Abstract
Central nervous system (CNS) insulin resistance is a condition in which the cells within the CNS do not respond to insulin appropriately and is often linked to aberrant CNS insulin levels. CNS insulin is primarily derived from the periphery. Aberrant CNS insulin levels can arise due to various factors including i) decreased endogenous insulin transport into the brain, across the blood-brain barrier (BBB), ii) reduced CNS sequestration of insulin, and iii) increased CNS degradation. While the sole route of endogenous insulin transport into the brain is via the BBB, there are multiple therapeutic routes of administration that have been investigated to deliver exogenous insulin to the CNS. These alternative administrative routes can be utilized to increase the amount of CNS insulin and aid in overcoming CNS insulin resistance. This review focuses on the intravenous, intracerebroventricular, intranasal, ocular, and intrathecal routes of administration and compares the impact of insulin delivery.
Introduction
Insulin in the central nervous system (CNS) has important regulatory effects on whole body metabolism and cognition. Specifically, insulin has been shown to act on every cell type within the CNS including neurons, astrocytes, oligodendrocytes, ependymal cells, brain endothelial cells, and microglia. Indeed, all CNS cell types express the insulin receptor (Arnold et al., 2018) suggesting the ability to respond to insulin. These cells are not insulin-dependent but are in fact insulin-responsive (Belfiore et al., 2009). The multi-functional action of insulin in the CNS is different from the well-known peripheral effects of regulating plasma glucose in that CNS insulin can regulate peripheral metabolism, food intake, and cognition through various signaling pathways that have been reviewed elsewhere (Banks et al., 2012; Stockhorst et al., 2004).
Whether insulin is synthesized locally within the CNS remains controversial. However, it is known peripherally-produced circulating insulin can enter the CNS via transport across the blood-brain barrier (BBB) (Banks and Kastin, 1998; Schwartz et al., 1991). It is also known that there are certain diseases and conditions in which CNS insulin levels are decreased or that insulin receptor resistance ensues (Table 1). Either resistance to or decreases in insulin in the CNS would result in a deficiency in the actions of CNS insulin. Fasting can significantly alter the amount of insulin present in the brain (Aime et al., 2012) and this has been shown to be due to changes in insulin transport across the BBB (Urayama and Banks, 2008). In addition, brain insulin levels are decreased with age and in people with Alzheimer’s disease (Frolich et al., 1998). This has also been shown for the level of insulin present in the cerebrospinal fluid (CSF) versus the blood, with Alzheimer’s disease patients having decreased CSF/serum insulin ratios (Craft et al., 1998). This latter point suggests three potential deficits in maintaining adequate CNS insulin levels: 1) decreased BBB transport, 2) reduced CNS sequestration, or 3) increased CNS degradation. A deficit at any of these processes could ultimately result in a deficiency in CNS insulin actions.
Table 1.
Overview of insulin accumulation in the CNS following various delivery routes.
| Delivery Route | Insulin Concentration | Region | Time | Reference |
|---|---|---|---|---|
| IV | 0.045 %inj/g | Whole brain | 2 min | Banks et al 1997b |
| ICV | 0.24 mg/g | Olfactory Bulb | 60 Min | Aime et al 2012 |
| INL | 0.265 %inj/g | Hypothalamus | 30 min | Rhea et al 2017 |
| Ocular | 0.24 pg insulin/μg protein | Thalamus | 10 min | Koevary et al 2003 |
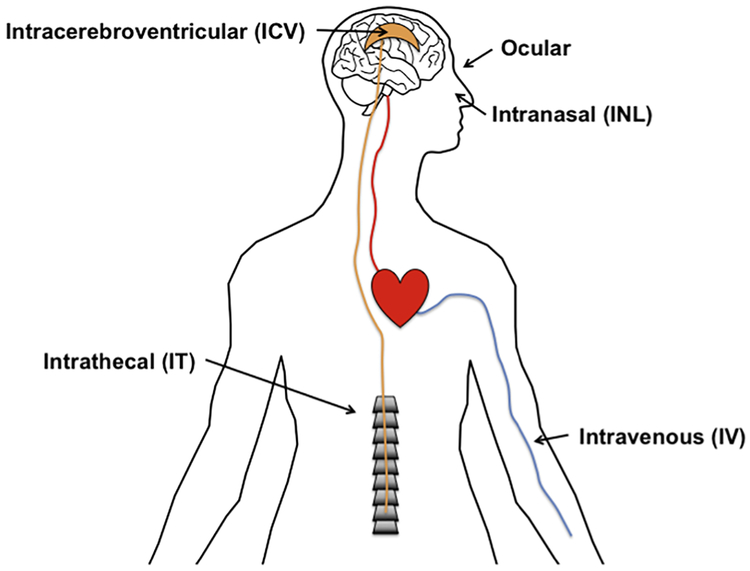
Regardless of whether insulin is produced by the brain, investigating ways to increase insulin in the CNS in these conditions is of great importance. This review will focus on various delivery routes that have been utilized both in pre-clinical and clinical models to increase CNS insulin (Figure 1). We will first describe the pharmacokinetics of each transit route, highlighting the maximal levels reached after delivery and timing, discuss states in which transport is altered with the given method, and lastly, point out the clinical or disease outcome with each delivery method.
Figure 1.
Administrative routes for exogenous insulin delivery in the treatment of overcoming CNS insulin resistance.
Delivery Methods
Intravenous Administration
Following intravenous (IV) administration, insulin must navigate the BBB to enter the CNS just like endogenous insulin. The BBB is a network of cells that strictly regulates the transport of substrates into and out of the brain. This obstacle prevents insulin from freely diffusing and thus requires an intact transport system. The rate (Ki) of human insulin transport across the mouse BBB is approximately 0.866 ± 0.086 μL/g-m (Banks et al. 1997a), but ranges from 0.2–1.7 μL/g-min based on previously published research (Banks et al., 2012). This transport was shown to be self-inhibited in a dose dependent manner with 0.12 μg insulin reducing the Ki for insulin to 50% (Banks et al., 1997a). Serum glucose was unaffected with the addition of 0.1 μg insulin per mouse suggesting the Ki for insulin is affected more by insulin levels than serum glucose. This also suggests that most of the blood-to-brain signaling mediated by insulin is unrelated to hypoglycemia. The peak amount of insulin present in the brain occurred 2 min after IV injection with a value of 0.045 % of the injected dose taken up per gram of brain. The BBB transport rate of insulin is similar to other peptides produced by the pancreas including amylin and pancreatic polypeptide, while the percent accumulating in the brain is somewhat less than these peptides. These discrepancies are important in instances where insulin is injected as a bolus rather than when blood levels are constant.
There is no change in the rate of insulin transport across the BBB with loss of the insulin receptor, suggesting insulin transport can occur independent of the insulin receptor (Rhea et al., 2018). It is likely human insulin crosses the mouse BBB by the same transporter as the endogenous mouse transporter as rat insulin was able to inhibit the transport of human insulin and vice versa (Banks et al., 1997b). The olfactory bulb has the fastest rate of transport, occurring about 2–6 times faster than the remaining brain (Banks et al., 1999). The rate for BBB insulin transport is influenced by various factors. There is no effect of verapamil (a P-glycoprotein inhibitor) and leptin on the Ki of insulin. The lack of effect of verapamil and leptin demonstrates that the P-glycoprotein and leptin transporter, respectively, is not involved in the BBB transport of insulin. With 100 mg/kg intraperitoneal pretreatment of aluminum, insulin Ki is reduced more than 70%. Indeed, this list of factors is continually growing from aluminum and dexamethasone pretreatment, strain of mouse, brain region, APP expression, hibernation, starvation, obesity, triglycerides, iron status, hyperglycemia, diabetic state, nitric oxide level, and activation of the innate immune system to also include cholecystokinin (Banks et al., 2012; May et al., 2016). A lack of effect of estrogen on insulin BBB transport has also been observed (May et al., 2016).
Not only does insulin have a biological effect within the CNS, but insulin also has several effects on brain endothelial cells as reviewed previously (Banks et al., 2012). It can directly affect transport of amino acids (Tagliamonte et al., 1976) and leptin (Kastin and Akerstrom, 2001) and increases the expression and function of P-glycoprotein (Liu et al., 2009). However, insulin does not seem to be important for BBB integrity (Kondo et al., 2004), although this lack of effect may not extend to diabetic animals in which insulin treatment combined with idebenone (a drug developed for the treatment of Alzheimer’s disease) improved BBB integrity (Sun et al., 2015). Insulin can differentially affect the distribution of amyloid β (Aβ1–40) and Aβ(1–42) (Swaminathan et al., 2018). Peripheral insulin administration can increase plasma clearance of Aβ(1–40) and decrease the clearance of Aβ(1–42). Brain elimination of Aβ(1–40) is decreased 3-fold when insulin is present while elimination of Aβ(1–42) is increased nearly 2-fold. Thus, the impact of insulin on brain endothelial cells and alteration of other transport systems should be taken into consideration when exploring ways to increase CNS insulin via the IV route.
In the SAMP8 mouse model of Alzheimer’s disease, insulin BBB transport is not different in the hippocampus between young and aged mice (Banks et al., 2000). When permeability of insulin at the BBB was investigated in the APP/PS1 mouse model of Alzheimer’s disease, it was found insulin BBB permeability was increased 1.7-fold in the hippocampus compared to adult B6SJL mice (Poduslo et al., 2001). Reports on the permeability of the BBB in Alzheimer’s disease vary in the literature which could explain the discrepancies in the differences in insulin transport across the BBB in the various models.
The beneficial effects of IV insulin in the CNS have been extended to human studies. IV application of insulin in humans was able to change cerebrocortical activity in lean subjects (Tschritter et al., 2006). In addition, it has been shown peripheral insulin sensitivity correlates with CSF/serum ratios. That is, the lower the ratio, the more insulin-resistant the subject was, independent of body weight (Heni et al., 2014). Importantly, there is a tight correlation between CSF and serum insulin levels in insulin-sensitive subjects while this correlation is diminished in insulin-resistant subjects suggesting impairment of transport into the brain. A study by Suzanne Craft and colleagues was one of the first to demonstrate that increasing plasma insulin levels improved memory in aged subjects with dementia. In this study the authors showed memory could be enhanced in people with dementia of the Alzheimer’s type by increasing plasma insulin levels via IV infusion while keeping glucose at a fasting level (Craft et al., 1996). Unfortunately, insulin cannot readily be given IV to increase brain insulin levels due to the risk for hypoglycemia unless blood glucose levels are kept constant as done in this study. Therefore, it is important to explore other ways in which to increase CNS insulin levels and limit peripheral side effects. Insulin delivery via intracerebroventricular, intranasal, ocular, and intrathecal routes will be discussed in the following sections.
Intracerebroventricular Administration
Intracerebroventicular (ICV) injections take advantage of delivery of insulin directly to the CSF for distribution of insulin throughout the cranial CSF. Similar injections can be made into specific brain regions, like the hypothalamus or hippocampus, rather than into the ventricle to deliver insulin regionally. ICV insulin reduces food intake and decreases body weight (Woods et al., 1979a). Central administration of insulin antibodies increases body weight (McGowan et al., 1992). The rate of diffusion for peptides in the CNS is extremely limited (Maness et al., 1996). Likewise, substances administered into the CSF have limited diffusion into brain beyond the periventricular region and do not diffuse well into spinal cord regions.
Non-fasted CSF insulin levels in a rat are about 17 μU/mL (Ono et al., 1983), approximately 3 pmol/L in normal, fasted aged humans (Craft et al., 1998), and 3.8 pmol/L in aged humans of unknown fasting state (Geijselaers et al., 2018). ICV infusion over 4.5 hours of 2.5 mU insulin led to over a 20-fold increase in insulin CSF levels (Ono et al., 1983).
Most substances after ICV injection enter the blood stream with the reabsorption of CSF and some have an accelerated efflux because of brain to blood transporters. In contrast to its saturable transport in the blood-to-brain direction, insulin has no saturable component to its brain-to-blood transport. There is no significant effect of excess insulin (10 nM). The half-time disappearance for insulin when delivered ICV is 26.4 min (Cashion et al., 1996). Aluminum (17.8 mg) and starvation (72 h) increases retention while fasting (16 h) and refeeding (fasted 56 h and fed 16 h) had no effect. The impact of starvation is consistent with the role of insulin in the CNS on feeding behavior.
Rats fasted for 30 hours have peak insulin signaling activity in the hypothalamus shown by phosphorylation of the insulin receptor, insulin receptor substrate 1 and 2, and AKT following 10 mU ICV insulin injection, although the amount of insulin present in this region was not measured in this study (Niswender et al., 2003). Aime et al showed a single 14 mU ICV insulin treatment increased olfactory bulb insulin levels in fasted rats from 0.10 ± 0.01 ng/g to 0.24 ± 0.05 ng/g, a level that is similar to satiated rats (0.21 ± 0.03 ng/g) (Aime et al., 2012). While this dose was not enough to alter food intake, it did decrease sniffing behavior in response to food odor. It was estimated the transport of insulin from the ventricle to the olfactory bulb is approximately 10× greater than the amount that reaches the olfactory bulb after intranasal delivery, which will be discussed below in more detail. In line with the impact of CNS insulin on metabolism, ICV insulin decreases motivation for sucrose in rats on a low fat diet but this effect is abolished when rats are fed a high fat diet (Figlewicz et al., 2006). These results suggest insulin can decrease food reward in the CNS. The effects are rapid and acute, occurring within minutes to hours and are not persistent. These data highlight the metabolic effect of insulin in the CNS and the pathways that could be disrupted in an insulin resistant state.
As for the effect of CNS insulin on memory, ICV insulin has been shown to improve consolidation of memory (Park et al., 2000). ICV delivery of 4 mU insulin immediately following training of the passive-avoidance task increased latency when tested 24 h after training. Despite this study being performed nearly two decades ago, there still remain numerous unanswered questions including the time course of the insulin effect and further characterizing the period during which insulin enhances memory consolidation. Another study investigated the memory effect of ICV insulin infusion and neurogenesis in aged rats using the Morris water maze (Haas et al., 2016). While memory was not altered following a 5-day daily ICV insulin infusion of 20 mU in aged (24 mo) rats, insulin signaling and neurogenesis was enhanced. It should be noted memory was improved in the young (4 mo) rats. The discrepancies between these two studies could be due to the cognitive task used or the age of the rats used (age not reported in the Park study). It is interesting that chronic ICV insulin infusion was not able to improve memory in the aged cohort whereas chronic treatment of intranasal insulin does result in memory improvements as discussed next.
Intranasal Administration
Intranasal (INL) administration provides a rapid and noninvasive method for the delivery of insulin to the brain, circumventing the problem of crossing blood-CNS barriers (Lalatsa et al., 2014; Salameh and Banks, 2014) and altering peripheral insulin levels (Nedelcovych et al., 2018; Salameh et al., 2015). This method of delivery occurs rapidly as insulin is detected in the olfactory bulb and brain as early as 2.5 minutes post application in wildtype (Rhea et al., 2017; Salameh et al., 2015) and SAMP8 mice (Rhea et al. 2017). INL insulin delivery is dependent on the insulin receptor (Rhea et al. 2017) unlike transport across the BBB (Rhea et al., 2018). Unlike IV delivery in SAMP8 mice, where insulin is not transported into the hippocampus (Banks et al., 2000), INL delivery of radioactive insulin was detected in all of the brain regions examined, including the hippocampus (Rhea et al., 2017; Salameh et al., 2015). However, similar to IV studies in the Alzheimer’s disease model (Banks et al., 2000), the highest levels were detected in the olfactory bulb. In the brains of wildtype mice, the hypothalamus had the highest amount of insulin present 30 min post INL administration (5.246 ± 0.1184 ng injected/g brain), which was 4× more abundant than the cortex (1.436 ± 0.0248 ng injected/g brain) (Rhea et al. 2017). This is potentially because of the higher density of insulin receptors in the hypothalamus compared to the cortex (Zahniser et al., 1984; Zhao and Alkon, 2001). Also, the same phenomenon occurred in young and aged SAMP8 mice, indicating that insulin transport is not altered in disease states like Alzheimer’s disease (Rhea et al. 2017).
Studies in both human and animal models have confirmed a link between peripheral insulin resistance and cognitive impairment and risks for Alzheimer’s disease (Blazquez et al., 2014; Cholerton et al., 2011; de la Monte and Tong, 2014). INL insulin, when administered to patients with Alzheimer’s disease, mild cognitive impairment, and even those who are cognitively intact, has shown marked improvements in numerous cognitive tests including the delayed story recall and Dementia Severity Rating Scale (Benedict et al., 2004; Craft et al., 2012; Reger et al., 2008a; Reger et al., 2008b). While insulin has been successful in improving cognition in individuals with Alzheimer’s disease and mild cognitive impairment, those who were APOE ε4 positive or female showed poorer recall following INL insulin administration compared to those who did not possess this allele or male (Claxton et al., 2013; Reger et al., 2006). These findings in regard to sex and APOE genotype were also apparent with the use of rapid-acting insulin, which is thought to have superior effects intranasally compared to regular insulin (Rosenbloom et al., 2014). Positron emission tomography (PET) studies of patients prior to and post INL insulin administration demonstrate an increase in 18F-fluorodeoxyglucose in the parietotemporal, frontal, precuneus, and cuneus regions (part of the occipital lobe) of the CNS compared to placebo controls, thus linking the improvement in cognitive function to processes in these brain regions (Craft et al., 2012). Cognitive enhancement has also been observed in animal models of Alzheimer’s disease following INL insulin delivery (Kamei et al., 2017; Mao et al., 2016; Salameh et al., 2015).
Brain insulin sensitivity is an important link between metabolism and cognitive dysfunction. In a recent study examining the acute effect of INL insulin on resting-state brain functional connectivity in lean and obese adults, the investigators found that INL insulin administration resulted in increased functional connectivity in the prefrontal cortex, the hippocampus, and the hypothalamus (Kullmann et al., 2017). In the hippocampus they found that brain connectivity was significantly correlated with visceral adipose tissue, while in the hypothalamus, brain connectivity was significantly correlated with peripheral insulin sensitivity. This demonstrates that brain insulin action may regulate eating behavior and facilitate weight loss through the modification of brain functional connectivity in regions involved in cognition and homeostatic function. Similar to the review on ICV insulin administration, these studies highlight the dual benefit of administering insulin to the insulin resistant CNS.
Ocular Administration
The eye poses unique opportunities and challenges when it comes to the delivery of pharmaceutics. Similar to INL administration, ocular delivery is a noninvasive delivery method. Studies originally done on the ocular delivery of insulin were performed as an alternative to insulin injections to regulate blood glucose levels and so were targeting delivery to the blood and not directly to the brain. For ocular absorption to occur, enhancers are required. Many agents such as saponin, EDTA and hyaluronate, and even conventional preservative agents (i.e. benzalkonium chloride and paraben), were shown to enhance insulin absorption (Hoffman and Ziv, 1997). In the absence of enhancers, ocular administration of insulin to rabbits showed no change in blood glucose levels (Srinivasan and Jain, 1998). Since the cornea is relatively impermeable (Prausnitz and Noonan, 1998), the bioavailability of ocular insulin is low (5–10%) compared to the availability following subcutaneous insulin (65%) (Goeders et al., 1987; Lee, 1990; Pillion et al., 1994). Therefore, even with enhancing agents, it was deemed the bioavailability was too low to affect blood glucose and this method was abandoned as a therapeutic alternative to insulin injections. However, the impact on CNS insulin levels in these studies was not measured.
In a more recent study using female Lewis rats, the accessibility of ocular applied insulin to the CSF and select brain regions was assessed. Insulin was administered as an eye drop by reconstituting it at a concentration of 0.75% in an isotonic bicarbonate buffer containing 0.5% of the permeation enhancer, polyoxyethylene 20 stearyl ether (Koevary et al., 2003). In this study, insulin was detected in the CSF and serum by 10 min post ocular insulin administration, with CSF levels dropping by 20–30 min before increasing again at 45 min. The highest levels were detected 10 min post application in the thalamus, specifically the medial geniculate nucleus (0.24 ± 0.06 pg insulin/μg protein) compared to the lateral geniculate nucleus (0.13 ± 0.03 pg insulin/μg protein) and the cortex (0.13 ± 0.04 pg insulin/μg protein). In comparison to subcutaneous insulin administration that would ultimately enter these brain regions by crossing the BBB, more insulin reached the lateral geniculate nucleus following ocular administration. Expectedly, the same was not true in the serum where levels were greater post subcutaneous administration (Koevary et al., 2003). Ten minutes following ocular administration, serum insulin levels increased nearly 6 fold over baseline. Due to the dual peak in CSF levels of insulin following ocular delivery, the authors theorize the first 10 min peak is from direct uptake of insulin into the CSF via the optic nerve while the second peak is from insulin transport across the BBB after entering the circulation via drainage through the nasal mucosa.
Little work has been done on the ocular route for brain delivery, and so it is noteworthy that this has route has been shown to be viable for at least one other peptide. Ocular administration of the neuroprotective peptide, PACAP-38, delivered in the enhancer benzalkonium chloride, has been used to investigate the benefits in retinal ischemia (Werling et al., 2016). In this study, radiolabeled PACAP-38 not only appeared in the retina but also in the whole brain. PACAP-38 appeared in the brain within 5 minutes and remained present over 2 hours. While little has been shown with ocular administration of peptides, this study provides insight on the beneficial effect in diseased conditions.
Likely due to the poor penetration of insulin into the CNS and increased systemic delivery following ocular delivery, this method has not been explored further, especially in the context of memory or in the use of Alzheimer’s disease models. However, with the continued research of enhancers in ocular delivery, this route could provide a promising avenue for increasing CNS insulin levels and overcoming CNS insulin resistance.
Intrathecal Administration
Intrathecal (IT) delivery involves a direct injection into the spinal canal or into the subarachnoid space so that it reaches the CSF, similar to ICV delivery. In general, the most common reasons for this particular administration is for spinal anesthesia, chemotherapy, and pain management. This delivery method is often overlooked because of its inability to deliver small, lipid-soluble drugs to the CNS. However, it has been shown the anorexogenic hormone leptin reaches the hypothalamus in baboons by 115 min following IT delivery (McCarthy et al., 2002). The biologically active concentration is estimated to be 8 ng/mL, which is 40 times greater than CSF levels of normal weight humans. In regards to delivering substrates such as leptin, the IT route of administration can rapidly achieve therapeutic and long-lasting levels. IT delivery has been quite successful in the transport of enzymes such as therapeutics for lysosomal storage diseases (Calias et al., 2012; Xu et al., 2011), the delivery of trastuzumab to the CNS in patients with meningeal carcinomatosis from breast cancer (Hofer et al., 2012; Mego et al., 2011), antisense delivery for spinal muscular atrophy type 1 (Aragon-Gawinska et al., 2018), and cyclodextrin delivery for Niemann-Pick disease (Calias, 2017). These large proteins, unlike small lipid soluble molecules, do not enter the blood by transcellular diffusion and so are “trapped” in the CSF space and can eventually make their way to the cranial CSF.
An advantage of this technique that can be extended to the other delivery methods includes that, while the CSF contains hundreds of proteins, it may have decreased enzymatic activity than in the serum leading to less degradation. Some disadvantages of using IT administration that can also be extended to other delivery methods, especially the ICV route, is the development of antibodies and the inability to accurately determine dosing due to CSF reabsorption rate variability among individuals. In addition, disease conditions such as obesity and the rapid growth rate of children can complicate proper catheter insertion (Follett et al., 2003).
While the distribution of insulin throughout the brain has not been measured following IT delivery, there are studies showing a beneficial effect with minimal side effects with this delivery method. IT insulin administration has been shown to rescue and regenerate sural nerves after crush injury. This indicates a direct neurotrophic effect of insulin in peripheral nerves (Toth et al., 2006). Also, systemic insulin injection enhances reinnervation of sciatic nerve transection (Xu et al., 2004). IT insulin has been used in the treatment of diabetic neuropathy, a condition with distal-to-proximal loss of nerve function (Grote and Wright, 2016).
While not direct delivery of insulin to the CNS via IT administration, a recent paper showed intracranial grafting of pancreatic islets was able to attenuate cognitive decline and peripheral metabolic dysfunction in an Alzheimer’s disease-like dementia animal model (ICV-STZ) (Bloch et al., 2018). ICV-STZ is able to induce sporadic Alzheimer’s disease-like dementia (Chen et al., 2013). In the study by Bloch et al, a small number of pancreatic islets were grafted into the cranial subarachnoid space of male Lewis rats (Bloch et al., 2018). Spatial learning and memory and long-term retention was assessed two months after islet transplantation by the Morris water maze. Islets were still viable and producing insulin two months later at the time of sacrifice leading to significantly increased CNS insulin levels especially in the hippocampus and frontal cortex (Bloch et al., 2015; Bloch et al., 2018). These improvements occurred without changes in peripheral fasting blood glucose and expression levels of the insulin receptor, suggesting post-insulin receptor alterations for memory enhancement.
Discussion
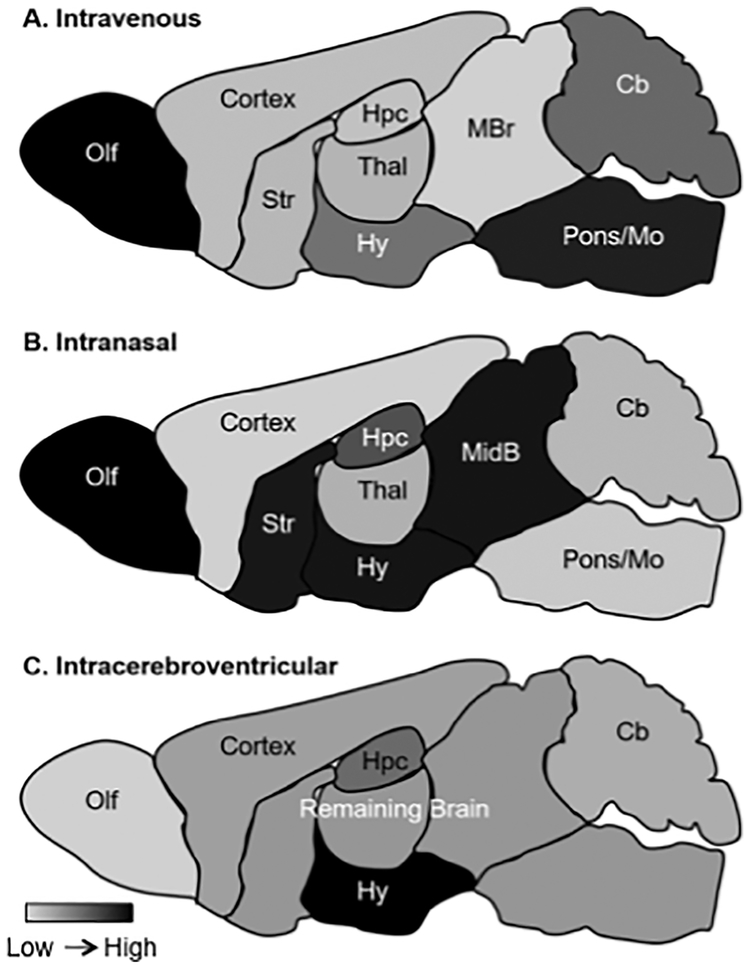
In this review, we have focused on administration techniques that can increase CNS insulin to overcome CNS insulin resistance. Where data was available, mostly in pre-clinical models, we reported how much insulin was delivered to the brain, and in some cases brain regions, following insulin administration to compare across the delivery methods (Figure 2, Table 2). While all delivery methods have advantages and disadvantages, some routes are currently more translatable to human investigations. For example, intranasal delivery of insulin has shown benefit in both young, cognitively intact individuals and AD patients.
Figure 2.
Relative distribution of insulin after various routes of administration. Black is the highest concentration and light gray the least.
Table 2.
Diseases and conditions known to exhibit decreased CNS insulin or insulin receptor levels.
| Condition/Disease | Prevalence in US | Decreased CNS Insulin/Receptor Levels |
|---|---|---|
| Alzheimer’s disease | 5.7 million | Craft et al Neurology 1998; Frolich 1998 |
| Aging (65+) | 46 million | Frolich et al 1998 |
| Pregnancy | 4 million live births per year | (Azar and Brooks, 2011; Daubert et al., 2007) |
| Fasting/Hibernation | N/A | (Florant et al., 1991) |
| Obesity | 160 million | (Kaiyala et al., 2000; Stein et al., 1987) |
| Peripheral Insulin Resistance | 100 million | Heni et al 2014 |
The greatest difference among the methods, even if they were all working optimally, is that they have different distribution patterns throughout the brain and enter the blood at different rates. For example, all those methods that deliver insulin to the CSF, most especially ICV and IT, also deliver insulin to the blood stream as the CSF is reabsorbed. The olfactory bulb is the area of greatest uptake after IV or INL administration, whereas it is presumed that ocular will favor the retina and IT the spinal cord.
Given these differences in CNS distribution as a function of route of administration, it may be that the optimal route for delivery of insulin to the brain may depend on the underlying disease being addressed. Would, for example, insulin with its mitogenic and vascular effects if given by the ocular route to treat the cognitive effects of diabetes have a positive or a negative effect on diabetic retinopathy?
Likewise, the different distributions will likely determine the “off target” effects of a cognitive dose of insulin. CNS insulin has been implicated in feeding (Woods et al., 1979b) and hepatic glucose production (Obici et al., 2002). As INL delivery produces hypothalamic levels that are relatively high in comparison to other brain regions, will this route have a disproportionately high impact on metabolism compared to other routes?
Much research is to be done to better understand the distribution and sequestration of insulin in the brain. While touched on but not thoroughly discussed, the transport paths of insulin that determine brain distribution following the various routes of delivery have not been well evaluated. The transport pathway for INL insulin has probably been most extensively studied and has been shown to occur through at least three routes involving the olfactory and/or trigeminal nerve, similar to the process of insulin-like growth factor 1 (Lochhead and Thorne, 2012). In addition to better understanding the distribution transport processes for insulin, finding ways to improve these processes will be of great value to better increase the absolute levels and efficacy of CNS insulin. This will ultimately allow us to more efficiently deliver insulin to the CNS, especially in insulin resistant states.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aime P, et al. , 2012. A physiological increase of insulin in the olfactory bulb decreases detection of a learned aversive odor and abolishes food odor-induced sniffing behavior in rats. PLoS One. 7, e51227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon-Gawinska K, et al. , 2018. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: A cohort study. Neurology. 91, e1313–e13118. [DOI] [PubMed] [Google Scholar]
- Arnold SE, et al. , 2018. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 14, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar AS, Brooks VL, 2011. Impaired baroreflex gain during pregnancy in concscious rats: role of brain insulin. Hypertension. 57, 283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, et al. , 1997a. Transport of insulin across the blood-brain barrier: Saturability at euglycemic doses of insulin. Peptides. 18, 1423–1429. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ, 1997b. Selective, physiological transport of insulin across the blood-brain barrier: Novel demonstration by species-specific enzyme immunoassays. Peptides. 18, 1257–1262. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, 1998. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 19, 883–9. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Pan W, 1999. Uptake and degradation of blood-borne insulin by the olfactory bulb. Peptides. 20, 373–8. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE, 2000. Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. J Gerontol A Biol Sci Med Sci. 55, B601–6. [DOI] [PubMed] [Google Scholar]
- Banks WA, Owen JB, Erickson MA, 2012. Insulin in the brain: there and back again. Pharmacol Ther. 136, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A, et al. , 2009. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 30, 586–623. [DOI] [PubMed] [Google Scholar]
- Benedict C, et al. , 2004. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 29, 1326–34. [DOI] [PubMed] [Google Scholar]
- Blazquez E, et al. , 2014. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne). 5, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K, et al. , 2015. Intracranial pancreatic islet transplantation increases islet hormone expression in the rat brain and attenuates behavioral dysfunctions induced by MK-801 (dizocilpine). Horm Behav. 72, 1–11. [DOI] [PubMed] [Google Scholar]
- Bloch K, et al. , 2018. Intracranial Transplantation of Pancreatic Islets Attenuates Cognitive and Peripheral Metabolic Dysfunctions in a Rat Model of Sporadic Alzheimer’s Disease. J Alzheimers Dis. [DOI] [PubMed] [Google Scholar]
- Calias P, et al. , 2012. CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS One. 7, e30341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calias P, 2017. 2-Hydroxypropyl-Beta-cyclodextrins and the blood-brain barrier: Considerations for Niemann-Pick disease type C1. Curr Pharm Des. 23, 6231–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion MF, Banks WA, Kastin AJ, 1996. Sequestration of centrally administered insulin by the brain: effects of starvation, aluminum, and TNF-alpha. Horm Behav. 30, 280–6. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. , 2013. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse). Mol Neurobiol. 47, 711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholerton B, Baker LD, Craft S, 2011. Insulin resistance and pathological brain ageing. Diabet Med. 28, 1463–75. [DOI] [PubMed] [Google Scholar]
- Claxton A, et al. , 2013. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis. 35, 789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, et al. , 1996. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 17, 123–30. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. , 1998. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 50, 164–8. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. , 2012. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 69, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert DL, Chung M-Y, Brooks VL, 2007. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol. 292, R2188–R2195. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, 2014. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol. 88, 548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, et al. , 2006. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 89, 611–6. [DOI] [PubMed] [Google Scholar]
- Florant GL, et al. , 1991. Seasonal changes in CSF insulin levels in marmots: insulin may not be a satiety signal for fasting in winter. American Journal of Physiology. 260, R712–R716. [DOI] [PubMed] [Google Scholar]
- Follett KA, et al. , 2003. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 6, 32–41. [DOI] [PubMed] [Google Scholar]
- Frolich L, et al. , 1998. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna). 105, 423–38. [DOI] [PubMed] [Google Scholar]
- Geijselaers SLC, et al. , 2018. Association of Cerebrospinal Fluid (CSF) Insulin with Cognitive Performance and CSF Biomarkers of Alzheimer’s Disease. J Alzheimers Dis. 61, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders LA, Esposito LA, Peterson ME, 1987. Absorption kinetics of regular and isophane (NPH) insulin in the normal dog. Domest Anim Endocrinol. 4, 43–50. [DOI] [PubMed] [Google Scholar]
- Grote CW, Wright DE, 2016. A role for insulin in diabetic neuropathy. Front Neurosci. 10, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas CB, et al. , 2016. Brain Insulin Administration Triggers Distinct Cognitive and Neurotrophic Responses in Young and Aged Rats. Mol Neurobiol. 53, 5807–5817. [DOI] [PubMed] [Google Scholar]
- Heni M, et al. , 2014. Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol. 51, 679–81. [DOI] [PubMed] [Google Scholar]
- Hofer S, et al. , 2012. Intrathecal trastuzumab: dose matters. Acta Oncol. 51, 955–6. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Ziv E, 1997. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin Pharmacokinet. 33, 285–301. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, et al. , 2000. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 49, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Kamei N, et al. , 2017. Effect of an Enhanced Nose-to-Brain Delivery of Insulin on Mild and Progressive Memory Loss in the Senescence-Accelerated Mouse. Mol Pharm. 14, 916–927. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, 2001. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology. 73, 237–42. [DOI] [PubMed] [Google Scholar]
- Koevary SB, et al. , 2003. Accumulation of porcine insulin in the rat brain and cerebrospinal fluid following ocular application. J Ocul Pharmacol Ther. 19, 377–84. [DOI] [PubMed] [Google Scholar]
- Kondo T, et al. , 2004. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood-brain barrier. Biochem Biophys Res Commun. 317, 315–20. [DOI] [PubMed] [Google Scholar]
- Kullmann S, et al. , 2017. Intranasal insulin enhances brain functional connectivity mediating the relationship between adiposity and subjective feeling of hunger. Sci Rep. 7, 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalatsa A, Schatzlein AG, Uchegbu IF, 2014. Strategies to deliver peptide drugs to the brain. Mol Pharm. 11, 1081–93. [DOI] [PubMed] [Google Scholar]
- Lee VH, 1990. New directions in the optimization of ocular drug delivery. J Ocul Pharmacol. 6, 157–64. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. , 2009. Insulin regulates P-glycoprotein in rat brain microvessel endothelial cells via an insulin receptor-mediated PKC/NF-kappaB pathway but not a PI3K/Akt pathway. Eur J Pharmacol. 602, 277–82. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG, 2012. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 64, 614–628. [DOI] [PubMed] [Google Scholar]
- Maness LM, et al. , 1996. Periventricular penetration and disappearance of ICV Tyr-MIF-1, DAMGO, tyrosine, and albumin. Peptides. 17, 247–50. [DOI] [PubMed] [Google Scholar]
- Mao YF, et al. , 2016. Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell. 15, 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AA, et al. , 2016. CCK increases the transport of insulin into the brain. Physiol Behav. 165, 392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TJ, et al. , 2002. Positron emission tomography shows that intrathecal leptin reaches the hypothalamus in baboons. Journal of Pharmacology and Experimental Therapeutics. 307, 878–883. [DOI] [PubMed] [Google Scholar]
- McGowan MK, Andrews KM, Grossman SP, 1992. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav. 51, 753–66. [DOI] [PubMed] [Google Scholar]
- Mego M, et al. , 2011. Intrathecal administration of trastuzumab with cytarabine and methotrexate in breast cancer patients with leptomeningeal carcinomatosis. Breast. 20, 478–80. [DOI] [PubMed] [Google Scholar]
- Nedelcovych MT, et al. , 2018. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem Neurosci. 9, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, et al. , 2003. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 52, 227–31. [DOI] [PubMed] [Google Scholar]
- Obici S, et al. , 2002. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 5, 566–572. [DOI] [PubMed] [Google Scholar]
- Ono T, Steffens AB, Sasaki K, 1983. Influence of peripheral and intracerebroventricular glucose and insulin infusions on peripheral and cerebrospinal fluid glucose and insulin levels. Physiol Behav. 30, 301–6. [DOI] [PubMed] [Google Scholar]
- Park CR, et al. , 2000. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 68, 509–14. [DOI] [PubMed] [Google Scholar]
- Pillion DJ, et al. , 1994. Alkylglycosides enhance systemic absorption of insulin applied topically to the rat eye. J Pharmacol Exp Ther. 271, 1274–80. [PubMed] [Google Scholar]
- Poduslo JF, et al. , 2001. Permeability of proteins at the blood-brain barrier in the normal adult mouse and double transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 8, 555–67. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Noonan JS, 1998. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 87, 1479–88. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. , 2006. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 27, 451–8. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. , 2008a. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 13, 323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, et al. , 2008b. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 70, 440–8. [DOI] [PubMed] [Google Scholar]
- Rhea EM, et al. , 2017. Intranasal Insulin Transport is Preserved in Aged SAMP8 Mice and is Altered by Albumin and Insulin Receptor Inhibition. J Alzheimers Dis. 57, 241–252. [DOI] [PubMed] [Google Scholar]
- Rhea EM, Rask-Madsen C, Banks WA, 2018. Insulin transport across the blood-brain barrier can occur independently of the insulin receptor. J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, et al. , 2014. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs. 28, 1185–9. [DOI] [PubMed] [Google Scholar]
- Salameh TS, Banks WA, 2014. Delivery of therapeutic peptides and proteins to the CNS. Adv Pharmacol. 71, 277–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh TS, et al. , 2015. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. J Alzheimers Dis. 47, 715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, et al. , 1991. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest. 88, 1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Jain SK, 1998. Insulin delivery through the ocular route. Drug Deliv. 5, 53–5. [DOI] [PubMed] [Google Scholar]
- Stein LJ, et al. , 1987. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of Obese Zucker rats. Endocrinology. 121, 1611–1615. [DOI] [PubMed] [Google Scholar]
- Stockhorst U, et al. , 2004. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav. 83, 47–54. [DOI] [PubMed] [Google Scholar]
- Sun YN, et al. , 2015. Effects of insulin combined with idebenone on blood-brain barrier permeability in diabetic rats. J Neurosci Res. 93, 666–77. [DOI] [PubMed] [Google Scholar]
- Swaminathan SK, et al. , 2018. Insulin differentially affects the distribution kinetics of amyloid beta 40 and 42 in plasma and brain. J Cereb Blood Flow Metab. 38, 904–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliamonte A, et al. , 1976. Possible role of insulin in the transport of tyrosine and tryptophan from blood to brain. Adv Exp Med Biol. 69, 89–94. [DOI] [PubMed] [Google Scholar]
- Toth C, et al. , 2006. Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience. 139, 429–49. [DOI] [PubMed] [Google Scholar]
- Tschritter O, et al. , 2006. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci U S A. 103, 12103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Banks WA, 2008. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 149, 3592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling D, et al. , 2016. Ocular delivery of PACAP1–27 protects the retina from ischemic damage in rodents. Invest Ophthalmol Vis Sci. 57, 6683–6691. [DOI] [PubMed] [Google Scholar]
- Woods SC, et al. , 1979a. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 282, 503–5. [DOI] [PubMed] [Google Scholar]
- Woods SC, et al. , 1979b. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 282, 503–505. [DOI] [PubMed] [Google Scholar]
- Xu QG, et al. , 2004. Insulin as an in vivo growth factor. Exp Neurol. 188, 43–51. [DOI] [PubMed] [Google Scholar]
- Xu S, et al. , 2011. Large-volume intrathecal enzyme delivery increases survival of a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 19, 1842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, et al. , 1984. Characterization and regulation of insulin receptors in rat brain. J Neurochem. 42, 1354–62. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL, 2001. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 177, 125–34. [DOI] [PubMed] [Google Scholar]