Abstract
Purpose
Validated models are needed to justify strategies to define planning target volumes (PTVs) for intact cervical cancer used in clinical practice. Our objective was to independently validate a previously published shape model, using data collected prospectively from clinical trials.
Methods and Materials
We analyzed 42 patients with intact cervical cancer treated with daily fractionated pelvic intensity modulated radiation therapy and concurrent chemotherapy in one of 2 prospective clinical trials. We collected online cone beam computed tomography (CBCT) scans before each fraction. Clinical target volume (CTV) structures from the planning computed tomography scan were cast onto each CBCT scan after rigid registration and manually redrawn to account for organ motion and deformation. We applied the 95% isodose cloud from the planning computed tomography scan to each CBCT scan and computed any CTV outside the 95% isodose cloud. The primary aim was to determine the proportion of CTVs that were encompassed within the 95% isodose volume. A 1-sample t test was used to test the hypothesis that the probability of complete coverage was different from 95%. We used mixed-effects logistic regression to assess effects of time and patient variability.
Results
The 95% isodose line completely encompassed 92.3% of all CTVs (95% confidence interval, 88.3%–96.4%), not significantly different from the 95% probability anticipated a priori (P=.19). The overall proportion of missed CTVs was small: the grand mean of covered CTVs was 99.9%, and 95.2% of misses were located in the anterior body of the uterus. Time did not affect coverage probability (P=.71).
Conclusions
With the clinical implementation of a previously proposed PTV definition strategy based on a shape model for intact cervical cancer, the probability of CTV coverage was high and the volume of CTV missed was low. This PTV expansion strategy is acceptable for clinical trials and practice; however, we recommend daily image guidance to avoid systematic large misses in select patients.
Summary
We sought to validate a strategy for planning target volume definition in patients with intact cervical cancer, based on a previously published shape model. Using daily cone beam computed tomography imaging from patients treated with intensity modulated radiation therapy, we found that 92.3% of target volumes were entirely encompassed within the 95% isodose structure, which was not significantly lower than our hypothesized probability of 95.0% (P=.19). Therefore, we consider this expansion strategy to be valid.
Introduction
Radiation therapy is an important component of treatment of cervical cancer, but it can result in significant toxicity, especially to the genitourinary, gastrointestinal, and hematologic systems (1). Advanced radiation therapy techniques, such as intensity modulated radiation therapy (IMRT), have the potential to reduce toxicity compared with conventional approaches. Dosimetric studies of IMRT have shown reduced dose to normal organs, including the bowel, bladder, rectum, and bone marrow (2–4). Furthermore, reports describing patients treated with IMRT have been encouraging in terms of toxicity and clinical outcomes (5–13).
However, the use of IMRT in the setting of intact cervical cancer has been controversial. An important and potentially limiting factor is the degree to which the cervix and uterus move both between and during treatment fractions. Large interfraction and intrafraction target motions could lead to underdosing and compromised clinical outcomes, whereas large planning margins to compensate for such motion can result in excess normal tissue dose, thereby increasing toxicity. How to define planning margins to optimize the tradeoff between target coverage and normal tissue sparing in the setting of intact cervical cancer is unclear.
Several groups have investigated interfractional and intrafractional cervical motion during radiation treatment and have reported relatively large movements (14–23). However, prior studies have generally been retrospective, involving less than daily online imaging and relatively small sample sizes, whereas well-powered studies to validate various proposed models independently and prospectively have been lacking. Although contouring guidelines for clinical target volume (CTV) delineation for cervical cancer exist (24, 25), guidelines for planning margins do not. Previously, Khan et al (23) proposed a shape model to describe interfractional CTV variation and estimated that an anisotropic expansion of 10 to 14 mm around the anterior surface at the level of the uterus, 5 to 10 mm along the interface of the CTV with the bladder and rectum, and 1 to 3 mm around the superior and lateral regions of the CTV would ensure a 95% probability of complete target coverage. These results served as the basis for anisotropic planning target volume (PTV) recipes used in several clinical trials (26–28), which have used expansions of 15 mm around the uterus and cervix, 10 mm around the vagina and parametria, and 5 to 7 mm around the nodal CTV (summarized in Table 1). Given the ongoing lack of consensus about the optimal strategy to define PTVs for intact cervical cancer, studies prospectively evaluating proposed methods would be of value. Therefore, the primary aim of our study is to evaluate the validity of this approach to PTV definition, using prospectively collected data from 2 clinical trials. This validation study was a prespecified aim for one of the trials.
Table 1.
Summary of PTV expansion strategy based on model of Khan et al (23).
| Region of CTV | Anisotropic expansion |
|---|---|
| Uterus and cervix (CTV1) | 15 mm |
| Vagina and parametria (CTV2) | 10 mm |
| Nodal CTV (CTV3) | 5–7 mm |
| CTVboost | 7 mm |
Abbreviations: CTV = clinical target volume; PTV = planning target volume.
Methods and Materials
Population and sampling methods
This analysis was approved by our Institutional Review Board. The population consisted of patients with cervical cancer receiving daily fractionated radiation therapy with concurrent chemotherapy. Eligible patients for this study had unresected, biopsy-proven stage IB to IVA cervical carcinoma registered for one of 2 prospective clinical trials at our institution. We also included sets of images from 2 patients who were ineligible for trial participation because of a hemoglobin level <10 g/dL but who were treated according to protocol and received daily cone beam computed tomography (CBCT) imaging. Patients treated post-operatively or with extended field radiation therapy were ineligible.
Simulation and treatment planning
All patients underwent simulation in the supine position from T12 to mid femur with a customized vacuum immobilization device (Vac-Lok; Med-Tech, Orange City, IA) by use of a 4-slice computed tomography (CT) scanner (Lightspeed; GE Medical Systems, Waukesha, WI) with a 2.5-mm slice thickness. Patients with adequate renal function received intravenous contrast. Patients were simulated with both a full bladder and empty bladder and treated in a consistent bladder-filling state (ie, either always full or always empty) according to the preference of the treating physician. If patients required re-simulation during treatment, the new planning CT scan was used for the analysis.
All patients were treated with IMRT followed by an intracavitary brachytherapy boost. A parametrial boost after intracavitary brachytherapy was optionally used at the discretion of the treating physician. The CTV was defined on the planning CT scan and consisted of 3 subvolumes: CTV1 (gross tumor, cervix, and uterus), CTV2 (upper half of the vagina and parametria), and CTV3 (pelvic lymph nodes, including the common iliac, external and internal iliac, and presacral lymph nodes). For patients with gross nodal disease, a boost volume was generated consisting of the diseased node. The PTV was generated by applying a 15-mm margin around CTV1, a 10-mm margin around CTV2, and a 5- to 7-mm margin around CTV3. If applicable, a 7-mm margin was applied around CTVboost to generate PTVboost. The bladder, rectum, bowel, pelvic bone marrow, and femoral heads were contoured on each planning CT scan as organs at risk. The prescription dose for patients without gross nodal disease was 45 Gy in 25 daily fractions to the PTV. The prescription dose for patients with gross nodal disease was 47.6 Gy in 28 daily fractions to the PTV and 2.0 to 2.12 Gy to PTVboost depending on adjacent normal tissue tolerance. Treatment plans were generated using the Eclipse treatment planning system (Varian, Palo Alto, CA) with either 7 to 8 static coplanar beams or 2 coplanar arcs, with 6- or 15-MV photons. Patients received 5 to 6 cycles of concurrent cisplatin (40 mg/m2 weekly), with or without concurrent gemcitabine (50–125 mg/m2 weekly), according to the trial protocol.
Daily CBCT
All patients were treated by use of a linear accelerator equipped with a gantry-mounted imager for obtaining CBCT scans before each fraction. Each patient was initially set up with tattoo markers, and on-board planar kilovolt (kV) x-ray imaging was used to align bony anatomy before treatment each day. Prior to delivery of the first fraction, a CBCT scan was acquired and reviewed by the therapists and treating physicians to ensure adequate target volume coverage. For subsequent fractions, the CBCT scan was reviewed by therapists before treatment delivery and offline by a physician after each delivery. In instances of poor target coverage, small (<3 mm) shifts were applied based on manual soft tissue alignment. If larger shifts (>3 mm) were required (eg, because of rectal filling), patients were removed from the treatment table to void prior to treatment. In cases of systematic miss, the patient underwent a resimulation.
The CBCT scan parameters were 125 kV (peak), 80 mAs, and 25 ms per frame. The images were taken at a source-image distance of 150 cm with 440 projections. The device was operated in half-fan mode with a bowtie filter to reduce scatter and adequately encompass the patient’s anatomy. The typical length of a CBCT scan was 16 cm in the superior-inferior direction, which was generally sufficient to encompass the bladder, rectum, upper vagina, presacral lymph nodes, parametria, cervix, and uterus.
Assessment of CTV coverage
CBCT scans for each fraction were rigidly registered to the planning CT scan based on alignment of bony anatomy, by use of the MIM platform (MIM Software, Cleveland, OH). The CTV1 and CTV3 contours from the planning CT scan were cast onto each registered CBCT scan and manually redrawn to account for organ motion and deformation, creating a new CTV1 and CTV3 for each fraction. The 95% isodose volume was generated from the planning CT scan for each patient and was then overlaid onto each registered CBCT scan for that patient. The investigators involved in defining the new target volumes were blinded to the location of the 95% isodose volume. The volumes of the new targets lying outside the 95% isodose cloud (if any) were then computed. We expected to observe an overall probability of complete target coverage within the 95% isodose volume of at least 95%.
Statistical considerations
The primary aim of the study was to determine the percentages of CTV1 and CTV3 that were fully encompassed within the 95% isodose volume across all patients and fractions. The null hypothesis was that there is a 95% probability that the 95% isodose volume will entirely encompass the union of CTV1 and CTV3 for any given fraction, based on the model described by Khan et al (23). We computed the proportion of scans with complete coverage for each patient and used a 1-sample t test to test the alternative hypothesis that the overall probability of coverage was significantly different from 95%. We specified a priori that we would consider the prior shape model valid if the grand mean of fully encompassed scans was not significantly less than 95%. We also performed sensitivity analyses on the primary outcome by using a nonparametric Wilcoxon rank sum test and by fitting a generalized estimating equation model.
On the basis of preliminary results from 15 patients, we estimated the sample standard deviation for this probability to be 13%. We calculated that a sample size of 42 patients would provide 80% power to detect a mean difference of 5% or more and would provide 90% power to detect a mean difference of 6% or more, with a 1-sided type I error of 5%. We used mixed-effects, random-intercept logistic regression modeling on the binary outcome of any missed CTV versus complete coverage at each visit, regressed on linear time and including a subject-specific random intercept. In addition, we modeled demographic and tumor characteristics to identify potential predictors of missed target volume.
To assess the reliability and interobserver agreement of the CBCT contouring, another radiation oncologist independently determined the extent of CTV coverage on 3 randomly selected scans per patient. We calculated the Cohen к statistic for the agreement between the investigators. The second investigator was blinded to the 95% isodose cloud as well as the contours generated by the first investigator. Paired t tests were used to evaluate differences between CTVs on the initial planning CT scans compared with volumes on CBCT scans. Data were prepared and analyzed using R (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria [http://www.r-project.org]) and SPSS (version 23; IBM, Armonk, NY).
Results
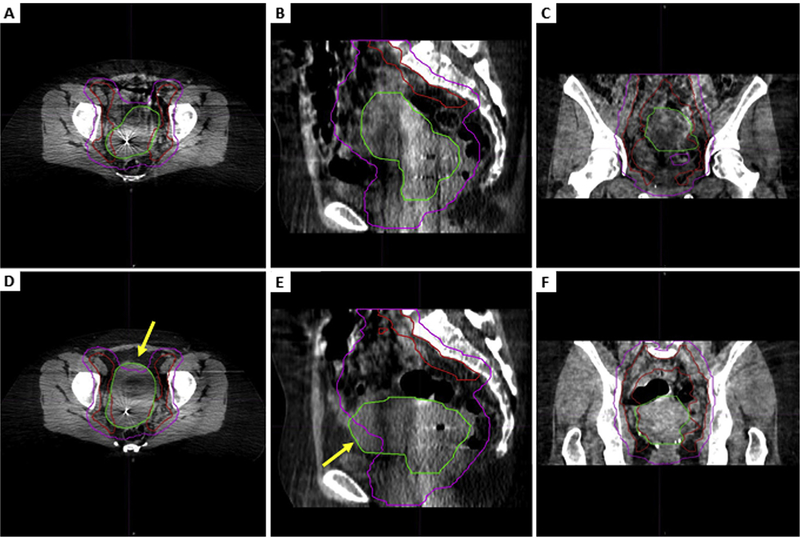
We analyzed a total of 1084 daily CBCT scans from 42 eligible patients (25.8 scans per patient; SD, 2.0). Figure 1 shows representative images of a completely encompassed CTV and a CTV that was partially missed. Because of limitations in the CBCT frame of view, the superior-inferior margins of the CBCT scans occasionally cut off portions of the CTV. This was most pronounced for CTV3, where a mean of 2.6 cm was cut off across the entire sample (SD, 2.1 cm; range, 0–9.9 cm). For CTV1, a mean of 0.04 cm (SD, 0.2 cm; range, 0–2 cm) was below the inferior border of the CBCT scan. However, 1015 of 1084 CTV1 contours (94%) were completely imaged.
Fig. 1.
Representative cone beam computed tomography slices showing a fully encompassed clinical target volume (CTV) compared with a partially missed CTV. Axial (A), sagittal (B), and coronal (C) views of CTV comprising gross tumor, cervix, and uterus (CTV1) (green) and CTV comprising pelvic lymph nodes (CTV3) (red) that are completely encompassed within the 95% isodose structure (pink) and axial (D), sagittal (E), and coronal (F) views from a scan with an anterior CTV1 miss in the uterine body. The arrows point to the missed CTV1. (A color version of this figure is available at www.redjournal.org.)
Demographic characteristics of the sample are shown in Table 2. The median age was 47.5 years (interquartile range, 40–57 years), and the mean body mass index was 28.5 (SD, 6.2). Most patients were white, had squamous cell carcinoma, and had a Karnofsky Performance Status of 100 prior to treatment.
Table 2.
Sample descriptive statistics
| Data (NZ42) | |
|---|---|
| Age, y | |
| Mean (SD) | 48.5 (12.3) |
| Median (IQR) | 47.5 (40–57) |
| Race or ethnicity | |
| Asian | 3 (7.1) |
| Black | 3 (7.1) |
| Hispanic | 13 (31.0) |
| White | 22 (52.4) |
| Other | 1 (2.4) |
| Mean body mass index (SD) | 28.5 (6.2) |
| Karnofsky Performance Status | |
| 100 | 33 (78.6) |
| 90 | 5 (11.9) |
| 80 | 2 (4.8) |
| NA | 2 (4.8) |
| Histology | |
| Squamous cell carcinoma | 32 (76.2) |
| Adenocarcinoma | 9 (21.4) |
| Adenosquamous carcinoma | 1 (2.4) |
| Grade | |
| 1 | 1 (2.4) |
| 2 | 16 (38.1) |
| 3 | 16 (38.1) |
| NA | 9 (21.4) |
| Stage | |
| IB2 | 12 (28.6) |
| IIA1 | 1 (2.4) |
| IIB | 15 (35.7) |
| IIIB | 14 (33.3) |
Abbreviations: IQR Z interquartile range; NA Z not available.
Results are presented as number (percentage) unless otherwise specified. Some percentages may not add up to 100% because of rounding.
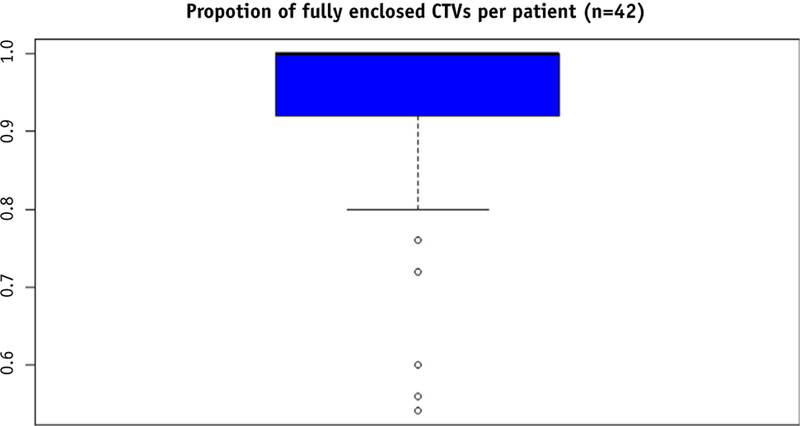
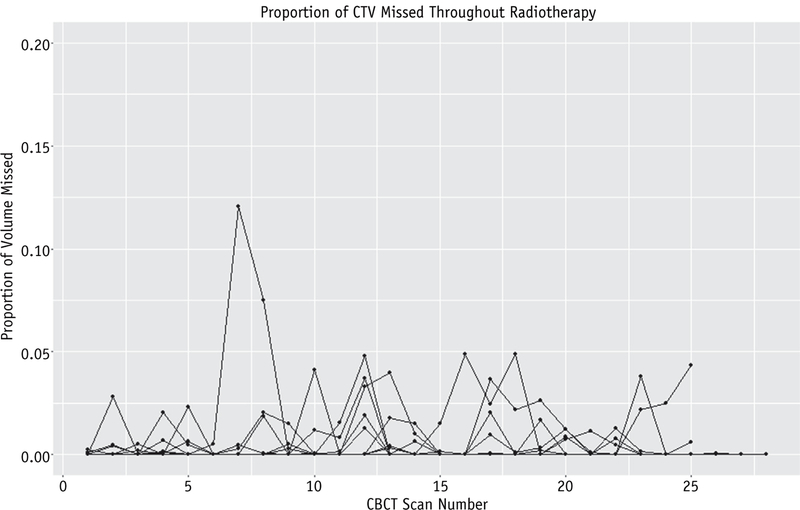
The 95% isodose line completely encompassed the CTV on 92.3% (95% confidence interval [CI], 88.3%–96.4%) of all daily CBCT scans for all patients, which was not significantly different from the 95% hypothesized a priori (P=.19). Because our primary outcome data were skewed (Fig. 2), with most percentages near the maximum of 100%, we performed sensitivity analyses by calculating a nonparametric Wilcoxon rank sum test and fitting a generalized estimating equation model. Both of these analyses were consistent with the t test; the choice of test did not affect the inference that our sample mean was not significantly different from 95% (P=.46 and P=.11, respectively). Figure 3 shows the daily proportion of CTV that fell outside the 95% isodose cloud throughout the course of external beam radiation therapy.
Fig. 2.
Box plot showing skewed distribution of primary outcome. Abbreviation: CTV Z clinical target volume.
Fig. 3.
Daily proportion of missed clinical target volume (CTV). Each line represents 1 patient (NZ42). Abbreviation: CBCT = cone beam computed tomography.
A total of 81 CBCT images showed at least 1 area of missed CTV; because 2 scans showed missed volume in 2 regions, there were 83 portions of missed CTVs in total (Table 2). Almost all of the scans that included a miss (95.2%) were in the region of the body of the uterus, whereas the cervix and lateral external iliac lymph nodes were each missed on 2 scans. The range of scans with any CTV miss per patient was 0 to 11, which translated to a coverage percentage ranging from 0% to 45.8%. Of 42 patients, 17 (40.5%) had at least 1 miss. On average, 1.9 scans per patient (SD, 3.2) showed a miss. Across all patients, the percentage of encompassed CTV was 99.9% (SD, 0.003). The volume of missed CTV was <1 cm3 in 22.2% of all of the misses and >10 cm3 in 24.7% of all misses.
We investigated potential differences between CTV contours that were generated by the treating physician on the planning CT scan and those delineated on the daily CBCT scans. A paired t test comparing the total volume of CTV from the planning CT scan with the CTV from the first CBCT scan showed no difference (P=.36). Similarly, a comparison of the CTV from the planning CT scan with the mean CTV from all CBCT scans for each patient showed no difference (P=.57). We also measured interobserver agreement of binary hit or miss status by calculating the Cohen k for measurements taken by an independent reviewer. The second investigator reviewed 3 scans per patient (11% of the entire sample). The Cohen k was 0.80 (95% CI, 0.65–0.94). Percent agreement between the 2 investigators was 94%.
Mixed-effects longitudinal logistic regression modeling showed an odds ratio of 0.994 for a miss per daily treatment fraction (95% CI, 0.960–1.028), which was not statistically significant (P=.71). However, the random intercept term was significant (P<.001), indicating that the misses tended to be clustered in some patients rather than randomly distributed between patients. We sought to identify potential predictors of miss by assessing age, ethnicity, body mass index, Karnofsky Performance Status, tumor histology, tumor grade, tumor stage, and initial CTV as covariates in the mixed-effects model. None of these predictors were statistically significant at a P value threshold of .05.
Discussion
The mean percentage of completely encompassed CTVs on daily CBCT imaging was not significantly lower than 95%. Therefore, CTV coverage was consistent with expectations from the model proposed by Khan et al (23), and we consider this expansion strategy to be valid. Previous reports have generally recommended margins of approximately 15 mm around the uterus and cervix, which is in line with the Khan et al model and with our results (15–18, 29). To our knowledge, this is the first well-powered, independent and hypothesis-driven validation of a model for PTV margin expansion in the setting of intact cervical cancer that takes advantage of prospectively collected daily imaging data.
The observed target volume misses were predominantly located in the anterior body of the uterus, with rare misses in the cervix and lymph nodes. Given the incidence of small misses, a reasonable strategy for improving target coverage could be to add a small expansion of 1 to 3 mm to the anterior uterine CTV. However, the clinical impact of relatively small misses in the body of the uterus is uncertain. Although most patients had no misses, some patients had several, and mixed-effects logistic regression showed that the misses were clustered in certain patients. We modeled the effect of time and assessed several potential predictors of missed CTVs, including initial CTV1 size. However, none of these variables were statistically significant. Given that misses were relatively rare events, perhaps the sample size is insufficient to identify such predictors. Other potential drivers of miss include bowel and bladder filling (30), which were not assessed in this study. Moreover, internal uterus motion, such as conversion between anteflexed and retroflexed states, likely plays a large role (29, 31). Baseline uterine position (ie, anteverted, midplane, retroverted) and/or intention to treat with a full or empty bladder could also affect coverage. Future work addressing the contribution of these anatomic considerations is warranted. Prior studies have shown regression in tumor and target volumes with time (14, 15, 20), but we found that time did not predict coverage likelihood. Assuming that tumor regression does not lead to increased mobility of the uterus and cervix, it should not affect coverage.
A limitation of this study is the range in quality of CBCT scans, which provide lower resolution than planning CT scans. However, daily CBCT scans have been used successfully in prior studies (20–22), and this technology is currently the most widely available option for daily online image guidance and soft tissue imaging. Despite some variability in scan quality, CTV delineation was in most cases straightforward. The frame of the CBCT scans occasionally cut off portions of the CTV, which led to the assumption that volumes outside the frame were concordant in coverage with what we were able to observe. Most of the volumes that were cut off were small and located in the superior aspect of CTV3. Given that the pelvic lymph nodes are closely associated with relatively fixed large blood vessels that are in turn relatively fixed to bone, we did not anticipate and did not observe a substantial number of nodal misses. Therefore, the presence of minimally truncated CTV3s is not likely to substantially affect our conclusion.
We compared the planning CT scanederived CTV with both the initial CBCT scanederived CTV and the mean CBCT scanederived CTV to assess for systematic size differences, but there was no difference found with either comparison. Furthermore, we calculated the Cohen к to explore consistency with primary outcome measurements and found it to be 0.8, which is considered strong agreement (32), along with a high raw percentage agreement. Disagreement occurred in scans where either the miss or the margin of coverage was very small or where CBCT scan quality was relatively lower, making close distinctions more difficult. Taken together, this methodology appears to be reliable.
In conclusion, target coverage was high for patients with intact cervical cancer treated with PTV expansions based on the model described by Khan et al (23) (specifically, a 15mm margin around the uterus and cervix, a 10-mm margin around the superior vagina and parametria, and a 5-mm margin around the nodal CTV, using daily bone-bone kV matching for setup). When misses occurred, they tended to be minimal in size and located in the anterior body of the uterus rather than the cervix. As a result, this expansion strategy is acceptable for use in clinical trials and practice. However, given uncertainty about risk factors for missing target volumes, we recommend daily image guidance (eg, kV or CBCT imaging) to avoid systematic large misses in select patients. This method does not preclude the use of an internal target volume, and future studies comparing alternative strategies for defining PTVs in this population, including the use of an internal target volume, would be useful.
Acknowledgments
This project was partially supported by the National Institutes of Health, grant TL1TR001443. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: none.
References
- 1.Green J, Kirwan J, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev 2005;CD002225. [DOI] [PMC free article] [PubMed]
- 2.Roeske JC, Lujan A, Rotmensch J, et al. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2000;48:1613–1621. [DOI] [PubMed] [Google Scholar]
- 3.Lujan AE, Mundt AJ, Yamada SD, et al. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2003;57:516–521. [DOI] [PubMed] [Google Scholar]
- 4.Mell LK, Tiryaki H, Ahn KH, et al. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:1504–1510. [DOI] [PubMed] [Google Scholar]
- 5.Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;52:1330–1337. [DOI] [PubMed] [Google Scholar]
- 6.Hasselle MD, Rose BS, Kochanski JD, et al. Clinical outcomes of intensity-modulated pelvic radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2011;80:1436–1445. [DOI] [PubMed] [Google Scholar]
- 7.Kidd EA, Siegel BA, Dehdashti F, et al. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose-positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2010;77:1085–1091. [DOI] [PubMed] [Google Scholar]
- 8.Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;54:1388–1396. [DOI] [PubMed] [Google Scholar]
- 9.Chen MF, Tseng CJ, Tseng CC, et al. Clinical outcome in post-hysterectomy cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy: Comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:1438–1444. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi AK, Sharma DN, Rath GK, et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: A prospective randomized study. Int J Radiat Oncol Biol Phys 2013;87:542–548. [DOI] [PubMed] [Google Scholar]
- 11.Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys 2013;86:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G, He F, Fu C, et al. Definitive extended field intensity-modulated radiotherapy and concurrent cisplatin chemosensitization in the treatment of IB2-IIIB cervical cancer. J Gynecol Oncol 2014;25: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang Y, Bydder M, Yashar CM, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys 2013;85:406–414. [DOI] [PubMed] [Google Scholar]
- 14.Beadle BM, Jhingran A, Salehpour M, et al. Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 2009;73:235–241. [DOI] [PubMed] [Google Scholar]
- 15.van de Bunt L, Jürgenliemk-Schulz IM, de Kort GA, et al. Motion and deformation of the target volumes during IMRT for cervical cancer: What margins do we need? Radiother Oncol 2008;88:233–240. [DOI] [PubMed] [Google Scholar]
- 16.Chan P, Dinniwell R, Haider MA, et al. Inter- and intrafractional tumor and organ movement in patients with cervical cancer undergoing radiotherapy: A cinematic-MRI point-of-interest study. Int J Radiat Oncol Biol Phys 2008;70:1507–1515. [DOI] [PubMed] [Google Scholar]
- 17.Taylor A, Powell ME. An assessment of interfractional uterine and cervical motion: Implications for radiotherapy target volume definition in gynaecological cancer. Radiother Oncol 2008;88:250–257. [DOI] [PubMed] [Google Scholar]
- 18.Haripotepornkul NH, Nath SK, Scanderbeg D, et al. Evaluation of intra- and interfraction movement of the cervix during intensity modulated radiation therapy. Radiother Oncol 2011;98:347–351. [DOI] [PubMed] [Google Scholar]
- 19.Lim K, Kelly V, Stewart J, et al. Pelvic radiotherapy for cancer of the cervix: Is what you plan actually what you deliver? Int J Radiat Oncol Biol Phys 2009;74:304–312. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi N, Lewis JH, Yashar CM, et al. Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys 2011;80:273–280. [DOI] [PubMed] [Google Scholar]
- 21.Heijkoop ST, Langerak TR, Quint S, et al. Quantification of intrafraction changes during radiotherapy of cervical cancer assessed with pre- and post-fraction cone beam CT scans. Radiother Oncol 2015;117:536–541. [DOI] [PubMed] [Google Scholar]
- 22.Langerak T, Mens JW, Quint S, et al. Cervix motion in 50 cervical cancer patients assessed by daily cone beam computed tomographic imaging of a new type of marker. Int J Radiat Oncol Biol Phys 2015; 93:532–539. [DOI] [PubMed] [Google Scholar]
- 23.Khan A, Jensen LG, Sun S, et al. Optimized planning target volume for intact cervical cancer. Int J Radiat Oncol Biol Phys 2012;83:1500–1505. [DOI] [PubMed] [Google Scholar]
- 24.Lim K, Small W Jr., Portelance L, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys 2011;79:348–355. [DOI] [PubMed] [Google Scholar]
- 25.Small W Jr., Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of California, San Diego. Study with intensity modulated radiation therapy with cisplatin to treat stage I-IVA cervical cancer Available at: https://clinicaltrials.gov/ct2/show/NCT01554397?termZintertecc&rankZ1. Accessed May 31, 2016.
- 27.University of California, San Diego. Intensity modulated radiation therapy with cisplatin and gemcitabine to treat locally advanced cervical carcinoma Available at: https://www.clinicaltrials.gov/ct2/show/NCT01554410?termZgemcitabineþcervicalþcancer&rankZ9. Accessed May 31, 2016.
- 28.National Cancer Institute. Radiation therapy and cisplatin with or without triapine in treating patients with newly diagnosed stage IB2, II, or IIIB-IVA cervical cancer or stage II-IVA vaginal cancer Available at: https://clinicaltrials.gov/ct2/show/NCT02466971. Accessed May 31, 2016.
- 29.Kaatee RS, Olofsen MJ, Verstraate MB, et al. Detection of organ movement in cervix cancer patients using a fluoroscopic electronic portal imaging device and radiopaque markers. Int J Radiat Oncol Biol Phys 2002;54:576–583. [DOI] [PubMed] [Google Scholar]
- 30.Buchali A, Koswig S, Dinges S, et al. Impact of the filling status of the bladder and rectum on their integral dose distribution and the movement of the uterus in the treatment planning of gynaecological cancer. Radiother Oncol 1999;52:29–34. [DOI] [PubMed] [Google Scholar]
- 31.Huh SJ, Park W, Han Y. Interfractional variation in position of the uterus during radical radiotherapy for cervical cancer. Radiother Oncol 2004;71:73–79. [DOI] [PubMed] [Google Scholar]
- 32.McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]