Abstract
Objective:
Despite considerable evidence that greater exposure to discrimination over the life course increases risk for systemic inflammation, little is known about the mechanisms responsible for this association. Here we examine the role of global sleep quality as a potential pathway by which self-reported experiences of discrimination contribute to inflammatory dysfunction in a multiethnic sample of middle-aged adults.
Methods:
Participants were 300 adults (36-85 years; 65% women) from Milwaukee, Wisconsin, a subset of the Midlife in the United States Study II (2004-2006). Racial/ethnic representation included African American (77.7%), Hispanic (12.7%), Asian/Pacific Islander (5.6%), and Native American (4.0%). Global sleep quality and perceptions of lifetime and daily discrimination were measured by questionnaire. A composite score of inflammation burden was computed as the sum of five makers including C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, E-selectin, and intracellular adhesion molecule-1 (ICAM-1).
Results:
Greater lifetime exposure to discrimination was associated with higher inflammation burden. This relationship remained significant after adjustments for potential confounding factors, including demographics, medication use, health behaviors, psychological distress, and daily discrimination. Mediation analyses suggested that poor global sleep quality was a key mechanism underlying the link between lifetime discrimination and inflammation burden.
Conclusion:
These results add to a growing literature on the effects of bias and unfair treatment experienced by people of color and other marginalized groups by demonstrating how such experiences may be particularly consequential for sleep and physiological functioning in midlife.
Keywords: biological processes, inflammation, lifetime discrimination, sleep, unfair treatment
Systemic inflammation is a key risk factor for early morbidity and mortality, and perceived discrimination has been suggested as a significant correlate of inflammation. Across clinical and population-based samples, heightened inflammatory responses have been shown to contribute to poor health outcomes (e.g., atherosclerosis, Type II diabetes, rheumatoid disease, osteoporosis) and elicit a number of pathogenic processes (e.g., oxidative stress, insulin resistance, plaque rupture, endothelial pathology) that play a major role in the risk of premature mortality (Cesari, Penninx, Newman et al., 2003; Epel & Lithgow, 2014; Miller, Chen, & Parker, 2011; Schneiderman, Ironson, & Siegal, 2008).
Among the limited studies considering an association between self-reported experiences of discrimination and inflammation, evidence is mixed. For example, positive associations between discrimination and inflammation have been reported in studies of young adults (Cunningham, Seeman, Kawachi et al., 2012), midlife adults (Stepanikova, Bateman, & Oates, 2017), low-income African American youth (Goosby, Malone, Richardson, Cheadle, & Williams, 2015), and older African Americans (Lewis, Aiello, Leurgans, Kelly, & Barnes, 2010). In other studies, significant associations between discrimination and inflammation were limited to specific inflammatory markers (Kershaw, Lewis, Diez Roux et al., 2016) and participant subgroups, including Caucasian men (Friedman, Williams, Singer, & Ryff, 2009), African American women reporting high levels of anticipatory racism threat (Nuru-Jeter, Chae, Price et al., 2013), and non-obese women (Beatty Moody, Brown, Matthews, & Bromberger, 2014). Finally, using data from the Dallas Heart Study, Albert et al (2008) found no relationship between reports of discrimination and inflammation among non-Hispanic black, non-Hispanic white, and Hispanic adults.
The mixed findings in the literature may reflect differences in the measure of discrimination used across studies, with some studies using lifetime discrimination, some using everyday discrimination, and some using a combination of these measures. Whereas measures of lifetime discrimination capture the accumulation of acute exposure to major experiences of discrimination across a variety of life domains such as being unfairly denied a promotion or being unfairly prevented from moving into a neighborhood, measures of everyday discrimination capture the range of chronic exposure to day-to-day experiences of discrimination such as being followed around in stores or being treated with less courtesy or respect than others (Pascoe & Smart Richman, 2009; Williams & Mohammed, 2009). Relative to nonminority racial groups, racial/ethnic minorities consistently report more experiences of major and everyday discrimination at every level of age, gender, and socio-economic status (Barnes, De Leon, Wilson et al., 2004; Forman, Williams, & Jackson, 1997; Kessler, Mickelson, & Williams, 1999; Lewis, Yang, Jacobs, & Fitchett, 2012). It is therefore no surprise that the literature examining the adverse effects of acute and chronic discrimination on health has predominately focused on members of historically marginalized groups in the United States, including racial/ethnic minorities.
Additionally, much of the research to date has focused on individual physiological indicators or preclinical endpoints of poor health. Given that the effects of psychosocial stress are typically nonspecific (Segerstrom & Miller, 2004), studies that assess only one or a few individual biomarkers cannot adequately capture the cumulative impact of acute and chronic exposure to discrimination. In comparison, a multisystemic biomarker approach is consistent with evidence that many people, particularly at later ages, suffer from multiple, co-occurring chronic conditions which are likely to contribute to increased risks for morbidity and mortality (Yancik, Ershler, Satariano et al., 2007). This accumulative process is also captured in life course risk models (Gee, Walsemann, & Brondolo, 2012; Lynch & Smith, 2005) and theories of weathering (Geronimus, Hicken, Keene, & Bound, 2006), which posit that exposure to continuous and repeated stressors over long spans of time increases vulnerability to disease-related outcomes in later life. Overall, studies examining links between reports of discrimination and systemic components of inflammation in racial/ethnic minority populations are critically lacking.
In addition to direct associations between discrimination and inflammation, the extant evidence suggests the possibility of indirect behavioral links from discrimination to inflammation. Specifically, several investigators have suggested that suboptimal sleep may represent a pathway through which discrimination affects mental and physical health (Hale & Do, 2007; Slopen & Williams, 2014; Tomfohr, Pung, Edwards, & Dimsdale, 2012). Chronic deficits in fundamental aspects of sleep—including sleep efficiency (i.e., initiating and maintaining sleep) and sleep quality (i.e., feeling rested and restored upon waking)—can have profound health effects that contribute to increased risks for central adiposity (Lewis, Kravitz, Janssen, & L., 2011), obesity (Knutson & Van Cauter, 2008), diabetes (Cappuccio, D’Elia, & Miller, 2010), hypertension (Gottlieb, Redline, Nieto et al., 2006), cardiometabolic risk (Curtis, Fuller-Rowell, El-Sheikh, Carnethon, & Ryff, 2017), heart disease (Phillips & Mannino, 2007), and mortality (Li, Zhang, Winkelman et al., 2014). Importantly, a recent systematic review of data from 17 studies found robust evidence that discrimination was associated with poorer sleep outcomes (Slopen, Lewis, & Williams, 2016). Moreover, increasing evidence suggests that diminished sleep salubrity plays a major role in the cellular expression of inflammatory cytokines (for a review, see Irwin, Carrillo, & Olmstead, 2010; Irwin, Olmstead, Ganz, & Haque, 2013). Taken together, these lines of inquiry suggest that sleep may be a key mechanism linking discrimination to inflammatory dysfunction. We tested this hypothesis in a multiethnic sample of middle-aged adults.
The current analyses extend prior studies of the association between discrimination and inflammation in four ways. First, this study builds on prior work by examining the relationship between perceptions of discrimination and inflammatory dysregulation in a racially diverse sample of middle-aged adults. Midlife may be an important point in the life span for examining these processes, because it ushers in a period of markedly rising risk for acute and chronic illness (House, Lantz, & Herd, 2005; Karlamangla, Singer, & Seeman, 2006). As such, there is a need to explore biological stress mechanisms among middle-aged racial/ethnic minorities, a population particularly at risk for a broad-spectrum of stress-related disorders, including diabetes, cardiovascular disease, hypertension, and obesity (Krieger, 1990; Kurian & Cardarelli, 2007; Williams & Collins, 1995). Second, the current study considers the role of confounding variables. Lewis et al. (2015) recently called for more systematic research on the role of psychological distress symptoms as a potential confounder in studies of discrimination and health. Moreover, increasing age, female sex, low socioeconomic status, medication use, and unhealthy behaviors represent key variables that may confound associations of discrimination with sleep and inflammation (Friedman, Christ, & Mroczek, 2015; Friedman, Hayney, Love et al., 2005; Friedman & Herd, 2010; Friedman, et al., 2009; Matthews, Zheng, Kravitz et al., 2010). To minimize confounding, we controlled for demographics (age, gender, education level), medication use (antihypertensive, cholesterol lowering, steroid, and antidepressant medications) health behaviors (smoking and alcohol use), and psychological distress (depression and anxiety symptomatology) in all analyses. Third, we examined the relationship between acute and chronic exposure to discrimination and inflammation. Based on prior work, we hypothesized that perceptions of lifetime and everyday discrimination would be equally predictive of inflammatory dysregulation (Friedman, et al., 2009; Kershaw, et al., 2016). Finally, we explored the extent to which the overall stress burden imposed by discrimination gives rise to poor global sleep quality which, in turn, impacts inflammatory risk. Based on previous research showing gender and age differences in the relations among discrimination, sleep, and inflammation (Cunningham, et al., 2012; Friedman, et al., 2009; Owens, Hunte, Sterkel, Johnson, & Johnson-Lawrence, 2017), we tested whether age and gender moderated the indirect effect of discrimination on inflammation.
Methods
Data and Analytic Sample
Data are from a multiethnic sample of middle-aged adults collected in Milwaukee County, Wisconsin as part of a supplement to the Midlife in the United States (MIDUS II, 2004-2006) study (Brim, Ryff, & Kessler, 2004). Survey data were collected via a computer-assisted personal interview (CAPI) protocol and with subsequent, mailed self-administered questionnaires. Additional details about the sampling procedure are described elsewhere (Ryff, Almeida, Ayanian et al., 2008a, 2008b). Respondents from the MIDUS II and Milwaukee samples were eligible to participate in the Biomarker Study if they participated in the telephone (or home interview for Milwaukee) and mail surveys, and lived in the continental United States.
The analytic sample for the current study consisted of 300 adults (65% women) aged 36-85 years at MIDUS II who participated in the Biomarker Study (Love, Seeman, Weinstein, & Ryff, 2010). Ethnic representation included African American (77.7%), Hispanic (12.7%), Asian/Pacific Islander (5.6%), and Native American (4.0%). Biomarker data were collected during an overnight visit at a regional medical center in Madison, WI between 2004 and 2009. Study participants provided a complete medical history, underwent a physical examination, and provided blood, urine, and saliva samples, along with cardiovascular and heart rate variability measurements. Fasting blood was collected at 07:00 h (before caffeine or nicotine consumption). Urine was collected during a 12-hr (19:00 h to 07:00 h) overnight stay (for details, see Love, et al., 2010). Data collection for the MIDUS, Milwaukee, and biomarker studies were approved by Institutional Review Boards at each participating site, and all participants provided informed consent.
Measures
Lifetime discrimination.
Reports of lifetime occurrences of discrimination were assessed across 11 settings that included academics (discouraged from continuing education, denied scholarship), employment (not hired or promoted, fired), financial services (denied a ban loan, prevented from renting or buying a home, given inferior service), and experiences of social hostility (forced out of a neighborhood, hassled by the police) (Kessler, et al., 1999). Respondents indicated how many times they experienced each event “because of such things as your race, ethnicity, gender, age, religion, physical appearance, sexual orientation, or other characteristics.” Due to high skewness in the data, we calculated a summary index of lifetime discrimination by recoding responses into 3 categories (none, 1-2 instances, 3 or more instances), similar to previous MIDUS studies of discrimination (Friedman, et al., 2009; Mays & Cochran, 2001).
Everyday discrimination.
Perceptions of everyday discrimination were assessed with the 9-item Detroit Area Study Everyday Discrimination Scale (Williams, Yu, Jackson, & Anderson, 1997). Respondents reported on the frequency of various forms of interpersonal unfair treatment in their daily lives. Items included: Being treated with less courtesy or respect than others; receiving poorer service than others at restaurants or stores; being called names, insulted, threatened, or harassed; having people act afraid of the respondent; having people act as if the respondent was dishonest, not smart, or not as good as they were. The frequency of each type of daily discrimination was assessed using a 4-point scale (1 = never; 2 = rarely; 3 = sometimes; 4 = often). Responses were averaged to form a summary score of everyday discrimination (Cronbach α’s for the 9-item index was .93).
Global sleep quality.
Subjective global sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI), a widely used and well-known survey instrument intended to measure sleep quality over the previous month. It consists of 19 items used to form 7 component scores: subjective sleep quality, sleep latency, sleep duration, habitual SE, sleep dysfunction, use of sleeping meds, and daytime dysfunction. Scores are coded and summed into a global score with a possible range of 0-21 (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Higher scores are indicative of greater overall sleep difficulty. The internal consistency of the global PSQI in the current sample was 0.71.
Inflammation burden.
Following previous work (Glei, Goldman, Shkolnikov et al., 2013; Kang & Marks, 2014; Yang, Gerken, Schorpp, Boen, & Harris, 2017), quartile values for items assessing inflammation were computed to create dichotomous variables for each item, where 1 = high risk quartile (i.e., high risk = being in the highest quarter of the distribution for IL-6, CRP, E-selectin, ICAM-1, and fibrinogen) and 0 = otherwise. A composite score of inflammation burden, designed to summarize dysregulation across multiple inflammatory markers, was computed by summing five of the dichotomous inflammatory markers: IL-6, CRP, E-selectin, ICAM-1, and fibrinogen.”
Covariates.
Socio-demographic, medical, health behavior, and psychosocial covariates were selected based on their potential for confounding the associations between discrimination and inflammation. Socio-demographic covariates included age (in years), gender, and educational attainment (continuous, using categories: 1 = no school/some grade school (1–6) to 12 = PhD, EdD, MD, DDS, LLD, JD, or other professional degree). Medical covariates included use of antihypertensive, cholesterol lowering, steroid, and antidepressant medications to lower clinical risk. Health behavior covariates included smoking status (coded as non-smoker, ex-smoker, or current smoker) and the presence of alcohol problems, as assessed by a five-item modified version of the Michigan Alcohol Screening Test (MAST; Selzer, 1971). Responses on the MAST were summed and then dichotomized (0 = no alcohol problems, 1 = otherwise).
Psychosocial covariates included depression and anxiety symptomatology, assessed in the Biomarker Study via the general distress depressive symptoms (12 items) and anxious arousal (11 items) subscales from the Mood and Anxiety Questionnaire (MAS-Q; Clark & Watson, 1991). The items assess distress symptoms commonly associated with depression (e.g., “Felt discouraged”; α = .91) and anxiety (e.g., “Was unable to relax”; α = .87). Responses are based on a 5-point Likert-like scale (1 = not at all and 5 = extremely).
Statistical Analyses
Regression analyses were used to examine the association between discrimination and inflammation burden, adjusting for the effects of covariates described above. Multiple imputation procedures were used to impute missing values on covariates (Graham, 2009; Royston, 2005). We imputed missing data using a fully conditional model, the Markov chain Monte Carlo (MCMC) approach. The primary advantage is that both continuous but also categorical variables can be imputed using either linear or logistic regression, respectively. The quality of the imputation was evaluated by examining trace plots. The plots indicated successful convergence of the MCMC algorithm for the mean and variance of all imputed variables. In total, we imputed 5 data sets and pooled parameter estimates and standard errors across imputations, following Rubin’s rules (1987).
The relationship between discrimination and inflammation burden was examined using a series of multivariate-adjusted models. Five models were fitted in all. In the base model, inflammation burden scores were regressed on discrimination measures (lifetime and everyday), omitting any covariates. This model provides a comparison of results obtained using multiple predictors to those obtained from simpler, univariate analyses in which the outcome is regressed separately on each predictor variable (Cohen, West, & Aiken, 2003, p. 425). Model 1 included socio-demographic factors (age, gender, education). Model 2 added medical covariates (antihypertensive, cholesterol lowering, steroid, and antidepressant medications). Health behaviors (smoking, alcohol problems) were added in Model 3, and psychosocial factors (depression and anxiety) were included in Model 4. Finally, we tested whether global sleep quality mediated the association between discrimination and inflammation burden by estimating bootstrap confidence intervals for the indirect association (Preacher & Hayes, 2008). All results are presented as unstandardized regression coefficients (B) with 95% confidence intervals (CIs) based on pooled parameter estimates and standard errors across five imputed data sets. Values of R2 are based on the application of Rubin’s rules (1987) after Fisher’s Z transformation following Harel (2009).
Results
Descriptive Statistics
Descriptive data are presented in Tables 1 and 2. Respondents were on average 53.9 years (SD = 10.5) of age and 65% female at the second MIDUS wave. The majority of respondents (58.7%) had some college education or at least a bachelor’s degree. On average, level of inflammatory risk was moderate in the sample (M = 2.03, SD = 1.43; observed range 0 - 5). Approximately a quarter of respondents reported at least one major discriminatory event in their lifetime, while over 33% reported 3 or more discriminatory lifetime events. Bivariate correlations among study variables are displayed in Table 2. Several demographic variables were associated with the study’s primary variables of interest. Specifically, male gender and higher educational attainment were associated with greater inflammation burden. Older age was associated with greater reports of lifetime occurrences of discrimination. Finally, lifetime discrimination and everyday discrimination variables were positively correlated with one another, and both of these variables were correlated positively with inflammation burden and PSQI global sleep quality.
Table 1.
Sample Characteristics
| Characteristic | n | % or Range | M | SD |
|---|---|---|---|---|
| Socio-demographic | ||||
| Age (years) | 300 | 36 - 85 | 53.90 | 10.54 |
| Male | 104 | 34.7% | ||
| Female | 196 | 65.3% | ||
| Education (%) | 233 | |||
| ≤ High school | 124 | 41.3% | ||
| Some college | 101 | 33.7% | ||
| ≥ University degree | 75 | 25.0% | ||
| Health behaviors | ||||
| Smoking | 237 | |||
| Non-smoker | 164 | 54.7% | ||
| Current smoker | 73 | 24.3% | ||
| Alcohol problems | 298 | 0 – 1 | .07 | .26 |
| Medication use (% yes) | ||||
| Blood pressure | 127 | 42.3% | ||
| Cholesterol | 65 | 21.7% | ||
| Steroid | 32 | 10.7% | ||
| Depression | 26 | 8.7% | ||
| Mental health | ||||
| Anxiety | 297 | 12.00 – 57.00 | 19.93 | 7.91 |
| Depression | 296 | 11.00 – 47.00 | 17.37 | 6.19 |
| Inflammation Biomarkers | ||||
| C-reactive protein | 291 | 0.14 – 31.70 | 3.87 | 4.81 |
| Interleukin-6 | 296 | 0.46 – 22.40 | 3.83 | 3.49 |
| Fibrinogen | 291 | 167.00 – 857.00 | 380.92 | 95.26 |
| E-selectin | 295 | 10.04 – 178.05 | 50.09 | 27.59 |
| Intracellular adhesion molecule-1 | 295 | 44.00 – 1076.59 | 282.63 | 153.65 |
| Inflammation burden | 300 | 0.00 – 5.00 | 2.03 | 1.43 |
| Lifetime discrimination | ||||
| Never | 67 | 22.3% | ||
| 1-2 instances | 76 | 25.3% | ||
| 3 or more | 100 | 33.38% | ||
| Everyday discrimination | 297 | 9.00 - 32.00 | 14.84 | 6.34 |
Table 2.
Descriptive Statistics and Correlations of Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Lifetime discrimination | --- | .42** | .29** | .18** | .21** | −.02 | .09 | .04 | .09 | .08 | −.05 | .07 | -.05 | .14** | .16** |
| 2.Everyday discrimination | --- | 24** | .16** | −.09 | .11 | .03 | −.01 | −.01 | .03 | −.05 | .07 | −.05 | .18** | .19** | |
| 3.PSQI global sleep quality | --- | .29** | −.14* | .03 | −.04 | −.01 | −.03 | .02 | −.22** | −.11 | .06 | .41** | .42** | ||
| 4.Inflammation burden | --- | −.03 | −.13* | −.15** | −.20** | .02 | −.05 | −.14* | −.02 | .13* | .14* | .15** | |||
| 5.Age | --- | −.08 | −.02 | −.42** | −.25** | −.09 | .01 | −.06 | .08 | −.17* | −.16** | ||||
| 6.Gender | --- | .03 | .10 | −.09 | .16** | .07 | −.09 | .06 | −.07 | −.06 | |||||
| 7.Education | --- | .07 | .03 | .05 | −.08 | .20** | −.06 | −.01 | −.01 | ||||||
| 8.Blood pressure medication | --- | .29** | .05 | .12* | −.03 | .01 | .03 | .01 | |||||||
| 9.Cholesterol medication | --- | .05 | −.02 | .05 | .03 | .02 | .03 | ||||||||
| 10. Corticosteriod medication | --- | .01 | .07 | .05 | −.02 | .02 | |||||||||
| 11. Depression medication | --- | .01 | .04 | −.23** | −.27** | ||||||||||
| 12. Current smoker | --- | −.08 | −.10 | −.11 | |||||||||||
| 13. Alcohol problems | --- | .05 | .03 | ||||||||||||
| 14. Anxiety | --- | .80** | |||||||||||||
| 15. Depression | --- |
Note.
p <.05;
p < .01; PSQI = Pittsburgh Sleep Quality Assessment
Discrimination and Inflammation Burden
Associations between lifetime discrimination and inflammation burden were highly consistent across all five models. Results of regression analyses are summarized in Table 3. The unadjusted model (Base model) indicated that lifetime discrimination was associated with higher levels of inflammation burden (B = .309, 95% CI = .094, .524). The adjusted base model (Model 1) indicated that net of socio-demographic factors, lifetime reports of discrimination predicted significantly higher levels of inflammatory risk (B = .280, 95% CI = .018, .542). Adding medical covariates (Model 2), health behaviors (Model 3), and psychosocial factors (Model 4) to the base model did not alter these results. The association between lifetime discrimination and inflammation burden was attenuated, but remained significant across all models (see Table 3). The full model accounted for approximately 18% of the variance in inflammatory dysfunction scores. Contrary to predictions, no significant effects emerged for everyday discrimination and inflammation burden.
Table 3.
Parameter Estimates from Unadjusted and Adjusted Regression Models Predicting Inflammation Burden
| Base Model | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | |
| Discrimination | |||||
| Everyday | .023 (−.005, .052) | .025 (−.003, .053) | .022 (−.006, .046) | .023 (−.004, .050) | .021 (−.007, .048) |
| Lifetime | .309 (.094, .524)** | .280 (.018, .542)* | .272 (.016, .527)* | .278 (.031, .525)* | .274 (.028, .521)* |
| Socio-demographics | |||||
| Age | .001 (−.014, .017) | −.010 (−.026, .007) | −.008 (−.024, .009) | −.006 (−.023, .011) | |
| Gender (Ref: Male) | −.437 (−.765, −.109)** | −.336 (−.664, −.008)* | −.366 (−.695, −.037)* | −.348 (−.679, −.017)* | |
| Education | −.104 (−.165, −.044)** | −.100 (−.160, −.041)** | −.095 (−.155, −.034)** | −.095 (−.156, −.034)** | |
| Medication use | |||||
| Blood pressure | −.632 (−.982, −.282)** | −.612 (−.959, −.264)** | −.609 (−.957, −.261)** | ||
| Cholesterol | .129 (−.262, .520) | .122 (−.266, .511) | .126 (−.263, .515) | ||
| Corticosteroid | −.152 (−.658, .338) | −.176 (−.670, .31) | −.179 (−.674, .317) | ||
| Depression | −.572 (−.648, .345) | −.594 (−1.134, −.054)* | .508 (−1.067, .050) | ||
| Health behaviors | |||||
| Smoking | −.049 (−.413, .315) | −.027 (−.396, .343) | |||
| Alcohol use | .752 (.171, 1.334)* | .739 (.156, 1.322)* | |||
| Psychosocial factors | |||||
| Depressive symptoms | .002 (−.030, .035) | ||||
| Anxiety symptoms | .013 (−.028, .055) | ||||
| R2 | .040 | .098 | .156 | .176 | .180 |
| F for change in R2 | 6.108** | 6.255*** | 4.921** | 3.412* | 0.703 |
Note. CI = confidence interval. Model parameters are based on pooled estimates from multiple imputation.
p < .05.
p < .01.
p < .001.
Mediation Analyses
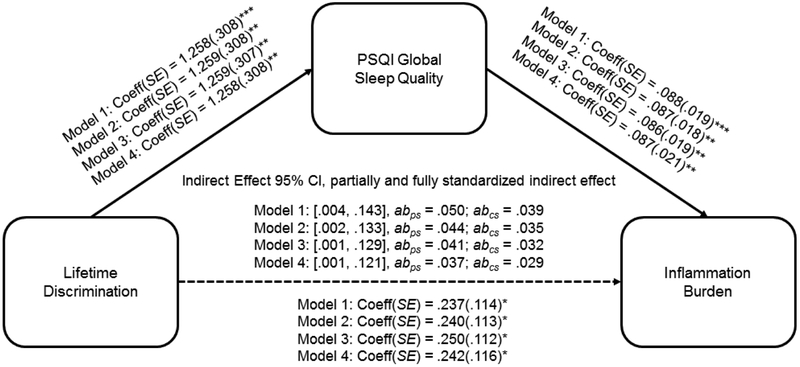
To estimate the indirect associations of lifetime discrimination and global sleep quality with inflammation burden, we used the PROCESS macro for SAS (Hayes, 2013). Bias-corrected bootstrap confidence intervals for the indirect association were estimated based on 5,000 bootstrap samples. Analyses revealed a significant indirect association between lifetime discrimination and inflammation burden through global sleep quality. As shown in Figure 1, the indirect association remained significant adjusting for socio-demographic factors (Model 1; 95% CI = .004, .143), medication use (Model 2; 95% CI = .002, .133), health behaviors (Model 3; 95% CI = .001, .129), and psychosocial factors (Model 4; 95% CI = .001, .121), suggesting that the association between lifetime discrimination and inflammation burden was driven by decreases in global sleep quality. Figure 1 also displays the partially standardized and completely standardized indirect effects. Across all models, the completely standardized effects were smaller than the partially standardized effects because they reference differences in standard deviations of inflammation burden across participants that differed by .7854 units on lifetime discrimination rather than a full standard deviation unit. As can be seen in Figure 1, the partially standardized and completely standardized indirect effects in the full model were .037 (95% CI = 002, .075) and .029 (95% CI = .001, .058), respectively, indicating that inflammation burden increased by .037 and .029 standard deviations for every one-unit increase in lifetime discrimination (on its 3-point scale) indirectly via PSQI global sleep quality.
Figure 1.
Direct and indirect associations between lifetime discrimination, PSQI global sleep quality, and inflammatory dysfunction. The sample size was 300. Coeff = coefficient; SE = standard error; CI = confidence interval. abps = partially standardized indirect effect; abcs = completely standardized indirect effect. Model 1 = analysis with demographic covariates; Model 2 = analysis adding in medication covariates; Model 3 = analysis adding in health behavior covariates; Model 4 = analysis adding in psychosocial covariates. Higher scores on continuous variables indicate greater standing on the variable (e.g., greater sleep difficulty). In these models, a CI that does not include 0 indicates a statistically meaningful association. *p < .05, **p < .01, ***p < .001
To further explore the relationship between lifetime discrimination and inflammation burden, we used conditional process modeling to test for moderated mediation as outlined by Hayes (2015). Specifically, we tested to see whether age and gender moderated the indirect effect of lifetime discrimination on inflammation burden. The Index of Moderated Mediation, a test of equality of the conditional indirect effect, was not significant for age (95% CI = −.005, .002) or gender (95% CI = −.114, .041), indicating that the indirect relationship from lifetime discrimination to inflammation burden through PSQI global sleep quality did not vary significantly as a function of age or gender.
Discussion
Extensive evidence suggests that cumulative exposure to discrimination increases risk for premature morbidity and mortality (Lewis, et al., 2015; Mays, Cochran, & Barnes, 2007; Williams & Mohammed, 2009). Findings from the current study provide support for the hypothesis that among racial/ethnic minorities, the repeated experience of discrimination over the life contributes to greater overall inflammation burden. Whereas previous work has focused on individual inflammatory markers (Cunningham, et al., 2012; Friedman, et al., 2009; Stepanikova, et al., 2017), this study extends prior research by examining the association between discrimination and systemic inflammation.
Contrary to expectation, perceptions of everyday discrimination were unrelated to inflammation in the current data. Although previous studies have documented positive associations between indices of everyday discrimination and inflammation (Beatty Moody, et al., 2014; Friedman, et al., 2009; Lewis, et al., 2010), these studies did not control for lifetime discrimination. Consistent with findings from the current investigation, a recent study by Stepanikova et al. (2017) found associations between daily discrimination and inflammation dissipated after controlling for lifetime discrimination. Thus, the adverse effects of chronic exposure to day-to-day discrimination may be reflected in an embedded history of lifetime stress exposure (Mays, et al., 2007; Williams & Mohammed, 2009). Personal histories of stressor exposure may occur at different points across the life course (early life traumas, cumulative stressor exposure during adulthood, and current chronic stressors). Studies that integrate personal histories of stress exposure and daily stress processes would be instrumental in understanding how discrimination impacts long-term health and well-being. Ultimately, longitudinal studies are needed to fully disentangle the temporal relationships among cumulative lifetime discrimination, everyday discrimination and inflammation.
An additional aim was to examine the role of key variables that may confound associations of discrimination with sleep and inflammation. Previous reviews have pointed to the importance of controlling for the effects of demographics, medication use, health behaviors (Friedman, et al., 2015; Friedman, et al., 2005; Friedman & Herd, 2010; Friedman, et al., 2009; Matthews, et al., 2010), and psychological distress symptoms (Lewis, et al., 2015; Pascoe & Smart Richman, 2009) in studies of discrimination and physical health. Notably, we found the effect of lifetime discrimination on inflammatory dysfunction remained significant after including demographics (age, gender, education level), medication use (antihypertensive, cholesterol lowering, steroid, and antidepressant medications) health behaviors (smoking and alcohol use), and psychological distress (depression and anxiety symptomatology) in the model, indicating that although statistical adjustment for these confounding factors attenuated the association between lifetime discrimination and inflammation burden, they do not completely explain the effect in the current data. Taken together, these findings build upon existing literature by pointing to the significance of lifetime exposure to discrimination in the lives of racial/ethnic minorities and by illustrating how social conditions external to the individual “get under the skin” to affect later health outcomes.
Finally, the mediating effect by global sleep quality suggests that decreased sleep salubrity may be an especially important pathway through which lifetime exposure to discrimination leads to systemic inflammation. This finding corroborates existing literature suggesting links between discrimination and inflammation (Cunningham, et al., 2012; Friedman, et al., 2009; Stepanikova, et al., 2017) as well as discrimination and global sleep quality (for a review, see Slopen, et al., 2016). This is the first study to demonstrate that global sleep quality mediates the association between lifetime and inflammation. This has major implications for interpreting why cumulative lifetime discrimination exposure predicts broad-based morbidity and mortality. Specifically, these results suggest that individuals exposed to greater lifetime discrimination may be less resilient than those less exposed in part because they sleep more poorly. Sleep is a modifiable health behavior that can be improved through behavioral intervention (Irwin, Cole, & Nicassio, 2006). Future research is required to determine whether implementing evidence-based behavioral treatments for sleep problems (e.g., relaxation and exercise) might attenuate the impact of discrimination on global sleep quality and downstream physiological processes. Additionally, health-care seeking behaviors (e.g., interactions with healthcare providers, adherence to treatment advice) is an important behavioral pathway that should be examined in future work, as self-reported experiences of discrimination have been shown to be associated with lower levels of health care seeking (for a review, see Williams & Mohammed, 2009) and access to health care among racial/ethnic minority members (Blendon, Aiken, Freeman, & Corey, 1989; Williams & Rucker, 2000).
Our conclusions are necessarily limited by some features of our methods and analyses. First, although lifetime discrimination was as assessed prior to the assessments of inflammatory markers, both inflammation and global sleep quality were assessed in the same measurement occasion. Thus, future studies with additional follow-up data are needed to determine whether global sleep quality acts as a true mediator of the association between lifetime discrimination and inflammation burden, rather than a confound. Second, our measures of discrimination were based on self-report and did not include comprehensive assessments of structural discrimination (e.g., residential segregation, socio-economic mobility). Beyond the measurement of discrimination, the measurement of sleep is also an issue for the current study. Specifically, the characteristics of our sleep measures were subjective, and it remains unclear whether standard subjective assessments (e.g., global sleep quality) and objective methods (e.g., actigraph, polysomnographic monitoring) are equivalent or whether they assess different underlying processes with potentially differing sleep etiologies (Kahn, Sheppes, & Sadeh, 2013). Thus, until our findings are replicated using objective sleep measures, they should be interpreted with caution. Finally, our study was limited to a relatively small sample of fairly educated multiethnic sample of middle-aged adults, and the findings cannot be assumed to generalize beyond this socio-demographic group. Additional research in this area is warranted.
Despite the study limitations, to our knowledge, the present analysis is among the first to consider the cumulative effects of discrimination on global sleep quality and multisystem indices of inflammatory dysregulation within a community-based sample of racially diverse middle-aged adults. The current findings also shed light on a behavioral mechanism—disturbances in global sleep quality—that appears to partially explain the link between lifetime discrimination and inflammation burden. Continuing to advance understanding of the life-course pathways from self-reported experiences of discrimination to physical health is critical for informing culturally sensitive interventions and services aimed at mitigating the cumulative toll of discrimination and narrowing racial/ethnic disparities in health.
Acknowledgments
This research was supported, in part, by Grant P01-AG020166 from the National Institute on Aging to conduct a longitudinal follow-up of the MIDUS (Midlife in the United States) investigation. The original study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development.
References
- Albert M, Ravenell J, Glynn R, Khera A, Halevy N, & de Lemos J (2008). Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas Heart Study. American Heart Journal 156, 1103–1109. [DOI] [PubMed] [Google Scholar]
- Barnes LL, De Leon CFM, Wilson RS, Bienias JL, Bennett DA, & Evans DA (2004). Racial differences in perceived discrimination in a community population of older blacks and whites. Journal of Aging and Health, 16, 315–337. [DOI] [PubMed] [Google Scholar]
- Beatty Moody DL, Brown C, Matthews KA, & Bromberger JT (2014). Everyday discrimination prospectively predicts inflammation across 7-Years in racially diverse midlife women: Study of women's health across the nation. Journal of Social Issues, 70, 298–314. doi: 10.1111/josi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendon RJ, Aiken LH, Freeman HE, & Corey CR (1989). Access to medical care for black and white Americans: A matter of continuing concern. Journal of the American Medical Association, 81, 261–278. [PubMed] [Google Scholar]
- Brim OG, Ryff CD, & Kessler RC (Eds.). (2004). How healthy are we?: A national study of well-being at midlife: Chicago, IL, US: University of Chicago Press. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, & Miller M (2010). Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care, 33, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, . . . Pahor M (2003). Inflammatory markers and cardiovascular disease American Journal of Cardiology, 92, 522–528. [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: Evidence and taxonomic implications. Journal of Abnormal of Psychology, 100, 316–336. [DOI] [PubMed] [Google Scholar]
- Cohen J, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.): Mahwah, NJ, US: Lawrence Erlbaum Associates, Publishers. [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, & Berkman LF (2012). Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 U.S. communities. Social Science & Medicine, 75, 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DS, Fuller-Rowell TE, El-Sheikh M, Carnethon MR, & Ryff CD (2017). Habitual sleep as a contributor to racial differences in cardiometabolic risk. Proceedings of the National Academy of Sciences, 114, 8889–8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, & Lithgow GJ (2014). Stress biology and aging mechanisms: Toward understanding the deep connection between adaptation to stress and longevity. Journal of Gerontology: Biological Sciences, 69, S10–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman TA, Williams DR, & Jackson JS (1997). Race, place, and discrimination. Perspectives on Social Problems, 9, 231–261. [Google Scholar]
- Friedman EM, Christ SL, & Mroczek DK (2015). Inflammation partially mediates the association of multimorbidity and functional limitations in a national sample of middle-aged and older adults: The MIDUS study. Journal of Aging and Health, 27, 843–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ, . . . Ryff CD (2005). Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Sciences, 102, 18757–18762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, & Herd P (2010). Income, education, and inflammation: Differential associations in a national probability sample (The MIDUS Study). Psychosomatic Medicine, 72, 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, & Ryff CD (2009). Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: The MIDUS study. Brain, Behavior, and Immunity, 23, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Walsemann KM, & Brondolo E (2012). A life course perspective on how racism may be related to health inequities. American journal of public health, 102, 967–974. doi: 10.2105/ajph.2012.300666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). 'Weathering' and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health, 96, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Shkolnikov VM, Jdanov D, Shkolnikov M, Vaupel JW, & Weinstein M (2013). Perceived stress and biological risk: Is the linkage stronger in Russians than in Taiwanese and Americans? Stress, 16, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Malone S, Richardson EA, Cheadle JE, & Williams DT (2015). Perceived discrimination and markers of cardiovascular risk among low-income African American youth. American Journal of Human Biology, 27, 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D, Redline S, Nieto F, Baldwin C, Newman A, Resnick H, & Punjabi N (2006). Association of usual sleep duration with hypertension: The sleep heart health study. Sleep, 29, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Graham JW (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Hale L, & Do PD (2007). Racial Differences in Self-Reports of Sleep Duration in a Population-Based Study. Sleep, 30, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel O (2009). The estimation of R2 and adjusted R2 in incomplete data sets using multiple imputation. Journal of Applied Statistics, 36, 1109–1118. [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press. [Google Scholar]
- Hayes AF (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50, 1–22. [DOI] [PubMed] [Google Scholar]
- House J, Lantz P, & Herd P (2005). Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans' Changing Lives Study). Journal of Gerontology: Psychological Sciences, 60, S15–S26. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, & Olmstead R (2010). Sleep loss activates cellular markers of inflammation: Sex differences. Brain, Behavior, and Immunity, 24, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole JC, & Nicassio PM (2006). Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychology, 25, 3–14. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Ganz PA, & Haque R (2013). Sleep disturbance, inflammation and depression risk in cancer survivors. Brain, Behavior, and Immunity, 30, S58–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M, Sheppes G, & Sadeh A (2013). Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology, 89, 218–228. [DOI] [PubMed] [Google Scholar]
- Kang S, & Marks NF (2014). Filial caregiving is associated with greater neuroendocrine dysfunction: Evidence from the 2005 National Survey of Midlife in the United States. Sage Open Medicine. doi: 10.1177/2050312113520152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, & Seeman TE (2006). Reduction in allostatic load in older Adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosomatic Medicine, 68, 500–507. [DOI] [PubMed] [Google Scholar]
- Kershaw K, Lewis TT, Diez Roux AV, Jenny NS, Liu K, Penedo FJ, & Carnethon M (2016). Self-reported experiences of discrimination and inflammation among men and women: The Multi-Ethnic Study of Atherosclerosis. Health Psychology, 35, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, & Williams DR (1999). The prevalence, distribution, and mental health correlates of percieved discrimination in the United States. Journal of Health and Social Behavior, 40, 208–230. doi: 10.2307/2676349 [DOI] [PubMed] [Google Scholar]
- Knutson KL, & Van Cauter E (2008). Associations between sleep loss and increased risk of obesity and diabetes. Annals of the New York Academy of Sciences, 1129, 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N (1990). Racial and gender discrimination: Risk factors for high blood pressure? [DOI] [PubMed] [Google Scholar]
- Kurian AK, & Cardarelli KM (2007). Racial and ethnic differences in cardiovascular disease risk factors: A systemic review. Ethnicity & Disease, 17, 143–152. [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, & Barnes LL (2010). Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain, Behavior, and Immunity, 24, 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annual Review of Clinical Psychology, 11, 407–440. doi: 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Kravitz H, Janssen I, & L. P(2011). Self-reported experiences of discrimination and visceral fat in middle-aged African-American and Caucasian women. American Journal of Epidemiology, 173, 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Yang FM, Jacobs EA, & Fitchett G (2012). Racial/ethnic differences in responses to the Everyday Discrimination Scale: A differential item functioning analysis. American Journal of Epidemiology, 175, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang X, Winkelman J, Redline S, Hu F, Stampfer M, . . . Gao X (2014). The association between insomnia symptoms and mortality: A prospective study of US men. Circulation, 129, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, & Ryff CD (2010). Bioindicators in the MIDUS National Study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22, 1059–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, & Smith GD (2005). A life course approach to chronic disease epidemiology. Annual Review of Public Health, 26, 1–35. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Zheng H, Kravitz HM, Sowers MF, Bromberger JT, Buysse DJ, . . . Hall M (2010). Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women? Study of women’s health across the nation sleep study. Sleep, 33, 1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays VM, & Cochran SD (2001). Mental health correlates of perceived discrimination among lesbian, gay, and bisexual adults in the United States. American Journal of Public Health, 91, 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, & Barnes NW (2007). Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology, 58, 201–225. doi: 10.1146/annurev.psych.57.102904.190212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137, 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuru-Jeter A, Chae DH, Price M, Telesford J, Mendoza-Denton R, & Woods-Giscombe C (2013). Anticipatory Racism Threat and Superwoman Schema: Elucidating the Relationship Between Racial Discrimination and Chronic Inflammation. CIRCULATION, 128.23591419 [Google Scholar]
- Owens S, Hunte H, Sterkel A, Johnson DA, & Johnson-Lawrence V (2017). Association between discrimination and objective and subjective sleep measures in the MIDUS adult sample. Psychosomatic Medicine, 79, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe EA, & Smart Richman L (2009). Perceived discrimination and health: A meta-analytic review. Psychological Bulletin, 135, 531–554. doi: 10.1037/a0016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, & Mannino DM (2007). Do insomnia complaints cause hypertension or cardiovascular disease? Journal of Clinical Sleep Medicine, 3, 489–494. [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Royston P (2005). Multiple imputation of missing values: Update. The Stata Journal, 5, 188–201. [Google Scholar]
- Rubin DB (1987). Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons. [Google Scholar]
- Ryff CD, Almeida DM, Ayanian JS, Carr DS, Cleary PD, Coe C, . . . Williams DR (2008a). Field Report--Midlife Development in the United States (MIDUS II): Milwaukee African American sample, 2005-2006. Ann Arbor, MI: Inter-university Consortium for Political and Social Research. [Google Scholar]
- Ryff CD, Almeida DM, Ayanian JS, Carr DS, Cleary PD, Coe C, . . . Williams DR (2008b). National Survey of Midlife Development in the United States (MIDUS II), 2004–2006. ICPSR04652-v6. Ann Arbor, MI: Inter-university Consortium for Political and Social Research. [Google Scholar]
- Schneiderman N, Ironson G, & Siegal SD (2008). Stress and health: Psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology, 1, 607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, & Miller GE (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130, 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML (1971). The Michigan Alcohol Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry, 127, 89–94. [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TL, & Williams D (2016). Discrimination and sleep: A systemtic review. Sleep Medicine, 18, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, & Williams DR (2014). Discrimination, other psychosocial stressors, and self-reported sleep duration and difficulties Sleep, 37, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Bateman LB, & Oates GR (2017). Systemic inflammation in midlfe: Race, socioeconomic status, and perceived discrimination. American Journal of Preventive Medicine, 52, S63–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr L, Pung MA, Edwards KM, & Dimsdale JE (2012). Racial differences in sleep architecture: The role of ethnic discrimination. Biological Psychology, 89, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Collins C (1995). U.S. socioeconomic and racial differences in health: Patterns and explanations. Annual Review of Sociology, 21, 347–386. [Google Scholar]
- Williams DR, & Mohammed SA (2009). Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine, 32, 20–47. doi: 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Rucker TD (2000). Understanding and addressing racial disparities in health care. Health Care Financing Reviews, 75–90. [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JJ, & Anderson NB (1997). Racial differences in physical and mental health: Socioeconomic status, stress, and discrimination. Journal of Health Psychology, 2, 335–351. [DOI] [PubMed] [Google Scholar]
- Yancik R, Ershler WB, Satariano W, Hazzard W, Cohen HJ, & Ferrucci L (2007). Report of the national aging task force on comorbidity. Journal of Gerontology: Medical Sciences, 62, 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Gerken K, Schorpp K, Boen C, & Harris KM (2017). Early-life socioeconomic status and adult physiological functioning: A life course examination of biosocial mechanisms. Biodemography and Social biology, 63, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]