Significance
Human immunodeficiency virus 1 (HIV-1) infection causes a life-long disease, due to the ability of the virus to integrate into the host genome and establish latent infection. While research has revealed a number of host restriction factors that block primary infection, much less is understood with regard to the host factors that promote or block reactivation of the integrated proviral HIV-1. In this study, we show that a member of the Apobec3A (apolipoprotein B MRNA editing enzyme catalytic subunit 3A) family, A3A, suppresses HIV-1 reactivation by recruiting chromatin-modifying enzymes to impose repressive marks around the long terminal repeat promoter region. Identification of host factors that control HIV-1 latency may provide clues for therapeutic interventions needed to remove the viral reservoir from the infected individual.
Keywords: epigenetic regulation, TRIM28, reactivation, viral latency, T cells
Abstract
HIV-1 integrates into the genome of target cells and establishes latency indefinitely. Understanding the molecular mechanism of HIV-1 latency maintenance is needed for therapeutic strategies to combat existing infection. In this study, we found an unexpected role for Apobec3A (apolipoprotein B MRNA editing enzyme catalytic subunit 3A, abbreviated “A3A”) in maintaining the latency state within HIV-1–infected cells. Overexpression of A3A in latently infected cell lines led to lower reactivation, while knockdown or knockout of A3A led to increased spontaneous and inducible HIV-1 reactivation. A3A maintains HIV-1 latency by associating with proviral DNA at the 5′ long terminal repeat region, recruiting KAP1 and HP1, and imposing repressive histone marks. We show that knockdown of A3A in latently infected human primary CD4 T cells enhanced HIV-1 reactivation. Collectively, we provide evidence and a mechanism by which A3A reinforces HIV-1 latency in infected CD4 T cells.
Despite combination antiretroviral therapy (ART), HIV-1 persists in infected individuals as a latent and integrated provirus (1–3). In resting CD4 T cells, the lack of active cellular transcription factors (4–9) and of HIV-1 Tat and its cellular cofactors (10–15) limits the initiation and elongation of viral transcription, respectively (16, 17). Understanding the mechanism of latency maintenance is key to therapeutic approaches aimed at a cure. Cellular restriction factors block various stages of the HIV-1 life cycle, including viral entry, reverse transcription, nuclear transport, and virion release (18). However, host factors that maintain HIV-1 latency are less well understood.
Apobec3A (apolipoprotein B MRNA editing enzyme catalytic subunit 3A, abbreviated “A3A”) is one of the restriction factors that suppress HIV-1 primary infection in macrophages by its cytidine deaminase activity (19). A3A also suppresses other viruses, including hepatitis B virus, human papillomavirus, Epstein–Barr virus, cytomegalovirus, and herpes simplex virus type 1, by inducing the lethal mutation in viral genomic DNA by cytidine deamination (20–23). Interestingly, although A3A has been reported to be a potent inhibitor of retroelements such as LINE-1, long terminal repeat (LTR), and Alu retrotransposons, its inhibition mechanism is cytidine deamination-independent and has not yet been elucidated (24–27).
To understand the role of A3A in HIV-1 latency, we examined the effect of A3A overexpression or knockdown on HIV-1 reactivation. We found that A3A reinforced HIV-1 latency in HIV-1 latently infected cell lines. A3A suppressed HIV-1 proviral transcription by binding to the LTR region and subsequently recruiting repressive complexes including KRAB-associated protein 1 (KAP1) and heterochromatin protein 1 (HP1), thereby inducing silencing through histone H3K9 methylation. We further demonstrate that A3A knockdown enhances HIV-1 reactivation in latently infected primary human CD4 T cells. These results reveal an unexpected role of A3A in preventing reactivation of HIV-1 and promoting the maintenance of the latency state.
Results and Discussion
Apobec3A Suppresses HIV-1 Reactivation in Latently Infected Cell Lines.
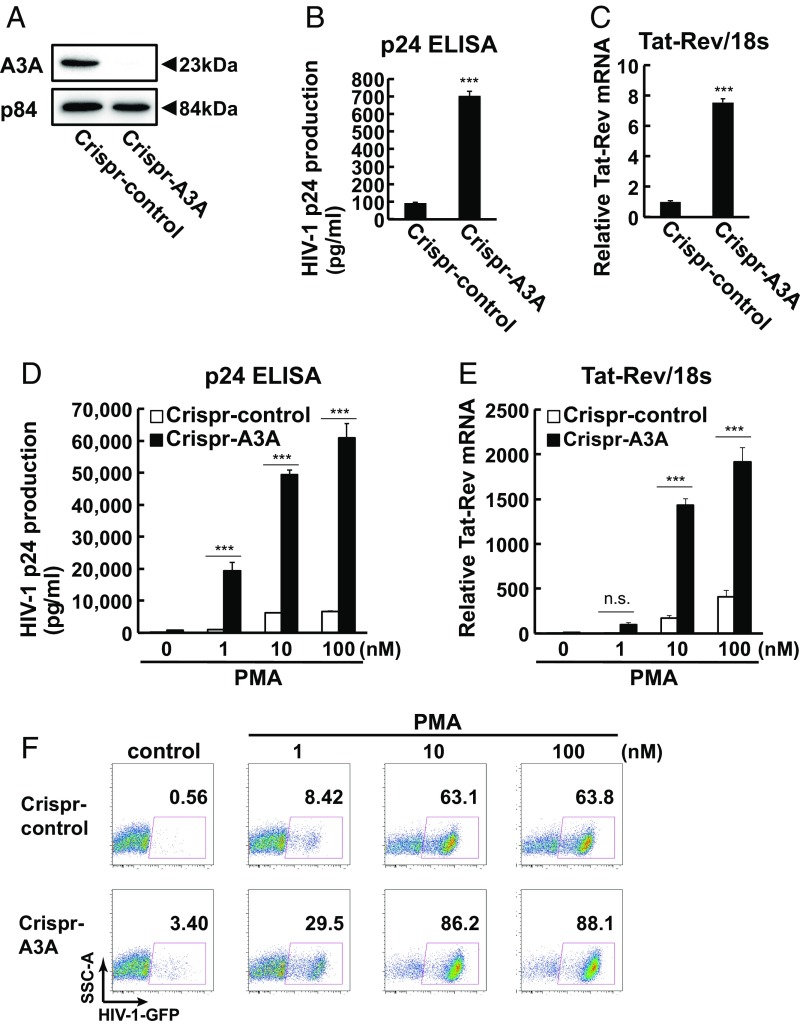
To examine the effect of A3A on HIV-1 latency, we began with siRNA knockdown and overexpression of A3A in HIV-1 latently infected cell line J-Lat10.6. J-Lat10.6 cells are a Jurkat cell line with a stably integrated HIV-1HXB2Δenv backbone that contains a GFP reporter gene in place of the nef gene. HIV-1 reactivation can be monitored through GFP expression and HIV-1 mRNA and replication-defective viral particle release in the culture medium (28). We found that knockdown of A3A led to spontaneous reactivation of HIV-1 in J-Lat10.6 latently infected cells (SI Appendix, Fig. S1 A and B). Overexpression of A3A suppressed phorbol myristate acetate (PMA)-induced HIV-1 reactivation (SI Appendix, Fig. S1 D and E). To confirm the role of A3A in the maintenance of HIV-1 latency, we generated A3A knockout J-Lat10.6 cells using CRISPR (Fig. 1A and SI Appendix, Figs. S2 and S3 A–C). Genetic deletion of A3A resulted in increased spontaneous HIV-1 reactivation, measured at both the mRNA and protein levels, compared with control guide RNA (gRNA) CRISPR-transduced cells in both a knockout cell clone and in three separate pools of A3A knockout cells (Fig. 1 B and C and SI Appendix, Fig. S2 A–D). PMA-induced HIV-1 reactivation was also enhanced in A3A knockout cells in a dose- and time-dependent manner (Fig. 1 D–F and SI Appendix, Fig. S3 D–F). Likewise, pooled A3A knockdown in a separate J-Lat line, J-Lat6.3, showed enhanced PMA-induced HIV-1 reactivation (SI Appendix, Fig. S2 E and F). These results demonstrated that A3A suppresses HIV-1 gene expression in latently infected cell lines.
Fig. 1.
Apobec3A regulates spontaneous HIV-1 reactivation. (A) A3A protein expression between CRISPR-control–transduced J-Lat10.6 (Crispr-control) and CRISPR-mediated A3A knockout J-Lat10.6 clones (Crispr-A3A). (B and C) HIV-1 p24 expression in culture medium and HIV-1 Tat-Rev mRNA expression between Crispr-control and Crispr-A3A J-Lat10.6 cell clones. Data are means ± SE (n = 3). ***P < 0.001 versus Crispr-control assessed by Student’s t test. (d–F) Crispr-control or Crispr-A3A J-Lat10.6 cell clones were treated with the indicated concentration of PMA for 6 or 18 h. HIV-1 reactivation was analyzed by p24 expression in culture medium at 18 h (D), HIV-1 Tat-Rev mRNA at 6 h (E), and flow cytometry at 18 h (F). Data are means ± SE (n = 3). ***P < 0.001 versus nontreated sample assessed by ANOVA with Tukey–Kramer’s test. n.s., not significant.
Cytidine Deaminase Activity Is Not Required for HIV-1 Latency Enforcement by A3A.
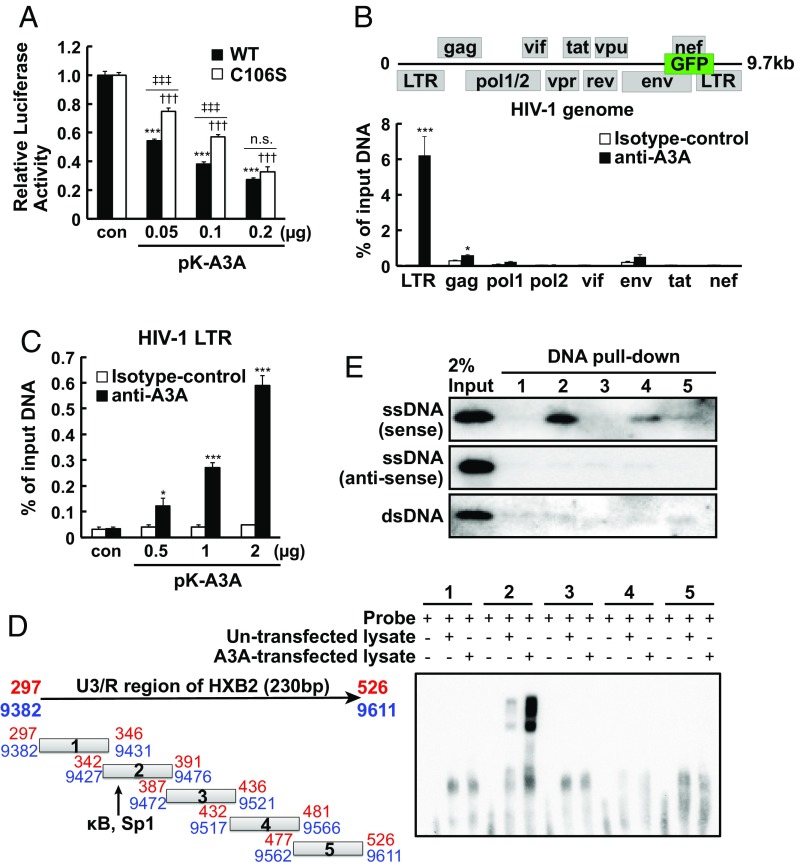
Next, we examined the requirement of cytidine deaminase activity of A3A in HIV-1 latency control. Some members of the Apobec3 family of cytidine deaminases, such as A3G, exert their inhibitory effects on primary HIV-1 infection through incorporation into virions, release into the cytosol, and deamination of newly synthesized viral cDNA in the target cells, thereby introducing lethal mutations into the HIV-1 genome (27). In contrast, A3A is excluded from being incorporated into virions (26), and acts within the nucleus of infected cells (19). In addition, A3A inhibition of adeno-associated virus and LTR and Alu retrotransposons is mediated in a deamination-independent manner (24–26). Our analysis showed that the cytidine deaminase mutant A3A C106S (26) lacked deaminase activity but suppressed HIV-1 LTR activity, albeit slightly less effectively than wild-type (WT) A3A (Fig. 2A and SI Appendix, Fig. S4 A and B). Further, PMA-induced HIV-1 reactivation was also suppressed by ectopic expression of A3A C106S, again slightly less effectively than WT A3A (SI Appendix, Fig. S4D). Thus, these data suggested that A3A suppresses HIV-1 gene expression in largely a cytidine deaminase-independent manner.
Fig. 2.
Apobec3A binds to HIV-1 LTR and suppresses its activity. (A) 293T cells were transfected with 0.2 μg of pGL2-HIV-1 5′ LTR plasmid and cotransfected with the indicated dose of A3A WT or C106S plasmids. Forty-eight hours after transfection, relative luciferase activity was determined by comparison with relevant controls. Data are means ± SE (n = 3). ***P < 0.001, †††P < 0.001 versus control, ‡‡‡P < 0.001 versus WT assessed by ANOVA with Tukey–Kramer’s test. n.s., not significant. (B) Endogenous A3A binding to the HIV-1 genome was analyzed by performing ChIP-qPCR in J-Lat10.6 cells. A3A binding to separate HIV-1 regions such as LTR, gag, pol1, pol2, vif, env, tat, and nef were examined using specific primers. Data are means ± SE (n = 3). ***P < 0.001, *P < 0.05 versus isotype control IgG assessed by ANOVA with Tukey–Kramer’s test. (C) Tzm-Bl cells were transfected with pK-A3A, and A3A binding to the HIV-1 LTR was analyzed by ChIP-qPCR. Data are means ± SE (n = 3). ***P < 0.001, *P < 0.05 versus isotype control IgG assessed by ANOVA with Tukey–Kramer’s test. (D) Nuclear extract from pcDNA3.1 or pK-A3A overexpressed 293T cells was incubated with five different biotinylated oligo-DNAs. Protein–DNA bindings were analyzed by EMSA. (E) Recombinant A3A was incubated with single-stranded (sense or antisense) or double-stranded five different biotinylated oligo-DNAs and streptavidin-coupled Dynabeads. A3A expression was examined by Western blotting.
A3A Binds to the NF-κB/Sp1–Binding Region of the HIV-1 LTR.
To probe the mechanism by which A3A enforces latency, we examined whether A3A binds the integrated HIV-1 genome. A3A was found in both the nucleus and cytosol (Fig. 3 C and D and SI Appendix, Fig. S4C), consistent with previous reports (25, 29). To analyze the binding of A3A on the HIV-1 genome, we conducted ChIP-qPCR using an anti-A3A antibody and primer set for the LTR region and HIV-1–coding gene segments within J-Lat10.6 cells (Fig. 2B). An anti-A3A antibody in ChIP-qPCR was validated by immunoprecipitation and Western blotting (SI Appendix, Fig. S9A). Endogenous A3A specifically bound the LTR of the integrated HIV-1 genome in J-Lat10.6 cells (Fig. 2B). Similarly, ectopically expressed A3A detected by anti-A3A antibody, as well as V5-tagged A3A (A3A-V5) detected by anti-V5 antibody, also associated with the 5′ LTR in a 5′ LTR/Luc–integrated Tzm-Bl cell line (Fig. 2C and SI Appendix, Fig. S5A) and inhibited PMA-induced and Tat-induced LTR activity (SI Appendix, Fig. S5 B–G). Together, these results showed that A3A binds to the LTR region of the integrated HIV-1 genome in latently infected cells.
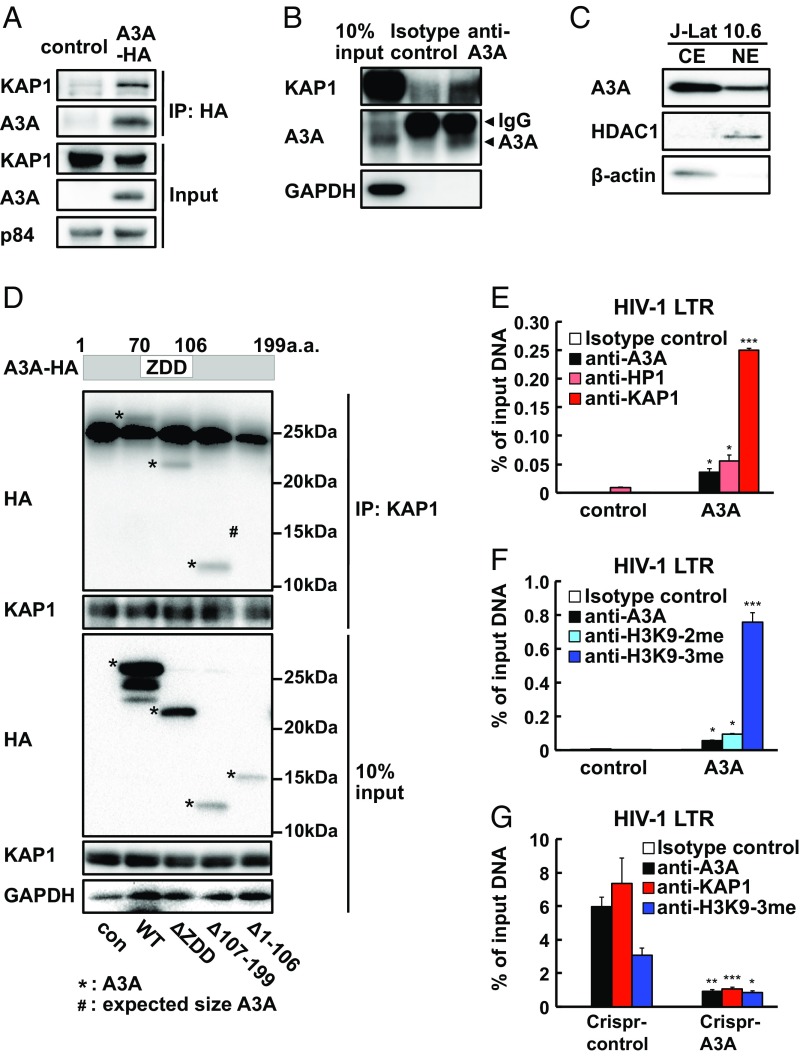
Fig. 3.
Apobec3A recruits KAP1 and HP1 to the HIV-1 LTR and induces heterochromatin formation. (A) 293T cells were transfected with A3A-HA. Forty-eight hours after transfection, total cell lysates were analyzed for protein interaction between A3A-HA and KAP1 by anti-HA immunoprecipitation and anti-KAP1 immunoblotting. (B) J-Lat10.6 cell lysate was analyzed for endogenous A3A and KAP1 interaction by anti-A3A immunoprecipitation and anti-KAP1 immunoblotting. (C) Nuclear (NE) and cytosol (CE) fractions from J-Lat10.6 were analyzed for A3A expression by Western blotting. (D) 293T cells were transfected with HA-tagged A3A-WT or A3A deletion mutants. Cell lysates were immunoprecipitated using an anti-KAP1 Ab and immunoblotted using an anti-HA antibody. (E and F) KAP1 and HP1 binding (E) and H3K9-2me and H3K9-3me histone modification (F) were examined by ChIP-qPCR targeting the LTR of HIV-1 in A3A-transfected Tzm-Bl cells. Data are means ± SE (n = 3). ***P < 0.001, *P < 0.05 versus control plasmid assessed by Student’s t test. (G) A3A, KAP1 binding, and H3K9-3me histone modifications were compared between Crispr-control and Crispr-A3A J-Lat10.6 cell clones. Data are means ± SE (n = 3). ***P < 0.001, **P < 0.01, *P < 0.05 versus WT assessed by Student’s t test.
To narrow in on the specific region within the LTR bound by A3A, we conducted an electrophoretic mobility-shift assay (EMSA) to determine whether proteins in either nontransfected or A3A-transfected cell lysates bind to five distinct dsDNA oligonucleotides spanning 230 bp within the U3 and R regions of the LTR. We observed a significant shift of the no. 2 oligonucleotide when cell lysates were added. The no. 2 oligonucleotide spans the NF-κB and Sp1 binding sites within U3 (Fig. 2D). Moreover, a DNA pull-down assay indicated that recombinant A3A specifically bound to the no. 2 sense single-stranded but not antisense single-stranded or double-stranded oligonucleotide (Fig. 2E). These results indicate that A3A directly binds the sense strand of the NF-κB– and Sp1-binding region of the HIV-1 LTR.
N-Terminal Region of A3A Is Required to Recruit KAP1 and Impose Repressive Histone Marks on HIV-1 LTR.
We next probed the mechanism by which A3A represses HIV-1 transcription. DNA methylation and repressive histone modifications are associated with silencing of proviruses (30–35). KAP1 is a well-known regulator of endogenous retroviral transcription (36), and is required for HIV-1 LTR repression (37). KAP1 engages histone modifiers such as HP1 and histone methyltransferase SETDB1 to induce di- and trimethylation of H3K9, silencing gene expression by heterochromatin formation (37). However, a study has also demonstrated a possible enhancer role of KAP1 for HIV-1 transcription (38). To determine the role of KAP1 in latency reactivation, we generated KAP1 knockout cells (SI Appendix, Fig. S6A). Genetic deletion of KAP1 resulted in both enhanced spontaneous and PMA-induced HIV-1 reactivation in J-Lat10.6 cells (SI Appendix, Fig. S6B). Therefore, in our hands, as well as reported by others (39–44), KAP1 has a repressive role in HIV-1 transcription.
Based on these data, we hypothesized that A3A binds to the LTR and recruits KAP1 to suppress HIV-1 transcription through imposing epigenetic silencing. To test this, we examined whether A3A interacts with KAP1. Both immunofluorescence microscopy and immunoprecipitation analysis showed that A3A expressed ectopically (in 293T cells; Fig. 3 A–C) and endogenously (in J-Lat10.6 cells; SI Appendix, Fig. S6 C and D) colocalized and interacted with KAP1. To identify the protein domains important for this interaction, we constructed A3A mutants that lack the zinc-dependent catalytic domain (ZDD) or C- or N-terminal domains (Fig. 3D). All mutant A3A proteins were expressed in transfected cells, albeit at varying degrees (Fig. 3D). Immunoprecipitation of A3A mutants showed that the N-terminal deletion of A3A rendered the molecule incapable of binding to KAP1 (Fig. 3D). Thus, these results indicated that the A3A N-terminal domain, but not the ZDD or the C-terminal domains, is required for KAP1 binding.
Based on the ability of A3A to recruit KAP1 to the LTR, we next analyzed whether A3A promotes H3K9 methylation of the LTR. A3A overexpression in Tzm-Bl cells enhanced not only A3A recruitment to the LTR but also increased KAP1 and HP1 recruitment (Fig. 3E). Consequently, A3A overexpression led to an increase in histone H3K9 di- and trimethylation on the 5′ LTR (Fig. 3F). Conversely, KAP1 recruitment and H3K9 trimethylation of the LTR was reduced in the A3A knockout J-Lat10.6 cell line (Fig. 3G). These results are consistent with the idea that A3A binds to the LTR region and promotes KAP1 and HP1 recruitment, thereby imposing histone H3K9 methylation and heterochromatin formation to repress HIV-1 transcription in latently infected cells.
A3A Inhibits HIV-1 Reactivation in Latently Infected Human Primary CD4 T Cells.
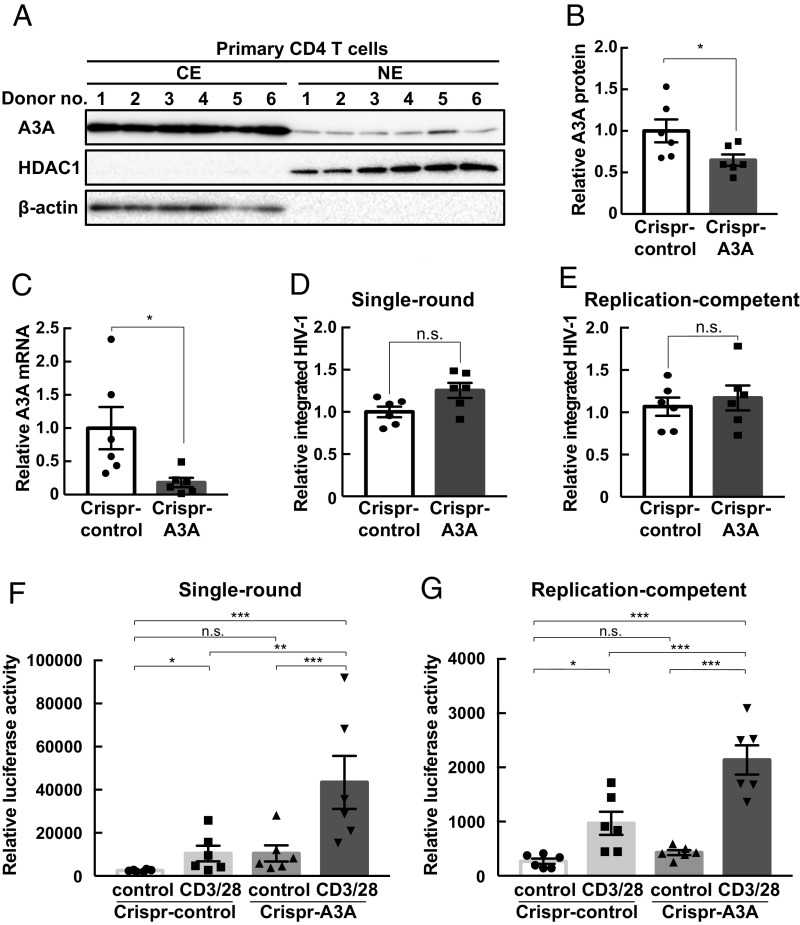
To probe the importance of these observations in primary human CD4 T cells, we isolated and expanded peripheral blood CD4 T cells from six healthy donors and transduced them with CRISPR-A3A lentivirus and pseudotyped single-round infectious NL4-3/Luc HIV-1 according to previous studies (45, 46). After culturing with IL-2 and puromycin for selection of transduced cells, CD4 T cells were activated by treatment with anti-CD3/anti-CD28 antibodies for 72 h. A previous report indicates that A3A mRNA is not expressed highly in naïve human CD4 T cells (26). To examine A3A protein expression, we conducted intracellular flow cytometry analysis. A3A protein was expressed in CD4 T cells, albeit at a lower level than in monocytes (SI Appendix, Fig. S7). Western blotting of lysates from monocytes, naïve CD4 T cells, and anti-CD3/anti-CD28–stimulated CD4 T cells revealed similar A3A protein expression in these cells (SI Appendix, Fig. S8). Additionally, fractionated lysates from CD4 T cells showed nuclear A3A protein expression, and microscopy analysis showed colocalization of A3A and KAP1 in the nucleus of primary CD4 T cells (Fig. 4A and SI Appendix, Fig. S9A). We also detected expression of A3A at the protein and mRNA levels in latently infected primary CD4 T cells (Fig. 4 C and D and SI Appendix, Fig. S9B). CRISPR-mediated A3A knockdown assessed by Western blotting and by RT-qPCR of CD4 T-cell lysate and total mRNA before reactivation showed ∼50% reduction in protein and mRNA expression (Fig. 4 B and C). An integration assay before reactivation indicated that A3A knockdown did not affect HIV-1 integration efficiency (Fig. 4D). However, A3A knockdown enhanced NL4-3/Luc–derived luciferase activity upon engagement of CD3 and CD28 in primary CD4 T cells (Fig. 4F and SI Appendix, Fig. S9C). Additionally, we confirmed these results in a widely used model of HIV-1 latency in primary human CD4 T cells by using replication-competent NL4-3/Luc HIV-1 followed by ART combination treatment (47, 48). Similar to the replication-defective HIV-1 latency model (Fig. 4 D–F and SI Appendix, Fig. S9C), the replication-competent model of HIV-1 latency imposed by ART also demonstrated enhanced reactivation when A3A was knocked down by CRISPR without changes in integration efficiency (Fig. 4 E and G and SI Appendix, Fig. S9D). These experiments showed, through the use of two separate models, that A3A reinforces HIV-1 latency in primary human CD4 T cells.
Fig. 4.
Apobec3A reinforces HIV-1 latency in primary human CD4 T cells. (A) CD4 T cells from six healthy donor PBMCs were expanded by incubation with CD3/28-Dynabeads antibodyand IL-2 with anti-human IL-12, anti-human IL-4, and TGF-β. Nuclear (NE) and cytosol (CE) lysates were fractionated and analyzed for A3A protein expression. Fractionation efficiency was confirmed by HDAC1 and β-actin expression. (B) Relative A3A protein expression was calculated vs. GAPDH protein expression in SI Appendix, Fig. S9A using ImageJ (NIH) and compared between CRISPR-control– and CRISPR-A3A–transduced CD4 T cells. *P < 0.05 versus control assessed by Student’s t test. (C) A3A mRNA expression was examined by RT-qPCR. Data are means ± SE (n = 6). *P < 0.05 versus control assessed by Student’s t test. (D and E) CD4 T cells from six healthy donor PBMCs were expanded by CD3/28-Dynabeads antibody and IL-2 with anti-human IL-12, anti-human IL-4, and TGF-β. Expanded CD4 T cells were spinoculated with pseudotyped NL4-3/luciferase and CRISPR-control or CRISPR-A3A lentivirus and cultured in the presence of puromycin. Integrated HIV-1 gene copy was analyzed by Alu-HIV PCR assay before reactivation (D). Significance between Crispr-control and Crispr-A3A was analyzed by Student’s t test. HIV-1 reactivation was analyzed by fold change of luciferase activity between control and CD3/28-Dynabeads antibody stimulation (E). n.s., not significant. (F and G) CD4 T cells from six healthy donors were expanded by CD3/28-Dynabeads antibody and IL-2 with anti-human IL-12, anti-human IL-4, and TGF-β, and spinoculated with replication-competent NL4-3/luciferase and Crispr-control or Crispr-A3A lentivirus. NL4-3/luciferase–infected cells were treated with raltegravir and nelfinavir to induced latency, and CRISPR-transduced cells were selected by puromycin treatment. Integrated HIV-1 gene copy was analyzed by Alu-HIV PCR assay before reactivation (F). ***P < 0.001, **P < 0.01, *P < 0.05 versus Crispr-control was assessed by ANOVA with Tukey–Kramer’s test. n.s., not significant between Crispr-control and Crispr-A3A was analyzed by Student’s t test. Replication-competent HIV-1 reactivation by CD3/28-Dynabeads antibody stimulation was examined by luciferase activity (G). Data are means ± SE (n = 6). ***P < 0.001, *P < 0.05 versus CRISPR-control assessed by ANOVA with Tukey–Kramer’s test. n.s., not significant.
Our study shows that A3A enforces latency by preventing HIV-1 reactivation through the recruitment of repressor complex KAP1 and HP1 to the LTR. A3A binds to KAP1, and promotes heterochromatin formation at the HIV-1 LTR. A3A knockdown enhances the reactivation of HIV-1 in HIV-1 latently infected primary CD4 T cells. Thus, our study reveals a previously unknown role of A3A in suppressing HIV-1 reactivation in latently infected cells, and provides a potential target for intervention for reversing latency. We show direct binding of A3A to a specific region within the HIV-1 LTR proviral DNA, namely the NF-κB and Sp1 binding sites. A3A bound to the sense strand DNA, and imposed blockade of HIV-1 reactivation independent of its cytidine deamination activity. A3A suppresses several retroelements independent of its cytidine deamination activity (24–26). Thus, A3A may function as an epigenetic modifier to regulate retroelement transcription in general.
Our results suggest that A3A binding to the HIV-1 LTR in the host genome occurs upon opening of the double-stranded DNA. There are many ssDNA-binding proteins that perform essential functions during telomere synthesis, transcription, DNA replication, recombination, and repair (49). Other examples of transcriptional regulators that bind to single-stranded DNA include FUSE-binding protein 1 (FUBP1) (50), runt-related transcription factor (RUNX)-1 and RUNX-3 (51), purine-rich negative regulatory α and β (52), and myelin gene expression factor-2 (53). Since most transcriptional regulators and epigenetic modifiers bind to dsDNA, future studies are needed to determine the mechanism by which A3A manages ssDNA binding to genomic DNA at the steady state. The obligatory binding of A3A to ssDNA suggests a need for initial transcription of the LTR to recruit A3A, followed by KAP1 recruitment and repression of proviral expression.
A crystal structure analysis of a complex of A3A with ssDNA bound in the active site revealed the residues within the ZDD that confer specificity toward CC/TC motifs (54). A3A binds to target sequences with polythymidine oligomers containing cytidine bases, 5′-TTTTTTTCTTTTTTT-3′, with the highest affinity (55). Of note, our DNA pull-down analysis showed the strongest binding of A3A to the oligonucleotide that contains the ACTTTC motif (SI Appendix, Table S4). We speculate that A3A may have affinity toward polythymidine containing cytidine that is found in the HIV-1 LTR and possibly other genomic DNA sequences. Whether other nuclear Apobec3 protein families possess specific sequence affinity and regulate gene expression remains to be determined.
In conclusion, our study demonstrates that human A3A is expressed in the nucleus by CD4 T cells and controls transcription of latent HIV-1. A3A binds directly to the LTR and imposes repressive histone marks by recruiting KAP1 and HP1 in a cytidine deaminase-independent manner. These data highlight the relevance of A3A in HIV-1 latency maintenance and reactivation, and provide a pathway that can be targeted for future therapeutic strategies.
Materials and Methods
Reagents and Antibodies.
PMA (phorbol 12-myristate 13-acetate) and LPS (Salmonella enterica serotype typhimurium) were purchased from Sigma-Aldrich. UltraPure salmon sperm DNA (15632011) was from Thermo Fisher Scientific. Glycogen (AB00670-00020) was from AmericanBio. Lenti-X concentrator was from Clontech. RediJect d-Luciferin Ultra Bioluminescent Substrate was purchased from PerkinElmer. Dynabeads Protein G, Dynabeads Human T-Activator CD3/CD28 (CD3/28-Dynabeads), and Dynabeads M-280 Streptavidin were purchased from Thermo Fisher Scientific. Recombinant A3A (TP320995) was purchased from OriGene. Anti-A3A (ab38641; for Western blotting, immunofluorescence, and flow cytometry), anti-KAP1 (ab22553), anti-H3K9 trimethylation (ab8898), and anti-V5 (ab9116) antibodies and normal rabbit IgG (ab172730) were from Abcam. Anti-H3K9 dimethylation (CS200587), anti-HP1gamma (CS203221), anti-Histone H3 (CS207299), anti–NF-κB p65 (CS204359), and normal mouse IgG (12-371) antibodies were from Millipore. Anti-HA (H3663) and anti–β-actin (A1978) were from Sigma-Aldrich. Anti-GAPDH (GTX627408-01) and anti-p84 (GTX70220-01) were from GeneTex. Anti-A3A (AP20219a, sc-130688; for IP and ChIP) and anti-HDAC1 (sc-7872) antibodies were from Abgent and Santa Cruz Biotechnology, respectively. HRP-conjugated anti-rabbit (7074) and anti-mouse (7076) antibodies were from Cell Signaling Technology. Brilliant Violet 605 anti-CD3 (300460), Alexa 700 anti-CD4 (300526), and FITC anti-CD14 (367116) antibodies were from BioLegend. Anti-rabbit Alexa Fluor 594 antibody was from Thermo Fisher Scientific. Anti-mouse Cy3 (715-165-151) and anti-rabbit Cy5 (111-176-144) antibodies were from Jackson ImmunoResearch. Anti–HIV-1 Tat rabbit antiserum (705) was provided by B. Cullen through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Plasmids, Cell Lines, Viruses, and siRNAs.
pcDNA3.1-A3A(WT) was provided by J. Ahn, University of Pittsburgh School of Medicine, Pittsburgh. pk-A3A WT and C106S plasmids were provided by J. Moran, University of Michigan Medical School, Ann Arbor, MI. pGL2-LTR-Luc WT, ΔκB, ΔSp1, and replication-competent NL4-3/Luc plasmids were provided by W. Greene, Gladstone Institute of Virology and Immunology, San Francisco. pcDNA3.1-A3A-V5/His plasmid was constructed by cutting the A3A-coding region from pcDNA3.1-A3A using Kpn1 and Xho1 and inserting into a pcDNA3.1-V5/His vector. ΔZDD, Δ107–199, and Δ1–106 A3A plasmids were generated by using a Q5 Site-Directed Mutagenesis Kit (New England Biolabs). The toxicity of the A3A constructs was analyzed by LDH assay (SI Appendix, Fig. S11 A and B). The oligonucleotide primers used for mutagenesis are shown in SI Appendix, Table S1. CRISPR-A3A (K0105505), CRISPR-KAP1 (K2465405), and CRISPR-control (K010) lentiviral plasmids were from Applied Biological Materials. pCMVR8.74 was provided by D. Torono through Addgene. pNL4-3.Luc.R−E−, pHEF-VSVG, pCEP4-Tat, J-Lat10.6 cells, J-Lat6.3 cells, and Tzm-Bl cells were provided by N. Landau, L. J. Chang, E. Verdin, T. Folks, J. C. Kappes, X. Wu, and Tranzyme, respectively, through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. pRL-TK (E2241) plasmids were purchased from Promega. 293T and 293FT cells were purchased from ATCC and Invitrogen, respectively. ON-TARGETplus Human SMARTpool si-A3A (L-017432-00-0005) and negative-control siRNA (con-si; D-001810-01-20) were purchased from GE Dharmacon.
ChIP-qPCR.
The ChIP-qPCR procedure was described previously (56, 57). Briefly, protein–DNA bound in cells was cross-linked by 1% formaldehyde treatment for 5 min. The reaction was stopped by adding 0.125 M glycine for 5 min. Cells were washed with PBS and lysed using a cell lysis buffer (50 mM Hepes, pH 7.4, 1 mM EDTA, 85 mM KCl, 10% glycerol, 0.5% Nonidet P-40, supplemented with protease inhibitor mixture). After centrifugation (1,200 × g, 4 °C, 5 min), the nuclear fraction of cells was suspended in nucleus lysis buffer (50 mM Tris⋅HCl, pH 8.0, 2 mM EDTA, 150 mM NaCl, 5% glycerol, 1% Triton-X 100, 0.1% SDS, supplemented with protease inhibitor cocktail). The nuclear suspension was sonicated using a Bioruptor (30-s sonic and 30-s cooling cycle 35 times) to recover a fragmented nucleosome. For immunoprecipitation of the nucleosome, 2 to 5 μg of anti-A3A, anti-KAP1, anti-HP1γ, anti-H3K9 trimethylation, anti-H3K9 dimethylation, and anti-Histone H3 antibodies and isotype control IgG was added and rotated overnight at 4 °C. After incubation with Dynabeads Protein G under rotation for 1 h at room temperature (RT), beads were washed with ChIP wash buffer 1 (20 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1% Triton-X 100, 0.1% SDS, 2 mM EDTA) twice, ChIP wash buffer 2 (20 mM Tris⋅HCl, pH 8.0, 500 mM NaCl, 1% Triton-X 100, 0.1% SDS, 2 mM EDTA) twice, ChIP wash buffer 3 (20 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 500 mM LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mM EDTA) once, and then using TE buffer (10 mM Tris⋅HCl, pH 8.0, 1 mM EDTA) once. After washing, the bead-bound nucleosome fraction was eluted using 150 μL of elution buffer (1% SDS, 0.1 M NaHCO3) at 65 °C for 15 min twice. The eluted sample was de-cross-linked by adding 18 μL of 5 M NaCl and incubated at 65 °C for 5 h. The DNA in the sample was precipitated by adding 2 μL 5 mg/mL glycogen and 2.5 times the volume of ethanol and incubated at −80 °C overnight. DNA was recovered by centrifugation and treated with Proteinase K for 1 to 2 h at 45 °C. Proteinase K-treated DNA was isolated by using a QIAquick PCR Purification Kit (Qiagen). For qPCR of immunoprecipitated DNA, targeting primers were used to analyze each protein-binding and histone modification at HIV-1 genes. The oligonucleotide primers used for ChIP-qPCR are listed in SI Appendix, Table S3. Specifically, the immunoprecipitation efficiency of the anti-A3A antibody was validated by immunoprecipitation and Western blotting (SI Appendix, Fig. S12A). Histone H3 binding was analyzed by ChIP-qPCR by using anti-Histone H3 antibody and primers for LTR (SI Appendix, Fig. S10 B and C).
NL4-3/Luc Pseudovirus, NL4-3/Luc Virus, and CRISPR Lentivirus.
NL4-3/Luc pseudovirus was recovered from the culture medium of pNL4-3.Luc.R−E−–, pCMVR8.74-, and pHEF-VSVG–transfected 293T cells. NL4-3/Luc replication-competent virus was recovered from the culture medium of pNL4-3/Luc–transfected 293FT cells. Lentivirus encoding CRISPR-A3A, CRISPR-KAP1, control-CRISPR gRNA, and CRISPR-Cas9 all in one was generated by following the manufacturer’s recommended protocol (Applied Biological Materials). Virus in culture medium was concentrated by using a Lenti-X concentrator (Clontech), and viral titer was measured by using an HIV-1 p24 ELISA.
HIV-1 Latently Infected Primary CD4 T-Cell Model.
HIV-1 latent infection was done following a previous report (45). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation (Lymphoprep; Stemcell Technologies) from buffy coats of six healthy anonymous donors (New York Blood Center). Naïve CD4 T cells were isolated by a naïve human CD4+ T-cell enrichment kit (17555; Stemcell Technologies). CD4 T cells were cultured with anti-human IL-12 (200 μg/mL), anti-human IL-4 (200 μg/mL), TGF-β (50 μg/mL), and CD3/28-Dynabeads for 3 d; the Dynabeads were then removed and cultured with IL-2 (30 IU/mL) for 4 d. CD4 T cells (5 × 105) were spinoculated with 50 ng of NL4-3/Luc pseudovirus and CRISPR-A3A lentivirus or control-CRISPR lentivirus by 800 × g for 2 h at RT. After spinoculation, cells were washed with prewarmed RPMI with IL-2 (30 IU/mL) and plated on a 12-well plate. Two days after incubation, cells were treated with 500 ng/mL puromycin for selection of CRISPR-transduced cells, and a portion of the cells was used for Western blotting to check the knockdown efficiency of CRISPR-A3A. Five days after puromycin treatment, cells were reactivated using CD3/28-Dynabeads. Three days after reactivation, cells were washed with PBS and lysed by passive lysis buffer for luciferase assay. HIV-1 reactivation efficiency was calculated by comparison with nonreactivated samples. HIV-1 latent infection using NL4-3/Luc replication-competent virus was carried out according to a previous report (47, 48). Briefly, CD4 T cells were spinoculated with 50 ng of NL4-3/Luc pseudovirus and CRISPR-A3A lentivirus or control-CRISPR lentivirus. Two days after incubation, CD4 T cells were treated with puromycin for selection of transduced cells. Three days later, CD4 T cells were treated with 1 μM raltegravir and 0.5 μM nelfinavir for 4 d. Viability of HIV-1–infected and ART-treated CD4 T cells was measured by auto cell counter (SI Appendix, Fig. S9E). CD4 T cells were treated with CD3/28-Dynabeads for 72 h, and HIV-1–driven luciferase activity was measured by luciferase assay compared with each nonstimulated control.
Statistical Analysis.
For statistical analysis, the data were analyzed by Student’s t test or ANOVA with either Tukey’s or Kramer’s multiple comparison test (JMP software; SAS Institute), as indicated in each figure legend. A P value of <0.05 is considered statistically significant.
Supplementary Material
Acknowledgments
We thank Alan N. Engelman (Harvard University) and Reuben Harris (University of Minnesota and HHMI) for critical reading of this manuscript. This work was supported by the Howard Hughes Medical Institute (A.I.). M.T. was a Japan Society for the Promotion of Science Fellow and in part supported by the Kanzawa Medical Research Foundation and Mochida Memorial Foundation for Medical and Pharmaceutical Research. E.S. was supported in part by T32 MSTP Fellowship 2T32GM007205-41.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819386116/-/DCSupplemental.
References
- 1.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 4.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 5.Böhnlein E, et al. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988;53:827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- 6.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinoshita S, et al. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 8.West MJ, Lowe AD, Karn J. Activation of human immunodeficiency virus transcription in T cells revisited: NF-kappaB p65 stimulates transcriptional elongation. J Virol. 2001;75:8524–8537. doi: 10.1128/JVI.75.18.8524-8537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganesh L, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 10.Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 11.Selby MJ, Peterlin BM. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990;62:769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 12.Jones KA, Peterlin BM. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann CH, Rice AP. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: Candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cujec TP, et al. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10:525–531. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Williams SA, Greene WC. Regulation of HIV-1 latency by T-cell activation. Cytokine. 2007;39:63–74. doi: 10.1016/j.cyto.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malim MH, Bieniasz PD. HIV restriction factors and mechanisms of evasion. Cold Spring Harb Perspect Med. 2012;2:a006940. doi: 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger G, et al. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 2011;7:e1002221. doi: 10.1371/journal.ppat.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucifora J, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren CJ, et al. APOBEC3A functions as a restriction factor of human papillomavirus. J Virol. 2015;89:688–702. doi: 10.1128/JVI.02383-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakaya Y, Stavrou S, Blouch K, Tattersall P, Ross SR. In vivo examination of mouse APOBEC3- and human APOBEC3A- and APOBEC3G-mediated restriction of parvovirus and herpesvirus infection in mouse models. J Virol. 2016;90:8005–8012. doi: 10.1128/JVI.00973-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suspène R, et al. Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J Virol. 2011;85:7594–7602. doi: 10.1128/JVI.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogerd HP, Wiegand HL, Doehle BP, Lueders KK, Cullen BR. APOBEC3A and APOBEC3B are potent inhibitors of LTR-retrotransposon function in human cells. Nucleic Acids Res. 2006;34:89–95. doi: 10.1093/nar/gkj416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogerd HP, et al. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc Natl Acad Sci USA. 2006;103:8780–8785. doi: 10.1073/pnas.0603313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H, et al. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Holmes RK, Malim MH, Bishop KN. APOBEC-mediated viral restriction: Not simply editing? Trends Biochem Sci. 2007;32:118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinomoto M, et al. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic Acids Res. 2007;35:2955–2964. doi: 10.1093/nar/gkm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verdin E, Paras P, Jr, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 32.Coull JJ, et al. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He G, Margolis DM. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams SA, et al. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e1000495. doi: 10.1371/journal.ppat.1000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlesinger S, Goff SP. Retroviral transcriptional regulation and embryonic stem cells: War and peace. Mol Cell Biol. 2015;35:770–777. doi: 10.1128/MCB.01293-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf G, Greenberg D, Macfarlan TS. Spotting the enemy within: Targeted silencing of foreign DNA in mammalian genomes by the Krüppel-associated box zinc finger protein family. Mob DNA. 2015;6:17. doi: 10.1186/s13100-015-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara RP, et al. KAP1 recruitment of the 7SK snRNP complex to promoters enables transcription elongation by RNA polymerase II. Mol Cell. 2016;61:39–53. doi: 10.1016/j.molcel.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horiba M, et al. OTK18, a zinc-finger protein, regulates human immunodeficiency virus type 1 long terminal repeat through two distinct regulatory regions. J Gen Virol. 2007;88:236–241. doi: 10.1099/vir.0.82066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishitsuji H, Abe M, Sawada R, Takaku H. ZBRK1 represses HIV-1 LTR-mediated transcription. FEBS Lett. 2012;586:3562–3568. doi: 10.1016/j.febslet.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Nishitsuji H, Sawada L, Sugiyama R, Takaku H. ZNF10 inhibits HIV-1 LTR activity through interaction with NF-κB and Sp1 binding motifs. FEBS Lett. 2015;589:2019–2025. doi: 10.1016/j.febslet.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Pengue G, Calabrò V, Bartoli PC, Pagliuca A, Lania L. Repression of transcriptional activity at a distance by the evolutionarily conserved KRAB domain present in a subfamily of zinc finger proteins. Nucleic Acids Res. 1994;22:2908–2914. doi: 10.1093/nar/22.15.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pengue G, Caputo A, Rossi C, Barbanti-Brodano G, Lania L. Transcriptional silencing of human immunodeficiency virus type 1 long terminal repeat-driven gene expression by the Krüppel-associated box repressor domain targeted to the transactivating response element. J Virol. 1995;69:6577–6580. doi: 10.1128/jvi.69.10.6577-6580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds L, et al. Repression of the HIV-1 5′ LTR promoter and inhibition of HIV-1 replication by using engineered zinc-finger transcription factors. Proc Natl Acad Sci USA. 2003;100:1615–1620. doi: 10.1073/pnas.252770699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lusic M, et al. Proximity to PML nuclear bodies regulates HIV-1 latency in CD4+ T cells. Cell Host Microbe. 2013;13:665–677. doi: 10.1016/j.chom.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Bosque A, Planelles V. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods. 2011;53:54–61. doi: 10.1016/j.ymeth.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins LJ, et al. Modeling HIV-1 latency in primary T cells using a replication-competent virus. AIDS Res Hum Retroviruses. 2016;32:187–193. doi: 10.1089/aid.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lassen KG, Hebbeler AM, Bhattacharyya D, Lobritz MA, Greene WC. A flexible model of HIV-1 latency permitting evaluation of many primary CD4 T-cell reservoirs. PLoS One. 2012;7:e30176. doi: 10.1371/journal.pone.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickey TH, Altschuler SE, Wuttke DS. Single-stranded DNA-binding proteins: Multiple domains for multiple functions. Structure. 2013;21:1074–1084. doi: 10.1016/j.str.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabenhorst U, et al. Single-stranded DNA-binding transcriptional regulator FUBP1 is essential for fetal and adult hematopoietic stem cell self-renewal. Cell Rep. 2015;11:1847–1855. doi: 10.1016/j.celrep.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 51.Tay LS, et al. RUNX poly(ADP-ribosyl)ation and BLM interaction facilitate the Fanconi anemia pathway of DNA repair. Cell Rep. 2018;24:1747–1755. doi: 10.1016/j.celrep.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 52.Gupta M, Sueblinvong V, Raman J, Jeevanandam V, Gupta MP. Single-stranded DNA-binding proteins PURalpha and PURbeta bind to a purine-rich negative regulatory element of the alpha-myosin heavy chain gene and control transcriptional and translational regulation of the gene expression. Implications in the repression of alpha-myosin heavy chain during heart failure. J Biol Chem. 2003;278:44935–44948. doi: 10.1074/jbc.M307696200. [DOI] [PubMed] [Google Scholar]
- 53.Haas S, Steplewski A, Siracusa LD, Amini S, Khalili K. Identification of a sequence-specific single-stranded DNA binding protein that suppresses transcription of the mouse myelin basic protein gene. J Biol Chem. 1995;270:12503–12510. doi: 10.1074/jbc.270.21.12503. [DOI] [PubMed] [Google Scholar]
- 54.Kouno T, et al. Crystal structure of APOBEC3A bound to single-stranded DNA reveals structural basis for cytidine deamination and specificity. Nat Commun. 2017;8:15024. doi: 10.1038/ncomms15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohn MF, et al. The ssDNA mutator APOBEC3A is regulated by cooperative dimerization. Structure. 2015;23:903–911. doi: 10.1016/j.str.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyata M, et al. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat Commun. 2015;6:6062. doi: 10.1038/ncomms7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satou Y, et al. The retrovirus HTLV-1 inserts an ectopic CTCF-binding site into the human genome. Proc Natl Acad Sci USA. 2016;113:3054–3059. doi: 10.1073/pnas.1423199113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.