LDR- or HDR-treated A549 cells were measured using real-time qRT-PCR. *p < 0.05 compared with control cells. (C) The 3′ UTR of SERPINE1 contains miR-30a- and miR-30b-binding sites. To verify the specificity of the miR-30a or miR-30b binding site, mutations were made in the expected binding region. The predicted secondary structures of SERPINE1 3′ UTR that bound to miR-30a or miR-30b are shown. (D) The luciferase activity was decreased upon miR-30a or miR-30b overexpression in the case of SERPINE1 wild-type 3′ UTR but was not affected in mutant 3′ UTR. *p < 0.05 compared with control. (E and F) PAI-1 mRNA and protein levels in NCI-H460 cells treated with miR-30a or miR-30b mimics were analyzed by real-time qRT-PCR (E) and western blotting (F). The number below the western blot bands indicates normalized expression (divided by α-tubulin expression) relative to control. *p < 0.05 compared with control cells; **p < 0.05 compared with irradiated cells. IR, ionizing radiation.
LDR-Induced miR-30a and miR-30b Increased HDR-Mediated Apoptosis
Next, a miR-30a inhibitor and a miR-30b inhibitor (whose sequences were complementary to miR-30a and miR-30b, respectively) were used to determine whether NCI-H460 cell apoptosis was upregulated by LDR-induced miR-30a and miR-30b. We confirmed that the miR-30a or miR-30b level was significantly decreased by treatment of its inhibitor (Figure S2). Radioresistant A549 and NCI-H292 cells transfected with the miR-30a or miR-30b inhibitors were treated with LDR followed by HDR, from which CM from miR-30a inhibitor-transfected cells (CM D) or CM from miR-30b inhibitor-transfected cells (CM E) were collected, respectively. The proportion of apoptotic cells in CM D- or CM E-treated NCI-H460 cells decreased after HDR (Figures 3A and 3B). These results suggested that PAI-1 levels increased due to the inhibition of miR-30a or miR-30b, which resulted in the CM D- or CM E-induced radioresistance of NCI-H460 cells. Next, we confirmed if long-term cell proliferation was regulated by LDR followed by HDR and the different types of CM. The colony-forming ability of the NCI-H460 cells treated with CM C was lower than that of the cells treated with CM B, implying that LDR followed by HDR sensitized the NCI-H460 cells (Figures 3C and 3D). Furthermore, NCI-H460 cells treated with CM D or CM E formed a higher number of colonies when compared to cells treated with CM C. These results indicated that the upregulated PAI-1 levels by inhibition of miR-30a or miR-30b stimulated tumor cell growth following HDR. Cumulatively, the results suggested that miR-30a and miR-30b could function as potential NSCLC radiosensitizers by inhibiting PAI-1.
Figure 3.
Incubation of NCI-H460 Cells with CM from LDR-Treated Radioresistant Cells Increased Apoptosis, Whereas It Decreased Long-Term Cell Proliferation
(A and B) Effects of CM containing miR-30a or miR-30b inhibitor and derived from A549 (A) or NCI-H292 (B) cells on the HDR-induced apoptosis of NCI-H460 cells were analyzed by Annexin V/PI staining assays. The percentage of Annexin V-positive apoptotic NCI-H460 cells are indicated in the graph. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells; ***p < 0.05 compared with irradiated and CM C-treated cells. (C and D) The long-term effects of CM containing miR-30a or miR-30b inhibitor and derived from A549 (C) or NCI-H292 (D) cells on the proliferation of NCI-H460 cells in response to HDR were measured by colony-forming assays. The number of NCI-H460 cell colonies relative to the control is indicated in the graph. The number of cell colonies of the control group was set as 1. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells; ***p < 0.05 compared with irradiated and CM C-treated cells. IR, ionizing radiation. CM A, CM from the unirradiated A549 or NCI-H292 cells; CM B, CM from the HDR-treated A549 or NCI-H292 cells; CM C, CM from A549 or NCI-H292 cells subjected to LDR followed by HDR; CM D, CM from miR-30a inhibitor-transfected A549 or NCI-H292 cells subjected to LDR followed by HDR; CM E, CM from miR-30b inhibitor-transfected A549 or NCI-H292 cells subjected to LDR followed by HDR.
PAI-1-Mediated Survival Signaling Pathways Were Inhibited by miR-30a and miR-30b
Next, we investigated whether miR-30a or miR-30b inhibited PAI-1-mediated survival-signaling pathways. According to our previous study, secreted PAI-1 activated Akt and ERK1/2, and inhibited caspase-3 activity.7 Here, we investigated Akt and ERK1/2 activation in NCI-H460 cells treated with CM A, B, C, D, or E followed by HDR. Phosphorylation of Akt and ERK1/2 was higher in CM B-treated NCI-H460 cells than in CM A-treated cells (Figure 4A). We also confirmed that NCI-H460 cells incubated with CM C showed decreased Akt and ERK1/2 phosphorylation when compared to cells incubated with CM B. However, treatment with CM D or CM E increased Akt and ERK1/2 activation, which indicated that LDR-induced miR-30a and miR-30b inhibited PAI-1 and its downstream survival signals (Figure 4B). Next, we investigated whether the different types of CM treatments influenced HDR-induced apoptosis. In irradiated NCI-H460 cells, CM B treatment decreased caspase-3/7 activity, whereas CM C treatment recovered it (Figure 4C). The expression of cleaved caspase-3 and poly (ADP-ribose) polymerase (PARP) was also indicated in the same manner (Figures 4D–4F). Cleaved caspase-3 and PARP levels, increased by CM C, were downregulated after incubation with CM D or CM E (Figures 4D and 4F). The results revealed that miR-30a and miR-30b increased PAI-1-mediated apoptosis caused by caspase-3 activation and PARP cleavage. Collectively, LDR-induced increase in miR-30a and miR-30b expression inhibited PAI-1 transcription and PAI-1-induced survival-signaling pathways and activated PAI-1-inhibited apoptosis-signaling pathways such as caspase-3 activation and PARP cleavage.
Figure 4.
PAI-Mediated Signaling Pathways Were Inhibited by Overexpression of miR-30a and miR-30b
(A and B) The extent of Akt and ERK1/2 phosphorylation in HDR-treated NCI-H460 cells varied with the types of CM used. The Akt and ERK1/2 phosphorylation was decreased by CM C treatment (A), but it was recovered by CM D or CM E treatment (B). (C and D) The activities of caspases in NCI-H460 cells treated with CM containing relatively high levels of PAI-1 (CM B, D, and E) or low levels of PAI-1 (CM A and C) were confirmed by using Caspase-Glo 3/7 assays (C) and western blotting (D). *p < 0.05 compared with control cells; **p < 0.05 compared with irradiated and CM A-treated cells; ***p < 0.05 compared with irradiated and CM B-treated cells. (E and F) The effects of CM A to C (E) or CM D and E (F) on PARP cleavage were analyzed via western blotting in NCI-H460 cells. The number below the western blot bands indicates normalized expression (divided by α-tubulin expression) relative to control. IR, ionizing radiation. CM A, CM from the unirradiated A549 or NCI-H292 cells; CM B, CM from the HDR-treated A549 or NCI-H292 cells; CM C, CM from A549 or NCI-H292 cells subjected to LDR followed by HDR; CM D, CM from miR-30a inhibitor-transfected A549 or NCI-H292 cells subjected to LDR followed by HDR; CM E, CM from miR-30b inhibitor-transfected A549 or NCI-H292 cells subjected to LDR followed by HDR.
PAI-1-Induced EMT and Migration Were Inhibited by miR-30a and miR-30b
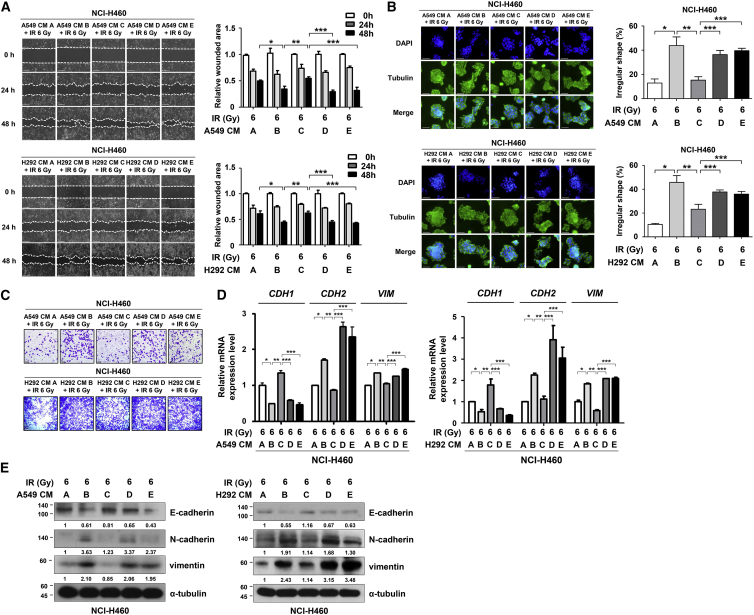
Since we determined that miR-30a and miR-30b promoted the apoptotic signaling pathways in NSCLC, we further investigated whether they could downregulate PAI-1-induced NSCLC aggressiveness. The migratory capacity of NCI-H460 cells exposed to HDR was determined via wound-healing assays. NCI-H460 cells incubated with CM C demonstrated reduced motility when compared to cells treated with CM B, which was recovered with CM D or CM E treatments (Figure 5A). Next, the morphological modifications were monitored using three-dimensional (3D) culture models.29 In this system, NCI-H460 cells treated with HDR and CM B showed invasive features in the Matrigel 3D matrix. However, irradiated NCI-H460 cells exhibited less aggressive morphologies after CM C treatment (Figure 5B). We confirmed that the PAI-1-induced increase in NCI-H460 cell migration was negatively regulated by PAI-1 inhibition (Figure 5C). Moreover, the mRNA and protein levels of EMT markers were also measured. E-cadherin (an epithelial marker) expression was also increased, whereas the expressions of N-cadherin and vimentin (mesenchymal markers) decreased after treatment with miR-30a- and miR-30b-rich CM, i.e., CM C (Figures 5D and 5E). Collectively, these results show that inhibition of PAI-1, resulting from LDR-induced miR-30a and miR-30b decreased the EMT and migration of NCI-H460 cells exposed to HDR.
Figure 5.
LDR-Induced Overexpression of miR-30a and miR-30b Repressed PAI-1-Induced EMT and Migration
(A) Effects of PAI-1 on HDR-induced migration in NCI-H460 cells were analyzed using wound-healing assays. Photomicrographs were taken at 100× magnification. Scale bars, 200 μm. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells; ***p < 0.05 compared with irradiated and CM C-treated cells. (B) The effect of different types of CM on the morphological changes in NCI-H460 cells were monitored using a 3D culture model. The cells were then permeabilized and stained: α-tubulin (green) and DAPI (blue). The results of the 3D culture was quantified with the spread and migration of spherioids (% of irregular spheres). Photomicrographs were taken at 200× magnification. Scale bars, 50 μm. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells; ***p < 0.05 compared with irradiated and CM C-treated cells. (C) HDR-induced migration of NCI-H460 cells was upregulated in PAI-1-rich CM (CM B, D, and E). Photomicrographs were taken at 100× magnification. Scale bars, 100 μm. (D and E) The effects of various types of CM with different PAI-1 levels on the mRNA and protein levels of EMT markers in NCI-H460 cells were analyzed using real-time qRT-PCR (D) and western blotting (E). The number below the western blot bands indicates normalized expression (divided by α-tubulin expression) relative to control. *p < 0.05 compared with irradiated and CM A-treated cells; **p < 0.05 compared with irradiated and CM B-treated cells; ***p < 0.05 compared with irradiated and CM C-treated cells. IR, ionizing radiation. CM A, CM from the unirradiated A549 or NCI-H292 cells; CM B, CM from the HDR-treated A549 or NCI-H292 cells; CM C, CM from A549 or NCI-H292 cells subjected to LDR followed by HDR; CM D, CM from miR-30a inhibitor-transfected A549 or NCI-H292 cells subjected to LDR followed by HDR; CM E, CM from miR-30b inhibitor-transfected A549 or NCI-H292 cells subjected to LDR followed by HDR.
Systemic Delivery of miR-30a and miR-30b Increased Radiosensitivity in Orthotopic Mouse Xenograft Model
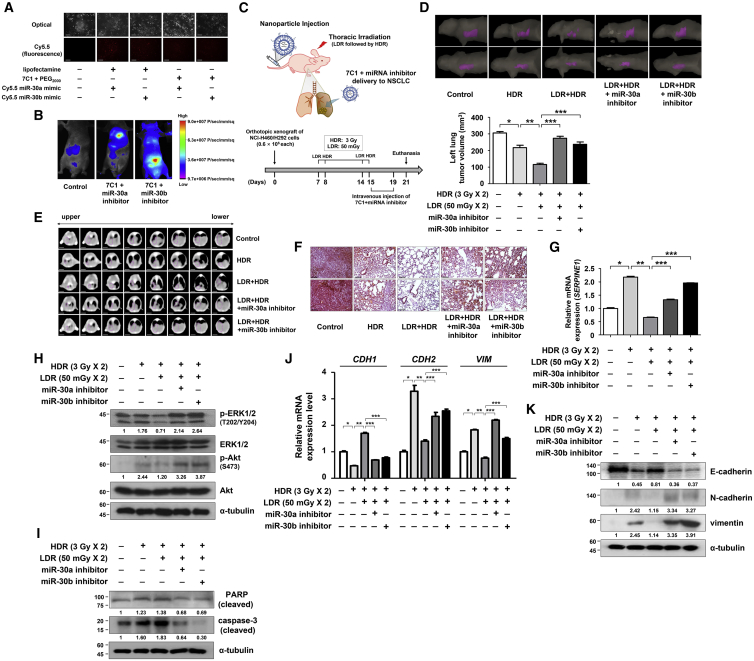
To evaluate the effects of LDR pretreatment and PAI-1 expression on the decreasing therapeutic resistance of NSCLC, 7C1 nanoparticles were used to encapsulate miR-30a or miR-30b inhibitor for in vivo delivery.30 The synthesized 7C1 was analyzed with proton nuclear magnetic resonance (1H-NMR) in CDCl3 (Figure S3A). We confirmed that the 7C1 nanoparticles and miRNA mimics were effectively delivered to the NCI-H460 cells and formed complexes (Figures 6A and S3B). The 7C1-miRNA inhibitor complexes were further confirmed to be specifically delivered to the mouse lungs (Figure 6B). Next, we established an orthotopic mouse xenograft model and investigated the effect of 7C1-miRNA inhibitors on in vivo radiosensitization (Figure 6C). We confirmed that the miR-30a or miR-30b level was significantly decreased by treatment of 7C1-miRNA inhibitors to mouse models (Figure S3C). After the experiment, the estimated tumor volume was significantly lowered by the HDR treatment (approximately 29% decrease in tumor volume; Figures 6D, 6E, and S3D). Moreover, LDR treatment followed by HDR resulted in the lowest tumor volume (approximately 62% decrease in tumor volume), and this tumor-inhibiting effect was reversed by the addition of miR-30a or miR-30b inhibitors (Figures 6D, 6E, and S3D). The tumor progression of orthotopic xenograft mouse models were verified using H&E staining in previous studies.31, 32 The H&E staining results of mouse lung tumors suggested that miR-30a or miR-30b inhibitors abrogated the tumor growth-inhibiting effect by the LDR pretreatments, thereby confirming the central role of PAI-1 in the radiation response of tumors (Figure 6F). Finally, by analyzing the orthotopically xenografted tumor, we showed that the HDR-mediated increase in the phosphorylation of ERK1/2 and Akt, and the levels of PAI-1 and EMT markers were significantly suppressed by LDR pretreatment (Figures 6G–6K). Taken together, these results show that LDR pretreatment or specific delivery of miR-30a or miR-30b could improve their therapeutic efficacy for treatment of NSCLC.
Figure 6.
Systemic Delivery of the miR-30a or miR-30b Inhibitors to the Lungs of the Orthotopic Xenograft Mouse Models Increased Sensitivity to Radiation
(A) The in vitro delivery of miR-30a or miR-30b contained in 7C1 nanoparticles was evaluated using optical and fluorescence microscopy. Photomicrographs were taken at 200× magnification. Scale bars, 100 μm. (B) Fluorescent imaging showing the successful delivery of the 7C1-miRNA inhibitor complexes to the lungs of the xenograft mouse models. (C) A schematic experimental diagram verifying the effect of miR-30a or miR-30b inhibitor delivery using orthotopic xenograft mouse models. (D) Representative CT images of the mouse lungs showing tumor regions (purple). The left lung tumor volume was evaluated. *p < 0.05 compared with control mice; **p < 0.05 compared with HDR-treated mice; ***p < 0.05 compared with mice treated with LDR followed by HDR. (E) Representative CT images of mouse lungs are shown. CT images of 1.5-mm slice thickness were contoured using Eclipse radiotherapy planning system. Scale bars, 5 mm. (F) Mouse lung tumors were stained with H&E to demonstrate the extent of the tumor progression. Photomicrographs were taken at 200× magnification. Scale bars, 100 μm. (G) SERPINE1 mRNA levels in xenografted lung tumors were determined using real-time RT-PCR. *p < 0.05 compared with control mice; **p < 0.05 compared with HDR-treated mice; ***p < 0.05 compared with mice treated with LDR followed by HDR. (H and I) ERK1/2 and Akt phosphorylation (H) and PARP cleavage and caspase-3 activation (I) in mouse lung tumors were evaluated via western blotting. (J and K) Expression of the EMT markers in mouse lung tumors was confirmed via real-time RT-PCR (J) and western blotting (K). The number below the western blot bands indicates normalized expression (divided by α-tubulin expression) relative to control. *p < 0.05 compared with control mice; **p < 0.05 compared with HDR-treated mice; ***p < 0.05 compared with mice treated with LDR followed by HDR.
Discussion
Our previous study indicated that the PAI-1 produced by radioresistant NSCLC cells induced the neighboring, relatively radiosensitive cells to become aggressive. In this study, we evaluated the effect of LDR in combination with HDR on the reduction of NSCLC aggressiveness. LDR increased the expression of miR-30a and miR-30b, which effectively inhibited PAI-1 expression and reduced its secretion. The LDR-mediated decrease in PAI-1 production reduced its survival-promoting effect on other tumor cells and increased tumor cell death, suggesting the possibility of a therapeutic strategy using LDR. Radiotherapy delivered to cancer cells, which is an indispensable therapeutic option, generally exposes cells to fractions of HDR. However, a small proportion of cancer cells survive after radiotherapy, which causes them to become more malignant. Therefore, much interest has been focused on identifying the molecular markers of radioresistance. Here, we present the report on the regulation of PAI-1 expression by miRNAs that were specifically upregulated by LDR. We also demonstrated the potential of utilizing these miRNAs in controlling the side effects of radiotherapy.
Our results indicated that LDR upregulated miR-30a and miR-30b, which then downregulated PAI-1 and PAI-1-induced signaling pathways. Additionally, miR-30a and miR-30b inhibited tumor cell proliferation, metastasis, and stem-like cell maintenance in many tissue types. For example, miR-30a targets Wnt5a to inhibit the stem-cell-like properties and progression of glioma and exhibits tumor-suppressive abilities by repressing the transmembrane-4-L-six-family protein in colorectal cancer.33, 34 Furthermore, miR-30b expression inhibited EMT in pancreatic cancer stem cells by targeting Snail and sensitizes HER2+ breast cancer to trastuzumab treatments by regulating cyclin E2.35, 36 Interestingly, our data showed that miR-30a and miR-30b overexpression led to improved radiotherapeutic efficacy by inhibiting PAI-1 expression (Figure 6). Moreover, our study also showed that miR-30a and miR-30b were induced by LDR and that they played a crucial role in NSCLC radiotherapy.
The lipid-based nanoparticle 7C1 is used to deliver small interfering RNA (siRNA) in vivo with lower molecular weight capabilities compared to traditional methods.30 Other studies have suggested that miRNAs could be formulated with the 7C1 nanoparticle and that the 7C1-miRNA treatment elicited significant effects, such as the activation of the immune system or delay of glioblastoma progression.37, 38 Dahlman et al.30 revealed that 7C1 lipid nanoparticles complexed with siRNA had diameter between 35 and 60 nm. In the present study, we also showed that the diameter of the miR-30a or miR-30b inhibitor encapsulated in 7C1 nanoparticles was about 50 nm (Figure S3B). Nanoparticles that are 30–60 nm in diameter are suitable for the membrane-wrapping process and efficient delivery into the target cells, whereas larger nanoparticles cause steric hindrance and receptor saturation, and smaller nanoparticles fail to drive the membrane-wrapping process.39 The low molecular weight of 7C1 nanoparticles is advantageous for the delivery of miRNAs, compared to other miRNA delivery systems used in previous studies, such as chitosan (over 100 nm in most cases) or poly(lactide-co-glycolide) particles (170 to 200 nm).40, 41 A previous study suggested that after injection of the 7C1 nanoparticle and ICAM-2 siRNA complex, the expression of ICAM-2 mRNA was initially decreased by 92% and suppressed between 73% and 85% for 21 days.30 Furthermore, the authors also identified that the target gene silencing effect by 7C1 nanoparticle was greater in the lung than in the liver, heart, or kidney. As the 7C1 nanoparticle remained in the mouse for about 7 days in our study (Figure 6C), it could be speculated that the 7C1-miRNA inhibitor complex was stable in vivo, and we verified the target PAI-1 mRNA expression level in the mouse lung (Figure 6G). Furthermore, as delivery of miR-30a and miR-30b to the lungs would elicit a similar effect to that of LDR in our study, it would decrease the psychological burden of patients with NSCLC that are receiving radiotherapy by reducing the total number of fractions. Instead of direct suppression of PAI-1 using a specific monoclonal antibody, we suggested a possible strategy of inhibiting PAI-1 by miRNA coated with 7C1 nanoparticle. Neutralizing antibodies have shown clear efficiency to treat human malignancies, but there have been several hurdles for efficient antibody-based cancer treatment, including difficulties for antibodies to penetrate the tumor tissue, immune escape by immunosuppressive microenvironment, and unintended immunogenic responses.42, 43, 44 Although miRNA-based approaches also have weaknesses like physiological instability and difficulties in delivery,42 we could stably deliver the miRNA inhibitors in NSCLC mouse model using 7C1 nanoparticles. Based on the understanding, we proposed that using PAI-1-targeting miRNAs delivered by 7C1 could be an option to enhance the efficacy of radiotherapy of NSCLC.
PAI-1 induced cell migration and metastasis in a variety of cancer types. Triple-negative breast cancer cells stimulated by TGF-β-secreted PAI-1, which accelerated their invasiveness by establishing a paracrine signaling mechanism with endothelial cells.45 He et al.46 revealed that the Wnt/β-catenin signaling pathway was directly upstream of PAI-1 and therefore increases its expression. Although we determined that miR-30a and miR-30b specifically bound to PAI-1 (Figure 2), it was possible that the miRNAs overexpressed by LDR negatively regulated the upstream signals of PAI-1. In fact, the Wnt/β-catenin/B cell lymphoma 9 pathway has been suggested to be a target of the miR-30 family, where miR-30a abrogates the TGF-β-induced EMT by targeting Snail1.47, 48 Considering these complex upstream mediators of PAI-1 and the importance of PAI-1 in cancer progression, the alterations of PAI-1 signaling by radiation need to be further investigated.
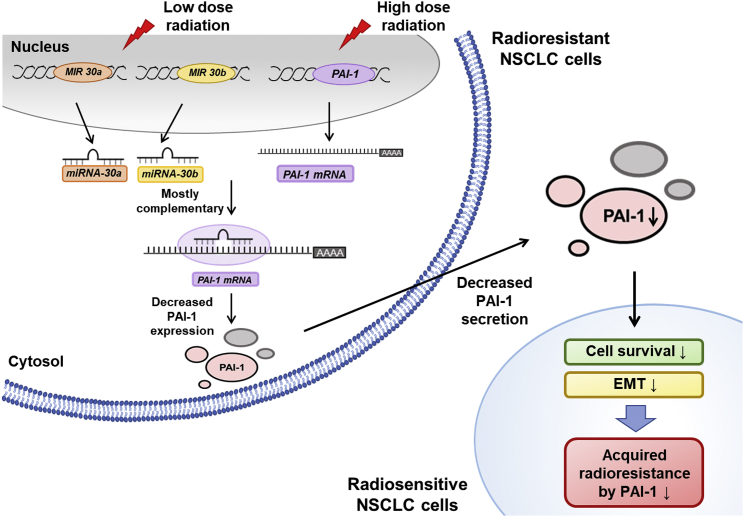
Residual cancer cells that survive radiotherapy often result in undesired aggressiveness and poor prognosis of the tumor. The results of the present study suggested that LDR-induced miR-30a and miR-30b targeted PAI-1 and elicited better clinical outcomes for NSCLC radiotherapy than conventional strategies did (Figure 7). We found that miR-30a and miR-30b were upregulated by LDR and that they inhibited expression of PAI-1, a radioresistance-inducing factor. In vivo orthotopic xenograft studies have also shown that tumor volumes were decreased by the systemic delivery of miR-30a and miR-30b in combination with LDR pretreatments to the NSCLC area. Cumulatively, the results of the present study suggested the possibility of introducing LDR or direct application of PAI-1-targeting miRNAs using nanoparticles into radiotherapy treatment plans for NSCLC in order to target PAI-1.
Figure 7.
A Schematic Diagram Illustrates How LDR-Induced Expressions of miR-30a and miR-30b Inhibit PAI-1 and Aggressiveness of NSCLC
Pretreatment of LDR to radioresistant cells lead to decreased PAI-1 expression and secretion. PAI-1 is known to be a key factor inducing radioresistance by activating survival signals in radiosensitive NSCLC cells. Reduced PAI-1 level in the microenvironment inhibits survival and EMT of radiosensitive cells. Therefore, utilizing LDR-induced miR-30a and miR-30b could be a novel strategy to improve radiotherapy.
Materials and Methods
Chemicals, Antibodies, and Reagents
Antibodies specific for PAI-1, tubulin, caspase-3, E-cadherin, N-cadherin, and vimentin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and Akt, phospho-Akt (Ser473), ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), and PARP were purchased from Cell Signaling Technology (Beverly, MA, USA). Cell culture medium (RPMI-1640), fetal bovine serum (FBS), glutamine, penicillin, and streptomycin were acquired from Gibco (Grand Island, NY, USA). Hsa-miR-30a-5p and hsa-miR-30b-5p inhibitor and fluorescently labeled miRNA mimics (Cy5.5-hsa-miR-30a and Cy5.5-hsa-miR-30b mimic) were synthesized by Bioneer (Daejeon, Republic of Korea).
Apoptosis Assay
The Annexin V-fluorescein isothiocyanate (FITC) kit (Enzo Life Science, Farmingdale, NY, USA) was used to detect apoptosis as previously described.49 Following the treatment of CM from NSCLC cells and HDR, cells (105–106 cells/mL) were harvested, washed with ice-cold PBS, and resuspended in 100 μL of ice-cold 1× binding buffer. Next, 25 ng of Annexin V-FITC and 250 ng of PI were added to the cell suspension and the cells were incubated on ice for 10 min in the dark. Finally, the stained cells were diluted to a final volume of 250 μL with 1× binding buffer and analyzed using a FACSVerse flow cytometer (BD Biosciences, San Jose, CA, USA).
3′ UTR Luciferase Assay to Establish SERPINE1 mRNA as a Direct Target of miR-30a and miR-30b
A 451-bp segment of the 3′ UTR of the SERPINE1 gene (positions 435–885 of 3′ UTR), which harbors the miR-30a or miR-30b binding site, was amplified (for primers, see Table S1) and cloned into the psiCHECK-2 vector (Promega, Madison, WI, USA). Mutant constructs of the miR-30a or miR-30b binding site were generated using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Palo Alto, CA, USA). Cotransfections of the wild-type SERPINE1 3′ UTR or the mutated SERPINE1 3′ UTR with the miR-30a or miR-30b mimics into the cells were accomplished with the Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Luciferase activity was measured 48 hr after the transfection using the Dual-Luciferase Reporter Assay System (Promega).
Caspase-3 Activity Assay
The Caspase-Glo 3/7 assay kit (Promega) was used to measure the activities of caspase-3/7 as previously described.50 Cells (2 × 104 cells/well) were seeded overnight in the 96-well plates and then incubated with the desired treatment of CM or irradiation. Subsequently, 100 μL of Caspase-Glo 3/7 reagent containing caspase-3/7 substrate was added to each well. After the contents of the wells were gently mixed at 300–500 rpm for 30 s, the plate was incubated at room temperature for 2 hr. Finally, the luminescence of each sample was measured using the Glomax multi detection system (Promega).
Wound-Healing Assay
Wound-healing assays were performed to measure changes in cell motility as previously described.51 In brief, cells were cultured to 70% confluence and treated with CM. After 24 hr, cells were exposed to HDR. The cell monolayers were then scratched with 200 μL pipette tips, after which NCI-H460 cells were further incubated with fresh media for 48 hr. Photomicrographs were then taken at 100× magnification using the Olympus IX71 inverted microscope (Olympus Optical, Tokyo, Japan).
Real-Time qRT-PCR
The expression levels of mRNAs, miRNAs, and EMT-related genes were analyzed by real-time qRT-PCR, as previously described.52 Aliquots of the master mix containing all of the reaction components with the primers were dispensed into a real-time PCR plate (Applied Biosystems, Foster City, CA, USA). All of the PCR reagents were from a SYBR Green core reagent kit (Applied Biosystems). The expressions of all genes were measured in triplicate in the reaction plate. The qRT-PCR was performed using the StepOne Real-Time PCR System (Applied Biosystems). It was performed by subjecting the samples at 95°C for 15 s and at 60°C for 1 min for 40 cycles followed by thermal denaturation. The expression of each gene relative to GAPDH mRNA was determined using the 2−ΔΔCT method. To simplify the data, values for the relative expression were multiplied by 102. The sequences of the primers used are shown in Table S2.
Western Blot Analysis
Following the experimental treatments, western blot analysis was performed as previously described.53 Detailed information is described in the Supplemental Materials and Methods.
3D Culture
Following the experimental treatments, 3D culture was performed as previously described.49 Detailed information is described in the Supplemental Materials and Methods.
Animal Care Protocol
Six-week-old female BALB/c athymic nude mice (Orient Bio, Seongnam, Republic of Korea) were used for the in vivo experiments. The protocols used were approved by the Institutional Animal Care and Use Committee of Pusan National University (Busan, Republic of Korea) and performed in accordance with the provisions of the NIH Guide for the Care and Use of Laboratory Animals. The mice were housed individually or in groups of up to five in sterile cages. They were maintained in animal care facilities in a temperature-regulated room (23°C ± 1°C) with a 12-hr light-dark cycle and quarantined for 1 week prior to the study. The animals had access to water and the standard mouse chow diet ad libitum.
Tumor Xenografts, In Vivo Imaging, and Histological Analysis in Nude Mice
The animals (n = 3 per group) were injected orthotopically with 0.6 × 106 NCI-H460 and NCI-H292 cells into the left lung, and tumors were then allowed to develop. Upon identification of a palpable tumor, miR-30a or miR-30b inhibitors encapsulated in 7C1 nanoparticles were administered via intravenous injection. The animals were also irradiated with LDR followed by HDR once a week for 2 weeks. The 3D-CT study of mouse lungs was performed using a 24-slice CT scanner (Somatom Sensation Open, Siemens Medical Solutions, Erlangen, Germany). The slice thickness was 1.5 mm for all CT images. All images were transferred to the radiotherapy-planning system (Eclipse, Varian Medical Systems, Palo Alto, CA, USA) for contouring and measuring tumor volumes. In vivo fluorescent images were collected by Maestro 2 multi-spectral imaging system (Perkin-Elmer, Waltham, MA, USA). At the end of the treatment period, the animals were euthanized and the tumors were used for biochemical studies or fixed with formalin, dehydrated, and embedded in paraffin. Next, sections were cut to 4 μm and stained with H&E following standard procedures.
Formation of 7C1 Nanoparticles with the miRNA Mimics or Inhibitors
The 7C1-M product (similar to 7C1 with longer alkyl chain lipid) was synthesized with PEI600 and 2-hexadecyloxirane as previously described.30 Typically PEI600 (0.2 g, 0.25 mmol) and 2-hexadecyloxirane (0.94 g, 3.5 mmol) were combined to 150 mL of absolute ethanol in a round-bottom flask with magnetic stirring. The mixture was refluxed at 90°C for 72 hr. After the reaction, the clear transparent solution was evaporated under the vacuum on a rotary evaporator. The product was re-dissolved in 10 mL of absolute ethanol and freeze-dried to obtain a white solid (0.8 g, yield 70.5%). The product was analyzed by 1H-NMR in CDCl3. The optimized formulations of 7C1 with miRNA inhibitors had a mass ratio of 7C1:PEG2000:miRNA inhibitors equal to 20:5:2. Nanoparticles were formulated with 30 μg of 7C1, 7.5 μg of PEG2000 in 60 μL of 90% ethanol, and 3 μg of miR-30a or miR-30b inhibitor in 20 μL diethyl pyrocarbonate (DEPC) water by mixing using sufficient pipetting. The 7C1-miRNA inhibitor complex was then injected intravenously with the dose of 1.5 mg/kg into tail vein of mice. Proper 7C1 nanoparticle formation was confirmed by cryogenic transmission electron microscope (cryo-TEM; Hitachi 7600 automatic analyzer, Hitachi, Tokyo, Japan). The samples for cryo-TEM were prepared using copper grids (200 mesh, Agar Grids) coated with a perforated film.
Author Contributions
G.P., B.S., J.K., and B.Y. designed the experiments. G.P., B.S., J.K., S.L., J.J., and J.-H.K. contributed to the data collection. G.P., B.S., J.K., G.-R.Y., H.Y., C.M., S.Y.N., and B.Y. analyzed and interpreted data. G.P., B.S., J.K., and B.Y. wrote the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (no. 20131610101840) and the Nuclear Safety Research Program through the Korea Foundation Of Nuclear Safety (KoFONS) using the financial resource granted by the Nuclear Safety and Security Commission (NSSC) of the Republic of Korea (no. 1805019).
Footnotes
Supplemental Information includes three figures, two tables, and Supplemental Materials and Methods and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.10.015.
Supplemental Information
References
- 1.Pao W., Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 2.Morgillo F., Della Corte C.M., Fasano M., Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinod S.K. International patterns of radiotherapy practice for non-small cell lung cancer. Semin. Radiat. Oncol. 2015;25:143–150. doi: 10.1016/j.semradonc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Kim W., Youn H., Kang C., Youn B. Inflammation-induced radioresistance is mediated by ROS-dependent inactivation of protein phosphatase 1 in non-small cell lung cancer cells. Apoptosis. 2015;20:1242–1252. doi: 10.1007/s10495-015-1141-1. [DOI] [PubMed] [Google Scholar]
- 5.Youn H., Son B., Kim W., Jun S.Y., Lee J.S., Lee J.M., Kang C., Kim J., Youn B. Dissociation of MIF-rpS3 complex and sequential NF-κB activation is involved in IR-induced metastatic conversion of NSCLC. J. Cell. Biochem. 2015;116:2504–2516. doi: 10.1002/jcb.25195. [DOI] [PubMed] [Google Scholar]
- 6.Son B., Lee S., Youn H., Kim E., Kim W., Youn B. The role of tumor microenvironment in therapeutic resistance. Oncotarget. 2017;8:3933–3945. doi: 10.18632/oncotarget.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang J., Kim W., Kwon T., Youn H., Kim J.S., Youn B. Plasminogen activator inhibitor-1 enhances radioresistance and aggressiveness of non-small cell lung cancer cells. Oncotarget. 2016;7:23961–23974. doi: 10.18632/oncotarget.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korpela E., Vesprini D., Liu S.K. MicroRNA in radiotherapy: miRage or miRador? Br. J. Cancer. 2015;112:777–782. doi: 10.1038/bjc.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 10.Keklikoglou I., Koerner C., Schmidt C., Zhang J.D., Heckmann D., Shavinskaya A., Allgayer H., Gückel B., Fehm T., Schneeweiss A. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene. 2012;31:4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 11.Tili E., Michaille J.J., Wernicke D., Alder H., Costinean S., Volinia S., Croce C.M. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc. Natl. Acad. Sci. USA. 2011;108:4908–4913. doi: 10.1073/pnas.1101795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werle B., Kotzsch M., Lah T.T., Kos J., Gabrijelcic-Geiger D., Spiess E., Schirren J., Ebert W., Fiehn W., Luther T. Cathepsin B, plasminogenactivator-inhibitor (PAI-1) and plasminogenactivator-receptor (uPAR) are prognostic factors for patients with non-small cell lung cancer. Anticancer Res. 2004;24:4147–4161. [PubMed] [Google Scholar]
- 13.Witzel I., Milde-Langosch K., Schmidt M., Karn T., Becker S., Wirtz R., Rody A., Laakmann E., Schütze D., Jänicke F., Müller V. Role of urokinase plasminogen activator and plasminogen activator inhibitor mRNA expression as prognostic factors in molecular subtypes of breast cancer. OncoTargets Ther. 2014;7:2205–2213. doi: 10.2147/OTT.S65344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mekkawy A.H., Pourgholami M.H., Morris D.L. Involvement of urokinase-type plasminogen activator system in cancer: an overview. Med. Res. Rev. 2014;34:918–956. doi: 10.1002/med.21308. [DOI] [PubMed] [Google Scholar]
- 15.Hogan N.M., Joyce M.R., Murphy J.M., Barry F.P., O’Brien T., Kerin M.J., Dwyer R.M. Impact of mesenchymal stem cell secreted PAI-1 on colon cancer cell migration and proliferation. Biochem. Biophys. Res. Commun. 2013;435:574–579. doi: 10.1016/j.bbrc.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Monaghan-Benson E., McKeown-Longo P.J. Urokinase-type plasminogen activator receptor regulates a novel pathway of fibronectin matrix assembly requiring Src-dependent transactivation of epidermal growth factor receptor. J. Biol. Chem. 2006;281:9450–9459. doi: 10.1074/jbc.M501901200. [DOI] [PubMed] [Google Scholar]
- 17.Rømer M.U., Larsen L., Offenberg H., Brünner N., Lademann U.A. Plasminogen activator inhibitor 1 protects fibrosarcoma cells from etoposide-induced apoptosis through activation of the PI3K/Akt cell survival pathway. Neoplasia. 2008;10:1083–1091. doi: 10.1593/neo.08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suarez-Carmona M., Bourcy M., Lesage J., Leroi N., Syne L., Blacher S., Hubert P., Erpicum C., Foidart J.M., Delvenne P. Soluble factors regulated by epithelial-mesenchymal transition mediate tumour angiogenesis and myeloid cell recruitment. J. Pathol. 2015;236:491–504. doi: 10.1002/path.4546. [DOI] [PubMed] [Google Scholar]
- 19.Hendee W.R., O’Connor M.K. Radiation risks of medical imaging: separating fact from fantasy. Radiology. 2012;264:312–321. doi: 10.1148/radiol.12112678. [DOI] [PubMed] [Google Scholar]
- 20.Doss M. Linear No-Threshold Model VS. Radiation Hormesis. Dose Response. 2013;11:480–497. doi: 10.2203/dose-response.13-005.Doss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong E.H., Song J.Y., Lee S.J., Park I.C., Um H.D., Park J.K., Lee K.H., Nam S.Y., Hwang S.G. Low-dose γ-radiation inhibits IL-1β-induced dedifferentiation and inflammation of articular chondrocytes via blockage of catenin signaling. IUBMB Life. 2014;66:128–137. doi: 10.1002/iub.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonner W.M. Low-dose radiation: thresholds, bystander effects, and adaptive responses. Proc. Natl. Acad. Sci. USA. 2003;100:4973–4975. doi: 10.1073/pnas.1031538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimova E.G., Bryant P.E., Chankova S.G. Adaptive response: some underlying mechanisms and open questions. Genet. Mol. Biol. 2008;31:396–408. [Google Scholar]
- 24.Bae S., Kim K., Cha H.J., Choi Y., Shin S.H., An I.S., Lee J.H., Lee S.J., Kim J.Y., Nam S.Y., An S. Low-dose γ-irradiation induces dual radio-adaptive responses depending on the post-irradiation time by altering microRNA expression profiles in normal human dermal fibroblasts. Int. J. Mol. Med. 2015;35:227–237. doi: 10.3892/ijmm.2014.1994. [DOI] [PubMed] [Google Scholar]
- 25.Das A.K., Sato M., Story M.D., Peyton M., Graves R., Redpath S., Girard L., Gazdar A.F., Shay J.W., Minna J.D., Nirodi C.S. Non-small-cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res. 2006;66:9601–9608. doi: 10.1158/0008-5472.CAN-06-2627. [DOI] [PubMed] [Google Scholar]
- 26.Ma D., Jia H., Qin M., Dai W., Wang T., Liang E., Dong G., Wang Z., Zhang Z., Feng F. MiR-122 Induces Radiosensitization in Non-Small Cell Lung Cancer Cell Line. Int. J. Mol. Sci. 2015;16:22137–22150. doi: 10.3390/ijms160922137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhang B., Chen J., Ren Z., Chen Y., Li J., Miao X., Song Y., Zhao T., Li Y., Shi Y. A specific miRNA signature promotes radioresistance of human cervical cancer cells. Cancer Cell Int. 2013;13:118. doi: 10.1186/1475-2867-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villadsen S.B., Bramsen J.B., Ostenfeld M.S., Wiklund E.D., Fristrup N., Gao S., Hansen T.B., Jensen T.I., Borre M., Ørntoft T.F. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br. J. Cancer. 2012;106:366–374. doi: 10.1038/bjc.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S., Kim D., Kang J., Kim E., Kim W., Youn H., Youn B. Surfactant Protein B Suppresses Lung Cancer Progression by Inhibiting Secretory Phospholipase A2 Activity and Arachidonic Acid Production. Cell. Physiol. Biochem. 2017;42:1684–1700. doi: 10.1159/000479418. [DOI] [PubMed] [Google Scholar]
- 30.Dahlman J.E., Barnes C., Khan O., Thiriot A., Jhunjunwala S., Shaw T.E., Xing Y., Sager H.B., Sahay G., Speciner L. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 2014;9:648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Z., Guo W., Ma X., Zhang B., Dong P., Huang L., Wang X., Wang C., Huo X., Yu W. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells. Mol. Cancer. 2014;13:203. doi: 10.1186/1476-4598-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen J., Wang B., Zhang T., Zhu N., Wang Z., Jin J., He Y., Hu M. Suppression of Non-Small Cell Lung Cancer Growth and Metastasis by a Novel Small Molecular Activator of RECK. Cell. Physiol. Biochem. 2018;45:1807–1817. doi: 10.1159/000487872. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Wu Z., Li L., Xie M. miR-30a inhibits glioma progression and stem cell-like properties by repression of Wnt5a. Oncol. Rep. 2017;38:1156–1162. doi: 10.3892/or.2017.5728. [DOI] [PubMed] [Google Scholar]
- 34.Park Y.R., Kim S.L., Lee M.R., Seo S.Y., Lee J.H., Kim S.H., Kim I.H., Lee S.O., Lee S.T., Kim S.W. MicroRNA-30a-5p (miR-30a) regulates cell motility and EMT by directly targeting oncogenic TM4SF1 in colorectal cancer. J. Cancer Res. Clin. Oncol. 2017;143:1915–1927. doi: 10.1007/s00432-017-2440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tormo E., Adam-Artigues A., Ballester S., Pineda B., Zazo S., González-Alonso P., Albanell J., Rovira A., Rojo F., Lluch A., Eroles P. The role of miR-26a and miR-30b in HER2+ breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Sci. Rep. 2017;7:41309. doi: 10.1038/srep41309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y., Wang Y., Wang L., Huang Y., Xu Y., Xu L., Guo Y., Lu J., Li X., Zhu M., Qian H. MicroRNA-30b targets Snail to impede epithelial-mesenchymal transition in pancreatic cancer stem cells. J. Cancer. 2018;9:2147–2159. doi: 10.7150/jca.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson R., Espinosa-Diez C., Kanner N., Chatterjee N., Ruhl R., Hipfinger C., Advani S.J., Li J., Khan O.F., Franovic A. MicroRNA regulation of endothelial TREX1 reprograms the tumour microenvironment. Nat. Commun. 2016;7:13597. doi: 10.1038/ncomms13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu D., Khan O.F., Suvà M.L., Dong B., Panek W.K., Xiao T., Wu M., Han Y., Ahmed A.U., Balyasnikova I.V. Multiplexed RNAi therapy against brain tumor-initiating cells via lipopolymeric nanoparticle infusion delays glioblastoma progression. Proc. Natl. Acad. Sci. USA. 2017;114:E6147–E6156. doi: 10.1073/pnas.1701911114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond.) 2016;11:673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragelle H., Vandermeulen G., Préat V. Chitosan-based siRNA delivery systems. J. Control. Release. 2013;172:207–218. doi: 10.1016/j.jconrel.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Yang X.Z., Dou S., Sun T.M., Mao C.Q., Wang H.X., Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J. Control. Release. 2011;156:203–211. doi: 10.1016/j.jconrel.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., Gao D.Y., Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv. Drug Deliv. Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillay V., Gan H.K., Scott A.M. Antibodies in oncology. N. Biotechnol. 2011;28:518–529. doi: 10.1016/j.nbt.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Weiner L.M., Surana R., Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W., Xu J., Fang H., Tang L., Chen W., Sun Q., Zhang Q., Yang F., Sun Z., Cao L. Endothelial cells promote triple-negative breast cancer cell metastasis via PAI-1 and CCL5 signaling. FASEB J. 2018;32:276–288. doi: 10.1096/fj.201700237RR. [DOI] [PubMed] [Google Scholar]
- 46.He W., Tan R., Dai C., Li Y., Wang D., Hao S., Kahn M., Liu Y. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J. Biol. Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J.J., Lin J., Zhu D., Wang X., Brooks D., Chen M., Chu Z.B., Takada K., Ciccarelli B., Admin S. miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res. 2014;74:1801–1813. doi: 10.1158/0008-5472.CAN-13-3311-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Q., Yang M., Lan H., Yu X. miR-30a negatively regulates TGF-β1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am. J. Pathol. 2013;183:808–819. doi: 10.1016/j.ajpath.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Son B., Kwon T., Lee S., Han I., Kim W., Youn H., Youn B. CYP2E1 regulates the development of radiation-induced pulmonary fibrosis via ER stress- and ROS-dependent mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313:L916–L929. doi: 10.1152/ajplung.00144.2017. [DOI] [PubMed] [Google Scholar]
- 50.Kim W., Youn H., Lee S., Kim E., Kim D., Sub Lee J., Lee J.M., Youn B. RNF138-mediated ubiquitination of rpS3 is required for resistance of glioblastoma cells to radiation-induced apoptosis. Exp. Mol. Med. 2018;50:e434. doi: 10.1038/emm.2017.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim W., Kim E., Lee S., Kim D., Chun J., Park K.H., Youn H., Youn B. TFAP2C-mediated upregulation of TGFBR1 promotes lung tumorigenesis and epithelial-mesenchymal transition. Exp. Mol. Med. 2016;48:e273. doi: 10.1038/emm.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Son B., Jun S.Y., Seo H., Youn H., Yang H.J., Kim W., Kim H.K., Kang C., Youn B. Inhibitory effect of traditional oriental medicine-derived monoamine oxidase B inhibitor on radioresistance of non-small cell lung cancer. Sci. Rep. 2016;6:21986. doi: 10.1038/srep21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang J., Kim W., Lee S., Kwon D., Chun J., Son B., Kim E., Lee J.M., Youn H., Youn B. TFAP2C promotes lung tumorigenesis and aggressiveness through miR-183- and miR-33a-mediated cell cycle regulation. Oncogene. 2017;36:1585–1596. doi: 10.1038/onc.2016.328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.