Abstract
Objective:
There is considerable variation in the travel required for a patient with head and neck squamous cell carcinoma (HNSCC) to receive a diagnosis. The impact of this travel on the late diagnosis of cancer remains unexamined, even though presenting stage is the strongest predictor of mortality. Our aim is to determine whether travel time affects HNSCC stage at diagnosis independently of other risk factors, and whether this association is affected by socioeconomic status.
Materials and Methods:
Cases were obtained from the CHANCE database, a population-based case-control study in North Carolina (n=808). The mean age was 59.6 and 72% were male. Stage at diagnosis was categorized as early (T1-T2) or advanced (T3-T4) T stage and the presence or absence of nodal metastasis. Multivariate logistic regression models were used to estimate odds ratios for stage-at-diagnosis based on travel time, after adjustment for variables including demographics, income, insurance status, alcohol, and tobacco use.
Results:
The adjusted odds ratio (OR) of advanced T-stage at diagnosis was 1.97 for each hour driven (95% CI 1.36 – 2.87). There was no association with nodal metastases. There was a significant interaction between travel time and income (p = 0.026) with a pattern of higher ORs for increased distance among lower income (<$20,000) patients compared to the ORs for higher income (>$20,000) patients.
Discussion:
Travel time was an independent contributor to advanced T stage at diagnosis among low income patients. This suggests travel burden may be a barrier to early diagnosis of HNSCC for impoverished patients.
Keywords: Head and Neck Neoplasms, Neoplasm Staging, Travel, Delayed Diagnosis, Income, Socioeconomic Status
Introduction
Squamous cell carcinoma of the head and neck (HNSCC) is the sixth most common cancer worldwide and the fifth most common cancer in the United States, affecting approximately 40,000 new patients annually.[1–3] It chiefly encompasses cancers of the oral cavity, oropharynx, and larynx. HNSCC also has a high mortality rate, with poorer survival than some other common malignancies such as breast, cervical, and colorectal cancers.[4]
The strongest predictor of mortality in HNSCC is the stage of the tumor at diagnosis.[5] For example, the 3-year survival rate for oral cavity carcinoma ranges from 74% for stage 1 cancer to 35% for stage 4.[6] Furthermore, patients with late-stage tumors typically require aggressive surgery and chemoradiation that can cause speech and swallowing dysfunction and poor quality of life. While more than half of HNSCC is diagnosed at a late stage,[6] early diagnosis may spare substantial morbidity and mortality.
Access-to-care has been associated with late presentation of HNSCC and other cancers.[7] A key determinant of access may be the distance that a patient must travel to obtain a diagnosis.[8–12] This distance may disproportionately affect HNSCC patients, who frequently have a low socioeconomic status and can lack resources for transportation.[13] Nonetheless, travel burden among HNSCC patients has never been examined.
More importantly, prior studies investigating travel for cancer patients have poorly accounted for socioeconomic status, using only regional measures or none at all.[8–12] However, it is possible that disadvantaged patients delay their presentation due to difficulty reaching farther-away providers. Research is needed to determine whether socioeconomic status affects travel burden as it could lead to interventions that aid early diagnosis.
Our objective in this study is to determine whether distance affects stage at diagnosis in a population-based cohort of HNSCC patients in North Carolina. We hypothesized that a longer travel time would be a barrier to diagnosis and associated with a later stage at diagnosis. Furthermore, this relationship would be independent of demographics, socioeconomic status, or residence in an urban or rural location. We finally postulated that distance may disproportionately affect patients with lower socioeconomic status.
Materials and Methods:
Population:
Data for analysis was obtained from a population-based case-control study in North Carolina - the Carolina Head and Neck Cancer Epidemiology Study (CHANCE).[14,15] North Carolina is the 9th most populous state in the United States, and the 28th largest by area. The state’s racial composition is 68.5% White, 21.5% Black or African American, and 8.4% Latin or Hispanic American.[16] The median household income is $46,639; it has the 14th highest poverty rate in the US at 17.6%.[17] The population is 66.1% urban, making it the 15th most rural US state.[16]
Cases were identified through rapid case ascertainment with the North Carolina Central Cancer Registry and were eligible if they had been diagnosed with a first primary squamous cell carcinoma of the oral cavity, pharynx, or larynx between January 1, 2002, and February 28, 2006, were ages 20 to 80 years at diagnosis, and resided in a 46-county region in central North Carolina. The Institutional Review Board (IRB) of the University of North Carolina approved this study.
The typical diagnosis and treatment pathway for a North Carolina HNSCC patient is provided for readers unfamiliar with treatment pathways in the US. After a mass is noted by a primary care provider, dentist, or hospital-based provider, the patient is typically referred to an otolaryngologist. The otolaryngologist biopsies the mass to diagnose a cancer. Each pathological cancer diagnosis is reported to the state cancer registry, per state law. Patients are then either treated at local hospitals or referred to larger tertiary care centers depending on the ability of their local specialists and the complexity of the cancer.
Patient Characteristics and Distances:
Demographics, behaviors, income, insurance, and other characteristics and indicators of socioeconomic status were assessed by trained nurse-interviewers using a structured questionnaire during an in-home visit. Cases were interviewed soon after cancer diagnosis (the average time between diagnosis and interview was 5.3 months).
Residential history was obtained from the patient interview and the address at their time of diagnosis was used. Geographic biopsy locations were abstracted from the pathology report containing the initial diagnosis of cancer (n = 51 hospitals). Linear (Euclidean) distances and driving times (network travel times) were calculated in ArcMap 10.5 (ESRI, 2017). The rurality of each address was determined from rural-urban commuting area (RUCA) codes based on census tracts; these were obtained from the United States Department of Agriculture Economic Research Service.[18] For patients with multiple home addresses (n = 127), the address closest to the biopsy location was chosen. There was no material change in the model results using the farther address.
Exclusion Criteria:
Within the CHANCE dataset (N = 1,389), we excluded patients whose medical records were missing the pathology report for the initial biopsy that diagnosed cancer (N = 311), patients without available home addresses (N = 115), and patients who declined to report income, race, tobacco or alcohol use (N = 45, 31, 27 and 2 respectively for each variable; 105 combined). Patients without an available biopsy report were more likely to have a lower T-stage at diagnosis (72% vs. 61%; p = 0.001); there were no differences in income or other measures of socioeconomic status. There were no significant differences in the T-stage for patients who declined to report their addresses, demographics, or behaviors. We further excluded patients with addresses greater than two hours away from the biopsy location (n = 46) due to the possibility that they changed their address between the time of their biopsy and their enrollment in the study. The analysis was subsequently re-run incorporating these fardistance patients with no material change in results.
Outcomes:
Outcomes were early (T1-T2) and late (T3-T4) T stage, and the presence or absence of nodal metastasis, at presentation. Stage at diagnosis was abstracted from medical records specifying the initial treatment plan. All staging used 7th edition AJCC guidelines.
Analysis:
Linear (Euclidean) distances and driving times (network travel times) were calculated in ArcMap 10.5 (ESRI, 2017). Cases were divided into quartiles based on the driving time between the case’s home address and the biopsy location. The range for each quartile was 1-12 min, 12-22 min, 22-42 min, and 42 – 119 min. Descriptive statistics were calculated for the 1st and 4th quartiles based on driving time; bivariate testing methods included two-sided t and chi-squared tests. An alpha of 0.05 was used for all testing.
Multivariable logistic regression models were used to determine the odds ratio and 95% confidence interval for advanced T stage or the presence of nodal metastases at presentation (the primary outcomes) in relation to distance quartile (the primary exposure). Age, sex, race, income, insurance status, education, alcohol, and tobacco use were incorporated into the model as covariates; each was used as a categorical variable.
Models were also created with interaction terms to examine for the multiplicative interactions between travel time and age, race, income, education, cancer site, and HPV status The cohort was then stratified by income, with separate models were constructed for low and high-income patients to examine the potential associations between distance and T stage at diagnosis in each group.
Results:
Population Characteristics:
The final study population consisted of 808 HNSCC patients. The mean age of the population was 59.6 (standard deviation (SD) 10.4); and 72% male. The median distances between the patient and their biopsy location were 2.5, 6.5, 14.8, and 34.9 miles, respectively for each quartile based on driving time (table 1). Median driving times were 7.2, 15.8, 31.0, and 61.3 minutes. The population was distributed across the state of North Carolina (figure 1); roughly 60% of the population lived within an urban area.
Table 1:
Travel times, linear distances, and rural-urban index for distance quartiles
| Driving Time (min) | Total Distance (mi) | Rural vs. Urban Address* |
||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Mean | SD | |
| 1st quartile (n=202) | 7.2 | 3.8 | 2.5 | 1.8 | 1.4 | 1.0 |
| 2nd quartile (n=202) | 15.8 | 4.8 | 6.5 | 2.8 | 1.6 | 1.3 |
| 3rd quartile (n=202) | 31.0 | 9.9 | 14.8 | 7.6 | 2.3 | 2.0 |
| 4th quartile (n=202) | 61.3 | 28.8 | 34.9 | 17.4 | 3.5 | 2.7 |
| Total (n=808) | 22.5 | 30.6 | 9.4 | 16.8 | 2.2 | 2.1 |
1 reperesents the most urban location and 10 represents the most rural. RUCA score was associated with travel time; R-squared 0.18, p < 0.001
Figure 1:
Geographic mapping of CHANCE patients
Distance-Quartile Comparison:
When compared to patients in the first (closest) quartile, patients in the 4th (farthest) quartile were significantly more likely to be male (83% vs. 71%; p = 0.007), white (75% vs. 63%; p = 0.01), and have an annual household income greater than $20,000 (63% vs. 51%; p = 0.030) (table 2). Residence in a more rural area was also associated with increasing driving time (R2 0.18; p < 0.001). There were no significant differences in age, education, insurance status, tobacco use, alcohol use, or HPV status based on distance quartile.
Table 2:
Comparison of 1st and 4th quartiles based on driving time (total n = 808)
| 1st quartile (n = 202) |
4th quartile (n=202) |
P-Value | |||
|---|---|---|---|---|---|
| Total n = 808 | No. | % | No. | % | |
| Age Category | |||||
| <50 (n=173) | 33 | 16% | 43 | 21% | 0.0771* |
| 50-65 (n=386) | 90 | 45% | 97 | 48% | |
| 65+ (n=249) | 79 | 39% | 62 | 31% | |
| Sex | |||||
| Male (n=630) | 144 | 71% | 167 | 83% | 0.007 |
| Female (n=178) | 58 | 29% | 35 | 17% | |
| Race | |||||
| White (n=600) | 127 | 63% | 151 | 75% | 0.01 |
| Black (n=208) | 75 | 37% | 51 | 25% | |
| Education | |||||
| Less Than High School (n=272) | 78 | 39% | 65 | 32% | 0.255** |
| High School Grad (n=234) | 58 | 29% | 60 | 30% | |
| Greater than High School (n=302) | 66 | 33% | 77 | 38% | |
| Income | |||||
| Income (relative to > 50 K) | 36 | 18% | 48 | 24% | |
| Income > $50,000 (n=224) | 68 | 34% | 80 | 40% | 0.012*** |
| Income $20,000 - $50,000 (n=285) | 98 | 49% | 74 | 37% | |
| Income < $20,000 (n=299) | 202 | 100% | 202 | 100% | |
| Insuracne Status | |||||
| Private (n=301) | 54 | 27% | 61 | 30% | 0 44**** |
| Medicaid/Medicare (n=271) | 95 | 47% | 66 | 33% | |
| None (n=114) | 29 | 14% | 30 | 15% | |
| Other (n=122) | 24 | 12% | 45 | 22% | |
| Smoking | |||||
| < 10 Years (n=154) | 28 | 14% | 36 | 18% | 0.282 |
| 10+ Years (n=654) | 174 | 86% | 166 | 82% | |
| Alcohol Use | |||||
| < 1 Drink / Week (n=104) | 23 | 11% | 22 | 11% | 0.877 |
| > 1 Drink / Week (n=704) | 179 | 89% | 180 | 89% | |
| Site | |||||
| Larynx/Hypopharynx (n=357) | 97 | 48% | 82 | 41% | 0.241 |
| Oral NOS (n=133) | 33 | 16% | 32 | 16% | |
| Oral cavity (n=77) | 25 | 12% | 24 | 12% | |
| Oropharynx (n=241) | 47 | 23% | 64 | 32% | |
| P-16 Associated (if tested) | |||||
| No (n=157) | 42 | 66% | 38 | 56% | 0.282 |
| Yes (n=107) | 22 | 34% | 30 | 44% | |
P-Value for 65+ vs. < 65
P-value for high-school education vs. none
P-value for > $20,000 vs. < $20,000
P-value for private vs. non-private insuracne
Unadjusted and Adjusted Models for Stage at Diagnosis
The proportion of patients presenting with an advanced T stage by quartile was 32%, 33%, 40%, and 47%, respectively. In an unadjusted model, each hour of driving time was significantly associated with twice the odds of advanced T-stage at diagnosis (95% CI 1.42 – 2.84). However, there was no significantly increased odds for the presence of nodal metastases (OR 1.10; 95% CI 0.77–1.54).
In a multivariable model incorporating demographics, socioeconomic status, and urban locations, the overall adjusted OR for high T-stage at diagnosis was 1.97 for each hour driven (95% CI 1.36 – 2.87). Patients in the third and fourth (farthest) quartiles were significantly more likely to present with an advanced T stage relative to patients in the first (for the 3rd quartile, adjusted OR 1.63, 95% CI 1.04 – 2.56; for the 4th, adjusted OR 2.08, 95% CI 1.29 – 3.34) (table 3).
Table 3:
Multivariate logistic regression model for odds of high T-stage at diagnosis (n = 808)
| Odds Ratio | 95% CI | P- Value |
|
|---|---|---|---|
| Driving Time (1st quartile as baseline) | |||
| 2nd quartile | 1.30 | 0.82 - 2.04 | 0.259 |
| 3rd quartile | 1.63 | 1.04 - 2.56 | 0.035 |
| 4th quartile | 2.08 | 1.29 - 3.34 | 0.003 |
| Age Category (Relative to < 50) | |||
| 50-65 | 0.74 | 0.50 - 1.10 | 0.138 |
| 65+ | 0.41 | 0.24 - 0.71 | 0.001 |
| Female sex (relative to male) | 1.02 | 0.68 - 1.51 | 0.932 |
| Non-white (vs. white) | 1.11 | 0.76 - 1.63 | 0.587 |
| Income (relative to > 50 K) | |||
| Income $20,000 - $50,000 | 1.05 | 0.68 - 1.61 | 0.825 |
| Income < $20,000 | 1.47 | 0.88 - 2.44 | 0.141 |
| Education (past high school) | 0.70 | 0.49 - 1.00 | 0.051 |
| Insurance (relative to private insurance) | |||
| Medicaid/Medicare | 1.51 | 0.90 - 2.53 | 0.120 |
| None | 2.75 | 1.63 - 4.64 | < 0.001 |
| Other | 1.50 | 0.88 - 2.55 | 0.136 |
| Site (Relative to larynx/hypopharynx) | |||
| Oral, NOS | 1.06 | 0.68 - 1.66 | 0.786 |
| Oral cavity | 1.34 | 0.78 - 2.29 | 0.293 |
| Oropharynx | 0.92 | 0.63 - 1.34 | 0.653 |
| Smoking (> 10 pack-years) | 1.29 | 0.84 - 1.97 | 0.241 |
| Alcohol use (> 1 drink / week) | 1.65 | 0.97 - 2.80 | 0.064 |
| Residence in Rural Area | 1.00 | 0.93 - 1.09 | 0.937 |
The only other associations with T stage at diagnosis were the lack of medical insurance (OR 2.27; 95% CI: 1.63 – 4.64) and an age over 65 (OR 0.41, p = 0.001; 95% CI: 0.24 - 0.71) (table 3). There was no association between distance and nodal disease at presentation (results not shown).
Distance and Income:
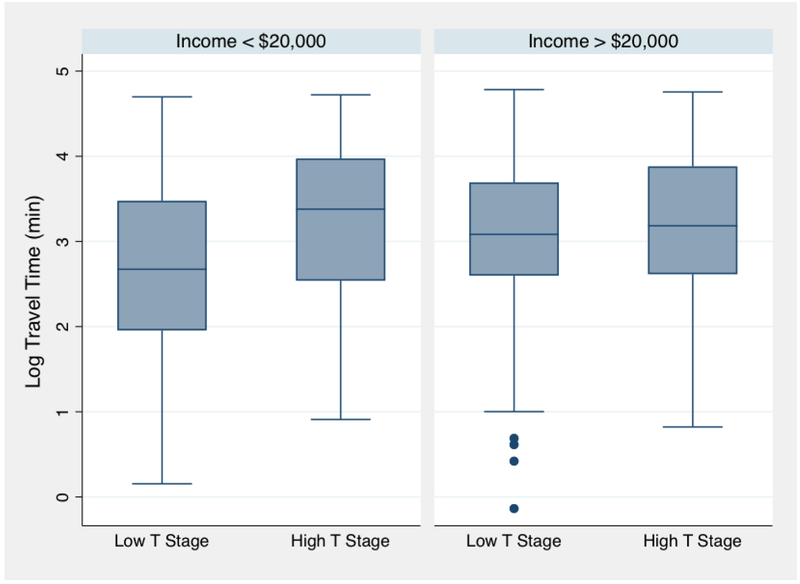
To test the hypothesis that distance would affect low and high-income patients differently, a model was constructed to include a travel time X income interaction term (with household income divided into < $20,000 and > $20,000). The interaction term was significant (p = 0.026). When the cohort was stratified by income, there was a pattern of higher ORs for increased distance among lower income (<$20,000) patients compared to the ORs for higher income (>$20,000) patients (figure 2; table 4). For example, the OR for between travel time and T stage among low income patients was OR 3.31 (95% CI 1.53 – 7.61) for 4th quartile relative to first compared to 1.49 (95% CI 0.79 – 2.80) for higher income patients.
Figure 2:
Log-transformed travel time and T-stage at diagnosis, stratified by income
Table 4:
Multivariate logistic regression for odds of high T stage at diagnosis, stratified by Income
| Income < $20,000 (N = 299) | Income > $20,000 (N = 509) | |||||
|---|---|---|---|---|---|---|
| Odds Ratio |
95% CI | P- Value |
Odds Ratio |
95% CI | P- Value |
|
| Driving Time (1st quartile as baseline) | ||||||
| 2nd quartile | 1.12 | 0.53 - 2.36 | 0.760 | 1.14 | 0.64 - 2.05 | 0.651 |
| 3rd quartile | 2.41 | 1.20 - 4.86 | 0.014 | 1.19 | 0.64 - 2.18 | 0.583 |
| 4th quartile | 3.31 | 1.53 - 7.16 | 0.002 | 1.49 | 0.79 - 2.80 | 0.220 |
| Age Category (Relative to < 50) | ||||||
| 50-65 | 0.92 | 0.45 - 1.87 | 0.819 | 0.67 | 0.41 - 1.08 | 0.102 |
| 65+ | 0.49 | 0.21 - 1.15 | 0.101 | 0.38 | 0.18 - 0.80 | 0.011 |
| Female sex (relative to male) | 0.98 | 0.53 - 1.81 | 0.954 | 1.12 | 0.66 - 1.91 | 0.675 |
| Non-white (vs. white) | 1.05 | 0.61 - 1.81 | 0.861 | 1.14 | 0.64 - 2.02 | 0.654 |
| Education past high school | 1.00 | 0.50 - 2.01 | 0.989 | 0.64 | 0.42 - 0.98 | 0.040 |
| Insurance (relative to private insurance) | ||||||
| Medicaid/Medicare | 1.41 | 0.49 - 4.04 | 0.526 | 1.39 | 0.68 - 2.85 | 0.362 |
| None | 2.67 | 0.91 - 7.88 | 0.075 | 2.85 | 1.40 - 5.81 | 0.004 |
| Other | 1.22 | 0.37 - 4.06 | 0.746 | 1.55 | 0.81 - 3.00 | 0.189 |
| Site (Relative to larynx/hypopharynx) | ||||||
| Oral, NOS | 1.38 | 0.66 - 2.86 | 0.392 | 0.98 | 0.55 - 1.74 | 0.941 |
| Oral cavity | 2.37 | 1.02 - 5.53 | 0.046 | 0.94 | 0.45 - 1.97 | 0.871 |
| Oropharynx | 1.20 | 0.65 - 2.21 | 0.568 | 0.80 | 0.49 - 1.32 | 0.380 |
| Smoking > 10 pack-years | 0.97 | 0.47 - 2.01 | 0.932 | 1.40 | 0.81 - 2.42 | 0.233 |
| Alcohol use > 1 drink / week | 2.22 | 0.89 - 5.51 | 0.086 | 1.49 | 0.76 - 2.92 | 0.241 |
| Rural Home Address | 1.05 | 0.91 - 1.21 | 0.521 | 0.97 | 0.88 - 1.08 | 0.625 |
Discussion:
In this study, we demonstrated that increased driving time was associated with an advanced T-stage at diagnosis for low-income HNSCC patients. This association was independent of other covariates such as medical insurance, indicators of socioeconomic status, and rural location. These findings suggest that distance may be a barrier to the early diagnosis of HNSCC, especially among disadvantaged patients.
Travel has been shown to be a barrier to the early diagnosis of several other cancers. Holmes et al. (2012) found that distance to a urologist was associated with diagnosis of high-risk prostate cancer.[9] Likewise, Huang et al. (2009) showed that distance to a mammography center was associated with more advanced breast cancer at diagnosis,[10] and similar findings have been reported in melanoma and colorectal cancer.[8,19] HNSCC may be especially susceptible to travel burden as diagnosis typically requires travel to an otolaryngologist. Moreover, HNSCC frequently affects low income patients, due in part to associations with tobacco and tobacco use, who may have additional difficulties in accessing care.[20–24] Nonetheless, this is the first study to find a link between stage-at-diagnosis and travel burden in HNSCC. In one similar study, Tan et al. (2016) has demonstrated that a remote location was associated with a delay between presentation and receipt of treatment among 158 Australian HNSCC patients.[24]
It is notable that there was a significant association between distance and T stage but not between distance and nodal metastases. HNSCC patients frequently present due to symptoms caused by the primary tumor and may be unaware of growing neck metastases. Likewise, prior studies on late presentation of HNSCC patients have only analyzed T stage.[7] Finally, the specific site influences the likelihood of nodal metastases for HNSCC due to variations in lymphatic drainage.[25]
In addition to distance, stage at diagnosis was influenced by age, education, and insurance status. A lack of medical insurance is known to be associated with late-presentation of multiple cancers.[26] Educational attainment has also been previously associated with late presentation of multiple cancers as well.[27,28] Overall, geographic distance may be one of the many barriers that low-income patients face in receiving a prompt diagnosis. Others include health literacy and medical insurance, and even the ability to leave work to seek care.
Advanced age has been associated with late presentation;[29–31] it is notable that it was associated with an earlier presentation in this population. Importantly, race was not significantly associated with advanced stage, and there was no interaction between race and distance. Several prior studies have noted race as a risk factor for advanced HNSCC, [20,32] although these did not account for socioeconomic status, which race may confound.[33,34]
This is the first study in any cancer site to find that distance disproportionately affects low-income patients. Previous studies either did not report socioeconomic status or used area-level variables, such as the median income of a cancer patient’s census tract.[8,10,19] Our population-based study used individual measures obtained by a trained interviewer. The increased precision of these markers likely allowed the detection of an interaction between distance and income.
The differential impact of income implies multiple possible mechanisms for the association between distance and stage at diagnosis. First, low socioeconomic status and limited resources for transportation may present barriers to patients seeking a timely diagnostic biopsy. This may lead to patients waiting until symptoms increase or until they require emergency care due to difficulty with swallowing or airway compromise. Second, patients with limited resources may lack access to screening for head and neck cancer through routine medical or dental visits.
Further research into the mechanism could provide insight for future interventions. These could include improved access to primary career and community efforts for screening among low-income patients without access to primary care. Additionally, care coordinators can be utilized to recommend accessible specialists, ensure follow up, and help with transportation.[35][36] Telemedicine may also have a role in bringing specialists into contact with patients who have transportation limitations.[24][37]
The main limitation of this study is that is represents data from a single state and may be less generalizable than national datasets. However, CHANCE is the largest population-based HNSCC study to date and provides granular social and demographic data that is limited in national cancer registry data. Another limitation is the exclusion of patients due to missing data on biopsy location, home address, demographics, or markers of socioeconomic status. There was no significant difference in the presenting T stage for patients who did not provide information. A lower T stage was seen in patients missing biopsy information, although there were no significant differences in any indicators of socioeconomic status. It is also important to note that there are many other variables intrinsic to the tumor that influence stage at diagnosis for any single patient. These include the site of the tumor (such as glottis vs. subglottis), and the growth rate of the cancer. Many of these variables were not directly measured or adjusted for in this study. However, these intrinsic factors are likely unrelated to income and geographic location, and so meaningful statistical patterns for income and geography can emerge when carefully evaluating data from many patients in a population-based context.
Conclusion:
In this study we identified travel time to diagnosing provider as a significant independent contributor to advanced T stage at diagnosis. We also found a greater impact of increased distance among low income patients with HNSCC. Our findings suggest that distance is a barrier to early diagnosis of HNSCC, especially among disadvantaged patients.
Highlights:
We examine whether travel time affects late presentation of cancer
Travel time among low-income patients was associated with T stage at presentation
Travel may be a barrier to early diagnosis of HNSCC for disadvantaged patients
Acknowledgements:
This study was supported in part by grants R01- CA90731 from the National Cancer Institute and T32 - DC005360-12 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
Disclosures and Conflicts of Interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Jemal A, Bray F, Ferlay J. Global Cancer Statistics: 2011. CA Cancer J Clin 1999;49:1,33–64. doi: 10.3322/caac.20107.Available. [DOI] [PubMed] [Google Scholar]
- [2].Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- [3].Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008;83:489–501. doi: 10.4065/83.4.48910.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- [5].Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: A site-specific analysis of the SEER database. Int J Cancer 2005;114:806–16. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- [6].American Joint Committee on Cancer American Joint Committe on Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- [7].Adrien J, Bertolus C, Gambotti L, Mallet A, Baujat B. Why are head and neck squamous cell carcinoma diagnosed so late? Influence of health care disparities and socio-economic factors. Oral Oncol 2014;50:90–7. doi: 10.1016/j.oraloncology.2013.10.016. [DOI] [PubMed] [Google Scholar]
- [8].Stitzenberg KB, Thomas NE, Dalton K, Brier SE, Ollila DW, Berwick M, et al. Distance to diagnosing provider as a measure of access for patients with melanoma. Arch Dermatol 2007;143:991–8. doi: 10.1001/archderm.143.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holmes JA, Carpenter WR, Wu Y, Hendrix LH, Peacock S, Massing M, et al. Impact of distance to a urologist on early diagnosis of prostate cancer among black and white patients. J Urol 2012;187:883–8. doi: 10.1016/j.juro.2011.10.156. [DOI] [PubMed] [Google Scholar]
- [10].Huang B, Dignan M, Han D, Johnson O. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in kentucky. J Rural Heal 2009;25:366–71. doi: 10.1111/j.1748-0361.2009.00245.x. [DOI] [PubMed] [Google Scholar]
- [11].Schroen AT, Brenin DR, Kelly MD, Knaus WA, Slingluff CL. Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Clin Oncol 2005;23:7074–80. doi: 10.1200/JCO.2005.06.032. [DOI] [PubMed] [Google Scholar]
- [12].Meden T, John-Larkin C, Hermes D, Sommerschield S Relationship Between Travel Distance and Utilization of Breast Cancer Treatment in Rural Northern Michigan. JAMA 2002;287:111. doi: 10.1001/jama.287.1.111-JMS0102-5-1. [DOI] [PubMed] [Google Scholar]
- [13].Johnson S, Corsten MJ, McDonald JT, Chun J. Socio-economic factors and stage at presentation of head and neck cancer patients in Ottawa, Canada: A logistic regression analysis. Oral Oncol 2010;46:366–8. doi: 10.1016/j.oraloncology.2010.02.010. [DOI] [PubMed] [Google Scholar]
- [14].Farquhar DR, Divaris K, Mazul AL, Weissler MC, Zevallos JP, Olshan AF. Poor oral health affects survival in head and neck cancer. Oral Oncol 2017;73:111–7. doi: 10.1016/j.oraloncology.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Divaris K, Olshan AF, Smith J, Bell ME, Weissler MC, Funkhouser WK, et al. Oral health and risk for head and neck squamous cell carcinoma: the Carolina Head and Neck Cancer Study. Cancer Causes Control 2010;21:567–75. doi: 10.1007/sl0552-009-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].US Census. 2010. Census n.d https://www2.census.gov/geo/maps/dcl0_thematic/2010_Profile/2010_Profile_Map_North_Carolina.pdf (accessed December 1, 2018).
- [17].Usa.com. U.S. Population in Poverty Percentage State Rank Based on ACS 2010-2014 data n.d http://www.usa.com/rank/us--population-in-poverty-percentage--state-rank.htm?hl=SC&hlst=SC&wist=&yr=9000&dis=&sb=ASC&plow=&phigh=&ps= (accessed December 1, 2018).
- [18].Morrill R, Cromartie J, Hart G. METROPOLITAN, URBAN, AND RURAL COMMUTING AREAS: TOWARD A BETTER DEPICTION OF THE UNITED STATES SETTLEMENT SYSTEM. Urban Geogr 1999;20:727–48. doi: 10.2747/0272-3638.20.8.727. [DOI] [Google Scholar]
- [19].Wan N, Zhan FB, Zou B, Wilson JG. Spatial Access to Health Care Services and Disparities in Colorectal Cancer Stage at Diagnosis in Texas. Prof Geogr 2013;65:527–41. doi: 10.1080/00330124.2012.700502. [DOI] [Google Scholar]
- [20].Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg 1998;124:951–62. [DOI] [PubMed] [Google Scholar]
- [21].Dl Conway, Brenner DR, McMahon AD, Macpherson LMD, Agudo A, Ahrens W, et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. IntJ Cancer 2015;136:1125–39. doi: 10.1002/ijc.29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282–7. [PubMed] [Google Scholar]
- [23].Dl Conway, Petticrew M, Marlborough H, Berthiller J, Hashibe M, Macpherson LMD. Socioeconomic inequalities and oral cancer risk: A systematic review and meta-analysis of case-control studies. Int J Cancer 2008;122:2811–9. doi: 10.1002/ijc.23430. [DOI] [PubMed] [Google Scholar]
- [24].Tan JY-A, Otty ZA, Vangaveti VN, Buttner P, Varma SC, Joshi AJ, et al. A prospective comparison of times to presentation and treatment of regional and remote head and neck patients in North Queensland, Australia. Intern Med J 2016;46:917–24. doi: 10.1111/imj.13138. [DOI] [PubMed] [Google Scholar]
- [25].Flint PW, Haughey BH, Lund VJ, Niparko JK, Robbins KT, Thomas JR, et al. Cummings Otolaryngology - Head & Neck Surgery. n.d. [Google Scholar]
- [26].Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol 2008;9:222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- [27].Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 2009;20:417–35. doi: 10.1007/sl0552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kaufman JS. Progress and pitfalls in the social epidemiology of cancer. Cancer Causes Control 1999;10:489–94. doi: 10.1023/A:1008958104748. [DOI] [PubMed] [Google Scholar]
- [29].Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health 1991;81:646–9. doi: 10.2105/AJPH.81.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Adrien J, Bertolus C, Gambotti L, Mallet A, Baujat B. Why are head and neck squamous cell carcinoma diagnosed so late? Influence of health care disparities and socio-economic factors. Oral Oncol 2014;50:90–7. doi: 10.1016/j.oraloncology.2013.10.016. [DOI] [PubMed] [Google Scholar]
- [31].Holmes FF, Erwin H. Cancer Stage-to-Age Relationship: Implications for Cancer Screening in the Elderly*. J Am Geriatr Soc 1981;29:55–7. doi: 10.1111/j.1532-5415.1981.tb01227.x. [DOI] [PubMed] [Google Scholar]
- [32].Sethi S, Lu M, Kapke A, Benninger MS, Worsham MJ. Patient and tumor factors at diagnosis in a multi-ethnic primary head and neck squamous cell carcinoma cohort. J Surg Oncol 2009;99:104–8. doi: 10.1002/jso.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaufman JS, Cooper RS, McGee DL. Socioeconomic Status and Health in Blacks and Whites: The Problem of Residual Confounding and the Resiliency of Race. Epidemiology n.d;8:621–8. doi: 10.2307/3702653. [DOI] [PubMed] [Google Scholar]
- [34].Williams DR, Yan Yu Y, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health. J Health Psychol 1997;2:335–51. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- [35].Desch CE, Grasso MA, McCue MJ, Buonaiuto D, Grasso K, Johantgen MK, et al. A Rural Cancer Outreach Program Lowers Patient Care Costs and Benefits Both the Rural Hospitals and Sponsoring Academic Medical Center. J Rural Heal 1999;15:157–67. doi: 10.1111/j.1748-0361.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- [36].Australian Cancer Society. Cancer forum. Australian Cancer Society; n.d. [Google Scholar]
- [37].Sabesan S, Kelly J, Evans R, Larkins S. A tele-oncology model replacing face-to-face specialist cancer care: perspectives of patients in North Queensland. J Telemed Telecare 2014;20:207–11. doi: 10.1177/1357633X14529237. [DOI] [PubMed] [Google Scholar]