Abstract
Study Objectives
Insomnia is a leading cause of disability in postmenopausal women. Multicomponent cognitive-behavioral therapy for insomnia (CBTI) is a first-line treatment for chronic insomnia, but support for its efficacy in treating menopause-related insomnia is scarce. The present study evaluated whether CBTI is an efficacious treatment for menopause-related chronic insomnia, and whether sleep restriction therapy (SRT)—a single component of CBTI—is equally efficacious compared with CBTI.
Methods
In a single-site, randomized controlled trial, 150 postmenopausal women (56.44 ± 5.64 years) with chronic DSM-5 insomnia disorder related to menopause were randomized to three treatment conditions: sleep hygiene education (SHE), SRT, or CBTI. Blinded assessments were performed at baseline, posttreatment, and 6 months after treatment. The Insomnia Severity Index (ISI) and sleep diaries served as primary outcomes.
Results
From baseline to posttreatment, ISI decreased 7.70 points in the CBTI group (p < .001), 6.56 points in the SRT group (p < .001), and 1.12 in the SHE group (p = .01). Although average sleep duration increased in all groups by 6 month follow-up, CBTI patients obtained 40–43 more minutes of nightly sleep than those who received SHE or SRT. Remission rates in the CBTI (54%–84%) and SRT (38%–57%) groups were higher than SHE patients (4%–33%) at posttreatment and 6 month follow-up. CBTI patients were generally more likely to remit than SRT patients.
Conclusions
CBTI and SRT effectively treat menopause-related insomnia disorder and are superior to SHE. Response to CBTI and SRT is similar, but CBTI outperforms SRT in improving sleep maintenance, which may increase likelihood of remission.
Clinical Trial Name: Behavioral Treatment of Menopausal Insomnia: Sleep and Daytime Outcomes. URL: clinicaltrials.gov. Registration: NCT01933295.
Keywords: insomnia, CBTI, sleep hygiene, menopause, sleep restriction
Statement of Significance
Sleep problems like insomnia are a chief complaint among women during and after the menopause transition. Hormone replacement therapy, hypnotics, and psychotropic medications have long been the only offered interventions for menopause-related insomnia. However, these medications have poor support as standalone treatments for menopausal insomnia and many carry risks for serious side effects. Recent evidence suggests that cognitive-behavioral interventions may alleviate insomnia symptoms associated with menopause. In this clinical trial, we showed that cognitive-behavioral therapy for insomnia (CBTI) and sleep restriction therapy (SRT) are superior treatments for menopause-related insomnia when compared with sleep hygiene education. Moreover, results of this study suggest that CBTI may produce higher likelihood of remission and more durable results than SRT for some women with menopausal insomnia.
Introduction
Insomnia is endemic to women transitioning through menopause [1–3]. In the United States, the highest rates of menopause-related insomnia complaints are among postmenopausal women at 43%–48% [1]. Menopause itself—via hormonal changes and related symptoms—often leads to sleep deterioration, thereby triggering the onset of insomnia disorder [2, 4]. As menopausal women with disturbed sleep endorse more comorbid chronic illnesses, greater alcohol consumption, higher stress, more depression, and overall worse health than good sleeping menopausal women [5, 6], it is imperative to provide efficacious treatments for menopause-related insomnia.
Despite being one of the most common menopause-related complaints, treatment options for menopausal insomnia have been rather limited. Most treatment options to improve sleep in menopause involve pharmacotherapy, but have produced mixed or weak results [3]. Despite hormonal imbalance having a suspected etiological role in menopausal insomnia, support for hormone replacement therapy (HRT) as an efficacious standalone insomnia treatment is scarce as results are often nonsignificant or, at best, statistically significant but clinically underwhelming [3, 7, 8]. As HRT has limited efficacy and carries serious health risks [9, 10], other forms of pharmacotherapy have been tested. Randomized controlled trials (RCTs) support the immediate efficacy of zolpidem [11], ramelteon [12], and eszopiclone [13] for menopausal insomnia, but data on long-term hypnotic use and treatment durability in this population have gone unreported. Furthermore, recent recommendations advise against hypnotics in this population due to the risks associated with long-term hypnotic use, particularly among older adults [3]. Other medications, including antidepressants, have demonstrated mixed benefits for sleep in menopause, but little evidence supports these as standalone treatments for insomnia disorder in this population [3].
Until recently, cognitive-behavioral interventions for menopause-related insomnia had not been investigated. Cognitive-behavioral therapy for insomnia (CBTI) is the most commonly administered nonpharmacological insomnia treatment among sleep specialists. Importantly, this is not true for nonspecialists who are more likely to deliver sleep hygiene education (SHE) as a standalone insomnia treatment. CBTI is a multicomponent treatment comprised of behavioral, cognitive, and educational components believed to target critical factors that maintain insomnia over time. As a first-line treatment for insomnia disorder [14], CBTI is equally effective as hypnotics for treating insomnia in the short-term, and more effective and better tolerated than hypnotics in the long-term [15]. Thus, it is surprising that CBTI for menopausal insomnia has only recently been investigated. As one of the MsFlash trials, perimenopausal and postmenopausal women with self-reported insomnia symptoms were randomized to telephone delivery of CBTI or menopause education control [16]. Follow-up data immediately after treatment then 16 weeks later showed substantial and durable reductions in insomnia symptoms in the CBTI group, but not the control condition. Despite these initial positive results, many gaps exist in our knowledge of cognitive and behavioral interventions for menopausal insomnia.
First, to firmly establish the efficacy of CBTI for menopause-related insomnia, it is imperative to examine only women whose insomnia onset or exacerbation coincides with the menopause transition. That is, as etiological factors likely differ between insomnia disorders that onset prior to the menopause transition vs onsets during or after menopause, investigating insomnia cases specifically associated with menopause transition will offer the clearest picture of treatment effects on actual menopause-related insomnia. Equally important is that patients should be diagnosed by a sleep specialist per current ICSD-3 [17] or DSM-5 [18] insomnia disorder criteria to ensure the validity of treatment effects. Second, although evidence suggests CBTI to be superior to menopause education in the treatment of menopausal insomnia, it remains unclear how CBTI compares with insomnia-focused interventions. CBTI treatment effects should be demonstrated as superior to real-world clinical practices, which often involve administering SHE as a standalone nonpharmacological treatment as usual. Thirdly, CBTI is just one treatment option for insomniacs preferring nonpharmacological intervention. Other nonpharmacological insomnia treatments have strong support for the treatment of insomnia, but have not been examined in menopause-related insomnia. Sleep restriction therapy (SRT) is an empirically supported standalone insomnia treatment [19]. Notably, sleep restriction is a primary component of CBTI. And as a standalone treatment, SRT often involves fewer sessions than CBTI, which can improve access to care by producing less patient burden.
The present study was a single-site RCT comparing CBTI, SRT, and SHE for the treatment of menopause-related DSM-5 insomnia disorder in a sample of 150 postmenopausal women [1]. We hypothesized that patients receiving CBTI or SRT would report greater improvements in insomnia symptoms and higher rates of remission immediately after treatment and at 6 month follow-up when compared with patients receiving SHE. In addition, we anticipated that the additional components of CBTI (i.e. stimulus control, cognitive therapy, progressive muscle relaxation, and sleep hygiene) would have substantial incremental value to treatment and reinforce longer-term adaptive sleep behaviors when compared with SRT. Therefore, we hypothesized that immediate posttreatment effects would be similar between the CBTI and SRT groups, but that CBTI would produce more durable treatment effects over the longer term as evidenced by substantially better sleep and higher remission rates for CBTI treatment compared with SRT at 6 month follow-up.
Methods
Participants and procedure
This study was conducted in a large 6-hospital health system in the state of Michigan. Participants were recruited from the health system in primary care and the sleep clinic, as well as from the community via newspaper advertisements and from a database of prior sleep center studies. To be eligible, women must have been postmenopausal (12 consecutive months without menses), reported wake after sleep onset (WASO; wakefulness in the middle of the night after falling asleep) of an hour or more on ≥3 nights per week, and met criteria for DSM-5 insomnia disorder that onset or was exacerbated during the perimenopausal or postmenopausal period per clinical interview with a registered nurse with specialty training in behavioral sleep medicine. Regarding our operationalization of menopause-related insomnia, participants had to endorse that current insomnia onset or worsened within ±6 months of menopause onset to be eligible. In addition, objective sleep disturbance had to be evident per mean WASO of ≥45 min across two overnight polysomnography (PSG) studies (adaptation night + baseline night, and neither night could have WASO of <30 min). Exclusionary criteria also included prior or current DSM-5 major depression per diagnostic interview, sleep–wake disorders other than insomnia [examined on PSG adaptation night (obstructive sleep apnea defined as apnea–hypopnea index of ≥15, periodic limb movements defined as arousal frequency of ≥15) and per patient report], and medications influencing sleep (prescription and nonprescription sleep aids, herbal supplements, and any antidepressants taken at night), although women receiving hormone therapy were permitted to participate.
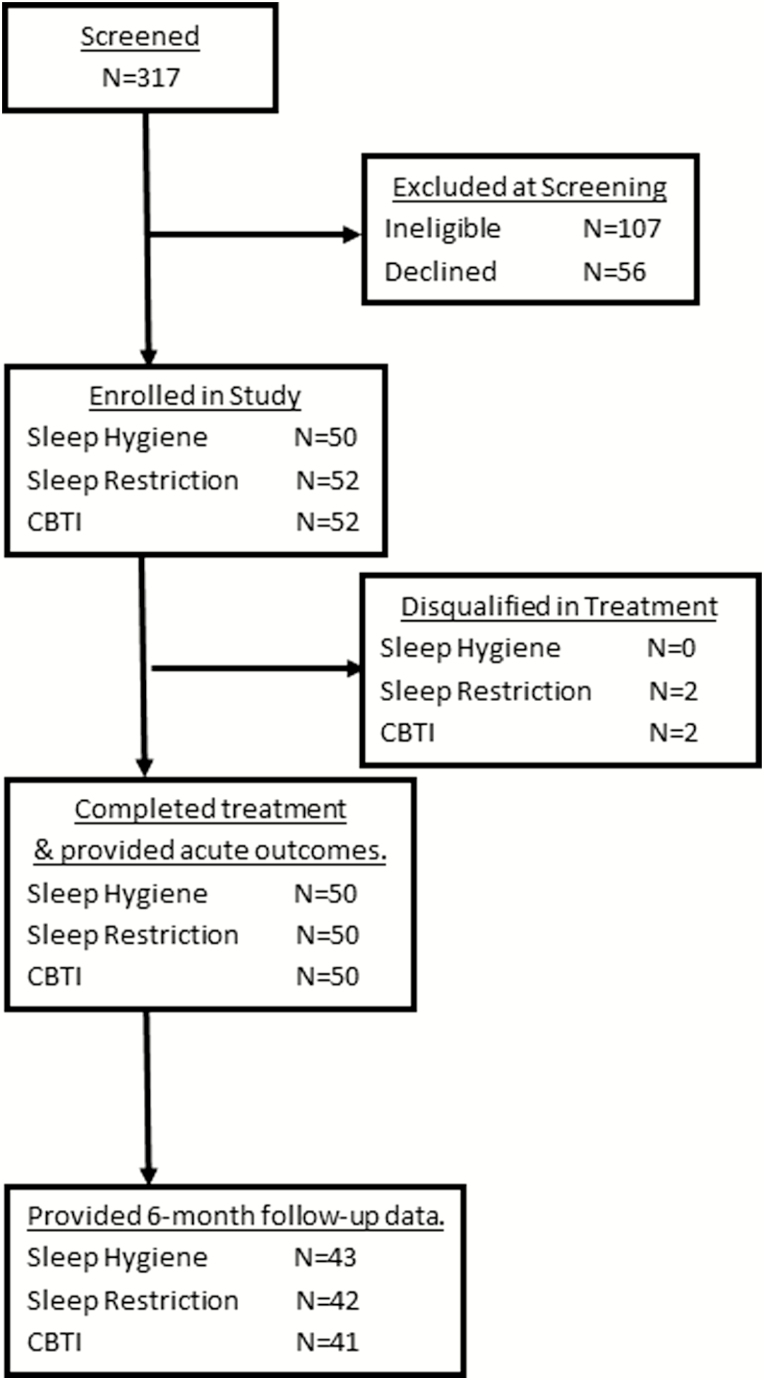
Refer to Figure 1 flow chart of study enrollment and participation. A total of 317 postmenopausal women were screened for eligibility. Of these individuals, 107 women were ineligible and another 56 declined to participate or had scheduling conflicts. Thus, 154 postmenopausal women were randomized to 1 of 3 treatment conditions: (1) SHE treatment as usual (N = 50), (2) SRT (N = 52), and (3) CBTI (N = 52). Randomization was conducted using 150 allocations (50 per group) that were ordered randomly and concealed in envelopes. Group allocation for each participant was then assigned using the order of concealed envelopes. Two participants in both the SRT and CBTI conditions were disqualified during treatment for changes in medication or new onset comorbid sleep disorder. These two allocations were replaced in random order by a research staff member not involved with this study, and recruitment included two more individuals to replace those who were disqualified. This resulted in 50 participants completing treatment in each of the three conditions. Although double-blind could not be achieved given the nature of the behavioral interventions, participants were not informed which treatments were considered control versus active, or of the specific hypotheses. Assessments of insomnia symptoms and sleep parameters were collected prior to treatment, at posttreatment (within 2 weeks of completing treatment), and 6 months after treatment completion. All 150 participants provided posttreatment outcome data, whereas 16% of treatment completers did not provide follow-up data 6 months later (Figure 1).
Figure 1.
Flow chart of study enrollment and participation.
Cognitive-behavioral therapy for insomnia
Women randomized to CBTI completed six face-to-face sleep therapy sessions with a registered nurse who specializes in behavioral sleep medicine. CBTI is a structured, multimodal treatment that targets sleep-disruptive behaviors and beliefs (see Perlis et al. [20]). Data from clinical trials consistently show that CBTI is as efficacious as pharmacological treatment in the short-term, but produces superior treatment response in the long-term [14, 15]. CBTI patients received 6 weekly sessions, which covered behavioral (sleep restriction and stimulus control) and cognitive (e.g. cognitive restructuring) components, as well as relaxation strategies (e.g. progressive muscle relaxation and autogenic training) and sleep hygiene. Because CBTI was a 6 week intervention, posttreatment outcomes were measured 6 weeks after pretreatment baseline. Fidelity monitoring for the nurse therapist included weekly supervision meetings with one of the two licensed PhD clinical psychologists, both of whom are certified in behavioral sleep medicine. Supervision meetings included discussions of cases, problem-solving, and listening to and providing feedback based on recorded therapy sessions.
Sleep restriction therapy
SRT is an effective standalone behavioral treatment for insomnia [19]. Although SRT actually predates CBTI, SRT is now commonly packaged as part of CBTI and is typically considered one of CBTI’s main active ingredients. As CBTI consists of SRT plus multiple other components, SRT is the briefer of the two interventions. Here, SRT was delivered as a 2 week intervention. Specifically, the initial face-to-face session consisted of reviewing patient sleep history, education and rationale for sleep restriction practices, and behavioral homework with a registered nurse who specializes in behavioral sleep medicine. Then four follow-up sessions (three phone contacts, each 3–4 days apart, followed by a second face-to-face session) were delivered across the following 2 weeks and were used to titrate sleep schedules based on sleep diary data. Because SRT was a 2 week intervention, posttreatment outcomes were measured 2 weeks after pretreatment baseline. Fidelity monitoring for the SRT condition was the same as described in the CBTI section above.
Sleep hygiene education (SHE), i.e., minimal intervention control condition
Women randomized to the online SHE condition received 6 weekly emails including general, nonpersonalized information on the following topics: the basics of endogenous sleep regulation; the impact of sleep on health problems such as obesity, diabetes, and hypertension; the effects of stimulants and other sleep-disruptive substances; the relationship between sleep, diet, and exercise; and tips on creating a sleep-conducive bedroom environment. Sleep hygiene is neither the primary cause nor a sufficient therapeutic target in insomnia disorder and therefore served as an ideal minimal intervention control condition and real-world comparator [21]. Because SHE was a 6 week intervention, posttreatment outcomes were measured 6 weeks after pretreatment baseline.
Measures
All self-report measures were collected with online surveys hosted by Qualtrics, LLC, and study personnel were blinded to these data. Insomnia symptoms and sleep parameters were collected using the Insomnia Severity Index (ISI) and the consensus sleep diary [22]. The ISI is a 7-item self-report measure of insomnia symptom severity [23]. A cutoff of ISI ≥ 11 indicates clinically significant self-reported insomnia severity in RCTs, whereas a cutoff of ISI ≤ 7 indicates remission [24]. The Ford Insomnia Response to Stress Test (FIRST) [25] measures trait-like stress-related sleep reactivity (i.e. tendency to experience sleep disturbance in response to stress) and was administered to characterize pretreatment sleep reactivity in this sample. Sleep diary data were collected for 2 weeks before treatment (pretreatment), the first 2 weeks after completing treatment (posttreatment), and for a final 2 weeks at 6 months after treatment (6 month follow-up). Diary data included sleep onset latency (SOL; in minutes), frequency of nighttime awakenings (number of awakenings), WASO (in minutes), sleep quality (SQ; 1–5 scale, higher scores indicating better quality), time in bed (TIB; time between bedtime and wake time), total sleep time (TST; TIB minus SOL and WASO and period between bedtime and lights out), and sleep efficiency (SE%; ratio of TST to TIB, with higher percentages indicating more TIB spent asleep with SE < 85% indicating inefficient sleep).
Analysis plan
Analyses were conducted using SPSS version 25. Overall demographics and pretreatment characteristics were first presented and cross-sectionally compared across the three treatment conditions to identify group differences before treatment. To test treatment effects, we first ran 3 × 2 repeated measures ANOVAs to examine Treatment × Time interactions for changes in sleep parameters (ISI and diary reports) from pretreatment to immediate posttreatment. With 150 participants across three groups (n = 50 in each), power analysis revealed over 90% power to detect medium-sized interactions. For diary data, we excluded statistical outliers on SOL (>120) and analyzed data from participants with full diary data at all three time points, resulting in analyzing diary data in 137 women. After testing for Treatment × Time interaction effects, paired samples t-tests were conducted within each condition to test for potential simple effects; significant results (p < .05) were then followed-up with Cohen’s d estimation of effect size specifically designed for paired samples t-tests, which accounts for the correlation between the pretreatment and posttreatment values [26]. In addition, a cross-sectional one-way ANOVA was used to compare mean levels for each treatment outcome to determine differences in symptom levels across groups. These analyses were then repeated to compare 6 month follow-up data with pretreatment symptomatology. Lastly, we compared remission rates across treatment conditions at posttreatment and 6 month follow-up based on ISI and diary-based quantitative criteria. Importantly, recent evidence suggests that remission based on global insomnia complaints may misrepresent individuals who continue to experience sleep disturbance; thus, incorporation of self-reported quantitative criteria into remission status has been strongly urged [27]. Thus, we examined insomnia remission per three different operationalizations: (1) ISI ≤ 7, (2) SE% ≥ 85%, and (3) SOL and WASO ≤ 30 min each. We evaluated remission rates by treatment for all three remission operationalizations.
Results
Screening and sample characteristics
See Table 1 for sample characteristics and symptom levels for the full sample and each of the treatment groups. Our sample was largely comprised of non-Hispanic White (52.0%) and non-Hispanic Black women (39.3%). The mean number of years since last menstrual period was 7.12 ± 7.04 years. Only four patients reported current HRT (2.7%), and 23.3% of the sample reported medical menopause due to hysterectomy (partial or complete), chemotherapy, or endometrial ablation. Sleep reactivity was high in the sample (FIRST: 21.86 ± 6.05), indicating that postmenopausal insomniacs have highly stress-reactive sleep systems. Prior to treatment, mean ISI scores were in the clinical range (ISI: 15.17 ± 3.98). Per sleep diaries, WASO ratings were in the clinical range for 75.2% of the sample (WASO > 30 min [28]), compared with just 42.3% of the sample struggling to fall asleep (sleep latency > 30 min [28]). Duration of sleep was short (TST: 5 hr 43 m ± 86 m) and mean SE% was < 85%, thereby indicating inefficient sleep (71% ±15%). Mean SQ ratings showed that participants largely reported having “fair” sleep at night. Comparisons of sociodemographic characteristics and pretreatment presentation revealed no differences across the three conditions. Participants who dropped out at 6 month follow-up did not differ from study completers on any pretreatment or posttreatment outcomes.
Table 1.
Sample characteristics prior to treatment (n = 150)
| Sample size | All participants | SHE | SRT | CBTI | |
|---|---|---|---|---|---|
| 150 | 50 | 50 | 50 | ||
| Age | 56.44 ± 5.64 years | 57.24 ± 5.55 years | 56.76 ± 5.39 years | 55.32 ± 5.90 years | F (2,147) = 1.58, p = .21 |
| Race | |||||
| White | 78; 52.0% | 26; 52.0% | 28; 56.0% | 24; 48.0% | |
| Black | 59; 39.3% | 20; 40.0% | 17; 34.0% | 22; 44.0% | |
| Hispanic or Latinx | 1; 0.7% | – | 1; 2.0% | – | |
| Multiracial | 1; 0.7% | – | 1; 2.0% | – | |
| Other | 2; 1.3% | 1; 2.0% | – | 1; 2.0% | |
| Did not answer | 9; 6.0% | 3; 6.0% | 3; 6.0% | 3; 6.0% | |
| Hormone replacement therapy | 4; 2.7% | 3; 6.0% | 1; 2.0% | 0; 0.0% | |
| Medical menopause | 35; 23.3% | 9; 18.0% | 12; 24.0% | 14; 28.0% | |
| Years since last menstruation | 7.12 ± 7.04 | 7.33 ± 7.79 | 6.93 ± 6.79 | 7.09 ± 6.65 | F(2,147) = 0.04, p = .96 |
| FIRST | 21.86 ± 6.05; 73.8% | 22.73 ± 6.53; 75.0% | 21.08 ± 4.95; 75.5% | 21.79 ± 6.58; 70.8% | F(2,147) = 0.90, p=.41 |
| Pretreatment | |||||
| ISI | 15.17 ± 3.98; 88.7% | 15.36 ± 4.36; 86.0% | 15.20 ± 3.67; 88.0% | 14.94 ± 3.97; 92.0% | F(2,147) = 0.14, p = .87 |
| Total sleep time | 5 hr 46 m ± 77 m | 5 hr 47 m ± 77 m | 5 hr 27 m ± 74 m | 6 hr 5 m ± 77 m | F(2,132) = 2.94, p = .06 |
| Sleep quality | 2.96 ± .54; Fair | 3.01 ± .50; Fair | 2.87 ± .54; Fair | 3.02 ± .57; Fair | F(2,134) = 1.14, p = .32 |
| Sleep latency | 30.83 ± 18.15; 42.3% | 28.37 ± 12.40; 34.1% | 34.86 ± 23.82; 46.8% | 29.06 ± 15.60; 45.7% | F(2,134) = 1.80, p = .17 |
| Nighttime awakenings | 2.75 ± 1.24 | 2.93 ± 1.35 | 2.59 ± .99 | 2.73 ± 1.35 | F(2,134) = 0.91, p = .40 |
| Wake after sleep onset | 57.72 ± 36.27; 75.2% | 61.83 ± 39.50; 79.5% | 62.33 ± 37.02; 78.7% | 49.07 ± 31.14; 67.4% | F(2,134) = 2.00, p = .14 |
| Sleep efficiency | 71% ± 15% | 72% ± 14% | 69% ± 14% | 75% ± 13% | F(2,132) = 2.79, p = .07 |
FIRST = ford insomnia response to stress test. Medical menopause = menopause due to medical treatments including complete or partial hysterectomy, chemotherapy, or endometrial ablation.
ISI % is proportion that is ISI ≥ 11. All other sleep parameters collected using the consensus sleep diary. One-way ANOVAs used to compare pretreatment means, with Bonferroni post hoc comparisons.
Treatment effects on insomnia symptoms and sleep diary parameters
Refer to Table 2 for full results on posttreatment outcomes.
Table 2.
Comparing CBTI vs SRT vs SHE on sleep parameters and nocturnal insomnia symptoms
| Posttreatment | Δ Pre- to posttreatment | 6 month Follow-up | Δ Pre- to 6 month follow-up | |
|---|---|---|---|---|
| ISI | F(2,147) = 37.33, p < .001 | F(2,147) = 34.32, p < .001 | F(2,128) = 23.68, p < .001 | F(2,128) = 18.60, p < .001 |
| SHE | 14.24 ± 4.49*,†; 78.0% | t(49) = −2.60, p = .01, d = .37 | 13.44 ± 4.64*,†; 80.0% | t(44) = −3.76, p < .001, d = .57 |
| SRT | 8.64 ± 4.18‡; 34.0% | t(49) = −11.64, p < .001, d = 1.66 | 8.12 ± 4.25‡; 25.6% | t(42) = −10.60, p < .001, d = 1.62 |
| CBTI | 7.24 ±4.18‡; 22.0% | t(49) = −10.13, p < .001, d = 1.43 | 6.95 ± 5.26‡; 25.2% | t(42) = −8.97, p < .001, d = 1.38 |
| Total sleep time | F(2,134) = 0.96, p = .39 | F(2,134) = −0.88, p = .42 | F(2,134) = 4.52, p = .01 | F(2,134) = −0.74, p = .48 |
| SHE | 6 hr 1 m ± 66 m | t(43) = 1.58, p = .12 | 6 hr 13 m ± 84 m† | t(43) = 3.01, p < .01, d = .45 |
| SRT | 5 hr 53 m ± 74 m | t(46) = 2.41, p = .02, d = .35 | 6 hr 10 m ± 74 m† | t(46) = 4.28, p < .001, d = .63 |
| CBTI | 6 hr 13 m ± 78 m | t(45) = 0.78, p = .44 | 6 hr 53 m ± 72 m†,‡ | t(45) = 3.91, p < .001, d = .58 |
| Sleep quality | F(2,134) = 7.53, p = .001 | F(2,134) = 10.58, p < .001 | F(2,134) = 12.58, p < .001 | F(2,134) = 15.41, p < .001 |
| SHE | 3.12 ± .64*,†; Fair | t(43) = 1.37, p = .18 | 3.12 ± .50*,†; Fair | t(43) = 1.51, p = .14 |
| SRT | 3.53±.63‡; Fair/Good | t(46) = 7.08, p < .001, d = 1.04 | 3.51±.51‡; Fair/Good | t(46) = 7.52, p < .001, d = 1.09 |
| CBTI | 3.63 ± .66‡; Good | t(45) = 6.12, p < .001, d = .91 | 3.66±.55‡; Good | t(45) = 8.86, p < .001, d = 1.31 |
| Sleep latency | F(2,134) = 5.73, p < .01 | F(2,134) = 7.65, p = .001 | F(2,134) = 0.65, p = .52 | F(2,134) = 2.72, p = .07 |
| SHE | 25.30 ± 18.31*,† | t(43) = −1.20, p = .24 | 21.65 ± 13.46 | t(43) = −3.42, p < .01, d = .52 |
| SRT | 18.35 ± 10.43‡ | t(46) = −5.96, p < .001, d = 1.14 | 20.84 ± 14.07 | t(46) = 5.34, p < .001, d = .90 |
| CBTI | 16.12 ± 10.09‡ | t(45) = −6.09, p < .001, d = .95 | 18.61 ± 11.75 | t(45) = 5.47, p < .001, d = .83 |
| Nighttime awakenings SHE SRT CBTI |
F(2,134) = 4.48, p = .01 2.62 ± 1.63* 1.84 ± .91‡ 2.11 ± 1.18 |
F(2,134) = 3.53, p = .03 t(43) = −2.65, p = .01, d = .43 t(46) = −7.34, p < .001, d = 1.07 t(45) = −4.68, p < .001, d = .69 |
F(2,134) = 3.28, p = .04 2.56 ± 1.49* 1.95 ± .89‡ 2.06 ± 1.18 |
F(2,134) = 1.83, p = .16 t(43) = −3.46, p < .01, d = .53 t(46) = −5.80, p < .001, d = .87 t(45) = −4.90, p < .001, d = .73 |
| Wake after sleep onset SHE SRT CBTI |
F(2,134) = 11.34, p <. 001 46.03 ± 32.61*,† 30.28 ± 21.89‡ 22.11 ± 15.32‡ |
F(2,134) = 3.24, p = .04 t(43) = −3.83, p < .001, d = .60 t(46) = −6.08, p < .001, d = .95 t(45) = −6.42, p < .001, d = 1.07 |
F(2,134) = 5.88, p < .01 48.02 ± 36.61† 35.75 ± 23.29 28.10 ± 21.39‡ |
F(2,134) = 2.00, p = .14 t(43) = −3.20, p < .01, d = .49 t(46) = −5.68, p < .001, d = .89 t(45) = −4.67, p < .001, d = .71 |
| Sleep efficiency SHE SRT CBTI |
F(2,134) = 6.46, p < .01 76% ± 14%*,† 83% ± 13%‡ 86% ± 14%‡ |
F(2,134) = 4.32, p = .02 t(43) = 2.13, p = .04, d = .36 t(46) = 6.31, p < .001, d = .93 t(45) = 4.97, p < .001, d = .78 |
F(2,134) = 5.56, p < .01 77% ± 15%† 81% ± 12% 86% ± 10%a |
F(2,134) = 2.78, p = .07 t(43) = 3.72, p < .01, d = .66 t(46) = 7.33, p < .001, d = 1.12 t(45) = 5.99, p < .001, d = .89 |
All other sleep parameters collected using the consensus sleep diary. In the posttreatment and 6 month follow-up columns, results from one-way ANOVAs comparing group means, with Bonferroni post hoc comparisons.
*Mean different from SRT group.
†Mean different from CBTI group. In the Δ Pre- to posttreatment and Δ Pre- to 6 month follow-up columns, F-statistic represents results from the Treatment × Time interaction in a 3 × 2 repeated measures ANOVA. t-statistics represent results from paired samples t-tests to examine simple effects within each treatment group.
‡Mean different from the SHE group.
Insomnia symptoms
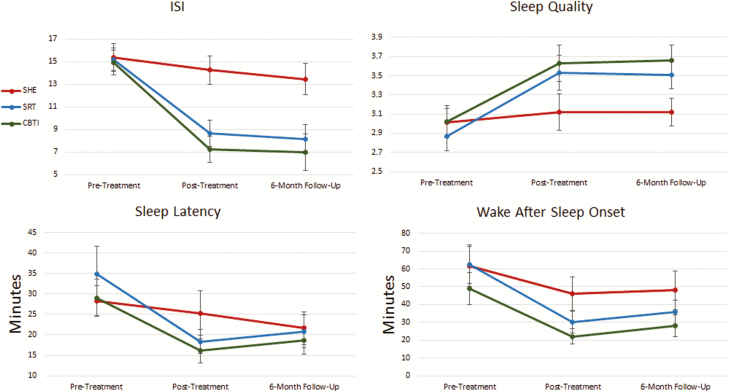
We first evaluated changes in our primary outcome measure: insomnia symptom severity (Figure 2). A repeated measures ANOVA evaluating changes in ISI scores from pretreatment to posttreatment showed a significant Treatment × Time interaction. Even so, follow-up paired samples t-tests showed that all three treatment groups reported reductions in ISI scores. However, large insomnia reductions were observed in the SRT (MeanT2−T1: −6.56, d = 1.66) and CBTI (MeanT2−T1: −7.70, d = 1.43) groups, whereas the SHE group experienced only a modest reduction of 1.12 points (d = .37). One-way ANOVA with post hoc Bonferroni comparisons showed that ISI scores were lower at posttreatment in the SRT and CBTI groups compared with the SHE group, but that the SRT and CBTI groups did not differ.
Figure 2.
Treatment effects of CBTI, SRT, and SHE on global insomnia, sleep quality, sleep latency, and wake after sleep onset.
Six months later, reductions in ISI from baseline were large in the SRT (MeanT3−T1: −7.02, d = 1.62) and CBTI (MeanT3−T1: −8.03, d = 1.38) groups, but moderate in the SHE group (MeanT3−T1: −2.22, d = .57). Comparing groups, ISI scores remained lower in SRT and CBTI patients than SHE patients, although ISI scores did not differ between SRT and CBTI groups (Table 2).
Total sleep time
Despite large acute improvements in insomnia symptom severity in two of the three groups, TST at posttreatment only increased in the SRT group, although modestly (d = .35). However, as there was a trend for pretreatment TST to be higher for CBTI women than for SRT women, this observed increase in TST for the SRT group could be regression to the mean, especially considering that SRT actively limits time spent in bed. Importantly, the three groups did not differ on TST at posttreatment (Table 2). When examining changes in TST at 6 month follow-up, paired samples t-tests showed that TST increased moderately in all groups from pretreatment baseline (SHE MeanT3−T1: +26 min; SRT MeanT3−T1: +43 min; CBTI MeanT3−T1: +48), yet TST was 40–43 min longer in the CBTI group than in the SRT and SHE groups. TST did not differ between SRT and SHE groups.
Sleep quality
Self-reported SQ followed a similar pattern as ISI scores (Table 2; Figure 2). A significant Treatment × Time interaction was observed, and paired samples t-tests showed large increases in SQ in SRT (d = 1.04) and CBTI (d = .91) patients, but no improvement in the SHE group (p = .18). Accordingly, posttreatment SQ was rated more positively in the SRT and CBTI groups than the SHE group, although the SRT and CBTI did not differ from one another.
Six months later, SQ was higher in the SRT and CBTI groups than in the SHE group (Table 2). Paired samples t-tests revealed large increases in SQ for the SRT (d = 1.09) and CBTI (d = 1.31) groups, but no improvement in the SHE group (MeanT3−T1: p = .14).
Sleep onset latency
SRT and CBTI patients reported reduced SOL at posttreatment (Table 2; Figure 2). A Treatment × Time interaction was significant such that large decreases in SOL were observed in the SRT (MeanT2−T1: −16.51 min, d = 1.14) and CBTI (MeanT2−T1: −12.94 min, d = .95) groups, whereas no significant change was observed in the SHE group (MeanT2−T1: −3.06 min, p = .24). Concordantly, posttreatment sleep latency was shorter in the SRT and CBTI groups than the SHE group, whereas the SRT and CBTI groups did not differ on sleep latency.
We also observed large reductions in SOL at 6 months after treatment in the SRT (MeanT3−T1: −14.02 min, d = .90) and CBTI (MeanT3−T1: −10.45 min, d = .83) groups, along with moderate reductions in latency to sleep in the SHE group (MeanT2−T1: −6.72, d = .52). However, the three treatment groups no longer differed on SOL at 6 month follow-up.
Sleep maintenance
Sleep maintenance issues, as captured by number of nighttime awakenings, WASO, and SE%, followed similar patterns (Table 2). A Treatment × Time interaction was observed for nighttime awakenings, such that SRT participants reported large reductions nighttime awakenings after completing treatment (MeanT2−T1: −.74, d = 1.07), whereas CBTI participants reported medium–large reductions (MeanT2−T1: −.60, d = .69). SHE reported only small reductions (MeanT2−T1: −.31, d = .43). Group comparisons showed that the SRT group reported fewer awakenings than the SHE group, although neither group differed from the CBTI group. A Treatment × Time interaction was also observed for WASO (Table 2; Figure 2). Both the SRT (MeanT2−T1: −32.04 min, d = 1.07) and CBTI (MeanT2−T1: −26.96 min, d = .95) groups reported large reductions in WASO at posttreatment, whereas the SHE group reported a moderate reduction (MeanT2−T1: −15.80, d = .60). Concordantly, SE% improvements were large for patients receiving SRT (MeanT2−T1: +15%, d = .93) and CBTI (MeanT2−T1: +10%, d = .78), whereas SE% increases in the SHE group (MeanT2−T1: +5%, d = .36) were more modest, F(2,134) = 4.32, p = .02. Posttreatment SE% was higher in the SRT (83% ± 13%) and CBTI (86% ± 14%) groups than the SHE group (76% ± 14%).
Although sleep maintenance improved across all treatment conditions, the results at 6 month follow-up were more positive in the SRT and CBTI groups. Reductions in nighttime awakenings were large in the SRT (MeanT2−T1: −.64, d = .87) and CBTI (MeanT2−T1: −.67, d = .73) groups, but moderate in the SHE group (MeanT2−T1: −.37, d = .53). Even so, fewer awakenings were reported by SRT patients than SHE patients. Results for 6 month outcomes for WASO and SE% were nearly identical such that large improvements in WASO and SE% were observed in SRT (SE% MeanT2−T1: +13%, WASO MeanT2−T1: −26.58 min) and CBTI (SE% MeanT2−T1: +11%, WASO MeanT2−T1: −20.97 min) patients, whereas moderate improvements were observed in the SHE group (SE% MeanT2−T1: +7%, WASO MeanT2−T1: −13.81 min). Notably, only CBTI patients reported better WASO and SE% than the SHE group, whereas SRT differed from neither of the other two treatment conditions. Moreover, mean levels of WASO and SE% in the CBTI group were in the normal range (<31 min for WASO and >85% for SE%), whereas these parameters were in the insomnia range for patients in the SRT and SHE groups.
Remission rates and comparative odds by treatment
Lastly, we examined insomnia remission per three different operationalizations: (1) ISI ≤ 7, (2) SE% ≥ 85%, and (3) SOL and WASO ≤ 30 min each. We first evaluated remission rates by treatment for all three remission operationalizations (Table 3). We then ran dummy-coded logistic regression (SHE as the reference group) to compare remission odds for CBTI and SRT with that of the SHE group. For ISI-based remission, both SRT and CBTI outperformed SHE at posttreatment and 6 month follow-up (Table 3). Although effect sizes appeared larger for the CBTI patients, logistic regression models comparing SRT and CBTI patients revealed no difference in remission odds at posttreatment or 6 month follow-up for the ISI-based remission.
Table 3.
Remission rates and odds at posttreatment and 6 month follow-up by treatment condition
| Remission rates and odds | ||||
|---|---|---|---|---|
| Posttreatment | OR, 95% CI | 6 month Follow-up | OR, 95% CI | |
| ISI ≤ 7 | ||||
| SHE | 2/50; 4.0% | – | 6/45; 13.3% | – |
| SRT | 19/50; 38.0% | 14.71, 3.20–67.62 | 24/43; 55.8% | 7.47, 2.60–21.44 |
| CBTI | 27/50; 54.0% | 28.17, 6.16–128.80 | 29/43; 67.8% | 13.28, 4.49–39.30 |
| SRT vs CBTI | CBTI: 1.92, .86-4.25 | SRT vs CBTI | CBTI: 1.78, .73–4.36 | |
| SE% ≥ 85% | ||||
| SHE | 14/46; 30.4% | – | 12/46; 26.1% | – |
| SRT | 25/48; 52.1% | 2.48, 1.07–5.79 | 19/47; 40.4% | 1.92, .80–4.630 |
| CBTI | 34/48; 70.8% | 5.55, 2.29–13.44 | 30/48; 62.5% | 4.72, 1.96–11.39 |
| SRT vs CBT | CBTI: 2.23, .96-5.18 | SRT vs CBTI | CBTI: 2.46, 1.08–5.61 | |
| SOL and WASO ≤ 30 min | ||||
| SHE | 16/48; 33.3% | – | 13/47; 27.7% | – |
| SRT | 28/49; 57.1% | 2.67, 1.17–6.08 | 23/49; 46.9% | 2.31, .99–5.42 |
| CBTI | 41/49; 83.7% | 10.25, 3.90–26.94 | 29/48; 60.4% | 3.99, 1.69–9.45 |
| SRT vs CBTI | CBTI: 3.84, 1.49–9.89 | SRT vs CBTI | CBTI: 1.73, .77–3.86 |
SE% = sleep efficiency; SOL = sleep latency; OR = odds ratio relative to the sleep education group; 95% CI = 95% confidence interval for the OR.
SRT vs CBTI represents results (OR and 95% CI) from logistic regression models comparing SRT (coded 0) and CBTI (coded 1).
Remission analyses based on SE% and SOL and WASO showed somewhat similar results to the ISI-based remission findings such that both SRT and CBTI outperformed SHE. However, these results also diverged from ISI-based remission results as evidence suggested that CBTI outperformed SRT at posttreatment and 6 month follow-up (see Table 3 for full results. Per SE%-based remission, higher odds of remission for CBTI patients than SRT patients was nonsignificant at posttreatment (OR = 2.23, p = .06). And at 6 month follow-up, this association was significant such that CBTI patients were over twice as likely to be in remission than SRT patients based on SE% (OR = 2.46, 95% CI = 1.08–5.61). SOL/WASO-based insomnia remission revealed a greater likelihood of remission for CBTI patients at posttreatment compared with SRT patients (OR = 3.84, 95% CI = 1.49–9.89), whereas remission odds between CBTI and SRT patients did not differ at 6 month follow-up (OR = 1.73, 95% CI = 0.77–3.86).
Discussion
In a sample of 150 postmenopausal women, we evaluated the efficacy of CBTI and SRT in comparison to SHE for chronic insomnia related to menopause. Both CBTI and SRT outperformed SHE and resulted in large reductions in insomnia symptoms after treatment. Improvements in sleep latency, sleep maintenance, and overall insomnia symptomatology were sustained 6 months later, reflecting durable treatment effects. Importantly, CBTI and SRT produced large improvements in most sleep parameters, indicating that both treatment options are appropriate for improving menopausal insomnia. Evidence suggested that only CBTI produced better long-term sleep maintenance outcomes than SHE control, whereas SRT did not differ from control on sleep maintenance outcomes. Furthermore, some remission metrics suggested that CBTI is associated with greater likelihood of remission than SRT. Taken together, these data suggest that CBTI may be a superior treatment option for most postmenopausal women with insomnia.
The primary complaint prior to treatment in our study was the inability to maintain sleep, thus confirming sleep maintenance difficulties as the cardinal feature of menopause-related insomnia [29–33]. Furthermore, our findings are highly consistent with the recent MsFLASH clinical trial showing that telephone delivery of CBTI effectively treats insomnia when compared with menopause education control in perimenopausal and postmenopausal women with high self-reported insomnia symptoms [16]. Indeed, CBTI treatment response effect sizes for improvements in ISI, SOL, WASO, and SE% are highly similar between the present study and those reported in the MsFLASH telemedicine CBTI trial [16]. When comparing our results with other MsFLASH trials for the treatment of menopausal insomnia, CBTI and SRT treatment effects in our study were 2–3 times larger than those produced by HRT (estradiol), antidepressant medication (escitalopram, venlafaxine), yoga, and exercise [34]. Taken together, these recent studies support face-to-face and telemedicine CBTI and SRT as efficacious and durable first-line treatments for menopausal insomnia.
The present study also adds to the literature in several key ways: This trial is the first to test CBTI efficacy in women with DSM-5 chronic insomnia disorder that onset or was exacerbated during or after the menopause transition as diagnosed by a behavioral sleep medicine specialist. This criterion was to ensure that the menopause transition triggered or worsened insomnia in our patients, rather than to focus on insomnia that preexisted menopause and simply persisted through the transition without change. Additionally, the present study is the first to demonstrate the superiority of CBTI for menopausal insomnia to an insomnia-focused minimal treatment control condition (i.e. SHE) that approximates a real-world comparator as sleep hygiene is often the nonpharmacological treatment as usual for insomnia. This finding confirms that menopause-related insomnia is a serious medical complaint that requires specialty intervention to treat adequately, and that simply improving sleep hygiene for women with menopausal insomnia has very little benefit by itself.
Our study is also the first to compare two nonpharmacological insomnia-focused treatments for menopausal insomnia: CBTI vs SRT. SRT originated as a standalone treatment and is empirically supported as an effective behavioral intervention for insomnia disorder [19]. As a component of CBTI, sleep restriction is considered one of the more active components [19, 35, 36]. And, to the best of our best knowledge, these two treatments have never been directly compared in an RCT, despite several insomnia trials each for CBTI and SRT. But along these lines, Epstein et al. compared SRT to SRT + stimulus control therapy (SCT, goal is to reduce cues associated with arousal and sleep incompatibility, see Bootzin and Perlis [37] for in-depth analysis of SCT), which are both CBTI components but do not comprise a full course of CBTI [36]. Patients receiving SRT showed large improvements in insomnia symptoms at 3 and 12 months after treatment, and gains were similar to those reported by patients who received combined SRT + SCT. However, remission rates were nearly twice as high in the SRT + SCT condition (43.9%) than in the SRT condition (22.7%).
Our findings were rather similar to Epstein’s dismantling results [36] such that CBTI and SRT produced large improvements in most sleep parameters in the acute period after treatment and 6 months later. Along these lines, remission rates based on the ISI did not differ between CBTI and SRT. However, remission rates based on SOL + WASO and SE% suggested that patients receiving CBTI may be more likely to remit than those receiving SRT; these data are consistent with Epstein’s remission findings comparing multicomponent insomnia treatment with SRT. Where our study findings diverge, however, is in our demonstration that multicomponent insomnia intervention—CBTI in our case—produces more durable long-term effects on sleep maintenance as evidenced by more efficient sleep, less time awake in the middle of the night, and longer nightly sleep duration compared to SRT. It is possible that the additional components in CBTI, by providing a broader set of personalized tools to utilize following treatment, allow patients to address future exacerbations of insomnia on their own, thereby preventing relapse. Indeed, our findings here are similar to Harvey’s deconstruction findings that CBTI and behavioral therapy for insomnia produce similar acute treatment effects, but that CBTI’s treatment gains are more durable than those produced by behavioral interventions alone [38]. We therefore propose that CBTI and SRT are both highly effective and appropriate standalone treatments for menopausal insomnia, but that CBTI may produce higher rates of insomnia remission and better long-term outcomes. Yet, it is also important to emphasize that SRT produced largely similar treatment effects in 2 weeks as compared to 6 weeks for CBTI, thus indicating SRT is an impressively effective acute insomnia intervention.
These comparative results are somewhat inconsistent with our a priori hypotheses, as we predicted long-term treatment response to be substantially better for CBTI patients than SRT patients. Despite SRT’s prior support as a standalone treatment [19, 35, 36], we anticipated that the additional components of CBTI (stimulus control, cognitive therapy, relaxation, and sleep hygiene) would have substantial incremental value to treatment and reinforce longer-term adaptive sleep behaviors when compared with SRT. Naturally, it is therefore incumbent on us to consider why CBTI did not outperform SRT to the extent that we might expect, based on the empirical support for the other components of CBTI [35].
One potential explanation is that a six-session regimen for CBTI is simply insufficient to allow some other components to produce substantial treatment effects. For instance, standard CBTI has been criticized for inadequately addressing insomniogenic thinking patterns [39]. Given that insomniacs and individuals at risk for insomnia show cognitive hyperarousal profiles similar to individuals with anxiety and depression [40–44], it is perhaps unsurprising that a single session of cognitive therapy (as part of a standard six-session CBTI regimen) may have little incremental value. In other words, in clinical practice, it is difficult to produce substantial and durable changes to entrenched maladaptive thinking patterns in a single session of cognitive therapy. But in a well-intentioned attempt to create a briefer and more affordable insomnia treatment and to address the shortage of sleep specialists qualified to deliver the treatment, CBTI has been reduced from a 10-session protocol [45] (or perhaps more in real-world practice, as guided by treatment response) down to commonly eight sessions and now down to six sessions or even fewer [15, 46, 47]. It is possible that certain components of CBTI have suffered as a result, particularly components that may require more time to gain traction. Indeed, evidence suggests that cognitive therapy for insomnia takes longer to produce gains than behavioral insomnia treatment [38]. And while many patients benefit and fully remit with brief insomnia therapies, other patients—perhaps with more complex etiology or comorbidity or some other distinguishing factors—may benefit from more extensive CBTI treatment regimens, like additional sessions of cognitive therapy. Recent evidence also supports the efficacy of mind-body approaches like mindfulness and yoga to reduce menopause-related insomnia symptoms [48, 49]. These noninsomnia focused treatments may serve as critical add-ons or adjunctive therapies for patients requiring higher levels of care. Such approaches are often utilized in clinical care and are the crux of precision medicine.
To improve personalized medicine, healthcare providers may move toward more flexible models, such as stepped-care, that match treatment to patient need. To maximize efficacy, women presenting with menopause-related insomnia to primary care or obstetrics–gynecology physicians may be referred to sleep medicine specialists who can first administer a brief dose of treatment delivered via telephone [16], video [50], or an automated internet-based interactive digital environment [51, 52]. Nonremitters can then “step up” to more intensive treatment including larger doses of CBTI or extending specific components depending on the nature of the refractory symptomatology.
Limitations and future directions
The present study should be interpreted in light of certain limitations. Our primary limitation concerns a lack of follow-up assessments beyond 6 months after treatment. Longer-term prospective data would improve our understanding of the durability of these effects in postmenopausal women. However, a recent study suggests that durability of CBTI is maintained 10 years after treatment [53]. Regarding generalizability, PSG verification of WASO is not required for insomnia diagnosis nor is it typically performed in clinical practice, which can limit the generalizability of our results to a broader patient population. Furthermore, our sample was recruited from the Metro Detroit area and certain racial and ethnic groups were either under- or completely unrepresented, such as individuals identifying as Hispanic, Asian, or Middle Eastern, which may limit generalizability. In addition, the three conditions had different treatment delivery modalities and dosing (SHE = 6 weekly emails; SRT = 2 in-person sessions and 3 phone calls over 2 weeks; CBTI = 6 weekly face-to-face sessions), which may have contributed to differences in treatment effects. Related, posttreatment outcomes for the CBTI and SHE treatments (i.e. 6 week treatment regimens) were collected 6 weeks after pretreatment baseline, whereas SRT (i.e. a 2-week treatment regimen) posttreatment outcomes were collected 2 weeks after pretreatment baseline. It is unclear if and how these differences in posttreatment data collection schedules may have affected results. Even so, delaying the posttreatment SRT collection until 6 weeks after baseline was decided against as there would have then been a 4 week delay between treatment completion and posttreatment evaluation. Finally, due to examining seven outcomes at two different posttreatment time points, the present study included multiple comparisons, which increases risk for type II errors.
Conclusions
CBTI and SRT are both viable and highly efficacious treatment options for postmenopausal women with insomnia. Patients receiving CBTI have remission rates of 54%–84% and SRT patients have remission rates of 38%–57%. Higher remission rates and greater improvements in sleep maintenance suggest that CBTI may be a superior treatment option for most women. Even so, SRT requires fewer treatment sessions than CBTI and produces similarly sized treatment effects as CBTI; therefore, SRT represents an especially attractive treatment option for patients limited in their ability to attend multiple weekly treatment sessions and may thus be an appropriate first-line option for consideration in the context of a stepped-care approach. Lastly, although SHE is a common insomnia-focused treatment and may slightly improve some aspects of sleep, our results roundly reject it as an appropriate standalone treatment for menopausal insomnia.
Funding
This study was funded by the National Institute of Nursing Research (R01 NR013959, PI: Drake). Dr. Cheng’s effort was supported by the National Heart, Lung, and Blood Institute (K23 HL138166, PI: Cheng).
Conflict of interest statement. Dr. Drake has received research support from Merck & Co., Eisai Co., Aladdin Dreamer, Jazz, Actelion, and Teva; and has served on speakers bureau for Merck & Co. Dr. Kalmbach has received research support from Merck & Co. Dr. Cheng has received research support from Harmony Biosciences. No other financial or nonfinancial interests exist.
Work Performed: Henry Ford Health System
References
- 1. Kravitz HM, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 2. Eichling PS, et al. Menopause related sleep disorders. J Clin Sleep Med. 2005;1(3):291–300. [PubMed] [Google Scholar]
- 3. Attarian H, et al. Treatment of chronic insomnia disorder in menopause: evaluation of literature. Menopause. 2015;22(6):674–684. [DOI] [PubMed] [Google Scholar]
- 4. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- 5. Woods NF, et al. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle midlife women’s health study. Sleep. 2010;33(4):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu HC, et al. Exploring quality of sleep and its related factors among menopausal women. J Nurs Res. 2005;13(2):153–164. [DOI] [PubMed] [Google Scholar]
- 7. Montplaisir J, et al. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause. 2001;8(1):10–16. [DOI] [PubMed] [Google Scholar]
- 8. Polo-Kantola P, et al. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178(5):1002–1009. [DOI] [PubMed] [Google Scholar]
- 9. Investigators WGftWsHI. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 10. Force UPST. Hormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the US preventive services task force. Ann Intern Med. 2005;142(10):855. [PubMed] [Google Scholar]
- 11. Dorsey CM, et al. Effect of zolpidem on sleep in women with perimenopausal and postmenopausal insomnia: a 4-week, randomized, multicenter, double-blind, placebo-controlled study. Clin Ther. 2004;26(10):1578–1586. [DOI] [PubMed] [Google Scholar]
- 12. Dobkin RD, et al. Ramelteon for the treatment of insomnia in menopausal women. Menopause Int. 2009;15(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soares CN, et al. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomized controlled trial. Obstet Gynecol. 2006;108(6): 1402–1410. [DOI] [PubMed] [Google Scholar]
- 14. Qaseem A, et al. ; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 15. Riemann D, et al. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13(3):205–214. [DOI] [PubMed] [Google Scholar]
- 16. McCurry SM, et al. Telephone-based cognitive behavioral therapy for insomnia in perimenopausal and postmenopausal women with vasomotor symptoms: a MsFLASH randomized clinical trial. JAMA Intern Med. 2016;176(7):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Medicine AAoS. International Classification of Sleep Disorders. 3rd ed. (ICSD-3). Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Pub; 2013. [Google Scholar]
- 19. Miller CB, et al. The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep Med Rev. 2014;18(5):415–424. [DOI] [PubMed] [Google Scholar]
- 20. Perlis ML, et al. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. Vol 1 New York, USA: Springer Science & Business Media; 2006. [Google Scholar]
- 21. Stepanski EJ, et al. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7(3):215–225. [DOI] [PubMed] [Google Scholar]
- 22. Carney CE, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bastien CH, et al. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 24. Morin CM, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drake C, et al. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. [DOI] [PubMed] [Google Scholar]
- 26. Morris SB, et al. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7(1):105–125. [DOI] [PubMed] [Google Scholar]
- 27. Pillai V, et al. Towards quantitative cutoffs for insomnia: how current diagnostic criteria mischaracterize remission. Sleep Med. 2016;26:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lichstein KL, et al. Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445. [DOI] [PubMed] [Google Scholar]
- 29. Shaver J, et al. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11(6):556–561. [DOI] [PubMed] [Google Scholar]
- 30. Kalleinen N, et al. Sleep and the menopause – do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 2008;14(3):97–104. [DOI] [PubMed] [Google Scholar]
- 31. Shaver JL, et al. Sleep and menopause: a narrative review. Menopause. 2015;22(8):899–915. [DOI] [PubMed] [Google Scholar]
- 32. Shin C, et al. Prevalence of insomnia and its relationship to menopausal status in middle-aged Korean women. Psychiatry Clin Neurosci. 2005;59(4):395–402. [DOI] [PubMed] [Google Scholar]
- 33. Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–1268. [DOI] [PubMed] [Google Scholar]
- 34. Guthrie KA, et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and self-reported sleep quality in women with hot flashes: a pooled analysis of individual participant data from four MSFLASH trials. Sleep. 2018;41(1):zsx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harvey L, et al. Insomniacs’ reported use of CBT components and relationship to long-term clinical outcome. Behav Res Ther. 2002;40(1):75–83. [DOI] [PubMed] [Google Scholar]
- 36. Epstein DR, et al. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. Sleep. 2012;35(6):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bootzin RR, et al. Stimulus control therapy. In: Behavioral Treatments for Sleep Disorders. London: Elsevier; 2011: 21–30. [Google Scholar]
- 38. Harvey AG, et al. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: a randomized controlled trial. J Consult Clin Psychol. 2014;82(4):670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harvey AG. A cognitive theory and therapy for chronic insomnia. J Cogn Psychother. 2005;19(1):41. [Google Scholar]
- 40. Baglioni C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. [DOI] [PubMed] [Google Scholar]
- 41. Fernandez-Mendoza J, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernández-Mendoza J, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4):397–403. [DOI] [PubMed] [Google Scholar]
- 43. Pillai V, Drake CL. Sleep and repetitive thought: the role of rumination and worry in sleep disturbance. In: Sleep and Affect. London: Elsevier; 2015:201–225. [Google Scholar]
- 44. Fernandez-Mendoza J, et al. Cognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res. 2014;23(5):489–498. [DOI] [PubMed] [Google Scholar]
- 45. Morin CM. Insomnia: Psychological Assessment and Management. New York, NY:Guilford Press; 1993. [Google Scholar]
- 46. Taylor DJ, et al. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry. 2014;26(2):205–213. [DOI] [PubMed] [Google Scholar]
- 47. Okajima I, et al. A meta‐analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9(1):24–34. [Google Scholar]
- 48. Afonso RF, et al. Yoga decreases insomnia in postmenopausal women: a randomized clinical trial. Menopause. 2012;19(2):186–193. [DOI] [PubMed] [Google Scholar]
- 49. Hachul H, et al. Complementary and alternative therapies for treatment of insomnia in women in postmenopause. Climacteric. 2014;17(6):645–653. [DOI] [PubMed] [Google Scholar]
- 50. Zia S, et al. Sleep telemedicine: an emerging field’s latest frontier. Chest. 2016;149(6):1556–1565. [DOI] [PubMed] [Google Scholar]
- 51. Christensen H, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333–341. [DOI] [PubMed] [Google Scholar]
- 52. Espie CA, et al. Use of the internet and mobile media for delivery of cognitive behavioral insomnia therapy. Sleep Medicine Clinics. 2013;8(3):407–419. [Google Scholar]
- 53. Castronovo V, et al. Long-term clinical effect of group cognitive behavioral therapy for insomnia: a case series study. Sleep Med. 2018;47:54–59. [DOI] [PubMed] [Google Scholar]