Abstract
Immunotoxins are potent anticancer agents with an unusual mechanism of action: inhibition of protein synthesis resulting in apoptotic cell death. Immunotoxins have produced many durable complete responses in refractory hairy cell leukemia, where patients rarely form antibodies to the bacterial toxin component of the immunotoxin. Patients with mesothelioma, however, have normal immune systems and form antibodies after one cycle, and tumor responses to the immunotoxin have not been observed in this disease. We describe the results of a trial in which major antitumor responses were seen in patients with advanced mesothelioma who received the anti-mesothelin immunotoxin SS1P, together with pentostatin and cyclophosphamide, to deplete T and B cells. Of 10 patients with chemotherapy-refractory mesothelioma, 3 have had major tumor regressions with 2 ongoing at 15 months, and 2 others responded to chemotherapy after discontinuing immunotoxin therapy. Antibody formation was markedly delayed, allowing more SS1P cycles to be given, but this alone does not appear to account for the marked antitumor activity observed.
INTRODUCTION
Malignant pleural mesothelioma is an aggressive disease with poor prognosis (1). In patients with unresectable disease, the combination of pemetrexed and cisplatin is the most effective therapy, but results in a median overall survival of only 12.1 months (2). There are no approved second-line treatments, and responses to second- and third-line treatments are rare in patients who fail chemotherapy (3). In peritoneal mesothelioma, surgical cytoreduction with intraperitoneal chemotherapy improves overall survival, but most of the patients ultimately die from disease progression (4).
The tumor differentiation antigen mesothelin is an attractive candidate for targeted therapy of mesothelioma because it is highly expressed on tumor cells and its expression in normal tissues is limited to mesothelial cells lining the pleura, peritoneum, and pericardium (5, 6). SS1P is a recombinant immunotoxin consisting of an anti-mesothelin variable fragment linked to PE38, a portion of Pseudomonas exotoxin A, and it is cytotoxic to mesothelin-expressing cell lines (7). The safety of SS1P was evaluated in two phase 1 clinical trials that included patients with previously treated mesothelin-expressing cancers (8, 9). These two trials evaluated different dosing schedules of SS1P, including administration of SS1P every other day for three or six doses or a 10-day continuous infusion schedule. Patients could receive additional cycles every 4 weeks in the absence of anti-SS1P antibodies or progressive disease. A total of 58 patients were treated on these phase 1 trials, including 36 patients with mesothelioma, 19 with ovarian cancer, and 3 with pancreatic cancer. In these phase 1 trials, SS1P showed only minor antitumor activity, with none of the 36 patients with mesothelioma having an objective partial tumor response. One reason for its low activity was that about 90% of patients developed neutralizing antibodies to SS1P, which prevented re-treatment, after only one cycle of therapy (8).
Efforts to decrease antibody responses to immunotoxins with cyclophosphamide, cyclosporine, or rituximab have been unsuccessful (10–12). We recently showed in mice that an immune-depleting and immunosuppressive regimen of pentostatin and cyclophosphamide could severely deplete host B and T immune cells with relative sparing of host myeloid cells, thereby safely abolishing the formation of antibodies to SS1P (13). On the basis of these results, we designed a pilot study to determine if pretreatment with pentostatin and cyclophosphamide could delay the human immune response to SS1P.
RESULTS
Patients
Between December 2011 and October 2012, 11 patients were enrolled: 9 with pleural mesothelioma and 2 with peritoneal mesothelioma. Their clinical characteristics are summarized in Table 1, and previous therapies received by these patients are shown in table S1. Patient 1, a 52-year-old man with pleural mesothelioma, who previously had an extrapleural pneumonectomy, experienced pleuritis after one dose of SS1P and was taken off the study because of concern for potential toxicity. He was not evaluable for response but was included in the safety analysis. All patients provided written informed consent before study enrollment.
Table 1.
Patient demographics, treatment received, and clinical outcome. M, male; F, female; PR, partial response; SD, stable disease; PD, progressive disease.
| Patient | Age (years)/ sex |
Tumor site* |
Tumor mesothelin expression† |
No. of previous therapies |
No. of SS1P cycles received |
Overall tumor response‡ |
Delayed tumor response§ |
Post-study chemotherapy |
Response to post-study chemotherapy |
Overall survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 50/M | Peritoneum | 100%, 3+ | 2 | 2 | PR (−44%) | Yes (7 months) | — | — | 18.2+ |
| 3 | 56/F | Pleura | 100%, 3+ | 2 | 6 | PR (−74%) | — | — | — | 17.1+ |
| 4 | 67/F | Pleura | 100%, 3+ | 3 | 2 | SD | — | Yes | PR (−55%) | 14 |
| 5 | 51/M | Peritoneum | 100%, 3+ | 4 | 4 | PR (−70%) | — | — | — | 14.5+ |
| 6 | 43/M | Pleura | 100%, 3+ | 4 | 2 | SD | — | — | — | 8.8 |
| 7 | 48/F | Pleura | 90%, 3+ | 2 | 2 | PD | — | — | — | 6.2 |
| 8 | 60/M | Pleura | 100%, 2+ | 6 | 2 | PD¶ | — | — | — | 5.7 |
| 9 | 52/F | Pleura | 70%, 2+ | 5 | 2 | PD | Yes (4 months)∥ | Yes | 85% decrease in tumor FDG uptake** | 10.6+ |
| 10 | 62/M | Pleura | 30%, 2+ | 2 | 2 | PD | — | — | — | 4.2 |
| 11 | 65/M | Pleura | 50%, 3+ | 1 | 2 | SD | — | Yes | No | 7.3 |
All patients had epithelial-type mesothelioma.
Mesothelin expression was determined by immunohistochemistry and presented as percentage of positive cells, and the degree of staining intensity (absent; 1+, mild; 2+, moderate; or 3+, strong) is shown.
For patients with tumor response, the percent decrease in the sum of target lesions is shown.
Months from study initiation to the time when response was first observed.
Patient 8 had progressive disease due to new brain metastasis but had a 10% reduction in target lesions.
Patient 9 had initial progressive disease but, at 4 months, had a 25% reduction in size of one of the target lesions and a marked decrease in metabolic activity by PET.
Patient 9 had an 85% reduction in tumor SULpeak/cm3 compared to baseline PET scan and stable disease by CT scan on post-study chemotherapy.
Safety
All adverse events that were possibly, probably, or definitively related to SS1P, pentostatin, or cyclophosphamide are summarized in table S2. No grade 4 toxicities ascribed to SS1P were observed; grade 3 toxicities included noncardiac chest pain, pleuritic pain, and back pain (9% each). Less serious SS1P-related adverse events included fatigue, noncardiac chest pain, edema, and hypoalbuminemia. Adverse events related to pentostatin or cyclophosphamide included grade 4 lymphopenia (100%), grade 3 anemia (9%), transaminitis (18%), and fever (9%). Despite immunosuppression, no patient developed bacterial, viral, or fungal infections.
Clinical activity
Of the 10 evaluable patients, 3 had durable partial responses, 3 stable disease, and 4 progressive disease (Table 1). Durable responses were observed in all three patients for many months after stopping treatment. One patient with stable disease and one patient with initial progressive disease had marked tumor responses to salvage chemotherapy.
Figure 1 illustrates the response in patient 3, a 56-year-old female with pleural mesothelioma whose best response to treatment was a 74% tumor shrinkage that had been maintained through last follow-up at 15 months. She was diagnosed with unresectable disease 2.5 years earlier and had received chemotherapy with pemetrexed plus cisplatin followed by gemcitabine plus carboplatin, with stable disease as the best response to both treatments. The patient had progressive disease when enrolled in the present study, and a computerized tomography (CT) scan was done on day 12 of therapy for shortness of breath, showing rapid tumor growth compared to CT scan done 3 weeks earlier (Fig. 1, A and B). Immunotherapy was continued despite apparent early radiographic progression. She went on to receive five more cycles of SS1P. CT scans performed after cycles 2, 4, and 6 demonstrated marked, sustained, and continued tumor shrinkage that was ongoing at her last follow-up imaging, 15 months from initiation of therapy (Fig. 1, C and D). She also had a complete metabolic response by [18F]fluorodeoxyglucose positron emission tomography ([18F]FDG PET), with a standardized FDG uptake value based on lean body mass (SUL) peak/cm3 of 0 at 11 months, compared to 57.8 before treatment.
Fig. 1. Tumor response in a patient with extensive pleural mesothelioma.
(A to C) Compared with CT obtained before treatment (A), patient 3 had a rapidly progressive disease on day 12, after only one dose of SS1P, which was administeredon day 10 (B). (C)However, the patient had a marked decrease in tumor burden at the end of cycle 2, which had been maintained through last follow-up at 15 months. Representative tumor involvement is indicated by red asterisks. (D) The magnitude of tumor response in this patient is shown by graphing the sum of the target lesions at time points when CT scans were obtained. The horizontal dashed line represents partial response, defined as a 30% reduction in the sum of target lesions from pretreatment CT scan.
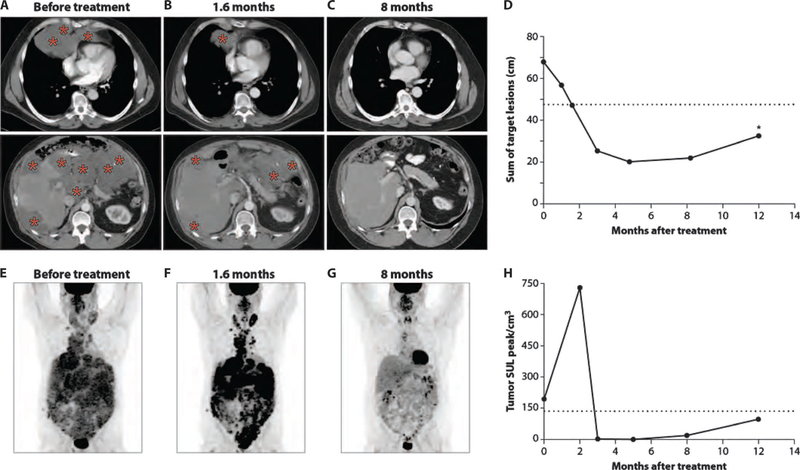
Figure 2 shows the tumor response in patient 5, a 51-year-old male with peritoneal mesothelioma, whose best response to treatment was a 70% tumor shrinkage at 5 months. He was diagnosed 15 months earlier and had received pemetrexed plus cisplatin with no response, followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. However, he developed extra-abdominal metastatic disease involving the neck, chest, abdomen, and pelvis 4 months after surgery. He was treated successively with pemetrexed plus cisplatin and an experimental agent on a phase 1 trial, but had progressive disease on each of these treatments. Representative CT images of his chest and abdomen before starting treatment are shown in Fig. 2A. Rapid reduction in the size of tumors in the neck, chest, abdomen, and pelvis was noted after only two cycles of SS1P (Fig. 2B). Continued tumor response was observed after cycle 4, when further treatment was discontinued because of development of anti-SS1P antibodies. A 70% tumor reduction was found at 5 months and maintained at 8 months (Fig. 2, C and D), but his tumor progressed again at 12 months. Figure 2E represents a PET scan showing increased tumor metabolic activity in the neck, chest, abdomen, and pelvis before treatment. Although marked tumor reductions were seen on CT scan after two cycles of SS1P (Fig. 2B), PET scan done at the same time point showed a further increase in tumor metabolic activity (Fig. 2F). However, subsequent PET scans showed a complete metabolic response (SULpeak/cm3 of 0) at 5 months and a near-complete metabolic response (SULpeak/cm3 of 19) at 8 months (Fig. 2G). The changes in tumor metabolic activity as measured by SULpeak/cm3 before therapy and at different time points after treatment are illustrated in Fig. 2H.
Fig. 2. Early and sustained regression of a widely metastatic peritoneal mesothelioma.
(A to C) Representative axial CT images of chest and abdomen for patient 5 before treatment (A), after receiving two cycles of therapy (1.6 months) (B), and at 8 months of follow-up (C). Representative tumor involvement is indicated by red asterisks. (D) The magnitude of tumor response in this patient is shown by graphing the sum of target lesions at time points when CT scans were obtained. The horizontal dashed line represents partial response, defined as a 30% reduction in the sum of target lesions from pretreatment CT scan. The asterisk at 12 months of follow-up indicates tumor progression. (E to G) [18F]FDG PET images from time points corresponding to (A) to (C), respectively. (H) Change in tumor metabolic activity as measured by SULpeak/cm3 before therapy and at different time points after treatment. The horizontal dashed line represents metabolic partial response, as described in (25).
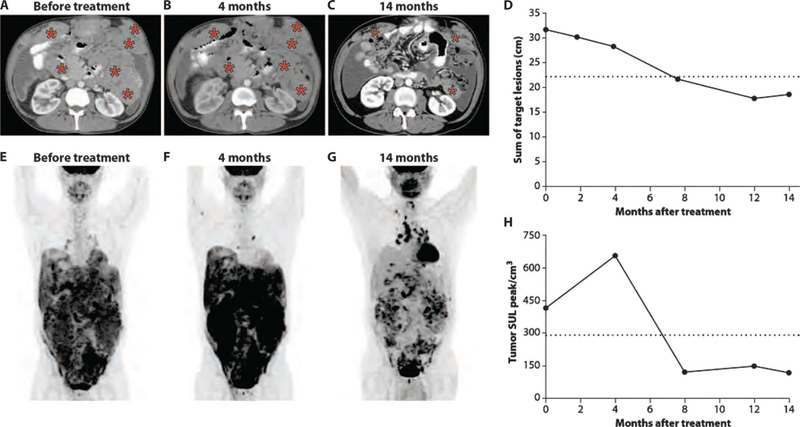
Delayed tumor response in patient 2, a 50-year-old male with peritoneal mesothelioma who was diagnosed 5 years prior, is illustrated in Fig. 3. He was treated with neoadjuvant pemetrexed plus cisplatin followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. The patient was lost to follow-up and came to medical attention more than 3 years later with a palpable mass in the left upper quadrant of the abdomen. He had extensive peritoneal involvement, which was unresectable. Figure 3A is a representative CT image of the upper abdomen at that time, showing tumor masses including subcutaneous tumors in the left and right upper abdomen. Only two cycles of SS1P were administered because of development of anti-SS1P antibodies, and a CT scan was performed after these two cycles showed stable disease. With no intervening treatment, the tumor remained stable at 4 months (Fig. 3B). However, substantial tumor shrinkage with objective partial response was observed at 7 months and was maintained at 14 months (Fig. 3C). The time course of tumor regression by CT scan is shown in Fig. 3D and demonstrates that the patient first achieved partial response 7 months after initiation of therapy, but then maintained this response without additional treatment. Figure 3E shows this patient’s tumor metabolic activity by PET scan before treatment, with a SULpeak/cm3 of 417. PET scan done at 4 months, when the patient had stable disease by CT (Fig. 3B), showed a significant increase in tumor metabolic activity with a SULpeak/cm3 of 660 (Fig. 3F). However, a PET scan done at 7 months showed a decrease in tumor metabolic activity, which was accompanied by partial response by CT imaging and had been maintained at 14 months (Fig. 3, G and H).
Fig. 3. Delayed tumor response in a patient with peritoneal mesothelioma.
(A to C) Representative CT images of the abdomen for patient 2 before treatment and at 4 and 14 months after therapy, respectively. There was no objective tumor regression at 4 months (B), but a CT obtained at 7 months showed a partial response that had been maintained at 14 months of follow-up (C). Tumor involvement is indicated by red asterisks. (D) The magnitude of tumor response in this patient is shown by graphing the sum of target lesions to time points at which CT scans were obtained. The horizontal dashed line represents partial response, defined as a 30% reduction in the sum of target lesions from pretreatment CT scan. (E to G) PET images from the time points shown in (A) to (C), respectively. (H) Change in tumor metabolic activity as measured by SULpeak/cm3 before therapy and at different time points after treatment. The horizontal dashed line represents metabolic partial response, as described in (25).
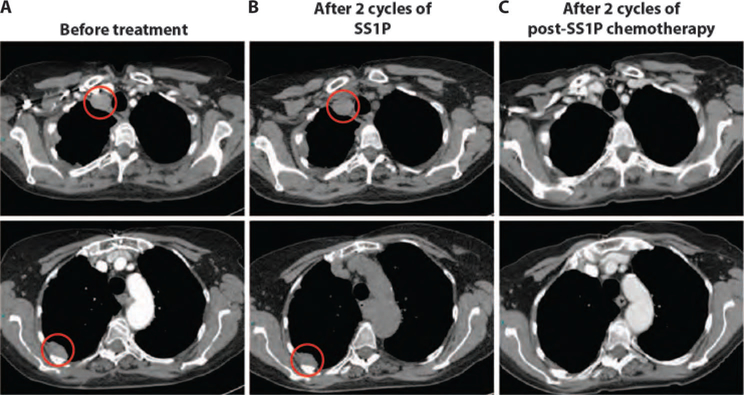
Two patients who received chemotherapy after completion of SS1P had uncharacteristic tumor responses (Table 1). Patient 4, a 67-year-old female, was diagnosed 16 months prior with pleural mesothelioma. She received neoadjuvant pemetrexed plus cisplatin and had stable disease after four cycles of treatment. She subsequently underwent right pleurectomy with decortication and received postoperative right thoracic radiation. Because of tumor progression, she was enrolled in the study and received two cycles of SS1P and was then taken off therapy because of development of anti-SS1P antibodies. CT scan after two cycles of SS1P showed stable disease (Fig. 4, A and B). She was then re-treated with pemetrexed plus cisplatin (3.5 months after SS1P), and CT scan showed a partial response with a 55% reduction in tumor after just two cycles of chemotherapy, which was confirmed on follow-up imaging (Fig. 4C).
Fig. 4. Tumor regression in a pleural mesothelioma patient treated with chemotherapy after SS1P.
(A to C) Representative axial CT images of the chest for patient 4 before treatment (A), after two cycles of SS1P (B), and after two cycles of post-SS1P chemotherapy (C). Areas of tumor involvement are indicated by red circles.
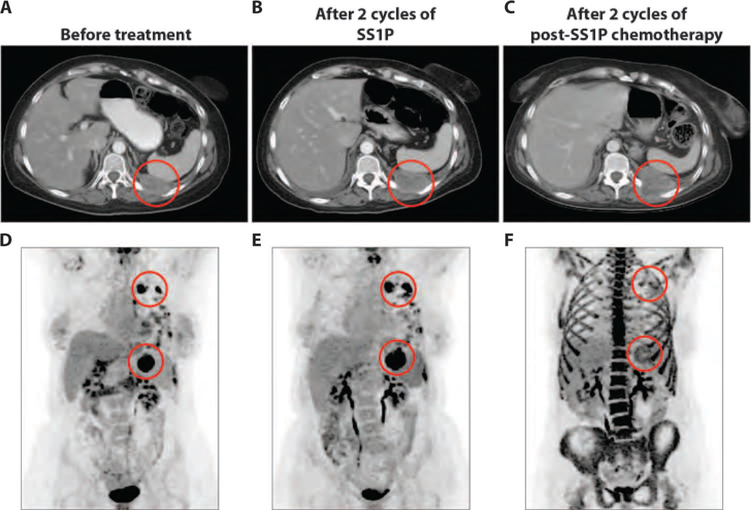
Patient 9, a 52-year-old female with pleural mesothelioma, was diagnosed 2.5 years prior and had received four chemotherapy regimens (see details in table S1) and local radiation to left chest. She had progressive disease after two cycles of SS1P therapy (Fig. 5, A and B) but later showed delayed antitumor activity, with a 25% decrease in one of the target lesions and marked reduction of its FDG uptake 2 months after completing SS1P. She then received chemotherapy with gemcitabine plus nab-paclitaxel. PET scan after two cycles of chemotherapy showed a marked reduction in tumor metabolic activity, with an 85% decrease in tumor SULpeak/cm3 compared to pretreatment PET scan. This reduction in tumor metabolic activity was not accompanied by a decrease in tumor size by CT scan (Fig. 5, C to F).
Fig. 5. Tumor response to post-SS1P chemotherapy in a patient with pleural mesothelioma.
(A to C) Representative axial CT images of the chest for patient 9 before treatment (A), after two cycles of SS1P (B), and after two cycles of post-SS1P chemotherapy (C). Areas of tumor involvement are indicated by red circles. (D to F) [18F]FDG PET images from time points corresponding to (A) to (C), respectively. Areas of tumor involvement are indicated by red circles. (F) Image showing a marked reduction of [18F]FDG uptake in the highlighted areas after post-SS1P chemotherapy, compared to after two cycles of SS1P (E). Intense bone marrow metabolic activity in the axial and appendicular skeleton in (F) is secondary to recent administration of granulocyte colony-stimulating factor to prevent chemotherapy-induced neutropenia.
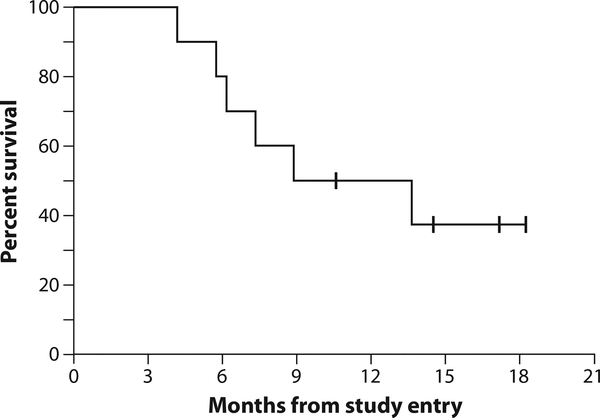
Of the three patients who had stable disease, the duration of stable disease could not be determined reliably for patients 4 and 6 because they went on to receive subsequent therapies without documented progressive disease. The duration of stable disease for patient 11 was 3 months. Six of the 10 evaluable patients have died, including 6 of 8 with pleural disease. Both patients with peritoneal mesothelioma were alive at 14 and 18 months. The median overall survival was 8.8 months, with a median potential follow-up of 12.7 months as of 13 June 2013 (Fig. 6).
Fig. 6. Kaplan-Meier plot of overall survival for all evaluable patients in the study.
As of last follow-up on 13 June 2013, two of eight patients with pleural mesothelioma and both patients with peritoneal mesothelioma are still alive.
Tumor mesothelin expression
On histological examination, all patients had epithelial mesothelioma that expressed mesothelin with 30 to 100% of tumor cells positive, typically with membrane and cytoplasmic staining of 2 to 3+ (Table 1). Figure 7 illustrates tumor mesothelin expression in the three patients who had durable tumor regressions after SS1P treatment.
Fig. 7. Tumor mesothelin expression.
(A to C) Mesothelin positivity (indicated by brown staining of tumor cells), as detected by immunohistochemistry with the anti-mesothelin monoclonal antibody 5B2, is shown for patients 2 (A), 3 (B), and 5 (C).
Blood counts and immune subsets
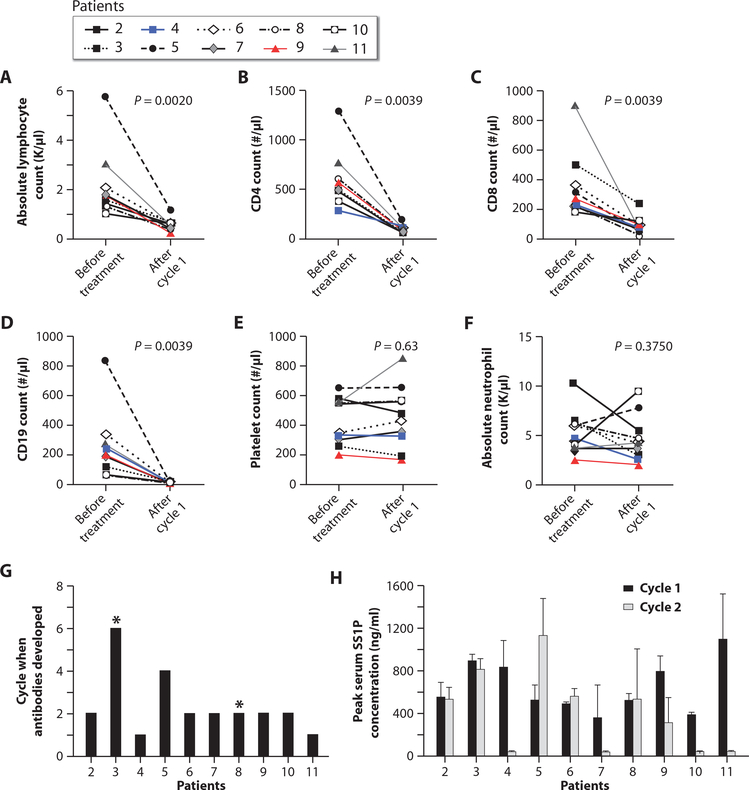
Pentostatin and cyclophosphamide produced lymphopenia without much myelosuppression. Figure 8 (A to D) shows significant decreases in absolute lymphocyte counts (ALC), CD4+ T cells, CD8+ T cells, and CD19+ B cells. There was no significant difference between baseline and end of cycle 1 for platelet counts (Fig. 8E) and absolute neutrophil count (ANC) (Fig. 8F).
Fig. 8. Blood counts, lymphocyte subsets, SS1P neutralizing antibodies, and serum SS1P concentrations.
(A to F) Effects of pentostatin and cyclophosphamide on ALC, CD4+ T cells, CD8+ T cells, CD19+ B cells, platelets, and ANC before treatment and at the end of cycle 1 (day 30). (G) The number of treatment cycles after which each patient developed anti-SS1P neutralizing antibodies. Asterisks indicate patients who did not develop antibodies. (H) Peak serum SS1P concentrations for each patient during cycles 1 and 2, calculated as mean serum SS1P concentration on days 1, 3, and 5 of each cycle. All patients had an SS1P serum concentration of >100 ng/ml during cycle 1. However, during cycle 2, four patients did not have an SS1P serum concentration of >100 ng/ml. Error bars indicate SD.
There was no association between baseline neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR) and response. Change in NLR from baseline to post–cycle 1 was not associated with response. Although limited by small sample size, the increase in PLR from baseline to post–cycle1washigherfornonresponders than for patients who had partial response (P = 0.033) (table S3).
Antibodies and serum SS1P concentration
Pentostatin and cyclophosphamide delayed the formation of neutralizing antibodies to SS1P. Figure 8G shows that only 2 of 10 patients developed anti-SS1P antibodies at the end of cycle 1 (day 30), whereas 5 of 10 patients (patients 2, 6, 7, 9, and 10) developed antibodies after cycle 2. Patient 3 did not develop antibodies after six cycles, and patient 8 did not develop antibodies after two cycles.
Because of time required to perform the antibody assay, the decision to administer SS1P for cycle 2 was based on assays doneonday24ofcycle1.Becausenoneof the patients had antibodies on day 24, all patients received cycle 2 of SS1P. Figure 7H shows that 4 of 10 patients had low serum SS1P concentrations during cycle 2. These results suggest that in addition to the two patients (patients 4 and 11) who developed antibodies at the end of cycle 1, two other patients (patients 7 and 10) developed anti-SS1P antibodies before receiving cycle 2, accounting for low serum SS1P levels in these patients. Notably, 6 of 10 patients had high SS1P blood levels (>100 ng/ml) during cycle 2, patient 3 had high levels during six cycles, and patient 4 during four cycles. The serum levels of SS1P were 100-fold higher than the concentration (1 ng/ml) at which cytotoxic activity was observed against mesothelin-expressing human tumor cells (6).
DISCUSSION
Here, we demonstrate that the regimen of pentostatin plus cyclophosphamide reduces neutralizing antibody formation to SS1P immunotoxin and facilitates the clinical antitumor effects of SS1P in a cohort of heavily pretreated chemotherapy-refractory patients with advanced mesothelioma. The antitumor effects we observed, which are rare in advanced mesothelioma, appear to be associated with increased overall survival, given the extremely poor prognosis of this cohort. These clinical trial results point to a direction in targeted cancer therapy, whereby improved clinical responses might occur through combining immunotoxin therapy with immune modulation.
The regimen of pentostatin and cyclophosphamide delayed the development of neutralizing antibodies to SS1P, thereby allowing multiple cycles of therapy with an immunotoxin that was previously generally limited to one therapeutic cycle. Because 6 of 10 patients who received a second cycle of SS1P had high serum concentrations of SS1P, preventing the formation of neutralizing antibodies improved our ability to deliver therapeutic doses of the immunotoxin. The immune modulation regimen we used allowed two patients to receive four or six cycles of SS1P. Theseresultsaresignificantlybetter(P = 0.0001)than our previous results when 88% of patients developed antibodies after one cycle and met the primary endpoint of this clinical trial which was to demonstrate whether the present regimen was associated with a delay in human immune response to SS1P (8). The outpatient pentostatin and cyclophosphamide regimen was safe, non-myelosuppresive, and not associated with opportunistic infection. As such, the regimen we have developed is targeted in terms of both its anticancer component (anti-mesothelin immunotoxin) and its host conditioning (lymphocyte-selective pentostatin plus cyclophosphamide).
We did not identify baseline patient characteristics associated with tumor response. It is unlikely that tumor histology was a determinant of response because all patients had epithelial mesothelioma with high tumor cell surface expression of mesothelin. In addition, there was no clear correlation between pretreatment tumor lymphocytic infiltrates or inflammatory-related biomarkers, such as NLR or PLR ratio, and response (14, 15). However, our data suggest that the most important parameter predictive of antitumor efficacy may be the ability to achieve high serum levels of SS1P. None of the four patients with low SS1P blood levels during cycle 2 had a tumor response, whereas four of six patients with high SS1P levels during cycle 2 had antitumor responses (P = 0.076).
Although the antitumor responses might be largely attributable to an ability to more effectively deliver immunotoxin, it is possible that additional therapeutic mechanisms might be operational. Further research in experimental animal models will be necessary to gain insight into such potential therapeutic mechanisms. Given that pentostatin has no reported activity in solid tumors and cyclophosphamide has no activity in mesothelioma (16, 17), it is unlikely that a direct chemotherapeutic antitumor effect occurred. However, an indirect antitumor effect may have occurred through a change in the immunologic milieu after pentostatin plus cyclophosphamide therapy. The observations consistent with this idea include (i) increased tumor metabolism concomitant with reduced tumor size by CT scan in some patients; (ii) delayed clinical responses even 7 months remote from immunotoxin administration; and (iii) marked response to chemotherapy after immunotoxin therapy. Of note, delayed tumor responses have also been observed in other immune-based therapies (18, 19), and increased tumor chemosensitivity has been observed after immunotherapy in other settings (20). However, further studies, including evaluation of immunological changes within the tumor, are needed to better understand if an immunological component contributed to the observed tumor responses.
In summary, our results show marked antitumor activity of SS1P in heavily pretreated patients with chemotherapy-refractory mesothelioma. The pentostatin plus cyclophosphamide regimen clearly contributes to therapeutic efficacy by permitting multiple cycles of SS1P therapy and, potentially, through indirect mechanisms arising from the altered host immunologic state. Because many common malignancies including pancreatic, ovarian, and lung cancers express mesothelin (6), targeting host tumor and host immunity through the current regimen may have a broad application in cancer therapy.
MATERIALS AND METHODS
Study design
The primary objective was to determine if the regimen of SS1P with pentostatin and cyclophosphamide is safe, feasible, and effective in delaying anti-SS1P neutralizing antibody formation. Secondary objectives included evaluation of tumor responses and SS1P pharmacokinetics. The protocol (ClinicalTrials.gov number, NCT01362790) was approved by the National Cancer Institute (NCI) Institutional Review Board and sponsored by the NCI Cancer Therapy Evaluation Program.
Patients
Eligible patients had histologically confirmed malignant pleural or peritoneal mesothelioma with epithelial or biphasic histology and had received at least one platinum-containing regimen with progressive disease at study entry. Other inclusion criteria included age >18 years; life expectancy >3 months; adequate hematologic, hepatic, and renal functions; an Eastern Cooperative Oncology Group performance status of 1 or less (21); and measurable disease. Exclusion criteria included symptomatic brain metastases, a history of HIV or hepatitis B or C virus infections, or previous treatment with immunotoxins. Patients with >75% neutralization of SS1P activity at 200 ng/ml using a bioassay were ineligible (8).
Treatment and safety assessment
The first cycle lasted 30 days, and subsequent cycles lasted 21 days. Patients received pentostatin (4 mg/m2) intravenously on days 1, 5, and 9 of cycle 1 and on day 1 of subsequent cycles and 200 mg of cyclophosphamide orally on days 1 to 12 of cycle 1 and on days 1 to 4 of subsequent cycles. SS1P (35 μg/kg) was given intravenously on days 10, 12, and 14 of cycle 1 and on days 2, 4, and 6 of subsequent cycles. Patients without tumor progression could receive up to six cycles, provided they had not developed antibodies to SS1P. All patients received sulfamethoxazole/trimethoprim for pneumocystis pneumonia prophylaxis and acyclovir for herpes simplex and varicella zoster virus prophylaxis until CD4+ T cell counts recovered to 200 cells/μl. Adverse event assessment was done using the NCI Common Terminology Criteria for Adverse Events, version 4.0 (22).
Evaluation of tumor response
Responses were evaluated by CT and PET imaging every two cycles and during follow-up. For pleural mesothelioma, tumor responses were measured using the modified Response Evaluation Criteria in Solid Tumors (RECIST) for mesothelioma; for peritoneal mesothelioma, RECIST 1.1 criteria were used (23, 24). Metabolic tumor responses were assessed using the PET Response Criteria in Solid Tumors (PERCIST), 1.0 (25). The tumor SULmax is the maximal SUL in a pixel of the tumor region. A 1-cm3 volume was applied to the tumor to determine the SULpeak/cm3 (MEDVIEW version 12.1.1, MedImage). For each patient, two to five tumor regions were analyzed for SULmax and SULpeak/cm3, corrected by subtracting the background FDG uptake in the liver, and then averaged.
Blood counts and immune subsets
The total blood counts, ALC, CD4+ and CD8+ T cells, and CD19+ B cells were measured at protocol-specified time points after treatment with pentostatin and cyclophosphamide.
SS1P neutralizing antibodies and pharmacokinetics
Neutralizing antibodies to SS1P were measured at study entry, day 24 of cycle 1, day 16 of subsequent cycles, and before each cycle with a bioassay (8). The decision to administer cycle 2 or subsequent cycles was based on the results of day 24 (for cycle 1) or day 16 (for cycle 2 or subsequent cycles) evaluation. After starting treatment, patients with less than 75% neutralization of SS1P activity at 1000 ng/ml received additional cycles. SS1P blood levels were measured with a bioassay (8).
Tumor mesothelin expression
Tumor specimens were evaluated for mesothelin positivity by immunohistochemistry with anti-mesothelin monoclonal antibody 5B2 (Novacastra). Mesothelin expression was evaluated for estimated percentage of positive tumor cells and staining intensity (absent; 1+, weak; 2+, moderate; or 3+, strong).
Statistical considerations
Because a previous phase 1 study of single-agent SS1P showed that 88% of patients develop neutralizing antibodies to SS1P 3 weeks after the first dose (8), the primary goal was to demonstrate whether the present regimen was associated with a fraction of antibody formation substantially below this. Ten patients were selected as the number to be evaluated, and the fraction that developed neutralizing antibodies was determined to assess whether SS1P was associated with a lower fraction than on the phase 1 study, specifically considering six or fewer patients with neutralizing antibodies to be a successful outcome. If 0 to 6 of 10 evaluable patients developed neutralizing antibodies, this would mean that there was a 90% or greater chance that the true probability of developing neutralizing antibodies was 45% or less, consistent with half of what had been found before. On the other hand, if the true probability of developing neutralizing antibodies was 80% or greater, there would only be a 12% probability or less of having 0 to 6 patients with neutralizing antibodies.
The Kaplan-Meier method was used to determine the probability of survival from on-study date until death or last follow-up. Follow-up was calculated from the date of study entry until 13 June 2013, the date of analysis.
The proportion of patients responding to the treatment was compared between those with and those without high SS1P blood levels in cycle 2, using Fisher’s exact test. The changes in ALC, CD4+ T cells, CD8+ T cells, ANC, and CD19+ B cells between days 0 and 30 were determined using the Wilcoxon signed rank test. All P values are two-tailed and presented without adjustment for multiple comparisons.
Supplementary Material
Table S1. Previous therapies received by patients.
Table S2. Adverse events related to SS1P, pentostatin, or cyclophosphamide.
Table S3. Association of NLR and PLR with tumor response.
Acknowledgments:
We thank our research nurses B. Schuler and Y. Mallory and nurse practitioner J. Nashed for taking care of the patients, and J. Zhang and B. Debrah for help in data collection and analysis.
Funding: This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Footnotes
Competing interests: I.P. is an inventor on patents on immunotoxins that have all been assigned to the NIH. Other authors declare that they have no competing financial interests.
Data and materials availability: SS1P was supplied by the Cancer Therapy Evaluation Program (CTEP), NCI, which holds the Investigational New Drug (IND) for the drug.
REFERENCES AND NOTES
- 1.Robinson BW, Lake RA, Advances in malignant mesothelioma. N. Engl. J. Med 353, 1591–1603 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U,Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P, Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol 21, 2636–2644 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Tsao A, Wistuba I, Roth JA, Kindler HL, Malignant pleural mesothelioma. J. Clin. Oncol 27, 2081–2090 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P,Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH, Cytoreductive surgery and hyperthermic intaperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J. Clin. Oncol 27, 6237–6242 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Chang K, Pastan I, Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. U.S.A 93, 136–140 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan R, Bera T, Pasta I, Mesothelin: A new target for immunotherapy. Clin. Cancer Res 10, 3937–3942 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury PS, Viner JL, Beers R, Pastan I, Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc. Natl. Acad. Sci. U.S.A 95, 669–674 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I, Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus i.v. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res 13, 5144–5149 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I, Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin. Cancer Res 15, 5274–5279 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oratz R, Speyer JL, Wernz JC, Hochster H, Meyers M, Mischak R, Spitler LE, Antimelanoma monoclonal antibody-ricin A chain immunoconjugate (XMMME-001-RTA) plus cyclophosphamide in the treatment of metastatic malignant melanoma: Results of a phase II trial. J. Biol. Response Mod 9, 345–354 (1990). [PubMed] [Google Scholar]
- 11.Selvaggi K, Saria EA, Schwartz R, Vlock DR, Ackerman S, Wedel N, Kirkwood JM, Jones H, Ernstoff MS, Phase I/II study of murine monoclonal antibody-ricin A chain (Xomazyme-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J. Immunother 13, 201–207 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Hassan R, Williams-Gould J, Watson T, Pai-Scherf L, Pastan I, Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin. Cancer Res 10, 16–18 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Mossoba ME, Onda M, Taylor J, Massey PR, Treadwell S, Sharon E, Hassan R, Pastan I, Fowler DH, Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin. Cancer Res 17, 3697–3705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ, High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin. Cancer Res 16, 5805–5813 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, Sharma R, Inflammation-based prognostic indices in malignant pleural mesothelioma. J. Thorac. Oncol 7, 587–594 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Klohs WD, Kraker AJ, Pentostatin: Future directions. Pharmacol. Rev 44, 459–477 (1992). [PubMed] [Google Scholar]
- 17.Ong ST, Vogelzang NJ. Chemotherapy in malignant pleural mesothelioma. A review. J. Clin. Oncol 14, 1007–1017 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ, Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 19, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hofi FS, Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res 15, 7412–7420 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan R, Gabrilovich DI, Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol. Immunother 62, 405–410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP, Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol 5, 649–655 (1982). [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program (CTEP) Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0 (National Cancer Institute, Bethesda, MD, 2009); http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf
- 23.Byrne MJ, Nowak AK, Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann. Oncol 15, 257–260 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S,Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J, New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Wahl RL, Jacene H, Kasamon Y, Lodge MA, From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med 50 (Suppl. 1), 122S–150S (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Previous therapies received by patients.
Table S2. Adverse events related to SS1P, pentostatin, or cyclophosphamide.
Table S3. Association of NLR and PLR with tumor response.