Abstract
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in patients with diabetes mellitus. Patients with diabetes have a higher prevalence of CAD and a larger magnitude of ischemia, and they are more likely to have silent myocardial ischemia and myocardial infarction. However, recent large cohort studies demonstrate that diabetic patients are not a homogenous group with similar high risk for cardiac events. In fact, more than 30% of asymptomatic diabetic patients do not have evidence of coronary atherosclerosis and have a very low annual cardiac event rate. Accordingly, there has been a recent paradigm shift as to whether the detection of subclinical coronary atherosclerosis through imaging can best guide therapeutic decision making. This review discusses the role of various cardiac imaging techniques for stratifying cardiovascular risk and optimizing therapy in asymptomatic diabetic patients.
Keywords: cardiac imaging, coronary artery disease, atherosclerosis, computed tomography coronary angiography, myocardial ischemia, coronary artery calcium scoring, CACS, diabetes, myocardial ischemia
INTRODUCTION
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in patients with diabetes mellitus. These patients have a higher prevalence of CAD and are more likely to have silent myocardial ischemia and myocardial infarction (MI) than nondiabetics.1,2 The goals for screening asymptomatic diabetic patients should be to (1) stratify risk beyond that estimated by clinical risk factors alone, (2) identify subclinical coronary atherosclerosis, (3) identify patients with significant obstructive CAD who have silent myocardial ischemia and are at higher short-term risk for events, and (4) guide patient management to improve long-term outcomes. Another important objective is to identify low-risk patients who may not require statin and aspirin pharmacotherapy and/or further diagnostic testing. Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend high-dose statin therapy for all patients with diabetes.3 Yet large cohort studies demonstrate that asymptomatic patients with diabetes are not a homogenous group, with > 30% having no evidence of coronary atherosclerosis let alone obstructive CAD. The following review discusses the role of cardiac imaging in risk-stratifying asymptomatic diabetic patients and guiding therapeutic decision making.
CORONARY ARTERY CALCIUM SCORING
The coronary artery calcium score (CACS) is a well-established noninvasive test for identifying patients with coronary atherosclerosis; it requires no patient preparation, is easily interpretable, and can be performed within seconds with very low radiation exposure (Figure 1). In a general asymptomatic population without prior CAD, the CACS reclassifies risk beyond clinical risk models alone and, unlike functional testing, identifies the entire spectrum of subclinical coronary atherosclerosis.4,5 A CACS of 0 predicts a very low annual risk for major adverse cardiac events (MACE) in both men and women.5–7 This is paramount because approximately 60% of patients screened will have a CACS = 0 (more so women than men at any given age). Such low-risk patients are unlikely to benefit from further cardiac testing or statin therapy.8,9 Conversely, an abnormal CACS identifies patients at increased risk for having myocardial ischemia10–12 and subsequent cardiac events, particularly when atherosclerosis is severe. A recent meta-analysis of 20 studies showed a 23.6% prevalence of ischemia on single photon emission computed tomography myocardial perfusion imaging in patients with a CACS > 400.13 Furthermore, CACS testing influences both patient and physician behavior regarding statin and aspirin use and is a strong motivator for reducing cardiac risk factors and decreasing unnecessary downstream testing.14–16
Figure 1.
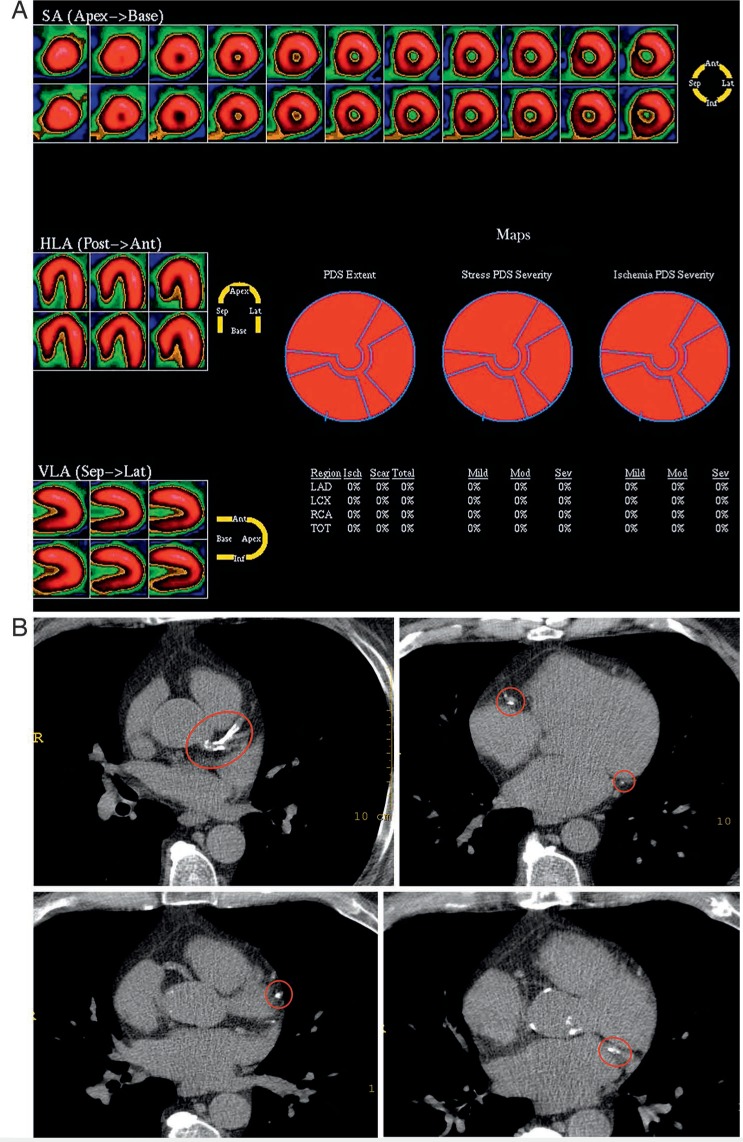
A 65-year-old man with a history of hypertension, hyperlipidemia, cigarette smoking, and recent onset atypical chest pain. Exercise treadmill test results showed exercise duration of 8.5 minutes with a maximal heart rate of 125 bpm and no symptoms or ST changes. Duke treadmill score was 8.5 (low risk). (A) Stress myocardial perfusion images were normal. (B) Despite a normal functional test, the coronary artery calcium score (CACS) was severe at 740, indicating high risk for subsequent cardiac events. Red circles highlight areas of coronary calcification. SA: short axis; HLA; horizontal long axis; VLA: vertical long access; PDS: perfusion defect size; LAD: left anterior descending; LCX: left circumflex; RCA: right coronary artery; TOT: total.
The CACS predicts overall MACE and all-cause mortality in both diabetic men and women.17–20 Those who are asymptomatic have a higher median CACS across all age groups, and CACS severity is sex independent.21,22 In an early study of 10,377 patients, Raggi et al. determined that annual all-cause mortality was similarly low for diabetic and nondiabetic patients who had a CACS = 0, whereas diabetic patients with a CACS > 0 had a significantly higher mortality rate.17 In the Diabetes Heart Study of 1,051 diabetic patients, those with a CACS = 0 had an annual mortality of 0.9% versus 2.7% for those with a CACS ≥ 1000.20
A recent meta-analysis of eight studies covering 6,521 patients reported a CACS < 10 in 29% of patients with diabetes18 who then had an annual hard cardiac event rate (death/nonfatal MI) of a 0.35%, a 6.8-fold reduction from the predicted 2.4% rate. The MESA investigators reported a CACS = 0 in 45% of patients with metabolic syndrome and 38% of patients with diabetes, with a low overall annual MACE in both groups.19 A longer follow-up analysis showed similar results but with MACE rates increasing as CACS severity increased in those with metabolic syndrome and particularly diabetes. The addition of CACS to clinical risk factors significantly improved net risk reclassification in both diabetic and nondiabetic patients and in those with metabolic syndrome.23
Although a CACS of 0 appears reassuring, other reports indicate that it may not confer the same low mortality risk in the long term.24 An observational study of 9,715 asymptomatic subjects undergoing CACS and 15-year follow-up found that diabetic patients with a CACS = 0 had a 2.5-fold higher hazard ratio for all-cause mortality compared to nondiabetics, although the annual mortality rates were still relatively low. Of interest, a significant increase in mortality within the diabetic cohort began > 5 years after the baseline normal CACS, suggesting that the “warranty period” for a CACS of 0 may be shorter for diabetic patients. Two studies in the general population have shown conversion from 0 to non-0 CACS in 16% of patients over 2.4 years and 25% over 5 years.25,26 In the latter study, the mean time of conversion was 4.1 + 0.9 years, suggesting that a repeat CACS at 4- to 5-year intervals may be prudent in patients with an initial CACS = 0.26
Data from the MESA investigators indicate that diabetic patients with a preexisting abnormal baseline CACS showed greater annual CACS progression compared with nondiabetic patients, regardless of sex.27 Poor glycemic control was a predictor of both conversion and progression. An increase in cardiac events mirrored the degree of change in CACS, with continued low event rates in nonprogressors but the highest event rates in patients at the highest tertile of progression and especially among those with metabolic syndrome or diabetes. This may explain the higher event rates seen at all levels of CACS > 0 in other studies comparing diabetic and nondiabetic patients. In further support of this finding, patients with diabetes/metabolic syndrome have a higher frequency of stress-induced myocardial ischemia compared to nondiabetic patients who have a severe or moderate CACS.28 Because myocardial ischemia predicts cardiac events, progression to even moderate CACS may be more worrisome in diabetic patients. These data support optimal glycemic control in all patients with diabetes and management of other cardiac risk factors to prevent plaque progression. Currently, CACS is considered an appropriate test in asymptomatic patients who are at intermediate or high clinical risk for CAD, a subgroup that includes the diabetic population.29
CT CORONARY ANGIOGRAPHY
Computed tomography coronary angiography (CTA) is highly accurate for diagnosing CAD,30,31 detecting ischemia,32–34 and predicting patient outcome based on the presence, extent, and severity of CAD.35–37 In the SCOT-HEART trial, CTA was significantly better than exercise treadmill testing (ETT) at reclassifying CAD diagnosis, leading to more aggressive treatment with statins and other therapies; also, patients undergoing CTA had better outcomes than those assessed by ETT alone.38 This is consistent with a recent observational study showing that statin therapy significantly improved outcomes in patients undergoing CTA who had extensive nonobstructive plaque.39
Although CTA has a potential advantage for detecting the entire spectrum of atherosclerotic plaque in asymptomatic diabetic patients, there are no data to suggest that it would perform better than a much simpler CACS. This is based on numerous studies in low-to-intermediate-risk patients with suspected CAD, wherein < 1.0% of patients had significant stenosis on CTA if the CACS was 0. At this juncture, CTA is not considered an appropriate test in asymptomatic patients.29
EXERCISE TREADMILL TESTING
Exercise treadmill testing predicts mortality in asymptomatic patients based on the presence of stress-induced ischemia, peak exercise capacity,40,41 post-exercise heart rate recovery,42 and the Duke Treadmill score (a composite of exercise duration, symptomatic status during exercise, and presence/extent of ischemia).43,44 Exercise capacity is one of the strongest predictors of survival in both asymptomatic men and women.40,41 In a recent study of 5,638 asymptomatic women, the Duke Treadmill score predicted total and cardiac mortality, but outcome was primarily driven by exercise capacity.45 Currently, ETT is considered “maybe appropriate” for asymptomatic patients at intermediate risk and “appropriate” for those at high global risk, providing that the baseline electrocardiogram is normal and the patient can exercise.29 Despite these recommendations, a normal ETT has a limited warranty period, suboptimal sensitivity and specificity for CAD detection, and does not identify subclinical coronary atherosclerosis.5,38
A recent observational study of approximately 1,000 generally asymptomatic patients compared the relative values of ETT and CACS for long-term risk stratification and determined that CACS severity best predicted risk5; it also improved risk prediction in the 85% to 90% of patients who had low-risk/normal ETT results, with subsequent event rates driven primarily by CACS severity. The results suggest that use of ETT in the general asymptomatic patient population has limited value.
However, because of its low cost, it may serve some value in diabetics with multiple other risk factors (i.e., the high-risk group) in situations where CACS is not available.
STRESS IMAGING
Stress myocardial perfusion imaging, stress echocardiography, and stress cardiac magnetic resonance imaging have all been shown to detect ischemia and predict outcome in patents with known or suspected coronary artery disease but are of limited clinical value in asymptomatic patients due to the low prevalence of a positive test result.12,13,46–58 Furthermore, they are inferior to CACS for predicting long-term risk due to their inability to detect subclinical coronary artery atherosclerosis and may therefore lead to a false sense of security if the test result is normal.12,13 However, targeted stress myocardial perfusion imaging is helpful in refining risk stratification in patients with a moderate (101–400) or severe (> 400) CACS because 25% to 40% will have significant silent myocardial ischemia.12,13,29
CLINICAL IMPLICATIONS
There is limited direct evidence showing improvement in patient outcomes through cardiac screening of asymptomatic patients, diabetic or otherwise. This is likely due to multiple issues including relatively short patient follow-up, low cardiac event rates, lack of structured protocol-directed treatment algorithms defined by imaging results, and the inability of most techniques to detect early coronary atherosclerosis. Most asymptomatic patients will have normal functional tests, but over half will have subclinical coronary atherosclerosis that will ultimately lead to a poor outcome.
Primary and secondary prevention trials have demonstrated significant reductions in cardiac event rates with statin therapy.59 In the JUPITER trial, 17,802 asymptomatic patients with elevated C-reactive protein as a marker of high risk were randomized to rosuvastatin or placebo. There was a 63% reduction in cardiac event rates in the treated population, primarily when low-density lipoprotein level was reduced to < 50 mg/dL.60,61 This was true irrespective of sex, age, body mass index, Framingham Risk Score, or presence of metabolic syndrome. Recently, the MESA investigators matched their patients to those in JUPITER and six other randomized primary prevention statin trials and demonstrated indirect evidence of benefit.9 Of the patients who would qualify for statin therapy based on clinical evidence, 44% had a CACS of 0. The annual event rates in patients with a CACS of 0 were only 0.39% for total atherosclerotic disease events and 0.17% for coronary heart disease events. In this analysis, 197 patients with a CACS of 0 would need statin treatment for 10 years to prevent one coronary heart disease event. However, the number of patients decreases to 56 for a CACS of 1 through 100 and only 28 for a CACS > 100. The CACS is the only noninvasive technique that detects early coronary atherosclerosis (other than CTA), effectively redefines risk, and can better discern which patients warrant statin therapy. This has been shown even within clinical groups recommended for statin therapy in the most recent ACC/AHA guidelines.8 The CACS appears to be an optimal initial screening test for defining the presence and extent of coronary atherosclerosis in asymptomatic diabetic patients and thereby guides patient management. In patients with a CACS of 0, statin therapy could be avoided with aggressive risk factor modification and repeat CACS testing at 3- to 4-year intervals. In patients with a CACS > 0, statin and aspirin therapy should be added to aggressive risk-factor modification, with functional testing reserved for those with silent myocardial ischemia who have a moderate to severe CACS score. Future large randomized trials should focus on how refining targeted treatment of patients with an abnormal CACS can lead to prevention of cardiac events.
KEY POINTS
The coronary artery calcium score (CACS) is currently the preferred noninvasive imaging test for stratifying risk and guiding therapeutic decision making in asymptomatic men and women, particularly those with diabetes.
Patients with a CACS of 0 have an exceedingly low annual event rate and can avoid statin therapy. Repeat CACS imaging is recommended at 3- to 4-year intervals.
Patients with a CACS > 0 should undergo aggressive risk factor modification that includes aspirin and statin therapy. Repeat CACS testing in such patients is not currently recommended.
Stress testing has limited value in asymptomatic patients with diabetes since it cannot detect subclinical coronary atherosclerosis, and a normal test result has only a 2- to 3-year warranty period. However, functional testing is recommended in the high-risk subset of patients with moderate (100–400) and severe (> 400) CACS to detect silent myocardial ischemia.
Footnotes
Conflict of Interest Disclosure: Dr. Mahmarian is a consultant and serves on the Speaker's Bureau for Astellas Pharma U.S., Inc.
REFERENCES
- 1.Emerging Risk Factors Collaboration et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London, England) 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morales DCV, Bhavnani SP, Ahlberg AW et al. Coronary risk equivalence of diabetes assessed by SPECT-MPI. J Nucl Cardiol. 2017 Dec 6; doi: 10.1007/s12350-017-1114-6. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt B):2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R, Möhlenkamp S, Moebus S et al. Coronary Risk Stratification, Discrimination, and Reclassification Improvement Based on Quantification of Subclinical Coronary Atherosclerosis. J Am Coll Cardiol. 2010 Oct 19;56(17):1397–406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Chang SM, Nabi F, Xu J et al. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long-term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: clinical implications in a multimodality imaging world. JACC Cardiovasc Imaging. 2015 Feb;8(2):134–44. doi: 10.1016/j.jcmg.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Detrano R, Guerci AD, Carr JJ et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med. Mar 27;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 7.Blaha M, Budoff MJ, Shaw LJ et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009 Jun;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Nasir K, Bittencourt MS, Blaha MJ et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015 Oct 13;66(15):1657–68. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen MB, Falk E, Li D et al. Statin Trials, Cardiovascular Events, and Coronary Artery Calcification: Implications for a Trial-Based Approach to Statin Therapy in MESA. JACC Cardiovasc Imaging. 2018 Feb 1;11(2):221–30. doi: 10.1016/j.jcmg.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman DS, Wong ND, Gransar H et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004 Aug 18;44(4):923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 11.He ZX, Hedrick TD, Pratt CM et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000 Jan 25;101(3):244–51. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 12.Chang SM, Nabi F, Xu J et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009 Nov 10;54(20):1872–82. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 13.Bavishi C, Argulian E, Chatterjee S, Rozanski A. CACS and the Frequency of Stress-Induced Myocardial Ischemia during MPI: A Meta-Analysis. JACC Cardiovasc Imaging. 2016 May;9(5):580–9. doi: 10.1016/j.jcmg.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Wong ND, Detrano RC, Diamond G et al. Does coronary artery screening by electron beam computed tomography motivate potentially beneficial lifestyle behaviors? Am J Cardiol. 1996 Dec 1;78(11):1220–3. doi: 10.1016/s0002-9149(96)00599-1. [DOI] [PubMed] [Google Scholar]
- 15.Taylor AJ, Bindeman J, Feuerstein I et al. Community-based provision of statin and aspirin after the detection of coronary artery calcium within a community-based screening cohort. J Am Coll Cardiol. 2008 Apr 8;51(14):1337–41. doi: 10.1016/j.jacc.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 16.Rozanski A, Gransar H, Shaw LJ et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011 Apr 12;57(15):1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004 May 5;43(9):1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 18.Kramer CK, Zinman B, Gross JL et al. Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. BMJ. 2013 Mar 25;346 doi: 10.1136/bmj.f1654. f1654. [DOI] [PubMed] [Google Scholar]
- 19.Malik S, Budoff MJ, Katz R et al. Impact of Subclinical Atherosclerosis on Cardiovascular Disease Events in Individuals with Metabolic Syndrome and Diabetes. The multi-ethnic study of atherosclerosis. Diabetes Care. 2011 Oct;34(10):2285–90. doi: 10.2337/dc11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S, Cox AJ, Herrington DM et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes Care. 2013 Apr;36(4):972–7. doi: 10.2337/dc12-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabelea D, Kinney G, Snell-Bergeon JK et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003 Nov;52(11):2833–9. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 22.Hoff JA, Quinn L, Sevrukov A et al. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol. 2003 Mar 19;41(6):1008–12. doi: 10.1016/s0735-1097(02)02975-3. [DOI] [PubMed] [Google Scholar]
- 23.Malik S, Zhao Y, Budoff M et al. Coronary Artery Calcium Score for Long-term Risk Classification in Individuals with Type 2 Diabetes and Metabolic Syndrome from the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol. 2017 Dec 1;2(12):1332–40. doi: 10.1001/jamacardio.2017.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valenti V, Hartaigh BÓ, Cho I et al. Absence of Coronary Artery Calcium Identifies Asymptomatic Diabetic Individuals at Low Near-Term But Not Long-Term Risk of Mortality: A 15-Year Follow-Up Study of 9715 Patients. Circ Cardiovasc Imaging. 2016 Feb;9(2):e003528. doi: 10.1161/CIRCIMAGING.115.003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kronmal RA, McClelland RL, Detrano R et al. Risk Factors for the Progression of Coronary Artery Calcification in Asymptomatic Subjects Results From the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007 May 29;115(21):2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 26.Min JK, Lin FY, Gidseg DS et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? J Am Coll Cardiol. 2010 Mar 16;55(11):1110–7. doi: 10.1016/j.jacc.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 27.Wong ND, Nelson JC, Granston T et al. Metabolic syndrome, diabetes, and incidence and progression of coronary calcium: the Multiethnic Study of Atherosclerosis study. JACC Cardiovasc Imaging. 2012 Apr;5(4):358–66. doi: 10.1016/j.jcmg.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ND, Sciammarella MG, Polk D et al. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003 May 7;41(9):1547–53. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 29.Wolk MJ, Bailey SR, Doherty JU et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014 Feb 4;63(4):380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Zhou T, Zhang R et al. Meta-analysis: diagnostic accuracy of coronary CT angiography with prospective ECG gating based on step-and-shoot, Flash and volume modes for detection of coronary artery disease. Eur Radiol. 2014 Oct;24(10):2345–52. doi: 10.1007/s00330-014-3221-y. [DOI] [PubMed] [Google Scholar]
- 31.Opolski MP, Staruch AD, Jakubczyk M et al. CT Angiography for the Detection of Coronary Artery Stenoses in Patients Referred for Cardiac Valve Surgery Systematic Review and Meta-Analysis. JACC CV Imaging. 2016 Sep;9(9):1059–70. doi: 10.1016/j.jcmg.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Nørgaard BL, Leipsic J, Gaur S et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014 Apr 1;63(12):1145–55. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Min JK, Leipsic J, Pencina MJ et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012 Sep 26;308(12):1237–45. doi: 10.1001/2012.jama.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nørgaard BL, Hjort J, Gaur S et al. Clinical Use of Coronary CTA-Derived FFR for Decision-Making in Stable CAD. JACC Cardiovasc Imaging. 2017 May;10(5):541–50. doi: 10.1016/j.jcmg.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Min JK, Dunning A, Lin FY et al. Age- and Sex-Related Differences in All-Cause Mortality Risk Based on Coronary Computed Tomography Angiography Findings Results From the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23, 854 Patients Without Known Coronary Artery Disease. JACC. 2011 Aug 16;58(8):849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 36.Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011 Mar 8;57(10):1237–47. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Hadamitzky M, Achenbach S, Al-Mallah M et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) J Am Coll Cardiol. 2013 Jul 30;62(5):468–76. doi: 10.1016/j.jacc.2013.04.064. CONFIRM Investigators. [DOI] [PubMed] [Google Scholar]
- 38.SCOT-HEART investigators CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015 Jun 13;385(9985):2383–91. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 39.Hulten E, Bittencourt MS, Singh A et al. Coronary artery disease detected by coronary computed tomographic angiography is associated with intensification of preventive medical therapy and lower low-density lipoprotein cholesterol. Circ Cardiovasc Imaging. 2014 Jul;7(4):629–38. doi: 10.1161/CIRCIMAGING.113.001564. [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989 Nov 3;262(17):2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 41.Mieres JH, Shaw LJ, Arai A et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005 Feb 8;111(5):682–96. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 42.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999 Oct 28;341(18):1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 43.Shaw LJ, Peterson ED, Shaw LK et al. Use of a Prognostic Treadmill Score in Identifying Diagnostic Coronary Disease Subgroups. Circulation. 1998 Oct 20;98(16):1622–30. doi: 10.1161/01.cir.98.16.1622. [DOI] [PubMed] [Google Scholar]
- 44.Alexander KP, Shaw LJ, Shaw LK, Delong ER, Mark DB, Peterson ED. Value of exercise treadmill testing in women. J Am Coll Cardiol. 1998 Nov 15;32(6):1657–64. doi: 10.1016/s0735-1097(98)00451-3. [DOI] [PubMed] [Google Scholar]
- 45.Gulati M, Amsdorf MF, Shaw LJ et al. Prognostic Value of the Duke Treadmill Score in Asymptomatic Women. Am J Cardiol. 2005 Aug 1;96(3):369–75. doi: 10.1016/j.amjcard.2005.03.078. [DOI] [PubMed] [Google Scholar]
- 46.Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004 Mar-Apr;11(2):171–85. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Nabi F, Chang SM, Xu J, Gigliotti E, Mahmarian JJ. Assessing risk in acute chest pain: The value of stress myocardial perfusion imaging in patients admitted through the emergency department. J Nucl Cardiol. 2012 Apr;19(2):233–43. doi: 10.1007/s12350-011-9484-7. [DOI] [PubMed] [Google Scholar]
- 48.Mahmarian JJ, Shaw LJ, Filipchuk NG et al. A Multinational Study to Establish the Value of Early Adenosine Technetium-99m Sestamibi Myocardial Perfusion Imaging in Identifying a Low-Risk Group for Early Hospital Discharge After Acute Myocardial Infarction. J Am Coll Cardiol. 2006;48:2448–57. doi: 10.1016/j.jacc.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 49.Giri S, Shaw LJ, Murthy DR et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002 Jan 1;105(1):32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 50.Acampa W, Cantoni V, Green R et al. Prognostic value of normal stress myocardial perfusion imaging in diabetic patients: A meta-analysis. J Nucl Cardiol. 2014 Oct;21(5):893–902. doi: 10.1007/s12350-014-9918-0. [DOI] [PubMed] [Google Scholar]
- 51.Marwick TH, Case C, Sawada S, Vasey C, Short L, Lauer M. Use of stress echocardiography to predict mortality in patients with diabetes and known or suspected coronary artery disease. Diabetes Care. 2002 Jun;25(6):1042–8. doi: 10.2337/diacare.25.6.1042. [DOI] [PubMed] [Google Scholar]
- 52.van der Sijde JN, Boiten HJ, Sozzi FB, Elhendy A, van Domburg RT, Schinkel AFL. Long-Term Prognostic Value of Dobutamine Stress Echocardiography in Diabetic Patients With Limited Exercise Capability: A 13-year follow-up study. Diabetes Care. 2012 Mar;35(3):634–9. doi: 10.2337/dc11-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenwood JP, Maredia N, Younger JF et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012 Feb 4;379(9814):453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu A, Wijesurendra RS, Liu JM et al. Diagnosis of Microvascular Angina Using Cardiac Magnetic Resonance. J Am Coll Cardiol. 2018 Mar 6;71(9):969–79. doi: 10.1016/j.jacc.2017.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Greenwood JP, Herzog BA, Brown JM et al. Prognostic Value of Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography in Suspected Coronary Heart Disease: Long-Term Followup of a Prospective, Diagnostic Accuracy Cohort Study. Ann Intern Med. 2016 May 10;165:1–9. doi: 10.7326/M15-1801. [DOI] [PubMed] [Google Scholar]
- 56.Zellweger MJ, Hachamovitch R, Kang X et al. Threshold, incidence, and predictors of prognostically high-risk silent ischemia in asymptomatic patients without prior diagnosis of coronary artery disease. J Nucl Cardiol. 2009 Mar-Apr;16(2):193–200. doi: 10.1007/s12350-008-9016-2. [DOI] [PubMed] [Google Scholar]
- 57.Blumenthal RS, Becker DM, Moy TF, Coresh J, Wilder LB, Becker LC. Exercise thallium tomography predicts future clinically manifest coronary heart disease in a high-risk asymptomatic population. Circulation. 1996 Mar 1;93(5):915–23. doi: 10.1161/01.cir.93.5.915. [DOI] [PubMed] [Google Scholar]
- 58.Young LH, Wackers FJT, Chyun DA et al. Cardiac Outcomes after Screening for Asymptomatic Coronary Artery Disease in Patients with Type 2 Diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009 Apr 15;301(15):1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Keefe JH, Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dL: lower is better and physiologically normal. J Am Coll Cardiol. 2004 Jun 2;43(11):2142–6. doi: 10.1016/j.jacc.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 60.Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol < 50 mg/dL with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) J Am Coll Cardiol. 2011 Apr 19;57(16):1666–75. doi: 10.1016/j.jacc.2010.09.082. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM, Danielson E, Fonseca FA et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]


