Abstract
Whole slide imaging (WSI) scanners and automatic image analysis algorithms, in order to be used for clinical applications, including primary diagnosis in pathology, are subject to specific regulatory frameworks in each country. Until May 25, 2018, in the European Union (EU), in vitro diagnostic (IVD) medical devices were regulated by directive 98/79/EC (in vitro diagnostic medical device directive [IVDD]). Main scanner vendors have obtained a Conformité Européenne mark of their products that in Europe were classified as General Class IVDD, so that conformity is only based on a self-declaration of the manufacturer. This contrasts with the initial classification of the US Food and Drug Administration (FDA) of WSI system as Class III medical devices, although the first digital pathology WSI system to be cleared by FDA was classified as Class II, with special controls. Other digital pathology solutions (automated cervical cytology slide reader) are considered of higher risk by US and European regulations. There is also some disparity in the classification of image analysis solutions between Europe and the United States. All IVD-MDs must be approved under the new European regulation (in vitro diagnostic medical device regulation) 2017/746 after May 26, 2024. This means the need of a performance evaluation, including a scientific validity report, an analytical performance report, and a clinical performance report. According to its clinical use (e.g., screening, diagnosis, or staging of cancer), a WSI slide scanner can be now classified as Class C device. A special regulation is applied to companion diagnostics. The new EU regulation 2017/746 contemplates the use of standard unique identifiers for medical devices and the creation of a European database on medical devices (Eudamed). Existing validation studies and clinical guidelines already available in the literature are a sound basis to avoid that this new regulation becomes a barrier for digital pathology development in Europe.
Keywords: Digital pathology, directive 98/79/EC, European Union, image analysis, regulation 2017/746, regulatory framework, whole slide imaging
INTRODUCTION
There is an increasing interest in the validation process of current digital pathology systems (DPSs) to be used in a regulated clinical and nonclinical environment. DPSs include whole slide imaging (WSI) scanners and automated image analysis solutions, among other systems.
This paper reviews the current status of the European Union (EU) regulation on in vitro diagnostic medical devices (IVD-MDs), with special interest in those aspects that can be of interest in the qualification and validation of a WSI system or image analysis software to be used in clinical practice.
Nowadays, a“Conformité Européenne (CE) mark” in the EU allows application of DPSs to be used with human tissue specimens.
PREVIOUS IN VITRO DIAGNOSTIC REGULATORY FRAMEWORK IN EUROPE FOR DIGITAL PATHOLOGY
As medical devices, WSI scanners are subject to specific regulatory frameworks.[1] In fact, most pathology slide scanners available in Europe are CE marked, according to directive 98/79/EC of the European Commission for in vitro diagnostic (in vitro diagnostic medical device directive [IVDD]) use [Table 1].[2] This allows the use of slide scanners within the EU for all significant clinical applications, including primary diagnosis in surgical pathology.
Table 1.
Digital pathology solutions that have received CE mark as compliant with directive 98/79/EC in vitro diagnostic directive*
| Vendor | Model | Description |
|---|---|---|
| 3DHistech | Pannoramic 250 Flash II, Pannoramic SCAN, Pannoramic MIDI, Pannoramic DESK | Brightfield slide scanner |
| 3DHistech | Pannoramic viewer | Digital slide viewer |
| 3DHistech | MembraneQuant, NuclearQuant | Automatic image analysis software |
| Bioview | DUET system and noninvasive sputum FISH (FDA) | Assisting in early detection of lung cancer in sputum |
| Bioview | Breast (FDA) | Automatic calculation of HER2 in FISH FFPE breast tissue sections |
| Bioview | Lung (FDA) | Automatic calculation of ALK in FISH FFPE lung tissue sections |
| Bioview | Hematology (FDA) | Automatic imaging and analysis of different areas on the same slide hybridized with different FISH probes |
| Hamamatsu | NanoZoomer-XR | Brightfield slide scanner |
| NanoZoomer-SQ | ||
| NanoZoomer S210 | ||
| NanoZoomer S60 | ||
| NanoZoomer S360 | ||
| Hamamatsu | NDP.view2 | Digital slide viewer |
| NDP.view2 Plus | ||
| Hamamatsu | NDP.serve3 | Digital slide image server |
| Inspirata | Omnyx Dynamyx | Pathology workflow |
| Leica | Aperio AT2, Aperio CS2, Aperio AT Turbo (FDA, 1) | Brightfield slide scanner for on screen diagnosis |
| Leica | Ariol | Brightfield, 7-channel fluorescence, and FISH slide scanner |
| Menarini | D-Sight 2.0 | Brightfield slide scanner |
| Menarini | D-Sight-F 3.0 | Brightfield, 6-channel fluorescence, and FISH slide scanner |
| Metasystems | Metafer (FDA, 2) | Brightfield, multiple-channel fluorescence, and FISH slide scanner |
| Objective Imaging | Glissando Slide Scanner | Scanner to aid pathology professionals creating, storing, and viewing digital whole slide images |
| PerkinElmer | The Nuance multispectral imaging system | Scanner and software for multispectral imaging |
| Philips | UFS digital pathology slide scanner | Brightfield slide scanner |
| Philips | Philips pathology solutions (FDA) | Diagnosis of routine pathology of paraffin-embedded tissue sections |
| Roche | Ventana DP 200 | Brightfield slide scanner |
| Roche | Ventana System for Primary Diagnosis (iScan Coreo slide scanner and Virtuoso software) (FDA, 3) | Scanner and software for routine pathology, including primary diagnosis with human tissue specimens |
| Roche | Ventana System for Primary Diagnosis (iScan HT slide scanner and Virtuoso software) (FDA, 4) | Scanner and software for routine pathology, including primary diagnosis with human tissue specimens |
| Sectra | Sectra’s solution for digital pathology | Software aimed to review cases digitally on a computer screen |
| Tribvn Healthcare | CaloPix | Software for on-screen diagnostics in routine pathology |
| Visiopharm | VDS Ki67 module for breast | Automated tumor/stroma separation and computation of the Ki67 labeling index |
| Zeiss | Axio Scan.Z1 | Brightfield slide scanner |
*See reference 4 for updated news on CE market digital pathology devices. (FDA) The system has received US FDA approval or clearance for clinical use. 1. Leica Biosystems Aperio ePathology eIHC IVD system received 510(k) clearance, including AT Turbo and CS2 scanner in 2014, for scoring ER, PR, and HER2 immunohistochemically stained slides, 2. In 1994, Metasystems IKAROS system received 510(k) clearance as automated chromosome analyzer. Metafer system has not received FDA clearance, 3. Roche Virtuoso System for IHC has received 510(k) clearance for ER (SP 1), PR (1E2), HER2 (4B5), KI-67 (30-9), and p53 (DO-7) digital read and image analysis applications, 4. Roche Ventana Virtuoso system using iScan HT scanner has received 510(k) clearance for manual scoring of digital images on a computer monitor of PR (1E2) IHC-stained slides. FDA: Food and Drug Administration, UFS: UltraFast Scanner, VDS: Virtual Double Staining, FISH: Fluorescent in situ hybridization, FFPE: Formalin fixed paraffin embedded, ER: Estrogen receptor, PR: Progesterone receptor, IHC: Immunohistochemistry, HER2: Human epidermal growth factor receptor 2, ALK: Anaplastic lymphoma kinase
Digital pathology software, as standalone software, such as WSI viewers or automated image analysis for specific immunohistochemistry (IHC) techniques can also receive CE mark IVD-MD [Table 1].
A complete digital pathology system consists of both hardware (microscope, camera, scanner, computer, and monitor) and software components that are necessary to fulfill all the functionality that replaces the conventional microscopy, such as image acquisition, processing, archiving, and retrieval.
In some cases, manufacturers (Philips and Roche are some examples) have requested the complete digital pathology system to be CE marked in the EU for routine pathology, including primary diagnosis with human tissue specimens. The Roche Ventana system for primary diagnosis consisting of Ventana Virtuoso software coupled with either the Ventana iScan Coreo or the Ventana iScan HT slide scanner was announced to receive CE mark on August 2014. Philips Digital Pathology Solutions have also acquired the CE mark for diagnosis of routine pathology, including, as it is announced in their website, for hematoxylin and eosin, IHC, and special stained formalin-fixed, paraffin-embedded tissue sections.[3]
According to the in vitro diagnostic directive 98/79/EC, digital pathology manufacturers usually declare digital pathology slide scanners as “General Class IVD” device. In this case, the conformity is only based on a self-declaration of the manufacturer.[4,5]
REGULATION (EUROPEAN UNION) 2017/746 OF THE EUROPEAN PARLIAMENT
The regulation (EU) 2017/746 of the European Parliament and of the Council of April 5, 2017, on IVD-MDs and repealing directive 98/79/EC and Commission Decision 2010/227/EU (in vitro diagnostic medical device regulation [IVDR]) is the new regulatory framework we should consider for all in vitro medical devices, including slide scanners and digital pathology software.[6]
A “regulation” is a binding legislative act, and it must be applied in its entirety across the EU. A “directive” is a legislative act that sets out a goal that all EU countries must achieve, but it is up to the individual countries to devise their own laws on how to reach these goals.[7]
IVD-MDs will require CE marking for their authorized distribution within the EU market. This will affect WSI scanning and image analysis systems, as IVD-MDs.
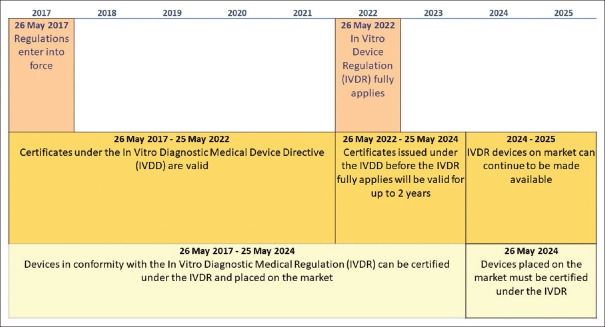
This new IVDR entered into force on May 25, 2017. For the EU in vitro diagnostic device regulation, there is a 5-year transition/implementation period. This means that the new regulations will apply across EU member states from 26 May 2022; until them, certificates under the previous IVDD will be valid. From May 25, 2022, to May 25, 2024, certificates issued under the previous IVDD before the new IVDR fully applies will be valid for up to 2 years. From May 26, 2024, all devices must be certified under the new EU IVDR [Figure 1].[8]
Figure 1.
Calendar for the European Regulation of in vitro diagnostic medical device
The EU has considered that is it necessary to clarify when software can be considered an IVD-MD, and the main criteria now are that when software, in its own right, either as a device or an accessory, is specifically intended by the manufacturer to be used for one or more of the medical purposes, it qualifies as an IVD-MD, while software for general purposes, even when used in a health-care setting, or software intended for well-being purposes is not an IVD-MD.[6]
The definition of in vitro has also been clarified, and in the new regulation, “IVD-MD” means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software, or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:[6]
Concerning a physiological or pathological process or state
Concerning congenital physical or mental impairments
Concerning the predisposition to a medical condition or a disease
To determine the safety and compatibility with potential recipients
To predict treatment response or reactions
To define or monitoring therapeutic measures.
Until recently, the directive 98/79/EC of the European Parliament and of the Council[2] was the Union regulatory framework for IVD-MDs. Regarding software, directive 2007/47/EC amended the definition of the term “medical device,” stating that “it is necessary to clarify that software in its own right, when specifically intended by the manufacturer to be used for one or more of the medical purposes set out in the definition of a medical device, is a medical device.”[9]
The main differences in the new definition of IVD-MD in the regulation (EU) 2017/746 are the reference to software, and the inclusion of information related to predisposition to a disease and treatment prediction.
The US Food and Drug Administration (FDA) has defined a medical device as “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals.”[10] Although there is not a specific mention to software in this definition, software elements that comprise a digital pathology or WSI system should also be included as part of a medical device.
According to the regulators, the main reason why the directive 98/79/EC was modified was to “ensure a high level of safety and health while supporting innovation.” In this sense, there are many references to safety measures and risk evaluation in the new regulation. Now, there are special requirements related to software and hardware in Chapters II and III of Annex I of regulation 2017/746.[6]
MAIN ACTORS IN THE NEW EUROPEAN UNION REGULATION
It is the responsibility of each member state to decide, on a case-by-case basis, whether or not a product falls within the scope of the regulation 2017/746. The European Commission, particularly in borderline cases, also has competences in that decision, after having consulted the Medical Device Coordination Group, the European Medicines Agency (EMA), or the European Chemicals Agency and the European Food Safety Authority, as relevant.[6]
A notified body (NB) is defined as a conformity assessment body designated in accordance with this regulation. They are designated by EU member states to carry out conformity assessment activities under this new regulation.[6]
The new regulation takes into consideration the international initiatives for medical devices, particularly, the Global Harmonization Task Force and the International Medical Devices Regulators Forum, a voluntary group of medical device regulators (http://www.imdrf.org/). As a fruit of these intergovernmental initiatives, the Global Medical Device Nomenclature (GMDN) was created. GMDN is maintained by the GMDN Agency (https://www.gmdnagency.org/).
The GMDN system was created in 1991, and it is translated into 25 languages. It is a 5-digit numeric GMDN Code cross-referenced to a specific Term Name and Definition. The GMDN term defines a lowest level of a generic device group. This allows a uniform device description, grouping all devices with similar generic features, to be more easily identified.[11] The following is an example widely used:
GMDN Term Name – “Scalpel, single-use”
GMDN Code – “47569”
GMDN Definition – “A sterile, hand-held, manual surgical instrument constructed as a one-piece handle and scalpel blade (not an exchangeable component) used by the operator to manually cut or dissect tissue. The blade is typically made of high-grade stainless-steel alloy or carbon steel and the handle is often made of plastic. This is a single-use device.”
Some of the items we can find in GMDN database, related to pathology, are:
Basic light microscope
Microscope objective
Fluorescence light microscope
Pathology information system
Medical image management software
Microscope slide digital imaging scanner IVD.
The term “microscope slide digital imaging scanner IVD” is defined as: “A mains electricity (AC-powered) device designed to be used in a histopathology laboratory to scan clinical specimens on microscope slides, at a microscopic level, to produce images in a digital format. Also known as a whole slide scanner or digital pathology slide scanner, it is a bench-top unit with slide tray slots, high-resolution cameras, a user interface, and integrated software.”
The new European regulation does not make any specific reference to GMDN, but it makes a detailed description of the need of a unique device identification (UDI) system.
A cooperation agreement between the International Health Terminology Standards Development Organisation and the GMDN agency and active GMDN Preferred Terms have been included in the SNOMED CT International Release.[12]
EUROPEAN MEDICAL DEVICES DATABASE
The new EU regulation 2017/746 includes as a main objective, “the creation of a European database on medical devices (Eudamed)”[6] that enable the public to be adequately informed about devices on the Union market.[6,13] This database will include information such as conformity assessment, NBs, and performance studies. For Class C and D devices, a summary of the main safety and performance aspects of the device and the outcome of the performance evaluation should be publicly available. Eudamed should use an internationally recognized medical device nomenclature free of charge to manufacturers and other stakeholders.[14]
There is a previous Eudamed project that was active between 2001 and 2006.[15]
A UDI system (“UDI system”), to facilitate traceability of devices, either using bar code, radio-frequency identification, or other system, is also described in regulation 2017/746.[6] This identification system has two components:
An UDI device identifier (“UDI-DI”) specific to a manufacturer and a device, providing access to information such as name and address of the manufacturer, medical device nomenclature code, risk class of the device, trade name, device model, need for sterilization, and status of the device
An UDI production identifier that identifies the unit of device production and if applicable, the packaged devices.
According to information currently available, it is not fully clear how GMDN and UDI will work together, but the EC proposes GMDN for the Eudamed database. The US FDA is using GMDN to implement UDI. This means that, according to information provided by the device manufacturer, from scanning the bar code, the GMDN term can be identified from the public UDI Database.[11]
Health institutions may be required to store and keep, preferably by electronic means, the UDI of the devices (in implantable Class III devices but also in others).[8]
CLASSIFICATION OF IN VITRO DIAGNOSTIC MEDICAL DEVICES
In order to understand the devices or systems that will be affected, in the regulation (EU) 2017/746, devices are divided into Classes A, B, C, and D, considering the intended purpose of the devices and their inherent risks. The classification rules are based on the vulnerability of the human body, considering the potential risks associated with the technical design and manufacture of the devices. The four classes of IVD-MD include the following rules:[6]
Class D
Detection of the presence of, or exposure to, a transmissible agent in blood, blood components, cells, tissues or organs, or in any of their derivatives
-
Devices intended to determine any of the following markers:
- ABO system (A [ABO1], B [ABO2], AB [ABO3])
- Rhesus system (RH1 [D], RHW1, RH2 [C], RH3 [E], RH4 [c], RH5 [e])
- Kell system (Kel1 [K])
- Kidd system (JK1 [Jka], JK2 [Jkb])
- Duffy system (FY1 [Fya], FY2 [Fyb]).
Class C
Blood grouping or tissue typing to ensure the immunological compatibility of blood, blood components, cells, tissue, or organs that are intended for transfusion or transplantation or cell administration, except those in Class D
For detecting the presence of, or exposure to, a sexually transmitted agent
For detecting the presence in cerebrospinal fluid or blood of an infectious agent without a high or suspected high risk of propagation
For detecting the presence of an infectious agent, if there is a significant risk that an erroneous result would cause death or severe disability to the individual, fetus or embryo being tested, or to the individual's offspring
For prenatal screening of women in order to determine their immune status toward transmissible agents
For determining infective disease status or immune status, where there is a risk that an erroneous result would lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient's offspring
To be used as companion diagnostics
To be used for disease staging, where there is a risk that an erroneous result would lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient's offspring
To be used in screening, diagnosis, or staging of cancer
For human genetic testing
For monitoring of levels of medicinal products, substances, or biological components, when there is a risk that an erroneous result will lead to a patient management decision resulting in a life-threatening situation for the patient or for the patient's offspring
For management of patients suffering from a life-threatening disease or condition
For screening for congenital disorders in the embryo or fetus
For screening for congenital disorders in newborn babies where failure to detect and treat such disorders could lead to life-threatening situations or severe disabilities
Devices intended for self-testing are classified as Class C, except for devices classified as Class B.
Class B
Devices for the detection of pregnancy, for fertility testing and for determining cholesterol level, and devices for the detection of glucose, erythrocytes, leucocytes, and bacteria in urine, which are classified as Class B
Devices which are controls without a quantitative or qualitative assigned value
Devices not covered by any other classification rules are classified as Class B.
Class A
Products for general laboratory use, accessories which possess no critical characteristics, buffer solutions, washing solutions, and general culture media and histological stains, intended by the manufacturer to make them suitable for IVD procedures relating to a specific examination
Instruments intended by the manufacturer specifically to be used for IVD procedures
Specimen receptacles.
There are also some general rules
If the device or an accessory is intended to be used in combination with another device, the classification rules shall apply separately to each of the devices or accessory
Software, which drives a device or influences the use of a device, shall fall within the same class as the device. If the software is independent of any other device, it shall be classified in its own right
Where a manufacturer states multiple intended purposes for a device and, as a result, the device falls into more than one class, it shall be classified in the higher class.
The US FDA device classification is also risk based and determined based on the intended use of the device, assuring safety (probable benefits outweigh any probable risks) and effectiveness (clinically significant results), according to device classification regulation chapters (e.g., 21 CFR 860.7, 21 CFR 862.9, 21 CFR 864.9).[15]
Table 2 compares the US FDA[16] and regulation (EU) 2017/746[6] classification for IVD-MDs.
Table 2.
US Food and Drug Administration and European classification for in vitro diagnostic medical devices
| US FDA | Regulation (EU) 2017/746 |
|---|---|
| Class III | Class D |
| Highest risk | High risk of infection or specific blood groupings |
| General controls | Manufacturer performs tests on each manufactured batch of devices |
| PMA | Expert panel/EU reference laboratory evaluates the manufacturer performance evaluation report |
| Clinical studies needed^ | Class C |
| Class II | Detection of nonhigh risk infectious agents, managing life-threatening disease or condition, companion diagnostics |
| Moderate risk | |
| General controls and special controls | Class D and C require |
| Premarket notification 510(k) | Notified body conformity assessment* |
| Substantial equivalence to a predicate | EU type-examination certificate |
| Quality management system assessment | |
| Safety and performance evaluation document should be publicly available, updated at least annually | |
| Clinical studies needed^ | |
| Synergies with EU database for clinical trials on medicinal product | |
| Postmarket surveillance: PSUR at least annually | |
| - | Class B |
| Many self-testing devices (glucose, erythrocytes, leucocytes, and bacteria in urine) | |
| Notified body conformity assessment* | |
| Class I | Class A |
| Low risk | General laboratory products |
| General controls (GMP) | Manufacturer conformity assessment |
| 510(k) exempt | |
| Registration and listing |
^Clinical studies are needed. The study must demonstrate that clinical interpretations (diagnoses) made based on the digital pathology images are comparable to those made using glass slides. In case technological characteristics are modified, clinical studies are not needed if substantial equivalence to the previous version can be demonstrated with attributes such as the intended use/indications for use, technology and design features, and safety and effectiveness, *When the device clinical performance which cannot be fully determined by analytical performance studies, literature, and/or previous experience gained by routine diagnostic testing, clinical performance studies are necessary to demonstrate compliance with the relevant general safety and performance requirements. GMP: Good manufacturing practices, PMA: Premarket approval, PSUR: Periodic safety update report, EU: European Union, FDA: Food and Drug Administration
CLASSIFICATION OF DIGITAL PATHOLOGY DEVICES
Conventional microscopes are considered by the FDA Class I devices (general controls, 21 CFR 864.3600 microscopes, and accessories)[16] and would be classified as Class A (instruments) in EU 2017/746 (IVDR).
In the directive 98/79/EC (IVDD), automated image analysis systems (microscope, automated, image analysis, and operator intervention, according to IAF Medical Device Nomenclature-MDN) are classified as Class 1, and according to the US FDA risk, they are considered Class II.[17] But if the system is being used to evaluate nuclear intensity and percentage positivity in IHC, it is considered Class 2a by the EU, and Class II by the US FDA.[17] An automated scanning microscope and image analysis for fluorescence in situ hybridization (FISH) assay is classified as Class IIB in the EU IVDD and as Class II according to the US FDA.
Gynecologic cytology imaging systems or automated cervical cytology slide reader (Hologic Imager, BD FocalPoint) as considered Class III devices by the US FDA.[16] They are Class IIb according to the EU IVDD,[17] and now, they would be classified as Class C (to be used in screening of cancer) in the EU IVDR.
In a few cases, the EU directive 98/79/EC considers some devices of higher risk than its classification by the US FDA. For instance, an automated slide stainer is a Class IIa device in the EU, and a Class I device in the USA.
WSI systems raised new questions of safety and effectiveness that are being answered through premarket approval, and they were initially classified by FDA as Class III medical devices.[16]
According to the new EU IVDR, digital pathology slide scanners and complete digital pathology slide systems should be considered as Class C IVD-MDs, since pathology microscopic examination is essential in screening, diagnosis, or staging of cancer and in many other situations that may result in a life-threatening situation for the patient.
In the evaluation of the Philips IntelliSite Pathology Solution, published in October 2017, the FDA decided to classify this system as Class II, with special controls, under regulation 21 CFR 864.3700.[18] In this case, the manufacturer presented a complete digital pathology system consisting of two subsystems and a display:
Philips UltraFast Scanner (UFS) (for software UFS1.7.1.1);
Philips Image Management System (IMS) (for software IMS2.5.1.1);
Philips Display (PS27QHDCR).
The special controls of this Class II device, according to the US FDA, refer not only to technical aspects (optics, processing software, etc.) but also that “the indications for use must specify the tissue specimen that is intended to be used with the WSI system and the components of the system.”[18]
There are other devices using digital microscopic images that the FDA consider Class II devices (FISH enumeration systems, IHC image analysis for Her2, estrogen receptor, etc., or manual reading of digital images for progesterone receptor, Her2, etc.) [Table 1] that would be also classified as Class C (to be used in diagnosis or staging of cancer) in the EU IVDR.
The European regulation pays special attention to companion diagnostics, since competent authorities designated by the member states in accordance with the EU IVDD or from the EMA, must be also considered.
Other countries, such as Canada, currently use a simple self-declaration process with vendors being obliged to provide postmarket follow-up information. It is foreseeable that similar regulatory changes may be implemented in these other countries, after Europe experience.
EVALUATING A COMPLETE DIGITAL PATHOLOGY SYSTEM OR ITS COMPONENTS
The manufacture is expected to give information to the NBs of the complete system, including “a description of major subsystems, analytical technologies such as operating principles and control mechanisms, dedicated computer hardware and software,” and “an overview of the entire system.”[6]
In the case of software, information about the algorithm should also be provided in the technical documentation to be presented by the manufacturer. This should also include evidence of the validation of the software, with all verification, validation, and testing performed in-house and applicable. It shall also consider all possible different hardware configurations or operating systems.
PATHOLOGY INFORMATION SYSTEMS
If the main purpose of the software is not the examination of a specimen, but collecting results obtained from one or several IVD devices (directly and/or manually) or archiving patient results, and transmits without modification, this information to a centralized database (e.g., laboratory information management system) or to health-care providers is not an IVD-MD. In case of basic operations of arithmetic (e.g., mean and conversion of units) and/or plotting of results in function of time and/or a comparison of the result to the limits of acceptance set by the user, it is not considered as an IVD-MD.[19]
What's Behind a Conformité Européenne Mark
A CE mark refers to the initials “CE” with a very specific form [Figure 2].[6]
Figure 2.
Conformité Européenne mark for medical devices
This means that the manufacturer has achieved an EU Declaration of Conformity for a specific device.
The European Union declaration of conformity shall contain the following information
Name, registered trade name or registered trademark, and if already issued, SRN referred to in Article 28 of the manufacturer, and if applicable, its authorized representative, and the address of their registered place of business where they can be contacted, and their location be established
A statement that the EU declaration of conformity is issued under the sole responsibility of the manufacturer
The basic UDI-DI as referred to in Part C of Annex VI
Product and trade name, product code, catalog number, or other unambiguous reference allowing identification and traceability of the device covered by the EU declaration of conformity, such as a photograph, where appropriate, as well as its intended purpose. Except for the product or trade name, the information allowing identification and traceability may be provided by the basic UDI-DI referred to in point 3
Risk class of the device in accordance with the rules set out in Annex VIII
A statement that the device that is covered by the present declaration is in conformity with this regulation and if applicable, with any other relevant Union legislation that provides for the issuing of an EU declaration of conformity
References to any “common specifications” used and in relation to which conformity is declared
Where applicable, the name and identification number of the notified body, a description of the conformity assessment procedure performed, and identification of the certificate or certificates issued
Where applicable, additional information
Place and date of issue of the declaration, name, and function of the person who signed it as well as an indication for, and on behalf of whom, that person signed, signature.[6]
CLINICAL PERFORMANCE AND PERFORMANCE STUDIES
Confirmation of conformity of a device with relevant general safety and performance requirements, and of the acceptability of the benefit-risk ratio, shall be based on:[6]
Scientific validity report: Relevant information on the scientific validity of devices measuring the same analyte or marker; scientific (peer-reviewed) literature; consensus expert opinions/positions from relevant professional associations; results from proof of concept studies; and results from clinical performance studies
Analytical performance report: Analytical sensitivity, analytical specificity, trueness (bias), precision (repeatability and reproducibility), accuracy (resulting from trueness and precision), limits of detection and quantitation, measuring range, linearity, cutoff, including determination of appropriate criteria for specimen collection and handling and control of known relevant endogenous and exogenous interference, cross-reactions, and information about the use of available reference measurement procedures and materials by the user. The analytical performance shall always be demonstrated on the basis of analytical performance studies
-
Clinical performance report: Diagnostic sensitivity, diagnostic specificity, positive predictive value, negative predictive value, likelihood ratio, and expected values in normal and affected populations. Demonstration of the clinical performance of a device shall be based on one or a combination of the following sources:
- Clinical performance studies. A clinical performance study plan is needed
- scientific peer-reviewed literature
- Published experience gained by routine diagnostic testing.
They should provide enough clinical evidence. It is up to the manufacturer to specify and justify the level of the clinical evidence necessary to demonstrate conformity with the relevant general safety and performance requirements. Manufacturers shall plan, conduct, and document a performance evaluation. The performance evaluation report shall include that clinical evidence.[6]
The performance study plan must be authorized. Member states shall refuse the authorization of the performance study if the requirements of this regulation are not met.[6]
According to new EU regulation, in the design of the clinical evaluation of an in vitro device, specific outputs (i.e., clinical benefit, diagnostic specificity, diagnostic sensitivity, etc.) must be considered.[6]
All devices must include a clinical performance study as part of the demonstration of conformity with the general safety and performance requirements, unless it is duly justified to rely on other sources of clinical performance data.[6]
An interventional clinical performance study is defined as a clinical performance study where the test results may influence patient management decisions and/or may be used to guide treatment. For those devices intended to be used in the context of interventional clinical performance studies or other performance studies involving risks for the subjects of the studies, the application form should be accompanied of analytical performance data and existing clinical data (scientific literature and other relevant clinical data). When devices include tissues, cells, and substances of human, animal, or microbial origins, it is also necessary to include detailed information on the tissues, cells, and substances and on the compliance with the relevant general safety and performance requirements and the specific risk management in relation to those tissues, cells, and substances.[6]
The performance evaluation report for Class C and D devices shall be updated when necessary but at least annually.[6]
Manufacturers are requested to establish a risk management system and a clinical evaluation process, and they should be regularly updated. This risk management system should include possible clinical risks to be addressed during clinical investigations, clinical evaluation, and postmarket clinical follow-up.[6]
The summary of safety and clinical performance for a device should include the context the specific conditions of diagnostic or therapeutic options of that device when compared to the diagnostic or therapeutic alternatives.[6]
Special requirements for performance studies must be met by interventional clinical performance study and performance studies involving companion diagnostics. This does not apply to performance studies involving companion diagnostics using only leftover samples, although such studies shall be notified to the competent authority.[6] These additional requirements are related to scientific and ethical aspects, such as the participation of an ethics committee in accordance with national law, the protection of vulnerable populations and subjects, informed consent, the risk threshold, and the degree of acceptable distress, among others. Regarding the scientific and technical aspects, the following special rules are defined for these devices:[6]
Where appropriate, biological safety testing reflecting the latest scientific knowledge, or any other test deemed necessary in the light of the device's intended purpose has been conducted
In the case of clinical performance studies, the analytical performance has been demonstrated, taking into consideration the state of the art
In the case of interventional clinical performance studies, the analytical performance and scientific validity have been demonstrated, taking into consideration the state of the art. Where, for companion diagnostics, the scientific validity is not established, the scientific rationale for the use of the biomarker shall be provided
The technical safety of the device with regard to its use has been proven, taking into consideration the state of the art as well as provisions in the field of occupational safety and accident prevention.
An electronic system to manage performance studies will be created. This system will be responsible for creating the single identification numbers for performance studies, and it will be used as an entry point for the submission of all applications or notifications for performance studies. It should be interoperable with the EU database for clinical trials on medicinal products for human use.[6]
In vitro medical devices should also be accompanied with a postmarket performance follow-up that must be understood as a continuous process that updates the performance evaluation.[6]
WHOLE SLIDE SCANNERS AND CLINICAL PERFORMANCE STUDIES
The decision summary of the FDA Evaluation of Philips IntelliSite Pathology Solution includes specific performance studies that are classified as:[18]
-
Analytical performance:
- Precision/reproducibility: A intra- and inter-system precision study with three pathologists evaluating seven histopathologic features (e.g., mitosis) at 3 different magnifications (×10, ×20, ×40)
Technical studies: They include several studies evaluating different components and functionalities (from slide feeder to turnaround time)
Human factor studies: Critical tasks required for operation of the device
Clinical studies: A study with the objective to demonstrate that using is noninferior to using optical (light) microscopy in surgical pathology FFPE tissue slides, with a total of 2000 cases consisting of multiple organ and tissue types. Cases were distributed over four sites, four pathologists per site.
There is not any experience until now with EU 2017/746, but according to the experience in the US and all the experience in the CE mark for IVD use of slide scanners and digital pathology software according to current directive 98/79/EC, it is foreseeable that manufacturers will need to carry out clinical performance studies, and not only analytical performance results will have to be included in the technical documentation to obtain a EU Declaration of Conformity.
CAP guidelines will be a valuable source to design these studies, as well as other international guidelines for the adoption of digital pathology.[20,21,22] “FDA has also published a guide for the clinical evaluation” of “Software as a Medical Device,”[23] where four risk categories are defined, and quality management rules are described. Furthermore, in Europe, scientific societies can play an important role in the understanding and practical implementation of this new regulation related to digital pathology.
Possible consequences in using not marked IVD-MDs for clinical diagnosis can be related, for the companies, with the possibility of being eligible in public tenders in the acquisition of DPSs, and for pathology departments, with the certification and accreditation processes.
Main differences between former IVD directive (IVDD) and current IVD regulation (IVDR) are that this must be fully applied across all EU countries; a new risk classification that will affect WSI and image analysis software; a clinical evidence requirement, including scientific validity, analytical performance, and clinical performance is needed; and the continuous surveillance during the lifecycle of the device. In digital pathology, this means that a manufacturer's self-certification of conformity under the IVDD will no longer be possible in most cases.[24]
At the end, the statement that most pathologists will probably prefer is what is included by the US FDA in their decision summary after the evaluation of a WSI system: “It is the responsibility of a qualified pathologist to employ appropriate procedures and safeguards to assure the validity of the interpretation of images obtained using this device.”[8]
Financial support and sponsorship
This work has been partially supported and funded by the EU FP7 program, AIDPATH project, grant number 612471, and EU FEDER ITI Grant for Cadiz Province PI-0032-2017.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2019/10/1/2/250754
REFERENCES
- 1.Long RE, Smith A, Machotka SV, Chlipala E, Cann J, Knight B, et al. Scientific and regulatory policy committee (SRPC) paper: Validation of digital pathology systems in the regulated nonclinical environment. Toxicol Pathol. 2013;41:115–24. doi: 10.1177/0192623312451162. [DOI] [PubMed] [Google Scholar]
- 2.European Parliament and Council. Directive 98/79/EC on in vitro diagnostic medical devices (IVDMD) [Last accessed on 2018 May 12];Official J Eur Union. 1998 331:1–46. Available from: https://www.ec.europa.eu/growth/ sectors/medical-devices/regulatory-framework_en . [Google Scholar]
- 3.Philips. Innovations – Digital Pathology. [Last accessed on 2018 May 14]. Available from: https://www. philips.co.uk/healthcare/education-resources/publications/hotspot/ digital-pathology .
- 4.CE Mark. [Last accessed on 2018 Dec 06, Last updated on 2016 Jul 18, Last cited on 2016 Jul 18]. Tissuepathology.com [Internet]. Illinois: Keith J. Kaplan, MD. Available from: http://www.tissuepathology.com/Tags/ ce-mark/
- 5.BSI. Guide to the in vitro Diagnostic Directive. 2012. [Last accessed on 2018 Dec 6]. Available from: https://www.bsigroup.com/meddev/LocalFiles/en-IN/Technologies/ BSI-md-ivd-diagnostic-directive-guide-brochure-UK-EN.pdf .
- 6.Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/ EU. [Last accessed on 2018 May 12];Official J Eur Union. 2017 60:176–332. Available from: https:// ec.europa.eu/growth/sectors/medical-devices/regulatory-framework_ en . [Google Scholar]
- 7.European Union. Regulations, Directives and other Acts. [Last update on 2018 May 19]. Available from: https://www.europa.eu/european-union/eu-law/legal-acts_ en .
- 8.Medicines and Healthcare products Regulatory Agency – MHRA. Guidance Medical Devices: EU Regulations for MDR and IVDR. Health Institutions. [Last update on 2018 Feb 05]. Available from: https://www.gov.uk/guidance/ medical-devices-eu-regulations-for-mdr-and-ivdr .
- 9.Directive 2007/47/EC of the European Parliament and of the Council of 5 September 2007. [Last accessed on 2018 Dec 06];Official J Eur Union. 2007 247:21–55. Available from: http://www.eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L: 2007:247:0021:0055:en: PDF . [Google Scholar]
- 10.Cornish TC, Mcclintock DS. Medicolegal and regulatory aspects of whole slide imaging-based telepathology. [Last accessed on 2018 May 16];Diagn Histopathol. 2014 20:475–81. Available from: https://www.diagnostichistopathology.co.uk/article/ S1756-2317(14)00173-X/ [Google Scholar]
- 11.Wasmuth M. Global Medical Device Nomenclature. Requirement for UDI. [Last accessed on 2018 Dec 06]. Available from: https://www.gs1.org/sites/default/files/gudid_ gmdn.pdf .
- 12.IHTSDO. SNOMED CT to GMDN Simple Map package Release Notes. 2017. Jan, [Last update on 2017 Feb 28]. Available from: https://www.confluence.ihtsdotools.org/display/RMT/SNOMED+CT+to+GMDN+Simple+Map+package+Release+Notes+-+January+2017 .
- 13.Obelis Group. What is Eudamed. [Last accessed on 2018 Dec 06]. Available from: http://www.obelis.net/ what-is-eudamed/
- 14.IDABC European eGovernment Services. EUDAMED: European Database on Medical Devices. [Last update on 2005 Mar 1. Last accessed on 2018 Dec 06]. Available from: http://www.ec.europa.eu/ idabc/en/document/2256/5637.html .
- 15.U.S. Food and Drug Administration. Premarket Notification 510(k) [Last update on 2018 Mar 27]. Available from: https://www.fda.gov/MedicalDevices/ DeviceRegulationandGuidance/HowtoMarketYourDevice/ PremarketSubmissions/PremarketNotification510k/default.htm .
- 16.Faison TA. FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts. Clinical Laboratory Improvement Advisory Committee Meeting Centers for Disease Control and Prevention. [Last accessed on 2018 Dec 06]. 15 February, 2012. Available from: ftp://ftp.cdc.gov/pub/cliac_ meeting_presentations/pdf/addenda/cliac0212/Tab_15_Faison_ CLIAC_2012Feb14_Whole_Slide_Imaging.pdf .
- 17.International Accreditation Forum, Inc. IAF Medical Device Nomenclature (IAF MDN) Including Medical Device Risk Classifications. [Last accessed on 2018 Dec 06]. 30 January, 2017. Available from: http://www.iaf.nu/ upFiles/IAFID132017_Issue_1_30012017.pdf .
- 18.U.S. FDA. Evaluation of Automatic Class III Designation for Philips IntelliSite Pathology Solution (PIPS) Decision Summary. [Last accessed on 2018 Dec 06]. Correction Date; October, 2017. Available from: https://www.accessdata.fda.gov/ cdrh_docs/reviews/DEN160056.pdf .
- 19.European Commission. Medical Devices: Guidance document. Qualification and Classification of Stand-Alone Software. MEDDEV 2.1/6. July, 2016. [Last accessed on 2018 Dec 06]. Available from: https://www.ec.europa.eu/docsroom/ documents/17921 .
- 20.García-Rojo M. International clinical guidelines for the adoption of digital pathology: A review of technical aspects. Pathobiology. 2016;83:99–109. doi: 10.1159/000441192. [DOI] [PubMed] [Google Scholar]
- 21.Buck TP, Dilorio R, Havrilla L, O’Neill DG. Validation of a whole slide imaging system for primary diagnosis in surgical pathology: A community hospital experience. J Pathol Inform. 2014;5:43. doi: 10.4103/2153-3539.145731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard C, Chandrakanth SA, Cornell IS, Dalton J, Evans A, et al. Canadian Association of Pathologists Telepathology Guidelines Committee. Guidelines from the Canadian Association of Pathologists for Establishing a Telepathology service for anatomic pathology using whole-slide imaging. J Pathol Inform. 2014;5:15. doi: 10.4103/2153-3539.129455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. Food and Drug Administration Software as a Medical Device (SAMD): Clinical Evaluation. Guidance for Industry and Food and Drug Administration Staff. 8 December, 2017. [Last accessed on 2018 Dec 06]. Available from: https://www.fda. gov/downloads/medicaldevices/deviceregulationandguidance/ guidancedocuments/ucm524904.pdf .
- 24.Bray J, O’Connor M, Cumming RD. CE Marking of In Vitro Diagnostic Medical Devices under the New EU Regulation. Regulatory Focus. Regulatory Affairs Professionals Society; November, 2017. [Last accessed on 2018 Dec 06]. Available from: https://www.ppdi.com/-/media/Files/PPDI-Files/news/PPD-In- The-News/2017-Nov-Regulatory-Focus-Diagnostic-medical-devices- Bray-OConnor-Cumming.ashx .