Abstract
Study Objectives
Females demonstrate heightened central sensitization (CS), a risk factor for chronic pain characterized by enhanced responsivity of central nervous system nociceptors to normal or subthreshold input. Sleep disruption increases pain sensitivity, but sex has rarely been evaluated as a moderator and few experiments have measured CS. We evaluated whether two nights of sleep disruption alter CS measures of secondary hyperalgesia and mechanical temporal summation in a sex-dependent manner. We also evaluated differences in measures of pain sensitivity.
Methods
Seventy-nine healthy adults (female n = 46) participated in a randomized crossover experiment comparing two consecutive nights of eight pseudorandomly distributed forced awakenings (FA [−200 min sleep time]) against two nights of undisturbed sleep (US). We conducted sensory testing the mornings following Night 2; the heat-capsaicin pain model was used to induce secondary hyperalgesia.
Results
FA reduced total sleep time (REM and NREM Stage 3) more profoundly in males. We observed divergent, sex-dependent effects of FA on secondary hyperalgesia and temporal summation. FA significantly increased secondary hyperalgesia in males and significantly increased temporal summation in females. Sex differences were not attributable to differential sleep loss in males. FA also significantly reduced heat-pain threshold and cold pressor pain tolerance, independently of sex.
Conclusions
Sleep disruption enhances different pain facilitatory measures of CS in males and females suggesting that sleep disturbance may increase risk for chronic pain in males and females via distinct pathways. Findings have implications for understanding sex differences in chronic pain and investigating sleep in chronic pain prevention efforts.
Keywords: sleep disruption, pain sensitivity, central sensitization, sex differences, temporal summation, secondary hyperalgesia, capsaicin, chronic pain, sex effects
Statement of Significance
Chronic pain disorders are costly, often intractable, and differentially affect females. Elucidation of mechanisms and modifiable risk factors is needed. Sleep disruption is one promising risk factor; cross-sectional studies suggest that sleep disturbance is associated with central sensitization, a process that amplifies pain. Experiments determining whether sleep disruption causes central sensitization are lacking. Findings from this experiment indicate that sleep disruption may augment central sensitization in males and females differently. In males, sleep disruption induced secondary hyperalgesia, a feature commonly associated with neuropathic pain. In females, it increased temporal summation, a phenomenon common in many chronic pain disorders. These data link sleep disruption to central pain–processing alterations associated with chronic pain risk and support studies targeting sleep to prevent chronic pain.
Introduction
Chronic pain and insomnia disorders differentially affect females and are major contributors to health care costs and disability [1–5]. Insomnia-related sleep disruption, including multiple and prolonged nightly awakenings, is one of the most disabling chronic pain comorbidities, affecting 50%–88% of people with chronic pain [6, 7]. Furthermore, epidemiological studies demonstrate that self-reported sleep disruption (i.e. sleep maintenance problems) confers a two-to-three–fold risk of developing a new chronic pain disorder, particularly in females [8]. Moreover, sleep disruption predicts the emergence, progression, and persistence of musculoskeletal pain [8–10], risk of transitioning from acute to chronic pain, and progression of localized regional pain to a widespread disorder [11, 12].
The mechanisms by which sleep disruption increases risk for chronic pain remain largely unknown [13]. Experimental studies demonstrate that sleep loss increases pain sensitivity measured via quantitative sensory tests of pain threshold and tolerance [14–18]. However, these measures convey minimal information about possible alterations in central or peripheral pain processing that might explain the paths linking sleep disruption and chronic pain. Moreover, the possibility that sex influences the effects of sleep disruption on these measures has largely been neglected [14–18].
In contrast, measures of central sensitization (CS), a core feature of chronic pain etiology [19, 20], provide an assessment of possible neuroplastic alterations and aberrant responsivity of central nervous system nociceptive neurons to normal or subthreshold afferent input. Quantitative sensory tests of CS in humans assess both pain facilitatory and inhibitory responses [21, 22] and hence are especially relevant to understanding the mechanisms of chronic pain in which both deficient pain inhibitory capacity and increased pain facilitation are often found [23]. Recent data also suggest that there are sex differences in vulnerability to CS, consistent with sex differences in chronic pain risk, which have implications for higher prevalence of fibromyalgia, migraine, chronic widespread pain, and persistent postoperative pain in females when compared with males [24–26].
Experimental sleep disruption may also alter measures of CS. For example, two studies, focusing exclusively on female cohorts and using CS measures of pain inhibition, demonstrated that two consecutive nights of sleep disruption impaired pain inhibition in healthy women [27, 28], and that one night of total sleep deprivation impaired pain inhibitory capacity in females, but not males [29].
Similarly, sleep disruption is suggested to alter CS measures of pain facilitation, including mechanical secondary hyperalgesia (2° HA) and temporal summation (TS). With respect to 2° HA, this measure has specific relevance to chronic neuropathic pain pathophysiology, which can be experimentally modeled by administration of subcutaneous or topical capsaicin [30], a selective vanilloid-receptor excitotoxin [31]. Briefly, the treated area is heated to sensitize primary afferents [30, 32, 33], and 2° HA develops to mechanical stimulation in the surrounding untreated skin, dependent on hyperexcitability of spinal dorsal horn interneurons [34–36] and expansion of the receptive fields of A-fibers [37]. A single cross-sectional study reported that short sleepers demonstrate increased 2° HA [38], but few studies have evaluated sex differences in 2° HA [39–41], and to the best of our knowledge, no study has assessed the effects of any form of experimental sleep loss on 2° HA and related sex differences.
TS is enhanced in a variety of chronic idiopathic and musculoskeletal pain disorders such as fibromyalgia and temporomandibular joint disorder [42, 43]. Heightened TS correlates with increased risk of chronic pain [43–47] and is implicated in the development of acute and chronic postoperative pain [48]. Briefly, TS can also be experimentally modeled by demonstrating enhancement of pain caused by repeated noxious stimulation, due to sensitization of second-order dorsal horn neurons in the spinal cord [49–51]. A single cross-sectional study found that insomnia severity is associated with heightened TS in females with knee osteoarthritis [52]. Unfortunately, experimental data linking sleep loss and TS are limited [29, 53], and no study has examined sex differences in TS in response to sleep disruption.
To address these gaps, this study used an experimental model of sleep disruption which produces a profile of multiple, prolonged nocturnal awakenings, similar to what is observed in chronic pain patients with insomnia [54]. The primary objective of the current investigation was to evaluate the effects of two nights of experimental sleep disruption (forced awakenings, FA) on CS measures of pain facilitation, 2° HA and TS. The secondary objective was to examine whether the effects of FA on 2° HA and TS responses differ by sex. Additionally, given the absence of research on biological variability of pain responses to sleep loss [29], we also explored effects of sleep disruption on quantitative sensory testing measures of heat-pain threshold, pressure pain threshold, cold pain tolerance, and skin flare reaction to heat-capsaicin while investigating potential sex differences.
Methods
Participants
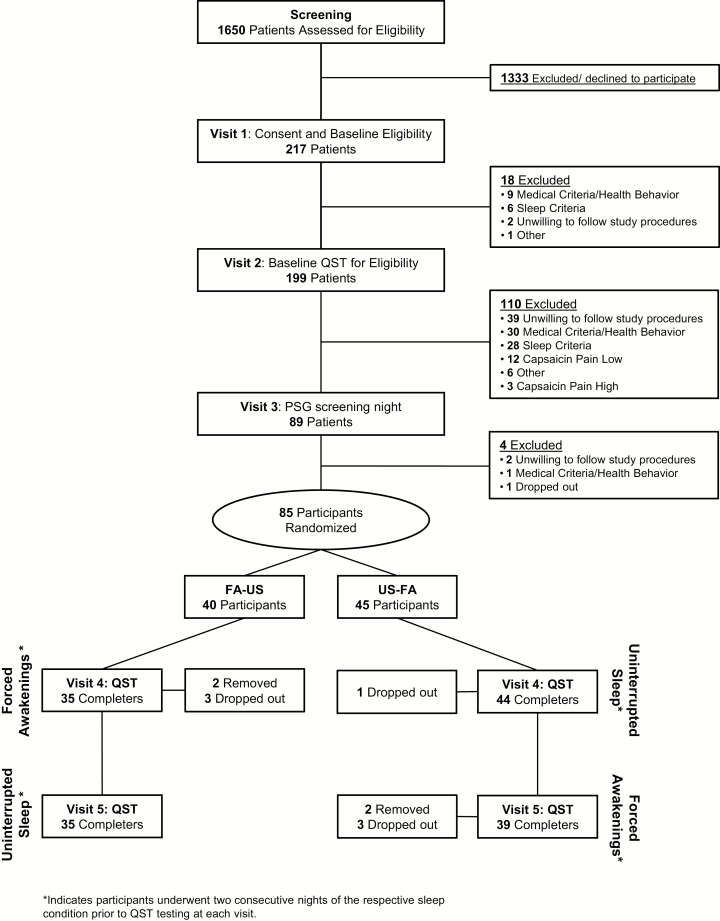
The current report presents data from 79 healthy, good-sleeping participants (46 females and 33 males) enrolled in a randomized controlled, single-blinded, crossover experiment, which was conducted over 5 days during two separate inpatient admissions to the Johns Hopkins Bayview Medical Center, Clinical Research Unit (CRU) in Baltimore, MD. Baseline demographics and clinical characteristics of the sample are presented in Table 1. Participants were recruited via community fliers, print, and electronic media. After passing a phone screen, participants completed three screening visits to determine eligibility. Inclusion and exclusion criteria are listed in Table 2. All participants completed informed consent. The protocol was approved by the Johns Hopkins University Institutional Review Board and complies with the Declaration of Helsinki. Figure 1 is a study design schematic and consort diagram describing participant flow through the protocol. Eighty-five participants were randomized. At Visit 4, prior to obtaining any QST data, four participants dropped out and two were removed (one for low blood pressure and one for cardiac arrhythmia found on polysomnography [PSG]).
Table 1.
Demographics
| Total participants | 79 |
| Age (Mean ± SD) | 27.18 ± 6.98 |
| Sex, n (%) | |
| Female | 46 (58.2%) |
| Male | 33 (41.8%) |
| Hormonal contraception, n (%) | |
| Yes | 12 (26.1%) |
| No | 34 (73.9%) |
| Race, n (%) | |
| Caucasian | 34 (43.0%) |
| African American | 26 (32.9%) |
| Asian | 10 (12.7%) |
| Multiracial | 6 (7.60%) |
| Other/decline to state | 3 (3.80%) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 13 (16.5%) |
| Education, n (%) | |
| High School/Some College | 26 (32.9%) |
| College Graduate | 41 (51.9%) |
| Graduate Studies | 12 (15.2%) |
| Employment, n (%) | |
| Student | 27 (34.2%) |
| Working for Pay | 38 (48.1%) |
| Unemployed | 13 (16.4%) |
| Homemaker | 1 (1.3%) |
| Clinical Variables, mean (SD) | |
| PSQI Total Score | 1.99 (1.43) |
| ESS | 3.63 (2.39) |
| BMI | 26.07 (4.72) |
| SBP | 110.56 (11.43) |
| DBP | 68.91 (9.41) |
PSQI = Pittsburgh Sleep Quality Index (higher values indicate worse sleep quality, <5= good sleeper); ESS = Epworth Sleepiness Scale (higher values indicate greater daytime sleepiness); BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria |
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
Figure 1.
Study consort diagram. Following initial screening procedures for eligibility, which included behavioral, health, and sleep assessments, participants were randomized to receive FA or Undisturbed Sleep (US) for their first inpatient visit (Visit 4). Following a minimum 2 week washout period, participants received the opposing sleep condition for their second inpatient visit (Visit 5). Participants who failed to pass screening assessments are listed along with rationale for their exclusion.
Study design and overview
Briefly, after a PSG screening/adaptation night, participants underwent two consecutive nights of either FA or undisturbed sleep (US). The order of this within-participant sleep condition was counter-balanced (randomized) with a minimum 2 week washout period of habitual sleep in their home environment, before completing the opposing sleep condition. For safety, participants were provided an opportunity for a third night of recovery sleep following the FA protocol (not included in the data analysis).
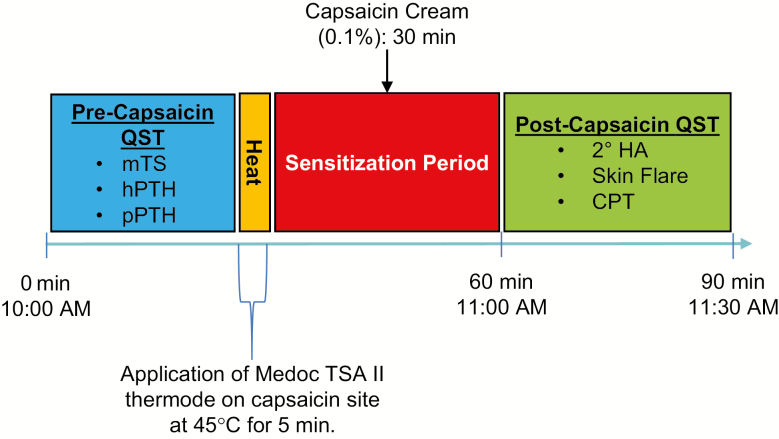
The day after the second night of both FA and US, participants completed the quantitative sensory testing protocol, starting with a precapsaicin assessment of mechanical TS, and heat and pressure-pain threshold testing (order randomized). Following completion of precapsaicin testing, participants then underwent the heat-capsaicin sensitization procedure, followed by postcapsaicin testing, including 2° HA, skin flare testing, and cold pressor pain tolerance (Figure 2).
Figure 2.
Quantitative sensory testing protocol. Following two nights of FA or Undisturbed Sleep (US), participants underwent daytime quantitative sensory testing. Precapsaicin quantitative sensory–testing measures included mechanical TS, hPTH, and pPTH. After 5 min of 45°C heat application via Medoc ATS II thermode to a predetermined location on the medial forearm, 0.1% capsaicin cream was applied and allowed to rest 30 min. Following removal of the capsaicin cream, postcapsaicin assessments included 2° HA, skin flare, and cold pressor pain tolerance testing (CPT).
Screening phase
At Visit 1, participants completed informed consent, a urine toxicology screen, anthropometry, and the following standardized questionnaires: Pittsburgh Sleep Quality Index [55], Epworth Sleepiness Scale [56], Brief Pain Inventory—short form [57], and a Health History Form [58]. The Epworth Sleepiness Scale and Pittsburgh Sleep Quality Index have well established cut-off criteria demarcating excessive daytime sleepiness [59] and poor sleep [60], respectively. The Health History Form obtained detailed information related to medical disorders, pain history, medications, and health behaviors (caffeine, exercise, etc.). Women completed detailed information related to their average menstrual cycle length, use of birth control, and first and last days of most recent menstrual cycle. The Structured Clinical Interview for DSM-IV Psychiatric Disorders Patient Questionnaire [61] identified the presence of current Axis I disorders, including substance abuse, for exclusion. The Structured Interview for Sleep Disorders [62] was used to identify and exclude the presence of any sleep disorder by history. The Time-line Follow-Back procedure [63] was used to obtain opioid use history. Participants were trained on standard electronic PDA Sleep and Pain Diaries [64–67], which they completed daily for 2 weeks at three periods during the study: (1) screening prior to Visit 2; (2) prior to the first inpatient stay (Visits 3 and 4); and (3) prior to the second inpatient visit (Visit 5) to confirm normal sleep and pain-free status. Morning entries queried information to calculate sleep continuity parameters. Evening entries queried for daytime pain [57], fatigue, naps, medications, and menstruation status. We estimated the menstrual phase of our female participants using the daily sleep diaries data and self-reported information about cycle length from their Health History Form. We identified the following phases: early follicular—participant was estimated to be in days 1–6 of her cycle; ovulatory phase—participant was estimated to be in days 14–16 of her cycle; and premenstrual phase (late luteal)—participant was estimated in days 22–28 of her cycle, or 84–89 if taking 90 day birth control medication.
At Visit 2, sleep diaries were reviewed and participants underwent a medical history and physical exam. Urine and blood samples were provided. Complete blood count with differential, comprehensive metabolic panel, toxicology, and pregnancy test (females) was performed and evaluated by a study physician to make final medical eligibility determinations. Participants were also familiarized and pretested with all of the quantitative sensory–testing procedures, including the heat-capsaicin procedure as described below. Pretesting is routinely performed, especially with the capsaicin pain model because a small percentage of participants experience no pain from the procedure. Pretesting also served to rule out participants who experienced such high levels of pain (>85/100) that sleep deprivation would likely make the procedure intolerable and induce drop out. As shown in Figure 1, 12 (6%) participants ruled out because they did not experience pain from capsaicin and three (1.5%) ruled out because pretesting capsaicin pain was too intense.
Twenty-four hours prior to and during CRU inpatient admissions (Visits 3–5), participants refrained from taking analgesics, hypnotics, and other centrally acting (e.g. caffeine) or anti-inflammatory agents. Visit 3 served as an adaptation night and additionally screened for occult sleep disorders. Registered PSG technicians conducted a nocturnal polysomnogram according to standard technical guidelines [68]. Participants were provided an 8 hr sleep opportunity in a private room. Lights out occurred at the participant’s average bedtime per diary. We used the American Academy of Sleep Medicine–recommended placement for electroencephalography, electrooculography, electromyography, and electrocardiography, acquiring electrophysiological signals using an Embla N7000 polysomnograph. Respiratory function and effort were measured via orinasal thermistor, nasal air pressure transducer, pulse oximetry, and abdominal and thoracic plethysmography belts. After Visit 3 (Night 1 of inpatient stay), we abbreviated the montage, removing anterior tibialis EMGs and all respiratory sensors. All records were scored according to the American Academy of Sleep Medicine guidelines, by a registered PSG technician and reviewed by a board certified in sleep medicine physician. Both were blind to study hypotheses. The morning after Night 1, the screening PSG was reviewed, and those participants exhibiting an apnea–hypopnea index score of >10 or periodic limb movement with arousal index of >15 were ruled out and referred for clinical care.
Experimental phase
Participants passing Visit 3 remained in the CRU and were randomized to receive either FA or US as their first sleep condition for Visit 4. Participants randomized to receive FA first (two continuous nights of disrupted sleep) completed their US sleep condition (Visit 5) after a minimum 2 week washout period. Similarly, participants first randomized to receive two nights of US returned a minimum of 2 weeks later to complete two nights of FA (Visit 5). Participants were not permitted to sleep outside their prescribed nocturnal schedule or leave the CRU during their inpatient stay (Visits 3–5). Nursing staff continuously monitored, maintained, and regularly documented wakefulness. Participants were provided a standard heart healthy diet free of fried, high-fat and high-sodium foods. Breakfast was served around 7:30 am, lunch at 12 pm (except during quantitative sensory testing, when lunch was served at 3 pm), and dinner by 5:30 pm.
Sleep conditions
Forced awakenings
The FA condition was conducted on each of two consecutive nights as described previously [27]. Briefly, an 8 hr sleep opportunity period starting from lights out was divided into eight, 1 hr intervals. One of the intervals was randomly determined to be a 60 min awakening, during which no sleep was permitted. Each of the remaining seven 60 min intervals were subdivided into tertiles (three 20 min blocks). A forced 20 min awakening was randomly scheduled to occur in either the first, second, or third tertile of each hour. During FA, PSG signals were monitored and nursing staff kept participants awake by having them sit up in bed with the lights on to reduce the chance of microsleep. The maximum total sleep time (TST) possible was 280 min.
Undisturbed sleep
An 8 hr (480 min) period of undisturbed sleep.
Daytime quantitative sensory testing procedures and protocol
Figure 2 depicts the precapsaicin pain testing, heat-capsaicin sensitization procedures, and postcapsaicin pain testing. Precapsaicin testing began roughly 2 ½ hr following breakfast and postcapsaicin testing was conducted at approximately 11 am, following the two consecutive nights of the US and FA sleep conditions. A pharmacological intervention and additional sensory testing sessions were conducted after the postcapsaicin testing session, as part of a different set of research aims, which will be presented in a future report. All technicians and staff conducting pain-testing procedures completed a rigorous training process that included interrater reliability testing (Κ > .80). All assessments were conducted by staff who were blind to participant conditions and hypotheses.
Precapsaicin quantitative sensory testing measures of mechanical TS and pain threshold
Trials of mechanical TS, heat-pain threshold, and pressure-pain threshold (pPTH) were conducted in a randomized order according to standardized testing procedures used in our laboratory.
Mechanical temporal summation
TS refers to the central nervous system enhancement of pain caused by repeated noxious stimulation [45]. To evaluate TS of mechanical pain, a custom-crafted, weighted pinprick stimulator with a flat contact area of 0.2 mm diameter, calibrated to deliver 512 mN of force was used. Pain ratings (“0” = “no pain” and “100” = “the worst pain imaginable”) were obtained in response to a single punctate stimulus and in response to a sequence of 10 identical punctate noxious stimuli using similar procedures as described previously [69]. One TS trial was performed bilaterally on the ventral surface of the forearm. After rating the single pinprick stimulus, the technician administered the train of identical 10 pinprick stimuli at a 1/s rate within an area of 1 cm2. After the train, patients rated the peak pain experienced during the sequence. A wind-up ratio (WUR) was calculated as the peak pain rating of trains divided by the pain rating of the single stimuli [69]. We assigned a minimum value of 1 in order to calculate the WUR in cases when a participant rated the initial or peak rating as zero.
Heat-pain threshold
Heat-pain threshold (hPTH) was assessed via a computer driven, peltier-element-based stimulator (Medoc TSA II, neurosensory Analyzer, Ramat Yishai, Israel) with a 9 cm2 advanced thermal stimulation thermode according to similar procedures detailed previously [58]. Briefly, hPTH was assessed on the ventral forearm using an ascending method of limits paradigm; from a 32°C baseline, the temperature was steadily increased at 0.5°C/s until the participant reported the first sensation of pain. We conducted two trials separated by >3 min. The thermode was affixed via Velcro straps to ensure even skin contact and repositioned to an adjacent site after each trial to avoid sensitization. We averaged the two temperatures from both trials to index hPTH.
Pressure-pain threshold
To measure pPTH, patients completed two trials at the metacarpophalangeal joint of thumb, bilaterally, according to procedures described previously [70]. A 2 min interval between trials was maintained. The technician used a digital algometer (SBMEDIC Electronics, Solna, Sweden) with a 1 cm [2] hard rubber probe and pinch handle attachment to apply pressure steadily at a constant rate of 30kPA/s, until the patient indicated that the stimulus “first feels painful.” An average of all four pressure measurements obtained was used in statistical analyses.
Heat-capsaicin sensitization and postcapsaicin quantitative sensory testing
We utilized procedures similar to established, published protocols [32, 71], using a Medoc TSA II, computer-driven device with a peltier thermode. The procedure involves a 35 min sensitization period (Figure 2). A 9 cm2 treatment site, the size, and shape of the thermode were randomly assigned and marked on either a lower or upper, nonoverlapping surface of the ventral forearm. Lower and upper treatment sites were counterbalanced across admissions. We selected sites that did not overlap with the site where hPTH was previously assessed. The thermode was secured on the skin with velcro straps and heated at 45°C for 5 min. An open square, raised adhesive frame patch (internal dimensions same as thermode), was then immediately applied to the borders of the treatment site and 0.35–0.40 g capsaicin cream (0.1% capsaicin, Capzasin HP for Arthritis Pain Relief) was evenly spread onto the skin, covered with Tegaderm, and permitted to absorb for 30 min. The raised frame prevents spillage or leakage of capsaicin outside the treatment site. Capsaicin was then removed and postcapsaicin testing was performed in the sequence and as described below.
Secondary hyperalgesia
We quantified the area of 2° HA to mechanical stimulation using a 5.18 (15.0 g) von Frey filament by stimulating along eight linear paths around the capsaicin treated site in 5 mm increments, at 1 s intervals [32]. The 15.0 g filament is a nonpainful stimuli, typically perceived as light touch. Stimulation started well outside the hyperalgesic area and continued towards the treated area until the participant reported a change in sensation. The border was marked on the skin with a pen and traced to acetate paper. The degree of 2° HA was quantified by calculating the surface area using a planimeter (Planix 10S).
Skin flare
Following removal of the capsaicin, the surface area of redness, or “flare,” induced by the capsaicin was traced from the forearm onto a sheet of acetate paper and measured using a digital planimeter (PLANIX 10S). Flare is a neurogenic inflammatory response (axon reflex vasodilation) associated with capsaicin [72].
Cold pressor–pain tolerance testing
Cold pressor tolerance (CPT) was conducted last, after 2° HA and skin flare testing due to potential carry-over effects of this procedure [58]. There was a 10 min time differential between skin flare testing and CPT. Participants immersed their hand of the noncapsaicin–treated arm up to their wrist in a circulating, cold water bath (4°C) for as long as possible, up to an uninformed 5 min (300 s) maximum time limit [73]. When the sensations became intolerable, participants removed their hand and the duration of submersion was recorded as the index of CPT.
Data analytic strategy
Mixed-effects modeling using restricted maximum-likelihood estimation was used to evaluate primary hypotheses due to the within-participant design, which allows for estimation of both intraindividual and interindividual variations [74]. A random intercept was included in all models. The distribution of residuals for each model was inspected for normality, and dependent variables were log-transformed (natural log) in the presence of non-normal residuals. This was necessary for two of the dependent variables: TS and cold water tolerance. The primary effect of interest in all models was the 2 × 2 interaction of sleep condition (US vs. FA) × sex (male vs. female). If the interaction was not significant, it was removed from the model and the main effects of sleep condition and sex were interpreted.
All models covaried age, race, ethnicity, and BMI, which have been shown to vary as a function of sleep [75–78]. Menstrual phases were covaried to account for variation across women in menstrual cycle at the time of testing. Nonsignificant covariates (p > .2) were removed from final models. Analyses were performed in R (version 3.3.3) and lme4 for mixed-effect estimation (version 1.1.13).
Results
Participant characteristics
Demographics and other participant characteristics of the 79 participants contributing at least partial quantitative sensory–testing data are presented in Table 2. The sample was young and racially diverse. Consistent with the inclusion criteria, participants were nonobese and had normal blood pressure and reported good sleep quality, as measured by the Pittsburgh Sleep Quality Index. Thirty-five per cent of the females reported taking hormonal contraception.
Manipulation check: effects of sleep disruption on sleep continuity and architecture
Review and analyses of sleep diary data demonstrated that participants reported similar average mean sleep latency (SL; 12.87 ± 12.08 min), wake after sleep onset (WASO; 6.76 ± 8.96 min), TST (455.3 ± 47.36 min), and sleep efficiency (SE; 95.8 ± 3.00%) during the screening period and the week prior to the US and FA conditions, all within the normal range for these parameters [79, 80].
Means and standard deviations of PSG-derived measures of sleep continuity and architecture, organized by sleep condition and sex, are presented in Table 3. Averaged over US Nights 1 and 2, male and female participants did not differ on any PSG-measured parameter of sleep continuity, including SL, WASO, TST, or SE, or on any measure of sleep architecture (Stages N1, N2, N3 nonrapid eye movement [NREM] [minutes or percentage of TST]), or REM (rapid eye movement) latency (p >.18). Confirming the efficacy of the manipulation, averaged across Nights 1 and 2, the FA condition significantly reduced TST and SE and increased WASO across participants (both sexes analyzed separately; p < .001). We found a significant sex × sleep condition interaction effect such that males evidenced a significantly greater loss of TST (p < .005) and decrement in SE (p < .05), as a result of the FA manipulation (averaged across nights) compared with females.
Table 3.
Effects of experimental sleep conditions on sleep continuity and architecture by sex
| US1 | US2 | FA1 | FA2 | Mean Difference (FA1,2)–(US1,2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | |
| Sleep continuity, N(SD) | 33 | 45 | 33 | 45 | 31 | 43 | 31 | 42 | N/A | N/A |
| SL | 15.32 (17.91) | 22.02 (38.48) | 16.07 (14.64) | 20.25 (24.98) | 27.84 (17.91) | 32.25 (38.48) | 22.15 (17.91) | 17.22 (38.48) | 9.91 (23.98)*** | 3.45 (24.76) |
| WASO | 29.28 (33.51) | 21.51 (19.95) | 26.98 (39.98) | 19.49 (20.28) | 240.81 (33.51) | 200.93 (19.95) | 196.83 (33.51) | 192.42 (19.95) | 189.58 (51.74)# | 176.12 (33.29)# |
| TST | 435.56 (36.9) | 434.86 (43.65) | 437.14 (41.51) | 433.49 (57.74) | 209.15 (36.9) | 246.8 (43.65) | 261.11 (36.9) | 269.27 (43.65) | −200.71 (47.12)# | −175.64 (45.49)#‡ |
| SE% | 90.71 (7.67) | 90.9 (8.89) | 91.03 (8.63) | 91.3 (7.76) | 43.78 (7.67) | 51.42 (8.89) | 54.39 (7.67) | 56.22 (8.89) | −41.68 (9.74)# | −37.22 (8.42)#† |
| Sleep architecture, N(SD) | 33 | 45 | 33 | 45 | 31 | 43 | 31 | 42 | NA | N/A |
| Stage 1 | 21.13 (25.91) | 16.09 (11.86) | 17.63 (8.29) | 14.68 (11.22) | 25.16 (25.91) | 21.58 (11.86) | 19.02 (25.91) | 18.91 (11.86) | 2.1 (12.77) | 4.13 (11.49)*** |
| Stage 2 | 209.99 (53.47) | 219.62 (50.48) | 198.99 (52.84) | 214.2 (48.94) | 103.4 (53.47) | 119.31 (50.48) | 115.75 (53.47) | 123.25 (50.48) | −96.25 (47.07)# | −96.75 (39.89)# |
| Stage N3 (SWS) | 111.87 (58.32) | 107.06 (39.89) | 116.71 (48.82) | 108.74 (40.19) | 55.18 (58.32) | 68.87 (39.89) | 73.53 (58.32) | 73.89 (39.89) | −47.8 (38.36)# | −35.41 (31.39)#† |
| REM | 92.57 (28.68) | 92.09 (29.06) | 103.8 (30.89) | 95.88 (25.67) | 25.41 (28.68) | 37.03 (29.06) | 52.81 (28.68) | 53.22 (29.06) | −58.77 (28.72)# | −47.6 (21.37)#† |
| REM Latency | 94.89 (46.35) | 95.11 (40.74) | 80.97 (38.63) | 80.98 (35.24) | 199.98 (46.35) | 174.99 (40.74) | 131.82 (46.35) | 142.66 (40.74) | 73.3 (78.66)# | 70.19 (71.97)# |
Values shown represent mean (SD) in minutes.
US1,2 = Undisturbed Sleep, Night 1 and Night 2, respectively; FA1,2 = Forced Awakenings Nights 1 and 2, respectively; M = male; F = female; SL = sleep onset latency; SE% = sleep efficiency percentage (TST/time in bed); Stages 1–3 = nonrapid eye movement stages 1–3; SWS = slow-wave sleep.
***p < .005; #p < .001; significance level of sleep condition (FA vs US with sex).
† p < .05; ‡p < 0.005; significance level of SEX BY sleep condition interaction.
With respect to sleep architecture, averaged across Nights 1 and 2, the FA condition significantly reduced the number of minutes spent in Stages N2, N3, and REM and significantly decreased REM latency in all participants (both sexes analyzed separately; p < .001).
Minutes spent in Stage N1 significantly increased from US to FA in females (p < .005) but not males. We found a significant sex × sleep condition interaction such that males evidenced significantly greater reductions in both Stage N3 and REM than females (averaged across Nights 1 and 2; p < .05).
Effects of sleep disruption on 2° HA
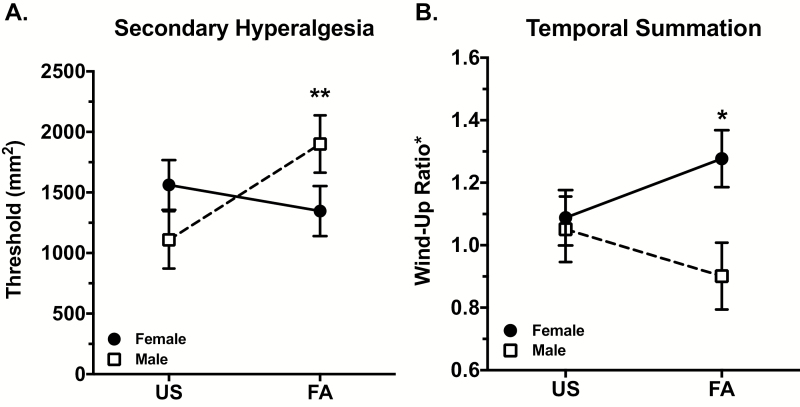
For all statistical models described below, Table 4 provides a summary of the full mixed-effects models, including intercepts, covariates, effect coefficients, and error terms. For 2° HA, initial tests revealed that race, ethnicity, age, and BMI were nonsignificant and failed to contribute to model fit and were removed from the final analyses. In the resulting model, there was a significant sleep condition × sex interaction (B = 1008.13, SE = 366.56, t = 2.75, p = .006). As displayed in Figure 3A, the interaction was characterized by an increase in 2° HA from US to FA that was evident in males but not females. Post hoc tests confirmed that FA induced a significant 792 mm2 increase in 2° HA in males (p = .008), with no significant effects in females (−215 mm2 decrease; p = .332). Because our manipulation check found that males demonstrated significantly greater decrements in TST, SE, REM sleep, and N3 during the FA challenge, we conducted secondary analyses to adjust for sleep at each condition in order to determine whether this differential sleep loss might explain the finding that males, but not females, demonstrate increased 2° HA due to FA. To accomplish this, we created four binary variables (dummy coded) indicating whether the participant had a specific sleep parameter in the top or bottom 25% for US and FA, respectively. We then included these dummy codes in the primary statistical models and evaluated whether they modified the magnitude or the significance of the sleep condition × sex interaction terms or any of the main effects. We did this for each of the PSG sleep parameters separately (TST, SE, REM minutes, and N3 minutes) for 2° HA. We found that neither the significance (all p’s < .005) nor the magnitude of the sleep condition × sex interaction changed substantively. We obtained similar findings when we included mean sleep parameters as covariates instead of the upper and lower quartile dummy codes. These analyses indicate that differential sleep by sex at each sleep condition did not alter the significant sleep condition × sex interaction found for 2° HA.
Table 4.
Summary of multivariate statistical models (intercepts, covariates, effects, and variance coefficients on QST outcomes)
| Heat-pain threshold | Cold pain tolerance | Pressure-pain threshold | Secondary hyperalgesia | Temporal summation | Skin flare | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P | Mean | P | Mean | P | Mean | P | Mean | P | Mean | P | |
| Intercept | 45.63 | 0.00 | 2.797 | 0.001 | 316.89 | 0.018 | 1562.57 | 7.87 × 10−14 | 1.088 | 6.22 × 10−34 | 3093.32 | 1.99 × 10−5 |
| Ovulation | −1.33 | 0.223 | N/A | N/A | 118.26 | <0.005 | N/A | N/A | N/A | N/A | N/A | N/A |
| PreMenstrual | −1.42 | <0.001 | N/A | N/A | −23.19 | 0.111 | N/A | N/A | N/A | N/A | N/A | N/A |
| Black | N/A | N/A | −0.937 | 0.001 | N/A | N/A | N/A | N/A | N/A | N/A | −557.41 | 0.02 |
| Hispanic | 1.83 | 0.022 | −0.431 | 0.232 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Age | −0.069 | 0.107 | N/A | N/A | −3.888 | 0.135 | N/A | N/A | N/A | N/A | N/A | N/A |
| BMI | N/A | N/A | 0.058 | 0.082 | 7.325 | 0.108 | N/A | N/A | N/A | N/A | −38.76 | 0.167 |
| Sleep Condition | −0.41 | 0.026 | −0.145 | 0.017 | −0.804 | 0.909 | −215.50 | 0.370 | 0.1888 | 0.045 | 246.96 | 0.086 |
| Sex | −0.59 | 0.333 | 0.137 | 0.612 | 36.02 | 0.331 | −454.35 | 0.154 | −0.037 | 0.790 | −523.20 | 0.020 |
| Sex × Sleep Condition | N/A | N/A | N/A | N/A | N/A | N/A | 1008.13 | *0.006 | −0.339 | *0.02 | N/A | N/A |
| Variance of Individual Intercepts | 6.175 | 1.264 | 23880.00 | 606800.00 | 0.1751 | 547100.00 | ||||||
| Residual Variance | 2.462 | 0.1387 | 6769.00 | 1186000.00 | 0.3885 | 760100.00 | ||||||
Values include mean and p-values for each quantitative sensory testing (QST) measure with the inclusion of select covariates to test for model fit. Covariates were included in the overall model if they were p ≤ .2, otherwise they were dropped.
N/A = not applicable (covariate dropped from the model). The two QST measures that displayed a significant Sex × Sleep Condition, with no significant covariates, were secondary hyperalgesia (p = .006) and temporal summation (p = .02).
Figure 3.
The effects of sleep disruption on measures of central sensitization by sex. For both 2° HA and mechanical TS, there was an overall significant sleep condition × sex interaction (*p < .05, **p < .01; Table 4). (A) Post hoc tests confirmed that FA induced a significant 792 mm2 increase from Undisturbed Sleep (US) in 2o HA selectively in males (p = .008), with no significant effects in females (p > .05). (B) Post hoc analyses revealed that females demonstrated a significant increase in wind-up ratio of .185 (p = .042) from US to FA. Males had no significant changes in TS (p > .05). Graphs display marginalized means ± standard errors derived from mixed-effects models adjusting for the effects of covariates found to contribute significantly to the model.
Effect of sleep disruption on mechanical TS
There was a significant sleep condition × sex interaction on TS [B = −.34, SE = .15, t = −2.33, p = .02]. As displayed in Figure 3B, the interaction was characterized by an increase in TS from US to FA that was evident in females but not males. Post hoc tests confirmed that FA induced a significant increase of .185 in WUR from US to FA (p = .042) in females, whereas no significant effects were observed in males (−.151; p = .191). Of note, analyses revealed that out of 611 data points collected, only 23 (3.8%) of those rated as having 0 both at the first and tenth weighted probe contact. Four participants reported greater pain at the initial probe contact compared with the tenth; however, the differences between them were small.
We utilized transformed data because raw WUR data have the potential to influence group means and to enforce symmetry of the models residuals. We conducted additional analyses using delta-change scores (peak—first prick), which is sometimes used instead of WUR and observed no change in consistency in the effect direction, shape, or significance of the findings (p < .05).
Because our manipulation check found that females demonstrated significantly less decrement in TST, SE, REM sleep, and N3 during the FA condition, we conducted secondary analyses similar to those performed on the 2° HA data, to evaluate whether sex differences associated with these sleep variables might explain the TS findings. We included the upper and lower quartile dummy codes for each of the four sleep parameters at FA and US in four separate models (one sleep parameter in each model). Similar to the 2° HA findings, neither the significance (all p’s < .004) nor the magnitude of the sleep condition × sex interaction changed substantively for TS. This indicates that the small, but significant differential resilience to FA in females does not account for the finding that females, under FA, demonstrated increased TS relative to males.
Menstrual phase characteristics and effects on the models
During the US condition, 10 women were menstruating, 10 women were estimated to be premenstrual, and no women were estimated to be ovulating. Under the FA condition, 12 women were menstruating, 10 were premenstrual, and two were estimated ovulating. To evaluate whether menstrual cycle might explain sex differences observed for 2° HA or TS, we incorporated each of these menstrual phases as covariates into the mixed-effects models. Ovulation was found to be a significant covariate for pPTH (p < .005), and premenstrual was found to be significant or heat-pain threshold (p < .001). Although there was a reduced number of women categorized in each menstrual phase group, we included these covariates in the heat and pPTH models because they significantly improved model fit by explaining a substantial amount of variance. Despite this, there was no significant interaction of sleep condition × sex for these measures. For the remaining quantitative sensory–testing measures, menstrual phases were nonsignificant covariates that failed to contribute to model fit and thus removed from the overall model.
Effects of sleep disruption on measures of pain threshold, tolerance, and skin flare
For all statistical models described below, Table 4 provides a summary of the full mixed-effects models, including intercepts, covariates, effect of coefficients, and error terms.
Heat-pain threshold
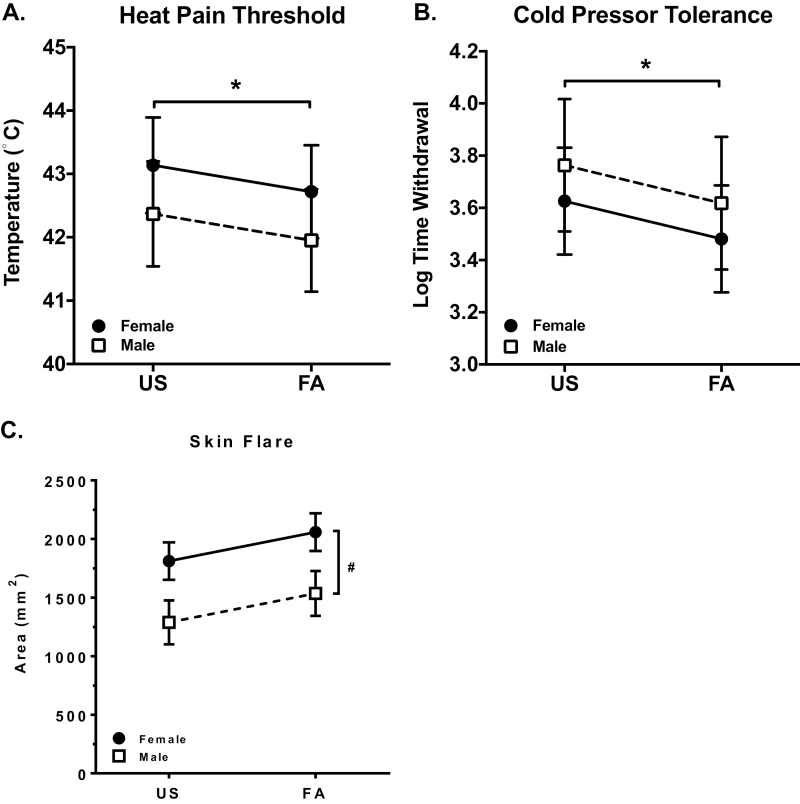
Initial tests revealed that race and BMI were nonsignificant and failed to contribute to model fit. The resulting model showed that hPTH differed as a function of sleep condition, with significantly lower thresholds (increased pain sensitivity) observed following FA compared with US (B = −.41, SE = .19, t = −2.23, p = .03). The sleep condition × sex interaction was not statistically significant (p = .12), although males trended toward lower hPTH following FA compared with females (Figure 4A). In this model, the premenstrual phase covariate was statistically significant (B = −1.42, p < .001) such that women estimated to be in the premenstrual phase of their cycle had lower heat-pain thresholds.
Figure 4.
Effects of sleep disruption on hPTH, CPT, and skin flare response by sex. Statistical models for each assessment are listed in Table 4. (A) Both hPTH, adjusted for age, ethnicity (Hispanic), phase ovulatory and phase premenstrual, and (B) CPT, adjusted for race (African American), ethnicity (Hispanic), and BMI, differed as a function of sleep, with participants displaying significantly reduced thresholds following FA compared with undisturbed sleep (US) (*p < .05). (C) Skin flare, adjusted for BMI and race (African American), differed as a function of sex, with females showing greater hyperemic response than males at both sleep conditions (#p < .05). Graphs display marginalized means ± standard errors.
Pressure-pain threshold
Initial tests revealed that race and ethnicity were not significant and failed to contribute to model fit. In the resulting model, neither sleep condition (p = .91) nor sex (p = .33) was significantly associated with pPTH. Additionally, the sleep condition × sex interaction was not significant (p = .36).
Cold-pain tolerance
Initial tests revealed that menstrual phase and age were nonsignificant and failed to contribute to model fit. The resulting model showed a significant main effect for sleep condition (B = −.15, SE = .06, t = −2.38, p = .02), reflective of a decrease in CPT following FA relative to US (Figure 4B). Neither sex (p = .61) nor the sleep condition × sex interaction (p = .39) was significant.
Skin flare response to heat-capsaicin
The mixed-effects model showed significant main effects for sex (B = −523.21, SE = 225.76, t = −2.32, p = .02), and a trend for a main effect of sleep condition (B = 246.96, SE = 144.05, t = 1.7, p = .08). The interaction of sleep condition × sex was not significant (p = .95). As shown in Figure 4C, females evidenced a greater hyperemic response than males, and both sexes evidenced a trend for an increase in hyperemic response following FA compared with US.
Discussion
The primary objective of this randomized crossover experiment was to evaluate whether sleep disruption due to FA, compared with undisturbed sleep (US), alters 2° HA and mechanical TS in a sex-dependent manner. We found divergent, sex-dependent effects of FA on 2° HA and TS such that sleep disruption significantly expanded the surface area of 2° HA in males, but not females, and significantly increased TS in females, but not males. These novel findings suggest that sleep disruption enhances CS and increases risk for chronic pain in both males and females via different central pain facilitatory pathways and compliment an emerging literature that sex also moderates the effects of sleep loss on CS measures of pain inhibition [27–29].
A secondary objective was to evaluate whether sex moderates the effects of sleep disruption on measures of pain sensitivity and tolerance. We found that sleep disruption significantly reduced hPTH and CPT similarly in both males and females, and observed no effect of FA on pPTh. We found a trend toward increased skin flare after FA, and a significant overall sex effect with females demonstrating greater hyperemia, irrespective of sleep condition.
Sleep disruption enhances 2° HA in males but not females
To the best of our knowledge, this is the first study to evaluate the effects of any form of experimental sleep loss on 2° HA, a measure of spinal CS [20]. These data extend a cross-sectional finding that healthy individuals reporting <6.5 hr/night demonstrate increased 2° HA compared with those sleeping ≥6.5 hr per night [38], by providing experimental evidence that sleep disruption augments 2° HA in males. Classic studies using anesthetic blockades demonstrate that 2° HA fails to develop without spinal involvement [35]. Studies using capsaicin have shown that 2° HA develops to mechanical, but not heat, stimulation in untreated skin [34, 35, 81, 82]. This enhanced sensitivity is dependent on the hyperexcitability of second-order spinal dorsal horn nociceptors [34–36], which expand the receptive fields of mechanosensitive (capsaicin insensitive) A-fibers to transduce pain from normally nonpainful mechanical stiumulation [37]. Spinal functional magnetic resonance imaging (fMRI) imaging shows that 2° HA corresponds to increased activity in spinal dorsal gray matter with concomitant supraspinal deactivation of descending inhibitory pathways in the rostral ventromedial medulla and dorsolateral pontine tegmentum [36]. The molecular mechanisms by which these nociceptors in the spinothalamic tract are sensitized to heat with low intensity stimulation of peripheral mechanoreceptors, outside the primary zone of capsaicin treated skin, have yet to be fully elucidated. Secondary hyperalgesia can be blocked by spinal administration of glutamate and/or substance p receptor antagonists, suggesting a major role for these neurotransmitters in this form of CS [83, 84]. Both preclinical and recent molecular imaging studies suggest that sleep deprivation enhances N-methyl-D-aspartic acid (NMDA) glutamate receptor function in the central nervous system, supporting the possibility that alterations in glutamatergic synaptic neuroplasticity may be one potential mechanism by which sleep disruption induces 2° HA [85–87]. Mechanistic studies using animal models are needed to investigate this possibility.
The finding that sleep disruption induces 2° HA is clinically significant because the sensory features of 2° HA, including pain sensitivity to light touch (allodynia) and referred pain, are similar to those observed in neuropathic pain conditions [81, 82, 88]. Indeed, the capsaicin model is the most widely used human model of neuropathic pain [30]. Consequently, our current data suggest that males with sleep disruption may be at heightened risk, compared with males without sleep disruption, for chronic neuropathic pain disorders. Insomnia-related sleep disruption is one of the most prominent symptoms associated with neuropathic pain, linked to greater pain intensity and persistence [89–92]. Together, these data and the current finding that sleep disruption may contribute to CS in males support the possibility that insomnia treatments, such as cognitive behavioral therapy [93], which consolidate and lengthen sleep, may prevent the development of chronic neuropathic pain in males and/or improve treatment response.
Few studies have evaluated whether there are general sex effects in heat-capsaicin–induced 2° HA and have yielded conflicting results. Jensen et al. found that females demonstrated heightened 2° HA sensitivity to brush stimulation but not von Frey stimulation [39]. Two studies of intradermally injected capsaicin found enhanced mechanical 2° HA in females compared with men [40, 41]. None of these studies assessed or controlled for sleep disturbances. Given the high prevalence of insomnia and insufficient sleep in neuropathic pain, our findings argue for the need to measure sleep as an important potential confounder in studies evaluating sex differences in pain sensitivity and 2° HA.
Interestingly, although the FA sleep manipulation robustly affected both sexes, we found that males demonstrated greater disruption in sleep architecture (stages REM and N3) and SE, losing 25 min more TST averaged over two nights of FA compared with females. The possibility that human females may be more resilient to sleep disruption is a novel finding requiring replication, which is not without preclinical precedent [94]. This raises the possibility that the observed effect of FA on 2° HA might be due to differential sleep loss in males. Our secondary analyses, however, demonstrated no significant alterations in the significance of the sleep condition by sex interaction when sleep parameters were controls in the models. This indicates that the degree of differential sleep loss does not account for the significant sex × sleep condition interactions on 2° HA. Future studies are needed to elucidate distinct physiological and molecular responses to sleep disruption that may explain why males develop 2° HA after sleep disruption, but females do not.
Sleep disruption enhances TS in females, but not males
We found that sleep disruption significantly increased TS selectively in females. To the best of our knowledge, this is the first study to evaluate whether experimental sleep disruption alters TS. In humans, TS refers to the enhancement of pain intensity caused by repeated noxious stimulation that is held at a constant intensity. Animal studies have shown that TS (wind-up) involves sensitization of second-order dorsal horn C-fibers in the spinal cord [49–51, 95]. Moreover, TS has been robustly implicated in pathophysiological models of chronic pain [21, 22]. TS is enhanced in a variety of idiopathic chronic pain disorders [43, 45–47] and predicts the development of chronic postoperative pain [48]. Cross-sectional studies have found that reduced SE and insomnia symptoms are associated with enhanced TS [96]. A knee osteoarthritis study found a sex effect, consistent with our findings; insomnia was associated with TS in females, but not males.
Two experimental studies, however, found that a single night of total sleep deprivation did not significantly alter TS in either sex [29, 53]. This suggests that the effect may be specific to sleep disruption. The impact of two nights of sleep disruption may be physiologically distinct from the effects of one night of total sleep deprivation. Future studies are needed to replicate and directly compare the moderating effects of sex on TS using different types of sleep loss.
The vast majority of the evidence indicates that TS is more pronounced in females [24, 25], and therefore, the current finding raises the possibility that one of the factors contributing to the higher prevalence of chronic pain in females may be disparities in sleep disruption [97, 98]. Despite the possibility that females may be more resilient to experimental sleep disruption, females have a nearly twofold prevalence of insomnia [99]. Together with the robust longitudinal literature indicating that sleep disturbances, particularly in females, are a risk factor for new onset chronic pain [8, 10, 100, 101], the current data provide a compelling rationale to target insomnia symptoms in chronic pain prevention efforts in females.
Our finding of divergent, sex-dependent effects of FA on 2° HA and TS has important implications for chronic pain research, as it indicates that greater focus on the mechanisms underlying the similarities and differences between these distinct forms of central pain facilitation may yield important clues related to sex differences in chronic pain pathophysiology. Sex hormones are known to differentially modulate spinal nociception via multiple molecular pathways including, but not limited to alpha (2)-adrenergic, kappa opioid, and NMDA-receptor mediated hyperalgesia and/or antinociceptive circuitry [102–104]. Both 2° HA and TS involve NMDA receptor activation [51, 105], which is upregulated by sleep deprivation. Mechanistic differences between 2° HA and TS are poorly understood, but available data indicate that they likely reflect distinct processes [106–108]. The most salient distinction between the two phenomena is that 2° HA is a heterosynaptic process, involving both A- and C-fibers, whereas TS is monosynaptic, induced via direct stimulation of C-fibers [108]. Preclinical work is need to replicate and disentangle the molecular mechanisms that may explain these findings.
Effects of FA on hPTH, pressure-pain threshold, cold pressor tolerance, and skin flare
A secondary objective was to evaluate possible sex differences in pain threshold and tolerance, and skin flare response to capsaicin. Prior studies investigating various forms of sleep loss have largely neglected the examination of sex effects. We found that FA (1) reduced hPTH and CPT in a sex-independent manner, (2) had no detectable impact on pPTH, and (3) trended toward increasing the skin flare response similarly in males and females.
The finding that FA decreased hPTH independently of sex is in line with several mixed sex studies [15, 53], and other studies observing increased sensitivity to suprathreshold heat pain stimuli [16, 17]. A recent study, however, found that one night of total sleep deprivation reduced hPTH in females, but not males [29]. It is unclear how to interpret this apparent inconsistency, though it could be related to differences in the two sleep loss paradigms. We also found that women estimated to be in the premenstrual phase of their cycle demonstrated significantly reduced hPTH. Prior studies, hampered by relatively small sample sizes, have yielded inconsistent results with respect to whether menstrual phase is associated with hPTH [109–111].
With respect to CPT, our data are consistent with a recent study that one night of total sleep deprivation increased cold pressor pain ratings during a 2 min cold pressor task equally among 14 males and 13 females [112]. Other total sleep deprivation studies have not detected any significant effect of sleep deprivation on CPT [29].
We did not detect any effect of FA on pPTH. To the best of our knowledge, this is the first study evaluating whether FA alters pPTH and therefore requires replication. Studies testing slow-wave sleep–specific fragmentation on pPTH have yielded both positive [18, 113] and negative findings [114, 115]. One possible explanation for these inconsistencies may be due to differences in the degree of slow-wave sleep disruption/loss over time across protocols.
Finally, regarding skin flare response in the capsaicin model, we found that females were significantly more sensitive to skin flare, irrespective of sleep condition. This is consistent with at least two prior studies [40, 41]. Skin flare reflects a peripheral neurogenic inflammatory response to skin irritation [116]. Two studies suggest that habitual short-sleep and slow-wave sleep disruptions, respectively, are associated with heightened skin flare [38, 113]. Of note, the slow-wave sleep disruption study induced skin flare by pinching the skin rather than using a chemical irritant. We found that both men and women trended equally toward increased skin flare after FA. It is unclear whether the weaker effects observed are due to differences between acute sleep fragmentation and habitual insufficient sleep or different methods of inducing the flare response. Conversely, more prolonged sleep fragmentation/greater slow-wave sleep disruption might be required to induce heightened skin flare.
Limitations and future directions
This study has a number of limitations that should be considered. Although the current study is perhaps the largest study to date investigating the effects of any form of sleep deprivation on quantitative sensory–testing measures, larger samples with broader age ranges are needed to elucidate the complex interactions between sex, age, pain sensitivity, and CS. The extant literature, including this study, is limited to relatively young adults. Larger cohorts and study designs directly comparing multiple sleep loss paradigms are needed to tease apart which dimensions of sleep disruption and sleep loss alter specific measures of CS and pain sensitivity. It should be noted that 2° HA and TS are indirect measures of CS and that acute, experimentally induced alterations in these measures may not reflect CS changes associated with chronic sleep loss. Similarly, it is important to consider that these findings are derived from healthy pain-free individuals, using rigorous inclusion/exclusion criteria to ensure safety and minimize potential confounds. They may not necessarily translate to clinical populations and individuals whose nociceptive systems are altered by clinical pain conditions, medications, or substance of abuse. In addition, it should be noted that we did not include measures of pain inhibition in this study due participant burden concerns.
Future studies determining sex differences in sleep disruption–induced alterations in pain inhibition are warranted in light of total sleep deprivation findings [29]. The use of neuroimaging modalities is also needed to understand how supraspinal structures that modulate spinal sensitization are functionally affected by sleep loss. Another limitation is the use of sleep diaries to estimate menstrual phase in women, which is subject to imprecision due to variable cycle lengths and hormone fluctuations. The only two prior sleep deprivation studies evaluating the effects of sex on sleep loss and pain sensitivity utilized a similar approach, but also restricted the study to a specific menstrual phase. This may provide more control, but also limits generalization of the findings to the specific phase studied. Human studies of pain sensitivity changes across the menstrual cycle are inconsistent [109–111, 117–120]. Future studies measuring hormones levels to provide a better estimate of cycle phase are needed. Despite these limitations, the current study provides compelling evidence that sleep disruption induces CS differentially in males and females. Studies identifying the molecular mechanisms of these effects are likely to contribute towards elucidating chronic pain pathophysiology. These experimental findings also support future intervention studies that target insomnia and sleep disturbance to prevent the risk of chronic pain and related disorders.
Funding
This project was funded by National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) R01DA0329922 (to M.T.S. and M.R.I.) and NIH T32 NS7020110 (to M.T.S. and B.R.); NIH K23 DA035915, NIH P30 NR014131, and Blaustein Pain Research Award (to P.H.F.); and NIH K23 DA029609 (to D.A.T.).
References
- 1. Sorge RE, et al. Sex Differences in Pain. J Neurosci Res. 2017;95(6):1271–1281. [DOI] [PubMed] [Google Scholar]
- 2. Krishnan V, et al. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383–389. [DOI] [PubMed] [Google Scholar]
- 3. Skaer TL, et al. Economic implications of sleep disorders. Pharmacoeconomics. 2010;28(11):1015–1023. [DOI] [PubMed] [Google Scholar]
- 4. Daley M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. [DOI] [PubMed] [Google Scholar]
- 5. Murray CJ, et al. ; U.S. Burden of Disease Collaborators The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith MT, et al. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. [DOI] [PubMed] [Google Scholar]
- 7. Follick MJ, et al. The sickness impact profile: a global measure of disability in chronic low back pain. Pain. 1985;21(1):67–76. [DOI] [PubMed] [Google Scholar]
- 8. Bonvanie IJ, et al. Sleep problems and pain: a longitudinal cohort study in emerging adults. Pain. 2016;157(4):957–963. [DOI] [PubMed] [Google Scholar]
- 9. Sanders AE, et al. Causal mediation in the development of painful temporomandibular disorder. J Pain. 2017;18(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta A, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology (Oxford). 2007;46(4):666–671. [DOI] [PubMed] [Google Scholar]
- 11. Castillo RC, et al. ; LEAP Study Group Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124(3):321–329. [DOI] [PubMed] [Google Scholar]
- 12. Smith MT, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138(3):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finan PH, et al. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lautenbacher S, et al. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. [DOI] [PubMed] [Google Scholar]
- 15. Kundermann B, et al. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66(6):932–937. [DOI] [PubMed] [Google Scholar]
- 16. Roehrs T, et al. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. [DOI] [PubMed] [Google Scholar]
- 17. Onen SH, et al. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10(1):35–42. [DOI] [PubMed] [Google Scholar]
- 18. Moldofsky H, et al. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38(1):35–44. [DOI] [PubMed] [Google Scholar]
- 19. Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev. 2015;11(2):70–85. [DOI] [PubMed] [Google Scholar]
- 20. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4(4):299–305. [DOI] [PubMed] [Google Scholar]
- 22. Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 2012;12(5):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradley LA. Pathophysiologic mechanisms of fibromyalgia and its related disorders. J Clin Psychiatry. 2008;69(Suppl 2):6–13. [PubMed] [Google Scholar]
- 24. Bartley EJ, et al. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Racine M, et al. A systematic literature review of 10 years of research on sex/gender and pain perception – part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153(3):619–635. [DOI] [PubMed] [Google Scholar]
- 26. Schug SA, et al. Risk stratification for the development of chronic postsurgical pain. Pain Rep. 2017;2(6):e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith MT, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. [DOI] [PubMed] [Google Scholar]
- 28. Iacovides S, et al. Sleep fragmentation hypersensitizes healthy young women to deep and superficial experimental pain. J Pain. 2017;18(7):844–854. [DOI] [PubMed] [Google Scholar]
- 29. Eichhorn N, et al. The role of sex in sleep deprivation related changes of nociception and conditioned pain modulation. Neuroscience. 2018;387:191–200. [DOI] [PubMed] [Google Scholar]
- 30. van Amerongen G, et al. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br J Clin Pharmacol. 2016; 82(4):903–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol. 2002;118(1):110–121. [DOI] [PubMed] [Google Scholar]
- 32. Petersen KL, et al. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10(7):1511–1516. [DOI] [PubMed] [Google Scholar]
- 33. Petersen KL, et al. Effect of remifentanil on pain and secondary hyperalgesia associated with the heat–capsaicin sensitization model in healthy volunteers. Anesthesiology. 2001;94(1):15–20. [DOI] [PubMed] [Google Scholar]
- 34. Torebjörk HE, et al. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LaMotte RH, et al. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66(1):190–211. [DOI] [PubMed] [Google Scholar]
- 36. Rempe T, et al. Spinal fMRI reveals decreased descending inhibition during secondary mechanical hyperalgesia. PLoS One. 2014;9(11):e112325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morris V, et al. Increased capsaicin-induced secondary hyperalgesia as a marker of abnormal sensory activity in patients with fibromyalgia. Neurosci Lett. 1998;250(3):205–207. [DOI] [PubMed] [Google Scholar]
- 38. Campbell CM, et al. Self-reported sleep duration associated with distraction analgesia, hyperemia, and secondary hyperalgesia in the heat-capsaicin nociceptive model. Eur J Pain. 2011;15(6):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen MT, et al. Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers. J Pain. 2006;7(3):211–217. [DOI] [PubMed] [Google Scholar]
- 40. Gazerani P, et al. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118(1-2):155–163. [DOI] [PubMed] [Google Scholar]
- 41. Gazerani P, et al. Site-specific, dose-dependent, and sex-related responses to the experimental pain model induced by intradermal injection of capsaicin to the foreheads and forearms of healthy humans. J Orofac Pain. 2007;21(4):289–302. [PubMed] [Google Scholar]
- 42. Sarlani E, et al. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J Orofac Pain. 2007;21(4):309–317. [PMC free article] [PubMed] [Google Scholar]
- 43. Staud R, et al. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1-2):165–175. [DOI] [PubMed] [Google Scholar]
- 44. Fillingim RB, et al. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75(1):121–127. [DOI] [PubMed] [Google Scholar]
- 45. Price DD, et al. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99(1-2):49–59. [DOI] [PubMed] [Google Scholar]
- 46. Maixner W, et al. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76(1-2):71–81. [DOI] [PubMed] [Google Scholar]
- 47. Bragdon EE, et al. Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96(3):227–237. [DOI] [PubMed] [Google Scholar]
- 48. Petersen KK, et al. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156(1):55–61. [DOI] [PubMed] [Google Scholar]
- 49. Lautenbacher S, et al. Tonic pain evoked by pulsating heat: temporal summation mechanisms and perceptual qualities. Somatosens Mot Res. 1995;12(1):59–70. [DOI] [PubMed] [Google Scholar]
- 50. Arendt-Nielsen L, et al. Wind-up and neuroplasticity: is there a correlation to clinical pain? Eur J Anaesthesiol Suppl. 1995;10:1–7. [PubMed] [Google Scholar]
- 51. Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. Eur J Pain. 2000;4(1):5–15. [DOI] [PubMed] [Google Scholar]
- 52. Petrov ME, et al. Disrupted sleep is associated with altered pain processing by sex and ethnicity in knee osteoarthritis. J Pain. 2015;16(5):478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schuh-Hofer S, et al. One night of total sleep deprivation promotes a state of generalized hyperalgesia: a surrogate pain model to study the relationship of insomnia and pain. Pain. 2013;154(9):1613–1621. [DOI] [PubMed] [Google Scholar]
- 54. Bjurstrom MF, et al. Polysomnographic characteristics in nonmalignant chronic pain populations: a review of controlled studies. Sleep Med Rev. 2016;26:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 56. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. [DOI] [PubMed] [Google Scholar]
- 57. Cleeland CS, et al. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2): 129–138. [PubMed] [Google Scholar]
- 58. Smith MT, et al. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32(6):779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17(8):703–710. [DOI] [PubMed] [Google Scholar]
- 60. Buysse DJ, et al. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- 61. Spitzer R, et al. Structured Clinical Interview for DSM-IV Axis 1 Disorders. Version 2. VA: American Psychiatric Association Publishing; 1997:1–132. [Google Scholar]
- 62. Schramm E, et al. Test-retest reliability and validity of the Structured Interview for Sleep Disorders According to DSM-III-R. Am J Psychiatry. 1993;150(6):867–872. [DOI] [PubMed] [Google Scholar]
- 63. Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, eds. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992:41–72. [Google Scholar]
- 64. Monk TH, et al. The Pittsburgh sleep diary. J Sleep Res. 1994;3(2):111–120. [PubMed] [Google Scholar]
- 65. Edinger JD, et al. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–1864. [DOI] [PubMed] [Google Scholar]
- 66. Smith MT, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. [DOI] [PubMed] [Google Scholar]
- 67. Haythornthwaite JA, et al. Development of a sleep diary for chronic pain patients. J Pain Symptom Manage. 1991;6(2):65–72. [DOI] [PubMed] [Google Scholar]
- 68. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, Illinois:American Academy of Sleep Medicine; 2007. [Google Scholar]
- 69. Rolke R, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. [DOI] [PubMed] [Google Scholar]
- 70. Lee YC, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken). 2011;63(3):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dirks J, et al. The heat/capsaicin sensitization model: a methodologic study. J Pain. 2003;4(3):122–128. [DOI] [PubMed] [Google Scholar]
- 72. Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30(1):5–11. [DOI] [PubMed] [Google Scholar]
- 73. Walsh NE, et al. Normative model for cold pressor test. Am J Phys Med Rehabil. 1989;68(1):6–11. [DOI] [PubMed] [Google Scholar]
- 74. Singer JD, et al. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. London: Oxford University Press; 2003. [Google Scholar]
- 75. Song Y, et al. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med. 2011;10(1):54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rao MN, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group Association between sleep architecture and measures of body composition. Sleep. 2009;32(4):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hall MH, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 78. Appelhans BM, et al. Sleep duration and weight change in midlife women: the SWAN sleep study. Obesity (Silver Spring). 2013;21(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 80. Consensus Conference P, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11(6):591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Treede RD, et al. Multiple mechanisms of secondary hyperalgesia. Prog Brain Res. 2000;129:331–341. [DOI] [PubMed] [Google Scholar]
- 82. Treede RD, et al. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38(4):397–421. [DOI] [PubMed] [Google Scholar]
- 83. Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci. 2001;933:142–156. [DOI] [PubMed] [Google Scholar]
- 84. Dougherty PM, et al. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994;72(4):1464–1475. [DOI] [PubMed] [Google Scholar]
- 85. Kopp C, et al. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26(48):12456–12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Holst SC, et al. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hefti K, et al. Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry. 2013;73(2):161–168. [DOI] [PubMed] [Google Scholar]
- 88. Woolf CJ, et al. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–1964. [DOI] [PubMed] [Google Scholar]
- 89. Mann R, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51(7):564–570. [DOI] [PubMed] [Google Scholar]
- 90. Kim SH, et al. Risk factors associated with clinical insomnia in chronic low back pain: a retrospective analysis in a university hospital in Korea. Korean J Pain. 2015;28(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Subedi A, et al. Effect of co-morbid conditions on persistent neuropathic pain after brachial plexus injury in adult patients. J Clin Neurol. 2016;12(4):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schaefer C, et al. Health status, function, productivity, and costs among individuals with idiopathic painful peripheral neuropathy with small fiber involvement in the United States: results from a retrospective chart review and cross-sectional survey. J Med Econ. 2014;17(6):394–407. [DOI] [PubMed] [Google Scholar]
- 93. Smith MT, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015;67(5):1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Koehl M, et al. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29(9):1224–1231. [DOI] [PubMed] [Google Scholar]
- 95. Li J, et al. Windup leads to characteristics of central sensitization. Pain. 1999;79(1):75–82. [DOI] [PubMed] [Google Scholar]
- 96. Bulls HW, et al. Depressive symptoms and sleep efficiency sequentially mediate racial differences in temporal summation of mechanical pain. Ann Behav Med. 2017;51(5):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Manber R, et al. Sex, steroids, and sleep: a review. Sleep. 1999;22(5):540–555. [PubMed] [Google Scholar]
- 98. Mong JA, et al. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Roth T, et al. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5(3):5–15. [DOI] [PubMed] [Google Scholar]
- 100. Sanders AE, et al. Subjective sleep quality deteriorates before development of painful temporomandibular disorder. J Pain. 2016;17(6):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Harrison L, et al. Exploring the associations between sleep problems and chronic musculoskeletal pain in adolescents: a prospective cohort study. Pain Res Manag. 2014;19(5):e139–e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Thompson AD, et al. Sex-specific modulation of spinal nociception by alpha2-adrenoceptors: differential regulation by estrogen and testosterone. Neuroscience. 2008; 153(4):1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lawson KP, et al. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain. 2010;151(3):806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McRoberts JA, et al. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148(4):1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Staud R, et al. Effects of the N-methyl-D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain. 2005;6(5):323–332. [DOI] [PubMed] [Google Scholar]
- 106. Yucel A, et al. Comparison of hyperalgesia induced by capsaicin injection and controlled heat injury: effect on temporal summation. Somatosens Mot Res. 2004;21(1):15–24. [DOI] [PubMed] [Google Scholar]
- 107. Pedersen JL, et al. Hyperalgesia and temporal summation of pain after heat injury in man. Pain. 1998;74(2-3):189–197. [DOI] [PubMed] [Google Scholar]
- 108. Magerl W, et al. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74(2-3):257–268. [DOI] [PubMed] [Google Scholar]
- 109. Riley JL 3rd, et al. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2-3): 181–187. [DOI] [PubMed] [Google Scholar]
- 110. Fillingim RB, et al. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med. 1997;59(5): 512–520. [DOI] [PubMed] [Google Scholar]
- 111. Sherman JJ, et al. Does experimental pain response vary across the menstrual cycle? A methodological review. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R245–R256. [DOI] [PubMed] [Google Scholar]
- 112. Larson RA, et al. Total sleep deprivation and pain perception during cold noxious stimuli in humans. Scand J Pain. 2016;13:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lentz MJ, et al. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26(7):1586–1592. [PubMed] [Google Scholar]
- 114. Arima T, et al. The relationship between selective sleep deprivation, nocturnal jaw-muscle activity and pain in healthy men. J Oral Rehabil. 2001;28(2):140–148. [DOI] [PubMed] [Google Scholar]
- 115. Older SA, et al. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25(6):1180–1186. [PubMed] [Google Scholar]
- 116. Coutaux A, et al. Hyperalgesia and allodynia: peripheral mechanisms. Joint Bone Spine. 2005;72(5):359–371. [DOI] [PubMed] [Google Scholar]
- 117. Bartley EJ, et al. Comparing pain sensitivity and the nociceptive flexion reflex threshold across the mid-follicular and late-luteal menstrual phases in healthy women. Clin J Pain. 2013;29(2):154–161. [DOI] [PubMed] [Google Scholar]
- 118. Kowalczyk WJ, et al. Sex differences and hormonal influences on response to mechanical pressure pain in humans. J Pain. 2010;11(4):330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Balter JE, et al. Mechanical pain sensitivity and the severity of chronic neck pain and disability are not modulated across the menstrual cycle. J Pain. 2013;14(11):1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Klatzkin RR, et al. Menstrual cycle phase does not influence gender differences in experimental pain sensitivity. Eur J Pain. 2010;14(1):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]