Significance
Extracellular matrix proteins have primarily been designated as supporting scaffolds for cells. This work presents the soluble extracellular matrix component tropoelastin as a powerful proproliferative and cell-attractive molecule that surpasses the potency of conventional growth factors and matrix proteins used in a mesenchymal stem cell (MSC) culture. Tropoelastin is also demonstrated to modulate MSCs both as a substrate coating and as a soluble additive in media, which significantly deviates from the classical dogma of cell anchorage-dependent structural roles of the matrix. We show that these activities of tropoelastin can be harnessed and establish a path to boosting the efficacy of and simplifying processes for clinical MSC expansion and therapeutic MSC recruitment.
Keywords: tropoelastin, mesenchymal stem cells, growth factor, expansion, migration
Abstract
We challenge the conventional designation of structural matrix proteins primarily as supporting scaffolds for resident cells. The extracellular matrix protein tropoelastin is classically regarded as a structural component that confers mechanical strength and resilience to tissues subject to repetitive elastic deformation. Here we describe how tropoelastin inherently induces a range of biological responses, even in cells not typically associated with elastic tissues and in a manner unexpected of typical substrate-dependent matrix proteins. We show that tropoelastin alone drives mesenchymal stem cell (MSC) proliferation and phenotypic maintenance, akin to the synergistic effects of potent growth factors such as insulin-like growth factor 1 and basic fibroblast growth factor. In addition, tropoelastin functionally surpasses these growth factors, as well as fibronectin, in allowing substantial media serum reduction without loss of proliferative potential. We further demonstrate that tropoelastin elicits strong mitogenic and cell-attractive responses, both as an immobilized substrate and as a soluble additive, via direct interactions with cell surface integrins αvβ3 and αvβ5. This duality of action converges the long-held mechanistic dichotomy between adhesive matrix proteins and soluble growth factors and uncovers the powerful, untapped potential of tropoelastin for clinical MSC expansion and therapeutic MSC recruitment. We propose that the potent, growth factor-like mitogenic and motogenic abilities of tropoelastin are biologically rooted in the need for rapid stem cell homing and proliferation during early development and/or wound repair.
Mesenchymal stem cells (MSCs) are used in therapeutic interventions for skeletal tissue injuries, myocardial infarctions, degenerative diseases, and organ failure (1) due to their inherent differentiation and regenerative potential, immunomodulatory properties, and migratory capacity toward sites of injury and disease (2). However, a significant hurdle hindering their widespread translation into clinical practice is the limited natural availability of these cells. Human bone marrow-derived MSCs comprise only 0.001–0.01% of the bone marrow mononuclear cell population (3). In contrast, a therapeutic dose for a single patient typically requires at least 1 to 2 million cells per kilogram of body weight (4, 5), due in part to the inefficient homing of administered MSCs (6). Evidently, there is strong demand for the ability to expand MSCs cost-effectively while maintaining stem cell properties closely linked with therapeutic efficacy (7). At the same time, there are clear benefits to identifying motogenic molecules that can effectively recruit MSCs to target sites and thus reduce therapeutic cell doses.
The growth and expansion of MSCs and other adherent therapeutic stem cells in general rely on interactions with soluble components in the culture medium, the surrounding cells, and the underlying substrate (5). These factors are acknowledged to function synergistically but not redundantly, such that an underlying substrate protein would not be expected to replace a soluble component. Accordingly, MSC expansion ex vivo has been enhanced by fortifying culture media with exogenous soluble factors and/or by coating culture surfaces with serum or ECM components. For example, MSC propagation can be amplified by supplementing basal media with additional serum proteins, hormones, or growth factors. Among these growth factors are TGF-β, EGF, PDGF, insulin-like growth factor-1 (IGF1), and, most commonly, basic fibroblast growth factor (bFGF) (5, 8). In particular, bFGF has a potent mitogenic effect toward MSCs (9–12) and is frequently used to supplement stem cell culture media with full or minimal serum content (5).
Culture substrates are typically coated with a range of connective tissue proteins, including fibronectin, collagen IV, vitronectin, and laminin (5, 13–15), due to their affinity to a wide range of cell integrin receptors (16). These coatings are classically considered to be important for cell retention on substrate surfaces and are commonly used in concert with serum- or growth factor-supplemented media to promote MSC adhesion, spreading, and expansion (5, 15).
Tropoelastin is an ECM component primarily located in elastic tissues and has been shown to promote the expansion of hematopoietic stem cells (HSCs) and MSCs when used as a substrate coating (17) or as part of the substrate bulk material (18). The ECM is proposed to modulate stem cell phenotype and activity, including proliferation, via its chemistry, topography, and mechanical properties (18). Consistent with this thinking, the proproliferative effects of tropoelastin on HSCs have been attributed to the extensional elasticity of the molecule, conveyed as mechanical signals to influence cellular gene expression (17). As evidence, inhibition of the HSC mechanotransduction machinery is shown to eliminate tropoelastin-mediated proliferation. Similarly, the increased proliferation of MSCs on elastin-blended materials is wholly ascribed to the elasticity and roughness of the substrate (18).
Similarly, ECM-mediated MSC migration is predicated on cell adhesion to matrix components such as fibronectin (19) and the recognition of substrate architecture and topography (20). Moreover, regulation of MSC homing is overwhelmingly attributed to diffusible cytokines and growth factors, including IGF1 and bFGF (21–23). These biophysical and biochemical signals are thought to present separate and often conflicting directional cues to cells (24).
Here we describe the novel proproliferative and cell-attractive effects of tropoelastin on MSCs. Surprisingly, we discover significant and unique capabilities of this protein in replacing exogenous growth factors and dramatically reducing the serum requirement in culture media. Moreover, in contrast to typical matrix protein–cell interactions, we demonstrate the ability of an ECM protein such as tropoelastin to robustly enhance MSC expansion as a soluble additive akin to growth factors. Furthermore, we reveal that tropoelastin strongly attracts MSCs when substrate bound, but unlike conventional haptotactic matrix proteins, it also promotes MSC chemotaxis in solution similarly to growth factors. We also identify an integrin-based mechanism underlying these potent functionalities of tropoelastin. We propose that tropoelastin holds powerful, untapped potential for the rapid, robust expansion of MSCs and the efficient recruitment of resident or exogenous MSCs toward target tissues.
Results
Surface-Bound Tropoelastin Can Replace IGF1 and/or bFGF in Media.
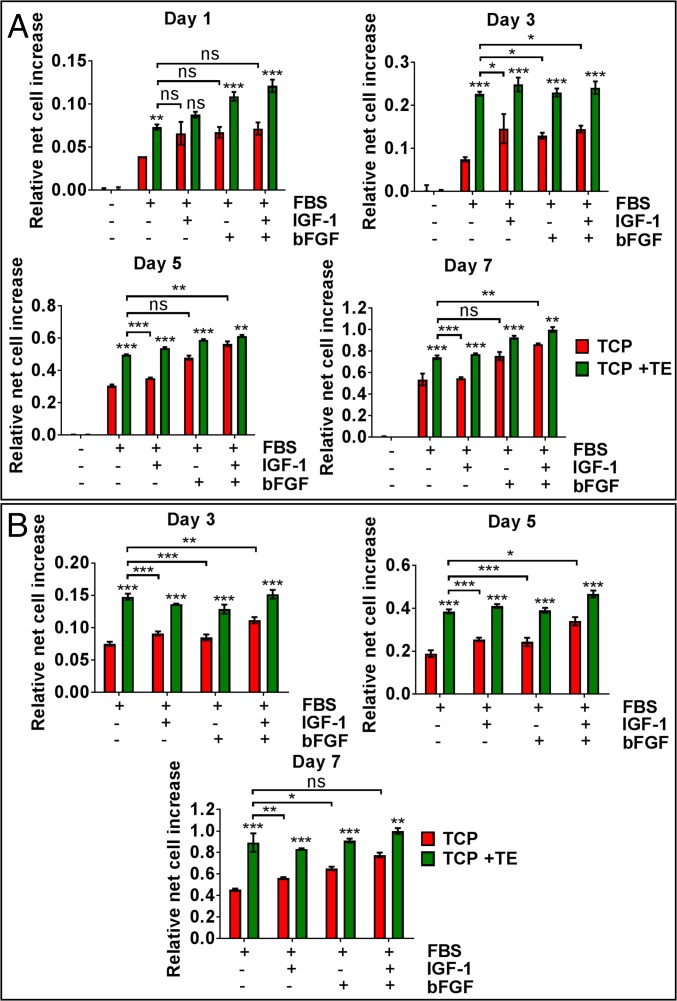
To determine the effect of substrate-bound tropoelastin on MSC proliferation, MSCs were cultured on bare or tropoelastin-coated tissue culture plastic (TCP) in various media formulations with and without 10% (vol/vol) FBS and optionally supplemented with IGF1 and/or bFGF (Fig. 1A). Cells proliferated over 7 d in all conditions except in serum-free basal media. Cell numbers on tropoelastin-coated TCP significantly increased over those on bare TCP, whether in full-serum media (39 ± 3% increase) or in media supplemented with IGF1 (41 ± 1% increase), bFGF (16 ± 2% increase), or IGF1 and bFGF (16 ± 3% increase). The highest cell numbers were observed in the presence of both surface-bound tropoelastin and soluble growth factors.
Fig. 1.
MSC proliferation on bare TCP or TCP coated with tropoelastin (TE) in media containing (A) 10% (vol/vol) FBS or (B) 7% (vol/vol) FBS, with and without IGF1 and/or bFGF growth factors. Panels show relative net cell increase at various days postseeding. Asterisks directly above the columns represent statistical differences between bare and tropoelastin-coated TCP in each media formulation: *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
As a comparison of the proproliferative activity of tropoelastin and growth factors, MSCs cultured on a tropoelastin substrate in full-serum media with no additional factors expanded 14 ± 2% less compared with cells on TCP in media with both IGF1 and bFGF (Fig. 1A). However, cells grown on tropoelastin in normal media proliferated 36 ± 3% more than cells on TCP in media with IGF1 and similarly to cells in media with bFGF. These findings indicate that substrate-bound tropoelastin not only improves MSC propagation in normal or growth factor-supplemented media but can also replace either IGF1 or bFGF while maintaining the same amplified level of cell expansion.
The addition of growth factors in culture media typically allows for a decrease in serum concentration without retarding MSC proliferation (25). Therefore, we wanted to determine the proproliferative benefits of substrate-bound tropoelastin in a reduced-serum environment normally compensated for by growth factors (Fig. 1B). In media containing 7% FBS, MSCs grown on TCP also exhibited proliferation over 7 d, although to a lesser extent than that previously observed in normal full-serum media. Substrate-bound tropoelastin dramatically promoted MSC proliferation in all reduced-serum conditions, not only in unsupplemented media (97 ± 19% increase) but also in media already containing IGF1, bFGF, or both growth factors (49 ± 1%, 40 ± 3%, or 29 ± 3% increase, respectively).
More remarkably, in these reduced-serum conditions, MSCs cultured on tropoelastin in unsupplemented media exhibited significantly greater expansion over 7 d relative to cells on TCP in media with either IGF1 or bFGF (59 ± 15% and 37 ± 13% increase, respectively) and were equivalent in abundance to cells in media with both growth factors (Fig. 1B). These results point to the ability of surface-coated tropoelastin to replace both IGF1 and bFGF in promoting MSC proliferation in a reduced-serum environment.
Tropoelastin Allows for Greater Serum Reduction Compared with Fibronectin or Growth Factors.
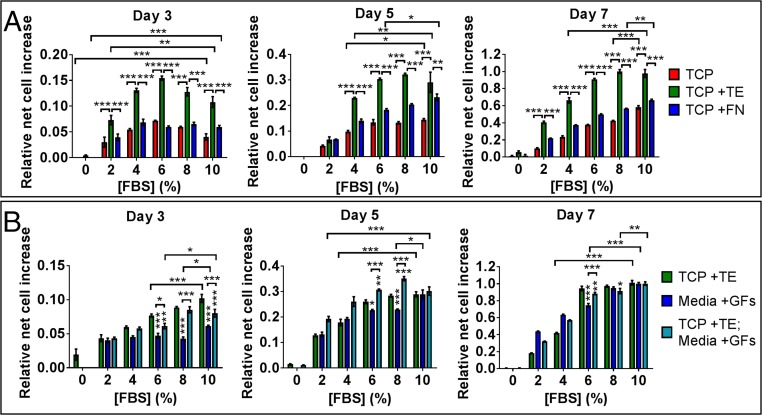
Due to the persistence of tropoelastin’s proproliferative activity in media with 7% (vol/vol) FBS, we investigated the maximum extent of serum reduction that would not affect tropoelastin-mediated MSC expansion (Fig. 2A). Cells were grown in decreasing amounts of FBS [0–10% (vol/vol) in media] on TCP and on TCP coated with tropoelastin or fibronectin. MSC numbers on bare and fibronectin-coated surfaces progressively declined with greater serum reduction. After 7 d, MSC proliferation on TCP and fibronectin decreased by 27 ± 1% and 15 ± 0.1%, respectively, following a mere 20% reduction in serum. In contrast, cell expansion on a tropoelastin substrate remained unaffected by up to a 40% decrease in serum. In media with 6% FBS, MSC proliferation on bare and fibronectin-coated TCP was inhibited by 35 ± 1% and 25 ± 1%, respectively, compared with that in normal media.
Fig. 2.
MSC proliferation in decreasing amounts of serum. (A) Cells were grown on bare, tropoelastin-coated or fibronectin (FN)-coated TCP in normal media. (B) Cells were cultured on tropoelastin-coated TCP in normal media, on TCP in media containing IGF1 and bFGF growth factors (GFs), or on tropoelastin-coated TCP in media supplemented with GFs. Panels show the relative net cell increase at 3, 5, and 7 d postseeding, normalized to the initial cell numbers at day 1. Asterisks directly above the columns represent statistical comparison with cells on tropoelastin-coated TCP in normal media: *P < 0.05; **P < 0.01; ***P < 0.001.
While fibronectin and tropoelastin promoted MSC propagation in full-serum media, the benefits of fibronectin were significantly diminished upon serum reduction. At these lower serum concentrations, that is, 2–8% (vol/vol) of the media composition, tropoelastin-coated surfaces consistently and significantly enhanced MSC proliferation compared with bare and fibronectin-coated surfaces by 135 ± 5 to 309 ± 12% and 76 ± 4 to 86 ± 6%, respectively. These findings strongly indicate that tropoelastin can uniquely compensate for substantial serum reduction in media without compromising MSC expansion levels.
The ability to promote high levels of stem cell growth in low-serum conditions, as demonstrated by tropoelastin, is a property typically ascribed to growth factors (25). On this basis, we compared this functionality of substrate-bound tropoelastin with that of IGF1 and bFGF (Fig. 2B). By 7 d postseeding, cell numbers on TCP in growth factor-containing media were unaltered in 8% (vol/vol) FBS, indicating that the combination of IGF1 and bFGF allows for slight (20%) serum reduction during culture. In 6% (vol/vol) FBS, however, cell numbers in media with growth factors were significantly decreased by 25 ± 2% compared with those in full-serum media. In contrast, cells on tropoelastin in the absence of growth factors sustained uncompromised levels of proliferation following a 40% decrease in serum. In 6% (vol/vol) serum, tropoelastin improved MSC proliferation by 27 ± 4% compared with IGF1 and bFGF in tandem, indicating that tropoelastin is functionally superior to the growth factors in stimulating MSC expansion in substantially reduced serum conditions.
Tropoelastin in Solution Promotes MSC Proliferation Similarly to Surface-Bound Tropoelastin.
We wished to determine whether the mitogenic activity of tropoelastin is conditional upon its immobilization to the culture substrate and the provision of mechanical cues. We therefore tested whether tropoelastin in solution achieves the same cell expansion benefits as the surface-bound protein. When tropoelastin was added to tissue culture wells that were preincubated with normal media, the protein remained in solution due to surface blocking by serum proteins (SI Appendix, Fig. S1A).
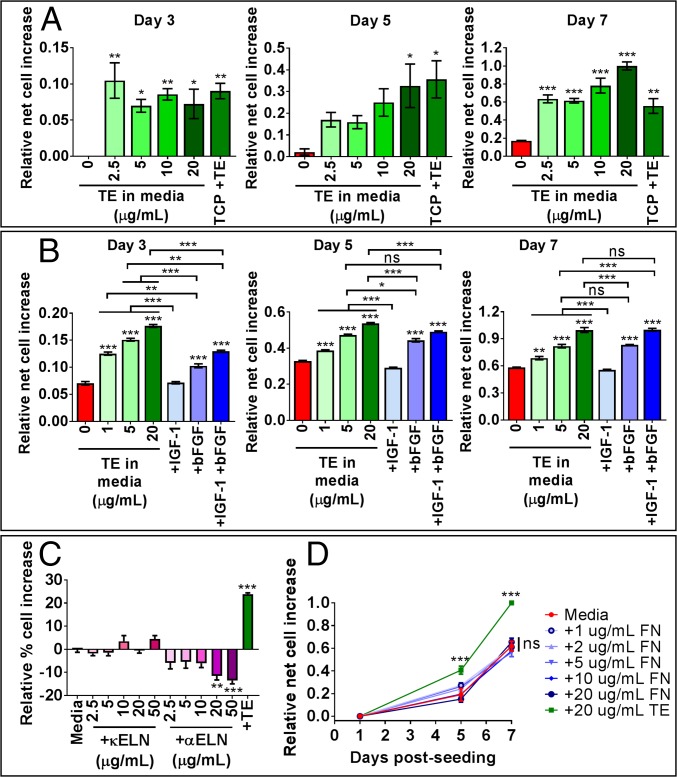
Soluble tropoelastin at concentrations as low as 1 μg/mL consistently promoted MSC proliferation over 7 d compared with normal media (SI Appendix, Fig. S1B). However, tropoelastin concentrations of at least 2.5 μg/mL were required to stimulate MSC proliferation to an extent comparable to that of substrate-bound tropoelastin (Fig. 3A). Increasing the solution concentration of tropoelastin to 20 μg/mL further improved MSC proliferation by 80 ± 8% over substrate-bound tropoelastin at 7 d postseeding. These results demonstrate that tropoelastin above a threshold concentration in solution significantly promotes MSC proliferation. Supplementation of media with 5 μg/mL tropoelastin is functionally equivalent to coating the culture substrate with tropoelastin and allows temporal control of the cell proliferation profile (SI Appendix, Fig. S1C). Evidently, tropoelastin can function as a signaling molecule in solution, similar to growth factors, to actively enhance MSC expansion.
Fig. 3.
MSC proliferation in media with tropoelastin in solution. (A) Cells were grown on TCP in media supplemented with increasing concentrations of soluble tropoelastin or on tropoelastin-coated TCP in normal media. Panels show relative net cell increase at 3, 5, and 7 d postseeding. Asterisks above individual columns depict statistical differences from the no-tropoelastin control. (B) Cells were cultured on TCP in normal media or in media supplemented with tropoelastin or growth factor(s). Panels show relative net cell increase at 3, 5, and 7 d postseeding. Asterisks directly above the data columns indicate statistical differences from the normal media control. (C) Cell proliferation for 7 d in normal media or in media supplemented with κELN, αELN, or tropoelastin. Asterisks indicate statistical differences from the normal media control. (D) Cells were grown for up to 7 d in normal media or media supplemented with fibronectin or tropoelastin in solution. Asterisks denote statistical differences from the normal media control. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Tropoelastin in Solution Can Replace IGF1 and bFGF in Full-Serum Media.
We investigated whether tropoelastin in solution, like substrate-bound tropoelastin, can mirror the effects of growth factors in eliciting a proliferative response from MSCs (Fig. 3B). We previously observed that substrate-bound tropoelastin can replace either IGF1 or bFGF in full-serum media. Media supplementation with IGF1 alone did not increase MSC numbers compared with normal media. As such, tropoelastin in solution at or above 1 μg/mL triggered significantly elevated cell proliferation over 7 d compared with normal media or media with IGF1. This level of increase is dose-dependent, ranging from 18 ± 5% with 1 μg/mL tropoelastin to 69 ± 7% with 20 μg/mL tropoelastin.
Soluble tropoelastin can likewise replace bFGF in media (Fig. 3B). During early-stage proliferation (3 and 5 d postseeding), soluble tropoelastin at and above 1 μg/mL surpassed bFGF by up to 74 ± 2% in promoting MSC expansion. At later time points (7 d postseeding), soluble tropoelastin at 5 μg/mL was comparable to bFGF; and at 20 μg/mL it was 18 ± 5% more potent than bFGF for MSC propagation.
Furthermore, while substrate-bound tropoelastin was functionally inferior to the cumulative benefit of IGF1 and bFGF in full-serum media, soluble tropoelastin at 20 μg/mL supported MSC expansion equivalently to both growth factors in full-serum media (Fig. 3B). These findings illustrate that tropoelastin in solution closely reflects the proproliferative capability of growth factors. At 5 μg/mL, tropoelastin can replace either IGF1 or bFGF, while a higher concentration of 20 μg/mL can adequately replace both growth factors without loss of MSC proliferative potential.
Soluble Elastin Fragments and Fibronectin Do Not Promote MSC Proliferation.
We wished to determine whether the potent mitogenic ability of tropoelastin in solution is similarly captured within fragments of the cross-linked protein. Cells were grown in normal media, in tropoelastin-supplemented media, or in media containing increasing amounts of soluble κ-elastin (κELN) or α-elastin (αELN) (Fig. 3C). These peptides are obtained from partial base or acid hydrolysis of native elastin and encompass a pool of heterogeneous elastin-derived sequences. Neither κELN nor αELN stimulated MSC proliferation above that in normal media. On the contrary, higher concentrations of αELN at 20–50 μg/mL suppressed cell expansion by up to 14 ± 1%. Therefore, we propose that the proproliferative effect of tropoelastin in solution requires the intact, full-length molecule.
This ability of tropoelastin to propagate cells in solution is unique for a matrix protein. Fibronectin promoted MSC expansion when coated on the substratum at concentrations as low as 2 μg/mL (Fig. 2A) but did not trigger any proliferative response when present in solution at up to 20 μg/mL (Fig. 3D). These results emphasize the singularity of tropoelastin’s dual capacity for modulating MSC proliferation as an underlying substrate and as a soluble factor.
MSCs Retain Cell Phenotype During Tropoelastin-Mediated Expansion.
An essential consideration when inducing MSC expansion is the maintenance of the native stem cell phenotype. Flow cytometry analyses indicated that cells cultured for 5 or 7 d on tropoelastin-coated surfaces, in full- or reduced-serum media with and without growth factors, exhibited characteristic MSC marker profiles (SI Appendix, Fig. S2A). At 5 d postseeding, more than 95% of cells in all media formulations expressed the positive MSC markers CD90, CD105, and CD73, while more than 98% lacked expression of hematopoietic stem cell markers CD34, CD45, CD11b, CD79a, and HLA-DR, in accordance with the MSC identification criteria set by the International Society for Cellular Therapy (26).
At 7 d postseeding, a decreased proportion of cells expressed all three MSC markers when grown on bare TCP in media containing only IGF1 or bFGF. Only 83.9 ± 0.7% of cells in IGF1-supplemented media and 92.9 ± 5.9% of cells in bFGF-supplemented media were positive for CD105. Likewise, only 89.1 ± 0.1% of cells in IGF1-containing media expressed CD73. These results point to the combined role of IGF1 and bFGF in maintaining the MSC phenotype during longer-term cell expansion.
Remarkably, substrate coating with tropoelastin restored the MSC marker expression levels of cells in these suboptimal media preparations to requisite thresholds. MSC phenotype was fully retained in all instances where substrate-bound tropoelastin was used to replace one or both growth factors in full-serum or reduced-serum media. Similarly, cells grown in media containing 20 μg/mL soluble tropoelastin also displayed characteristic CD90+, CD105+, CD73+, and lineage-negative expression profiles (SI Appendix, Fig. S2B).
Concomitant with the retention of cell surface markers, MSCs expanded in the presence of substrate-bound or soluble tropoelastin as a replacement for growth factors in normal or reduced-serum media also exhibited the capacity for multilineage differentiation (SI Appendix, Fig. S3). When induced with adipogenic media, these MSCs developed characteristic intracellular lipid droplets that appeared bright red with Oil Red O staining. When induced with osteogenic media, they formed mineralized calcium deposits visualized as red nodules by Alizarin Red S staining. When induced with chondrogenic media in micromass pellet culture, MSCs showed glycosaminoglycan-rich regions stained blue green by Alcian Blue, which were indicative of cartilage formation. These histological features were absent in noninduced samples. Taken together, these findings strongly support the ability of tropoelastin to preserve MSC phenotype and multipotent behavior throughout the amplified expansion process.
Tropoelastin Modulates MSC Attachment and Spreading via αv Integrins.
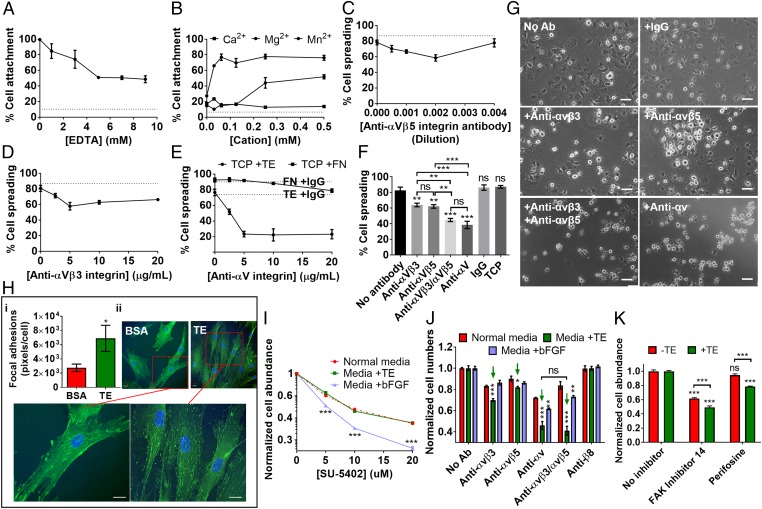
To determine the involvement of integrin receptors in tropoelastin modulation of MSC behavior, we analyzed the divalent cation dependence of tropoelastin–MSC interaction. Addition of the chelator EDTA significantly inhibited MSC attachment to substrate-bound tropoelastin in a dose-dependent manner (Fig. 4A). In the presence of 5 mM EDTA, MSC binding to tropoelastin was maximally reduced by 48.9 ± 0.5%. Furthermore, MSCs displayed minimal (20.0 ± 2.1%) adhesion to tropoelastin in a cation-free environment (Fig. 4B). The subsequent addition of up to 0.5 mM Ca2+ did not improve MSC binding (13.7 ± 1.0%); Mg2+ promoted moderate (51.8 ± 2.6%) cell attachment, while Mn2+ restored (76.1 ± 3.2%) cell adhesion to tropoelastin. This selective cation dependence is characteristic of an integrin-mediated cell binding mechanism (27).
Fig. 4.
Integrin-mediated effects of tropoelastin on MSC adhesion, spreading, and proliferation. (A) Cell adhesion to substrate-bound tropoelastin in the presence of EDTA. (B) Cell binding to tropoelastin in cation-free buffer with increasing doses of exogenous Mg2+, Ca2+, and Mn2+ divalent cations. (C–E) Cell spreading on tropoelastin with increasing concentrations of an (C) anti-αvβ5, (D) anti-αvβ3, or (E) pan anti-αv integrin antibody. Cell spreading on fibronectin with and without the anti-αv integrin antibody is shown as a control. (F) Cell spreading on tropoelastin in the presence of optimal inhibitory concentrations of anti-αvβ3, anti-αvβ5, combined anti-αvβ3 and anti-αvβ5, and anti-αv integrin antibodies. Cell spreading on TCP and that on tropoelastin in the absence of antibodies or with a nonspecific mouse IgG antibody are also included as controls. Asterisks above the data columns refer to statistical differences from the no-antibody control. (G) Representative images of MSC spreading on tropoelastin, with and without integrin-blocking antibodies. (Scale bar: 100 μm.) (H) Confocal microscope images of MSCs adhered on tropoelastin- or BSA-coated TCP, stained for focal adhesion vinculin (green) and cell nuclei (blue). The relative density of focal adhesion staining per cell is indicated. (Scale bar: 20 μm.) (I and J) MSC proliferation in the presence of (I) FGFR and (J) integrin inhibitors. Cells were grown on TCP in normal media, in media with 20 μg/mL tropoelastin, or in bFGF-supplemented media for 7 d. (I) Increasing doses of the FGFR inhibitor, SU-5402, were added to the media during the proliferation period. Cell numbers were normalized against samples without SU-5402. Cell numbers in media containing tropoelastin or bFGF were compared with those in normal media at each inhibitor concentration to account for the nonspecific toxicity of SU-5402. (J) Optimal inhibitory concentrations of anti-αvβ3, anti-αvβ5, anti-αvβ5 and anti-αvβ3, or anti-αv were added to the media over 7 d. Controls without antibodies or with an antibody against a nonexpressed integrin (anti-β8) were included. Green arrows indicate cells grown in the presence of tropoelastin and αv integrin subunit antibodies. Asterisks above individual columns denote significant differences from cells in normal media at each antibody condition. (K) MSC proliferation after 7 d in the presence of an FAK inhibitor (FAK inhibitor 14) or a PKB/AKT inhibitor (perifosine). Cell numbers were normalized against uninhibited samples. Asterisks above individual columns represent comparison with the no-inhibitor control. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
As further confirmation of the role of integrins in MSC interactions with tropoelastin, specific integrin-blocking antibodies impeded MSC spreading on a tropoelastin substrate (Fig. 4 C–G). The anti-αvβ5 and anti-αvβ3 integrin antibodies inhibited cell spreading on tropoelastin in a dose-dependent manner until optimal blocking concentrations were reached (Fig. 4 C and D). This inhibition was heightened with a pan anti-αv integrin subunit antibody (Fig. 4E). Antibody specificity was validated by the minimally inhibited spreading on fibronectin (78.8 ± 2.3%), which is known to alternatively interface with α5 and αv integrins, compared with the no-antibody (92.5 ± 2.6%) and IgG (90.1%) controls. At optimal antibody concentrations, the anti-αvβ5 and anti-αvβ3 antibodies significantly decreased MSC spreading on tropoelastin by 24.9 ± 2.7% and 22.7 ± 2.8%, respectively (Fig. 4F). The combined addition of anti-αvβ5 and anti-αvβ3 further inhibited spreading by 46.0 ± 2.5%, which was similar to the 53.6 ± 5.6% inhibition by the anti-αv antibody. Cell spreading on tropoelastin was unaffected by a nonspecific IgG antibody or in the absence of antibodies. Representative images of MSCs seeded on tropoelastin showed that in the absence of integrin-blocking antibodies, the majority of cells possessed a spread morphology characterized by a flattened, phase-dark cell body (Fig. 4G). In contrast, in the presence of anti-integrin antibodies, a markedly higher proportion of cells appeared unspread with a rounded, phase-bright morphology. In addition, vinculin staining of substrate-bound MSCs revealed a number of dot-like focal complexes and streak-like focal adhesions at the cell center and periphery. Cells adhered to tropoelastin possessed 1.5 ± 0.7-fold increased focal adhesions per cell compared with those on BSA (Fig. 4H). Taken together, these results support the role of αv integrins in mediating MSC interactions with tropoelastin.
Tropoelastin Modulates MSC Expansion via αv Integrins.
We found that soluble tropoelastin-mediated MSC expansion is attenuated by integrin blocking but not by growth factor receptor inhibition. The proliferative advantages of growth factors were primarily attributed to bFGF rather than IGF1; therefore, bFGF was selected as the functional parallel to tropoelastin. The addition of SU-5402, a fibroblast growth factor receptor (FGFR) inhibitor, hindered MSC proliferation over 7 d in a dose- and time-dependent manner (Fig. 4I and SI Appendix, Fig. S4A). The extent of inhibition varied significantly among cells cultured in normal media, media containing bFGF, and media containing tropoelastin in solution. The most profound inhibition, up to a 78.9 ± 0.7% reduction in overall cell numbers compared with the no-inhibitor control, consistently occurred with cells grown in bFGF-supplemented media. In contrast, the reduced cell proliferation in tropoelastin-supplemented media was similar to that in normal media and can likely be ascribed to the nonspecific effects of SU-5402 (Fig. 4I). These results suggest that, unlike bFGF, soluble tropoelastin stimulates MSC propagation via an FGFR-independent pathway.
We also explored the cell proliferative consequences of blocking integrin receptors, specifically αvβ3, αvβ5, or all αv subunit integrins, over 7 d (Fig. 4J and SI Appendix, Fig. S4B). Antibody inhibition of αv integrin activity universally diminished MSC proliferation to varying degrees, regardless of culture media composition. However, the decrease in cell expansion was consistently greater for cells in tropoelastin-supplemented media than cells in normal media or in bFGF-supplemented media. Compared with the no-antibody control, inclusion of the anti-αvβ3 or anti-αvβ5 antibody significantly inhibited tropoelastin-mediated MSC proliferation by 30 ± 1.3% and 18.1 ± 0.9%, respectively. Addition of both anti-αvβ3 and anti-αvβ5 antibodies decreased cell expansion by 58.9 ± 4.2%, which was similar in magnitude to the 54.1 ± 3.7% reduction in cell numbers by the pan anti-αv antibody. A control antibody against β8 integrins, which are not expressed by MSCs, did not affect cell proliferation. These findings strongly indicate that tropoelastin in solution, similar to the substrate-bound protein, interacts with MSCs via integrins. Furthermore, αv integrins, specifically αvβ3 and αvβ5 in tandem, are involved in the propagation of proproliferative signals from tropoelastin during MSC expansion. Accordingly, specific inhibition of downstream signaling molecules, that is, focal adhesion kinase (FAK) by FAK inhibitor 14 and protein kinase B (PKB/AKT) by perifosine, significantly reduced tropoelastin-mediated proliferation by 50.7 ± 2.0% and 21.3 ± 0.5%, respectively (Fig. 4K). This decrease is significantly more profound than that caused by the nonspecific effects of these inhibitors and confirms the role of the integrin–FAK–PKB/AKT pathway in transducing tropoelastin-activated mitogenic signals in MSCs.
Interestingly, MSC proliferation in bFGF-supplemented media was also negatively impacted by the presence of integrin-blocking antibodies, although not to the same extent as that observed in cultures with tropoelastin (Fig. 4J). Significant inhibition relative to cells in normal media occurred only in the presence of anti-αv or both anti-αvβ3 and anti-αvβ5 antibodies. These results further suggest that bFGF-mediated MSC proliferation is at least also partially dependent on αv integrin signaling.
Substrate-Bound and Soluble Tropoelastin Attract MSCs.
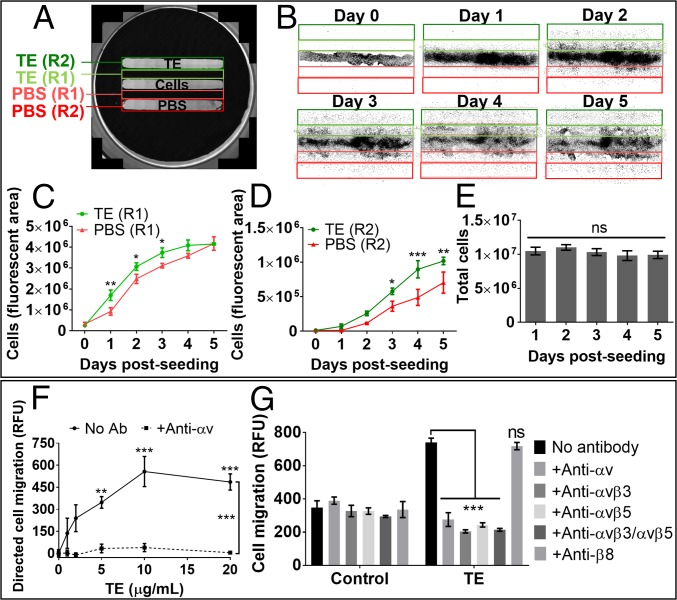
We wanted to investigate the potential of tropoelastin to attract MSCs, which would facilitate the tropoelastin–cell interactions for cell expansion. Cells seeded in a central region were equidistantly flanked by regions optionally coated with tropoelastin (Fig. 5A). MSCs preferentially migrated toward the surface-bound tropoelastin compared with the no-protein control over 5 d (Fig. 5B). This haptotactic gravitation toward tropoelastin manifested even at early time points (1–3 d postseeding), in which the region between the cells and tropoelastin was significantly more populated than the corresponding region between the cells and the PBS control (Fig. 5C). By 5 d postseeding, 45 ± 8% more cells had migrated to the tropoelastin-coated region compared with the control (Fig. 5D). The higher cell abundance associated with tropoelastin was not due to the increased proliferation of migrated cells, as suggested by similar total cell numbers over the experimental period (Fig. 5E).
Fig. 5.
Migration of MSCs toward tropoelastin. (A) Image showing the design of the migration assay. Cells were seeded in the middle chamber equidistant from flanking chambers containing substrate-bound tropoelastin or PBS. The well surface was divided into labeled regions within which cell numbers were measured as an indication of positional cell migration. (B) Binary images of the labeled regions over 5 d, showing the spread of cell migration. Each black dot represents one cell nucleus as visualized under fluorescence microscopy. (C) Comparative cell abundance within the regions that are adjacent to the areas coated with tropoelastin or PBS. (D) Comparative cell abundance within the regions coated with tropoelastin or PBS. (E) Total cell abundance within all regions over the experimental period. (F) Cell migration toward increasing concentrations of tropoelastin as a diffusible chemoattractant in the bottom chamber of a Boyden chamber assay. Cells were incubated with or without 5 μg/mL anti-αv integrin antibody in the top chamber. Cell migration was normalized to the level of unstimulated migration exhibited by no-tropoelastin controls. Asterisks above data points represent significant differences from the no-tropoelastin control. (G) Cell chemotaxis to normal or tropoelastin-supplemented media in the presence of integrin-blocking antibodies. Controls without antibodies or with an antibody against a nonexpressed integrin (anti-β8) were included. Asterisks represent significant differences from the no-antibody control. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; RFU, relative fluorescence unit.
Similarly, MSCs also migrated toward a diffusible gradient of tropoelastin in a Boyden chamber setup. Tropoelastin in solution induced a dose-dependent chemotactic response, which was abolished in the presence of the anti-αv integrin antibody (Fig. 5F). Antibodies that block all αv, either αvβ3 or αvβ5, or both αvβ3 and αvβ5 integrins effectively diminished tropoelastin-directed MSC migration to levels attributed to random cell mobility (Fig. 5G). In contrast, the control anti-β8 antibody did not affect MSC chemotaxis toward tropoelastin (SI Appendix, Fig. S5A). Moreover, the αv-inhibitory antibodies did not alter levels of undirected cell migration in which no chemoattractant was present, nor did they inhibit chemotaxis toward IGF1 or bFGF growth factors (SI Appendix, Fig. S5B).
These results demonstrate the strong motogenic ability of substrate-bound and soluble tropoelastin and the necessary and specific involvement of both αvβ3 and αvβ5 integrins in this process. This integrin dependence further implicates a method of MSC homing distinct from that used by chemotactic growth factors.
Discussion
The ability to efficiently and cost-effectively expand therapeutic cells such as MSCs is of significant clinical and commercial interest (28). Here we report that tropoelastin by itself not only markedly augments MSC proliferation but also parallels or surpasses the performance of specific growth factors. Among the growth factors used in MSC culture are IGF1 (29, 30) and bFGF (9, 10, 31), both of which are also part of commercially available MSC growth media. As a surface coating, tropoelastin promotes cell proliferation significantly better than IGF1, which alone does not increase cell numbers compared with normal media. This finding is consistent with reports that IGF1 facilitates MSC migration and early-stage growth (29) but does not improve long-term MSC proliferation (30). In addition, substrate-bound tropoelastin is functionally comparable to bFGF in full-serum media and superior in reduced-serum media in stimulating a proliferative response. The high capacity of tropoelastin to stimulate proliferation allows the replacement of IGF1 or bFGF in full-serum media and both IGF1 and bFGF in reduced-serum media without compromising the expansion potential of MSCs. Furthermore, supplanting growth factors with a stable recombinant protein such as tropoelastin also alleviates some of the challenges associated with the use of growth factors, such as their limited availability from animal tissues (9), high cost, and relative instability in media (31).
The potency of tropoelastin observed even in reduced-serum media points to its potential to replace a proportion of serum during MSC culture. Serum is included in MSC growth media as it not only promotes cell attachment due to the presence of base membrane proteins such as collagens, fibronectin, laminin, and vitronectin but also induces proliferation due to growth factors, hormones, and lipids (5, 8). Therefore, the ability of tropoelastin to compensate for serum reduction is consistent with its known cell adhesive function (32), combined with its high mitogenic activity reflective of growth factors. Tropoelastin remarkably allows up to a 40% reduction of serum content in culture media, a unique property not exhibited by other ECM proteins. We show that fibronectin, which is often used as an adhesion molecule in stem cell culture (14, 16), had diminished benefits even in 20% reduced-serum media.
Substantial serum compensation by tropoelastin mirrors another benefit typically associated with growth factors (5). We demonstrate that this ability of tropoelastin exceeds that of IGF1 and bFGF combined, which allowed for up to 20% reduction in serum. Interestingly, surface-bound tropoelastin did not allow significant serum reduction when growth factors were also present, suggesting that separate pathways may be activated by relative cell exposure to the soluble growth factors and to the substrate-bound tropoelastin. The use of tropoelastin to reduce reliance on serum during MSC expansion is clinically beneficial. Serum can carry contaminants that pose infection risks and, as an animal-derived product, can trigger adverse immune responses (25).
The functionality of tropoelastin, as with other matrix proteins, has conventionally been attributed to signals triggered upon cell adhesion to the molecule, whereby cell surface receptors such as integrins transduce the mechanical stimuli into chemical signals to effect a cellular response (33). Consistent with this paradigm, the proproliferative potential of tropoelastin has been ascribed solely to the elasticity, roughness, and cell adhesiveness of the molecule (17, 18). Contrary to this thinking, here we show that tropoelastin in solution above a concentration of 1 μg/mL also significantly enhances MSC expansion. At 20 μg/mL, tropoelastin in solution functionally supersedes the surface-bound protein and parallels the synergistic effect of IGF1 and bFGF in full-serum media. Our findings indicate that the mitogenic activity of tropoelastin can be independent of its effect on substrate elasticity and topography. While MSC progression through the cell cycle is anchorage-dependent (34), cells do not need to specifically attach and spread on the effector protein such as tropoelastin for proproliferative signaling to occur.
Furthermore, the modulatory behavior of tropoelastin in solution is most likely independent of mechanotransductive processes. As an individual molecule, the length of tropoelastin at ∼20 nm (35) would preclude mechanical connections with multiple cells. Within our experimental conditions, tropoelastin also cannot assemble into larger cell-linking constructs since the timescale of the proliferation assays at 7 d is significantly shorter than the minimum 12–14 d needed for elastic fibers to be formed. Also, the highest concentration of soluble tropoelastin used in these assays (20 μg/mL) is 50-fold below the critical concentration threshold for tropoelastin self-assembly (36).
Tropoelastin is a rare example of a full-length adhesive matrix protein that can moderate cell behavior as a soluble factor. In contrast, we show that fibronectin in solution does not promote MSC proliferation, possibly due to poor cell recognition as its cell receptor binding sites become exposed only upon adsorption to a surface such as a collagen matrix (37). The effects of tropoelastin in solution are likely enabled by the inherent accessibility (35, 38) of its cell binding regions (32, 39, 40). Before this work, soluble signaling factors derived from ECM proteins, including fibronectin, laminin, collagen, and elastin, were thought to be limited to peptides released by partial proteolysis, termed matrikines (41, 42). Presumably, these matrikines interact with cells via proteolytically exposed cell binding motifs. We find that the MSC modulatory properties of tropoelastin are distinctly different from that of elastin fragments and likely require the synergistic involvement of multiple cell-interactive regions (32, 39, 40) within a full-length molecule, such as the AAAAAAAAAAKAAKYGAAAGL sequence contained within domains 16–17 (40) and the C-terminal GRKRK motif in domain 36 (39).
The in vitro generation of MSCs can impact cell phenotype, which in turn can affect function and therapeutic potential (43, 44). Therefore, it is imperative that the tropoelastin-mediated amplification of MSC proliferation does not compromise stem cell properties. We found that cells expanded in the presence of substrate-bound or solution-based tropoelastin express characteristic surface markers and can undergo trilineage differentiation, consistent with the International Society for Cellular Therapy’s definition criteria for MSCs. This ability of tropoelastin to maintain MSC phenotype during expansion equates to that of growth factors in tandem. At sufficiently high concentrations, bFGF alone preserves MSC marker expression and delays proliferation-associated changes to stemness (9); however, long-term use increases differentiation and decreases expression of surface markers, including CD105 (11). Consistent with this finding, we show that media supplementation with IGF1 or bFGF alone reduces levels of CD105 and/or CD73, which are expected to be constitutively expressed by MSCs (43). The inclusion of tropoelastin remarkably protects against this phenotypic variation within the MSC population.
Phenotypic maintenance of stem cells is signaled from either soluble factors or adhesion proteins (17). Before this work, tropoelastin had been asserted to promote stemness via MSC sensing of substrate elasticity (17, 18). However, the similar protective function of tropoelastin in solution again strongly indicates an alternative anchorage-independent signaling mechanism akin to that of growth factors.
We discover that tropoelastin can directly interact with MSCs via cell surface integrins αvβ3 and αvβ5. These integrins are known to be expressed by bone marrow-derived MSCs (45), are recognized by domains 16–17 and domain 36 within tropoelastin, and have been implicated in tropoelastin interactions with other cell types such as fibroblasts (32, 39). When activated, integrins cluster as part of focal adhesions, detected in our studies by staining for a core focal adhesion protein, vinculin. Focal adhesions link extracellular matrix proteins to the actin cytoskeleton and transmit not only mechanical but also chemical signals from the cell environment (46).
While tropoelastin can directly mediate MSC attachment and spreading via integrins, we explored the alternative hypothesis that it may elicit MSC proliferation indirectly, particularly when in solution, or directly, albeit via a nonintegrin pathway. For instance, tropoelastin may potentiate the mitogenic activity of endogenous (31) or serum-derived (8) growth factors such as bFGF, as many ECM proteins can bind growth factors and increase localization to their receptors (47). Alternatively, tropoelastin may itself activate FGFR, as intrinsic domains within some ECM proteins can serve as noncanonical ligands for growth factor receptors (48). In these instances, addition of the FGFR inhibitor SU-5402 should negate the proproliferative function of both tropoelastin and bFGF. However, MSC expansion by tropoelastin, unlike that by bFGF, was not affected beyond the nonspecific inhibition associated with SU-5402 toxicity (49) and can therefore proceed via an FGFR-independent pathway. On this basis, we can exclude the sole involvement of bFGF as the effector protein or FGFR as the signaling receptor in tropoelastin-mediated MSC proliferation. Moreover, antibody and small-molecule inhibition of tropoelastin-mediated cell proliferation indicates the participation of αv integrins, namely, αvβ3 and αvβ5, and downstream signaling mediators such as FAK and PKB/AKT in this process. Activation of the integrin, FAK, and PKB/AKT cascade is known to regulate MSC responses, including proliferation (50, 51). Integrins have been shown to bind both immobilized and soluble ligands sufficiently to initiate signaling events (52, 53), suggesting a common mechanism by which substrate-bound and soluble tropoelastin direct MSC events. However, we cannot discount the involvement of other cell receptors, such as the elastin binding protein, in mediating the modulatory effects of tropoelastin in solution.
A similar dual mode of action is observed in tropoelastin-directed MSC migration, in which surface tropoelastin possesses the haptotactic nature of adhesive ECM proteins (19), while soluble tropoelastin mirrors the chemotactic ability of chemokines and growth factors (21). While these signals are thought to be independent and potentially conflicting (24), tropoelastin can uniquely provide both biophysical and biochemical directional stimuli to elicit a potentially stronger MSC homing response. This motogenic ability of tropoelastin, which has also been reported with other cell types (54), can be exploited in biomedical applications to recruit resident or administered MSCs for improved therapeutic outcomes.
Tropoelastin-mediated MSC recruitment is also reliant on protein interactions with αv integrins. The abolishment of this process by antibodies that block either αvβ3 or αvβ5 strongly suggests the requisite involvement of both integrins. Integrin subunits previously implicated in MSC homing have been limited to α4, α5, or β1 (22, 55) and are primarily regulated by chemokine activation of cognate receptors (22). Tropoelastin–αv integrin interactions represent a newfound mechanism underpinning MSC migration. Furthermore, the noninhibitory effect of αv-blocking antibodies on growth factor-mediated chemotaxis suggests separate, specific modes of MSC recruitment by tropoelastin and growth factors, at least on the cell surface level.
Integrin activation by ligand occupancy initiates multiple signaling cascades, including serine/threonine kinase, small GTPase, and inositol lipid pathways that mediate cell survival, adhesion, spreading, proliferation, and migration (15, 34, 56). Several of these pathways are also activated by bFGF binding to its FGF receptor in MSCs (25, 57). Furthermore, the association of αv integrins with growth factor receptors is thought to be required for sustained growth factor activation of downstream proliferative signals (33). In support, blocking αv integrins inhibits cell growth even in the presence of growth factors (58), which reflects our findings that αv integrin inhibition also attenuates bFGF-mediated MSC expansion. The overlap of intracellular signaling cascades shared by integrins and FGF receptors represents a possible mechanism by which tropoelastin parallels and can therefore replace the mitogenic, protective, and motogenic functions of growth factors such as bFGF (Fig. 6).
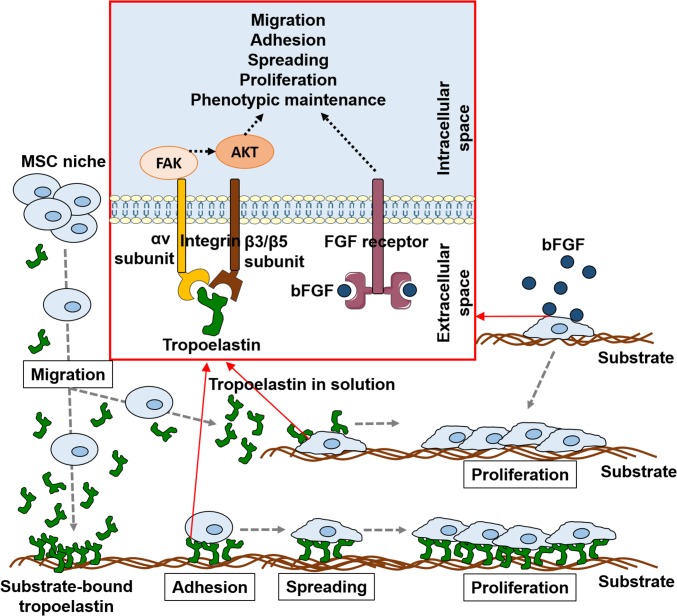
Fig. 6.
Model of tropoelastin modulation of MSC behavior. Substrate-bound or soluble tropoelastin attracts MSCs to migrate toward it. MSCs adhere and spread to the tropoelastin substrate, which triggers rapid cell expansion while simultaneously preserving MSC surface marker expression and trilineage differentiation potential. Unlike the majority of anchorage-dependent matrix proteins, tropoelastin in its soluble form likewise promotes MSC proliferation and phenotypic maintenance. These signals from tropoelastin are conveyed via cell surface integrin receptors, specifically αvβ3 and αvβ5, and propagated via FAK and PKB/AKT to induce potent motogenic and mitogenic MSC responses that mirror those of soluble growth factors such as bFGF.
The functionalities of tropoelastin, particularly in terms of MSC migration, propagation, growth factor replacement, and serum compensation, appear to be unique to this protein, despite the similar ability of other ECM proteins to bind integrins. It is thought that not all ECM–integrin interactions promote cell cycle progression equally, despite similar capabilities for cell adhesion and cytoskeletal organization (34). For example, the αvβ3 integrin can specifically associate with adapter proteins downstream of growth factor receptors and cooperatively activate and sustain long-term mitogenic pathways, allowing αvβ3 ligands such as tropoelastin to enhance cell proliferation more potently than nonligands (59). Moreover, matrix proteins such as fibronectin may adhere up to 20 types of integrins (60), which can drive opposing effects on cell proliferation and attenuate or avert the target cell response (16, 34). The narrow integrin selectivity of tropoelastin may therefore contribute to its specific outcomes on MSC behavior.
The potent mitogenic and motogenic effects of tropoelastin on MSCs is surprising since it is not natively present in the stem cell niche (61) unlike bFGF (62). We propose that this growth factor-like behavior of tropoelastin becomes biologically relevant in instances requiring rapid MSC homing and elevated MSC proliferation, namely, during embryonic development and wound repair, which coincide with the only periods in which free tropoelastin abounds in the extracellular environment. During the fetal to neonatal stages, peak tropoelastin synthesis occurs (63) alongside widespread bFGF expression (64), which may recruit MSCs and drive their propagation for normal development. The known inhibitory effects of bFGF on tropoelastin production during development (65) may indeed be a regulatory mechanism to safeguard against uncontrolled stem cell numbers resulting from the cumulative effects of bFGF and tropoelastin. During injury, up-regulated tropoelastin secretion may supplement the low level of bFGF in tissues (66) to rapidly stimulate MSC migration and proliferation integral to wound healing (67).
Further investigations are needed to define the intracellular responses to tropoelastin and understand the biological impetus for its potent MSC modulation. Nevertheless, this work demonstrates the potential of incorporating tropoelastin into materials to promote growth and maintain function of administered MSCs or to recruit endogenous MSCs to a target site for tissue repair. This work also lays a firm groundwork for the use of tropoelastin as a potent substrate coating or media additive for MSC culture, particularly in large-scale clinical expansion systems where the significant increase in cell yield and simultaneous reduction in serum and growth factor requirements translate to substantial cost savings. Tropoelastin can be produced in commercial quantities at good manufacturing practice grade for approximately US$0.70/mg. For a typical 5 L cell expansion system covering 2 m2 of culture area, replacing bFGF, fibronectin and 40% of the serum content with tropoelastin during each weekly expansion cycle results in annual savings of over US$86,000 in reagent costs alone. We propose that this approach represents a significant impact on cell manufacturing economics and poses a real potential to substantially reduce the cost of cell therapies.
Materials and Methods
Cell Culture.
Human bone marrow-derived MSCs obtained from American Type Culture Collection (ATCC) were cultured in normal media, which consists of alpha-minimum essential medium (α-MEM) (Lonza) with 10% (vol/vol) FBS (Life Technologies) and 2.4 mM l-glutamine (Lonza), at 37 °C in a humidified normoxic incubator up to a maximum of 10 population doublings. Where indicated, the normal medium was supplemented with 15 ng/mL IGF1 (Life Technologies) and/or 125 pg/mL bFGF (Life Technologies), equivalent to the growth factor concentrations in the ATCC-recommended media. Cells were passaged once they reached 70–80% confluence. Where indicated, tissue culture plastic wells were coated with 20 μg/mL recombinant human tropoelastin (Elastagen) or 2 μg/mL fibronectin (Sigma) in PBS (10 mM phosphate, 150 mM NaCl, pH 7.4) at 4 °C overnight. The protein solution was removed, and wells were washed three times with PBS to remove unbound protein before cell seeding. Where indicated, normal medium was supplemented with 2.5–20 μg/mL tropoelastin (Elastagen), 2.5–50 μg/mL of κELN (soluble human skin elastin from Elastin Products Company), or 2.5–50 μg/mL αELN (soluble human lung elastin from Elastin Products Company). To prevent protein adhesion on the tissue culture substrate, wells were preincubated with full-serum media for 5 h to enable surface blocking by serum proteins before cell seeding in supplemented media (details in SI Appendix).
Cell Proliferation.
Subconfluent flasks of MSCs were treated with 0.05% (vol/vol) trypsin-EDTA (Sigma) at 37 °C for 5 min to lift off adherent cells from the culture vessel. Trypsin was neutralized with two volumes of serum-containing growth media. Cells were centrifuged at 270 × g for 5 min and resuspended in the required media. Cells were seeded at a density of 5,000 cells/cm2 on bare or protein-coated 48-well tissue culture plastic wells, in normal or supplemented media. Media were changed every 2 d. After specific time points, cells were fixed with 3% (vol/vol) formaldehyde at room temperature for 20 min, washed with PBS, then stained with 0.1% (wt/vol) crystal violet in 0.2 M MES buffer for 1 h. Excess stain was washed off four times with reverse osmosis water. The retained stain was solubilized with 10% (vol/vol) acetic acid, and sample absorbance values indicative of cell abundance were read at 570 nm. Sample absorbance values were subtracted by baseline values (corresponding to cell numbers in serum-free media or cell numbers on day 1 postseeding) and expressed as a fraction of the highest absorbance among all samples on day 7 postseeding.
EDTA Inhibition and Cation Add Back.
MSCs were seeded at a density of 1.5 × 105 cells/cm2 on tropoelastin-coated wells in serum-free α-MEM containing 0–9 mM EDTA (Sigma). The cells were incubated for 1 h at 37 °C, then washed with cation-free PBS to remove unbound cells. Bound cells were fixed, stained, and measured for absorbance at 570 nm, as described for the proliferation assays. The percentage of cell attachment was determined relative to a set of standards with known cell numbers. For cation add-back assays, MSCs were washed with cation-free PBS, centrifuged at 270 × g for 5 min, and resuspended in cation-free PBS. The cells were seeded at a density of 1.5 × 105 cells/cm2 on tropoelastin-coated wells in the presence of 0–0.5 mM cation (Mg2+, Ca2+, or Mn2+) and incubated for 45 min at 37 °C. Bound cells were fixed and stained, and cell attachment was quantified as previously described.
Cell Spreading.
MSCs were seeded at a density of 7.5 × 104 cells/cm2 on tropoelastin-coated wells in serum-free α-MEM for 1.5 h at 37 °C. Cells were fixed and visualized by phase contrast microscopy under 10× magnification with a Zeiss Axio Vert.A1 microscope. Images were taken on an AxioCam ICm1 monochrome camera. Cells were categorized as spread (i.e., cells which exhibit a phase-dark, flattened morphology) or unspread (i.e., cells which appear round and phase-bright). Cell spreading was quantified by counting the percentage of spread cells in each field of view. Three fields of view were obtained at similar positions for each sample replicate, covering ∼10% of the total sample area.
Immunofluorescent Staining.
MSCs were seeded on TCP coated with 20 μg/mL tropoelastin or 10 mg/mL BSA for 1 d. Focal adhesions were detected with a fluorescently tagged anti-vinculin monoclonal antibody, while cell nuclei were stained with DAPI using a Focal Adhesion Staining Kit (Merck Millipore). Samples were visualized and imaged with an Olympus FV1000 confocal microscope at the Australian Centre for Microscopy & Microanalysis, University of Sydney. Focal adhesion density per cell was calculated by dividing the number of pixels corresponding to stained vinculin by the number of cells in each field of view, then averaged for each sample.
Integrin, FGFR, FAK, or PKB/AKT Inhibition.
To block specific integrin activity, up to 20 μg/mL of anti-αv or anti-αvβ3 integrin antibodies (Abcam) or up to 1:250 dilution of anti-αvβ5 integrin antibody (Abcam) were added to the media during MSC spreading or proliferation assays. Optimal inhibitory concentrations were selected for the anti-αv (5 μg/mL), anti-αvβ3 (5 μg/mL), and anti-αvβ5 (1:500 dilution) integrin antibodies. An anti-β8 integrin (5 μg/mL) (Abcam) or a nonspecific mouse IgG (5 μg/mL) (Sigma) was also included as a negative antibody control. To block FGFR activity, up to 20 μM SU-5402 FGFR inhibitor (Sigma) was added to the media during MSC proliferation. The integrin and FGFR inhibitors were replenished during every media change. To block FAK or PKB/AKT activity, up to 10 μM FAK inhibitor 14 (Sigma) or perifosine (Sigma) was added to the culture media during cell proliferation in the presence or absence of tropoelastin. Optimal inhibitory concentrations of FAK inhibitor 14 (2.5 μM) or perifosine (10 μM) were selected.
Cell Migration.
Polydimethylsiloxane (PDMS) was casted into 3D printed molds to create a circular shape with three rectangular cutouts, in which the middle rectangle was equidistant from the flanking rectangles. The PDMS stencil was placed inside a well plate and pressed tightly against the well surface to create a watertight seal. The top and bottom rectangular chambers were filled with tropoelastin solution (20 μg/mL) and PBS, respectively, and air-dried overnight. MSCs (1.2 × 106 cells/cm2) were seeded into the middle chamber and allowed to attach for at least 2 h. The PDMS stencil was removed, and the culture well was covered with normal media. For up to 5 d, cells were stained daily with NucBlue Live ReadyProbes Reagent (Life Technologies) for 15 min, washed once with PBS, covered with normal media, and imaged under fluorescence at 360/460 nm using a Nikon Ti-E Live Cell Microscope. Cell migration into regions defined by tropoelastin or PBS coating was quantified via relative fluorescent areas using ImageJ software.
Chemotaxis was measured using a fluorometric 96-well Boyden chamber assay system (QCM Chemotaxis Cell Migration Assay; Millipore) according to the supplier’s instructions. Normal, tropoelastin-supplemented, or growth factor-supplemented medium was added to the lower chamber of the well plate, while MSCs were seeded at 14,300 cells/cm2 in normal media into the upper chamber. Where indicated, integrin-blocking antibodies were added at optimized concentrations to the upper chamber with the cells. Cells that migrate through the permeable membrane into the lower chamber were detached and quantified. Normalized cell migration was calculated by subtracting the extent of undirected cell migration (where no chemoattractant was added to the lower chamber) from each experimental result.
MSC Verification.
MSCs expanded in various substrate and media conditions were phenotypically and functionally verified by surface marker expression via flow cytometry and osteogenic, adipogenic, and chondrogenic differentiation (details in SI Appendix).
Statistical Analyses.
All data were reported as mean ± SEM (n = 3). Statistical comparisons were calculated using ANOVA. Significance was set at P < 0.05. Statistical significance is denoted in figures by asterisks: *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We acknowledge Sanaz Maleki and Rosie Cheong-Soos from the histopathology laboratory at the University of Sydney and Dr. Michael Kuligowski from the Brain and Mind Centre at the University of Sydney. We acknowledge the facilities and technical assistance of the Advanced Cytometry Facility at the University of Sydney, as well as the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Australian Centre for Microscopy & Microanalysis at the University of Sydney. Part of this research was funded by the Cooperative Research Centre for Cell Therapy Manufacturing.
Footnotes
Conflict of interest statement: A.S.W. is the founding scientist of Elastagen Pty. Ltd., now sold to Allergan, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812951116/-/DCSupplemental.
References
- 1.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Connick P, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salzig D, et al. Attachment, growth, and detachment of human mesenchymal stem cells in a chemically defined medium. Stem Cells Int. 2016;2016:5246584. doi: 10.1155/2016/5246584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73–87. doi: 10.4252/wjsc.v8.i3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trainor N, Pietak A, Smith T. Rethinking clinical delivery of adult stem cell therapies. Nat Biotechnol. 2014;32:729–735. doi: 10.1038/nbt.2970. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Panchalingam KM, Rosenberg L, Behie LA. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012;2012:123030. doi: 10.1155/2012/123030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee TH, Kim WT, Ryu CJ, Jang YJ. Optimization of treatment with recombinant FGF-2 for proliferation and differentiation of human dental stem cells, mesenchymal stem cells, and osteoblasts. Biochem Cell Biol. 2015;93:298–305. doi: 10.1139/bcb-2014-0140. [DOI] [PubMed] [Google Scholar]
- 10.Bertolo A, et al. Growth factors cross-linked to collagen microcarriers promote expansion and chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2015;21:2618–2628. doi: 10.1089/ten.TEA.2015.0029. [DOI] [PubMed] [Google Scholar]
- 11.Gharibi B, Hughes FJ. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012;1:771–782. doi: 10.5966/sctm.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 13.Rojewski MT, et al. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 2013;22:1981–2000. doi: 10.3727/096368912X657990. [DOI] [PubMed] [Google Scholar]
- 14.Yun Y-R, et al. Engineering of self-assembled fibronectin matrix protein and its effects on mesenchymal stem cells. Int J Mol Sci. 2015;16:19645–19656. doi: 10.3390/ijms160819645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindner U, et al. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 16.Martino MM, et al. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holst J, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, et al. The influence of elasticity and surface roughness on myogenic and osteogenic-differentiation of cells on silk-elastin biomaterials. Biomaterials. 2011;32:8979–8989. doi: 10.1016/j.biomaterials.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J Cell Sci. 2011;124:1288–1300. doi: 10.1242/jcs.076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakian R, et al. Native extracellular matrix preserves mesenchymal stem cell “stemness” and differentiation potential under serum-free culture conditions. Stem Cell Res Ther. 2015;6:235. doi: 10.1186/s13287-015-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki Y, et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119–129. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- 22.Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon D, et al. Study on chemotaxis and chemokinesis of bone marrow-derived mesenchymal stem cells in hydrogel-based 3D microfluidic devices. Biomater Res. 2016;20:25. doi: 10.1186/s40824-016-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol. 2014;15:813–824. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.Zhang K, Chen J. The regulation of integrin function by divalent cations. Cell Adhes Migr. 2012;6:20–29. doi: 10.4161/cam.18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanley PJ, et al. Manufacturing mesenchymal stromal cells for phase I clinical trials. Cytotherapy. 2013;15:416–422. doi: 10.1016/j.jcyt.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YL, et al. Effects of insulin-like growth factor-1 on the properties of mesenchymal stem cells in vitro. J Zhejiang Univ Sci B. 2012;13:20–28. doi: 10.1631/jzus.B1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, et al. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Wijesinghe SJ, et al. Affinity selection of FGF2-binding heparan sulfates for ex vivo expansion of human mesenchymal stem cells. J Cell Physiol. 2017;232:566–575. doi: 10.1002/jcp.25454. [DOI] [PubMed] [Google Scholar]
- 32.Lee P, Bax DV, Bilek MMM, Weiss AS. A novel cell adhesion region in tropoelastin mediates attachment to integrin αVβ5. J Biol Chem. 2014;289:1467–1477. doi: 10.1074/jbc.M113.518381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivaska J, Heino J. Interplay between cell adhesion and growth factor receptors: From the plasma membrane to the endosomes. Cell Tissue Res. 2010;339:111–120. doi: 10.1007/s00441-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz MA, Assoian RK. Integrins and cell proliferation: Regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 35.Baldock C, et al. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc Natl Acad Sci USA. 2011;108:4322–4327. doi: 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo GC, Keeley FW, Weiss AS. Coacervation of tropoelastin. Adv Colloid Interface Sci. 2011;167:94–103. doi: 10.1016/j.cis.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: Expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014;134:895–901. doi: 10.1038/jid.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo GC, et al. Subtle balance of tropoelastin molecular shape and flexibility regulates dynamics and hierarchical assembly. Sci Adv. 2016;2:e1501145. doi: 10.1126/sciadv.1501145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bax DV, Rodgers UR, Bilek MMM, Weiss AS. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J Biol Chem. 2009;284:28616–28623. doi: 10.1074/jbc.M109.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee P, Yeo GC, Weiss AS. A cell adhesive peptide from tropoelastin promotes sequential cell attachment and spreading via distinct receptors. FEBS J. 2017;284:2216–2230. doi: 10.1111/febs.14114. [DOI] [PubMed] [Google Scholar]
- 41.Duca L, Floquet N, Alix AJP, Haye B, Debelle L. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49:235–244. doi: 10.1016/j.critrevonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Maquart F-X, Pasco S, Ramont L, Hornebeck W, Monboisse J-C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol. 2004;49:199–202. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: Implications for basic research and the clinic. Stem Cells. 2014;32:1713–1723. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- 44.Kim N, Cho S-G. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niehage C, et al. The cell surface proteome of human mesenchymal stromal cells. PLoS One. 2011;6:e20399. doi: 10.1371/journal.pone.0020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carisey A, et al. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol. 2013;23:271–281. doi: 10.1016/j.cub.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- 48.Schenk S, et al. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krejci P, et al. NF449 is a novel inhibitor of fibroblast growth factor receptor 3 (FGFR3) signaling active in chondrocytes and multiple myeloma cells. J Biol Chem. 2010;285:20644–20653. doi: 10.1074/jbc.M109.083626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Crawford R, Chen C, Xiao Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev. 2013;19:516–528. doi: 10.1089/ten.TEB.2012.0672. [DOI] [PubMed] [Google Scholar]
- 51.Prowse ABJ, Chong F, Gray PP, Munro TP. Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaric J, Rüegg C. Integrin-mediated adhesion and soluble ligand binding stabilize COX-2 protein levels in endothelial cells by inducing expression and preventing degradation. J Biol Chem. 2005;280:1077–1085. doi: 10.1074/jbc.M410006200. [DOI] [PubMed] [Google Scholar]
- 54.Mithieux SM, Wise SG, Weiss AS. Tropoelastin–A multifaceted naturally smart material. Adv Drug Deliv Rev. 2013;65:421–428. doi: 10.1016/j.addr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 56.Dedhar S. Cell-substrate interactions and signaling through ILK. Curr Opin Cell Biol. 2000;12:250–256. doi: 10.1016/s0955-0674(99)00083-6. [DOI] [PubMed] [Google Scholar]
- 57.Ng F, et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 58.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of beta 1 and alpha v beta 3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 59.Soldi R, et al. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogel V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 61.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 62.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swee MH, Parks WC, Pierce RA. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J Biol Chem. 1995;270:14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez AM, Hill DJ, Logan A, Maher PA, Baird A. Distribution of fibroblast growth factor (FGF)-2 and FGF receptor-1 messenger RNA expression and protein presence in the mid-trimester human fetus. Pediatr Res. 1996;39:375–385. doi: 10.1203/00006450-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Brettell LM, McGowan SE. Basic fibroblast growth factor decreases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol. 1994;10:306–315. doi: 10.1165/ajrcmb.10.3.8117449. [DOI] [PubMed] [Google Scholar]
- 66.Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.