Significance
Malignant pleural mesothelioma (MPM) is an aggressive cancer with poor prognosis and limited treatment options. MPM remains a serious public health problem, and novel therapeutic strategies are urgently needed. The antitumor properties of growth hormone-releasing hormone (GHRH) antagonists have been demonstrated in different cancers; however, their influence in MPM remains unexplored. Our work shows that GHRH antagonists MIA-602 and MIA-690 reduce survival, proliferation, and migration of human MPM cell lines and primary MPM cells in vitro by modulating apoptotic and oncogenic pathways. In vivo, GHRH antagonists inhibited the growth of MPM xenografts and blunted the production of growth factors in tumors. Overall, the inhibitory activities described in this study suggest that GHRH antagonists may be considered for development of therapies for MPM.
Keywords: growth hormone-releasing hormone, GHRH receptor, GHRH antagonists, malignant pleural mesothelioma
Abstract
Malignant pleural mesothelioma (MPM) is an aggressive malignancy associated with exposure to asbestos, with poor prognosis and no effective therapies. The strong inhibitory activities of growth hormone-releasing hormone (GHRH) antagonists have been demonstrated in different experimental human cancers, including lung cancer; however, their role in MPM remains unknown. We assessed the effects of the GHRH antagonists MIA-602 and MIA-690 in vitro in MPM cell lines and in primary MPM cells, and in vivo in MPM xenografts. GHRH, GHRH receptor, and its main splice variant SV1 were found in all the MPM cell types examined. In vitro, MIA-602 and MIA-690 reduced survival and proliferation in both MPM cell lines and primary cells and showed synergistic inhibitory activity with the chemotherapy drug pemetrexed. In MPM cells, GHRH antagonists also regulated activity and expression of apoptotic molecules, inhibited cell migration, and reduced the expression of matrix metalloproteinases. These effects were accompanied by impairment of mitochondrial activity and increased production of reactive oxygen species. In vivo, s.c. administration of MIA-602 and MIA-690 at the dose of 5 μg/d for 4 wk strongly inhibited the growth of MPM xenografts in mice, along with reduction of tumor insulin-like growth factor-I and vascular endothelial growth factor. Overall, these results suggest that treatment with GHRH antagonists, alone or in association with chemotherapy, may offer an approach for the treatment of MPM.
Malignant pleural mesothelioma (MPM) is a rare and aggressive cancer affecting the pleura (typically associated with exposure to asbestos), with poor prognosis and a median survival ranging from 3 to 12 mo. The incidence of MPM is expected to increase in the next decades because of the long latency from time of asbestos exposure to development of the disease (1). There is no effective treatment for MPM and since surgery is controversial, often a multimodal approach is applied that includes chemotherapy and sometimes radiotherapy, even at early stages. The first-line option for inoperable MPM is chemotherapy with cisplatin and pemetrexed (PEM); however, this is mainly palliative and has a short and insufficient efficacy (1, 2). Therefore, new therapeutic strategies are urgently needed. Pathologically, MPM comprises three main histologic types: epithelioid, sarcomatoid, and biphasic, which has both epithelioid and sarcomatoid components (3). The pathogenesis of MPM is multifactorial. Inhaled asbestos fibers migrate to the pleural space, causing DNA damage and abnormal repair of mesothelial cells, acute inflammation with the release of inflammatory cytokines, recruitment of macrophages and neutrophils, activation of various kinases, an increase in expression of protooncogenes, and proliferation of airway epithelial cells (4).
Growth hormone-releasing hormone (GHRH) is a neuropeptide produced in the hypothalamus. In the anterior pituitary, GHRH regulates the synthesis and secretion of growth hormone (GH) and the proliferation of pituitary cells, by binding to pituitary type GHRH receptor (pGHRH-R) (5). GHRH also exerts direct activities in extrapituitary cells and tissues (6, 7), which include, among others, cardioprotection (8, 9), regeneration of pancreatic islets (10), wound healing (11), and survival and antiapoptotic effects (12, 13). Moreover, GHRH functions as an autocrine/paracrine growth factor in nonmalignant cells and in tumors through mechanisms mediated by GHRH-R and its splice variant type 1 (SV1) (5, 6, 14). The stimulatory loop, formed by tumor-derived GHRH and its receptors, can be blocked by GHRH antagonists, resulting in inhibition of tumor growth in experimental models. In fact, GHRH antagonists suppress in vitro and in vivo the proliferation of many experimental human cancers, including lung cancer (14–17).
Recently, new GHRH antagonists of the Miami (MIA) series have been synthetized, such as MIA-602 and MIA-690, with greatly increased anticancer activity, higher binding affinity, and low endocrine effect on the GH/insulin-like growth factor I (IGF-I) axis. These analogs display potent inhibitory actions in thyroid, lung, gastric, renal, prostate, and endometrial cancer, as well as in glioblastoma, colorectal adenocarcinoma, lymphoma, and retinoblastoma (16–25). To date, however, the role of GHRH antagonists in MPM remains to be determined.
In this study, we evaluated the potential inhibitory activities of MIA-602 and MIA-690 on the survival and proliferation of human MPM cell lines and primary MPM cells. The effects on apoptosis and cell migration, along with mitochondria metabolism, were also analyzed in MPM cell lines. Finally, the antitumor activities of GHRH antagonists were assessed in vivo, in mice xenografted with human MPM cells.
Results
Expression of pGHRH-R, SV1, and GHRH in MPM Cells.
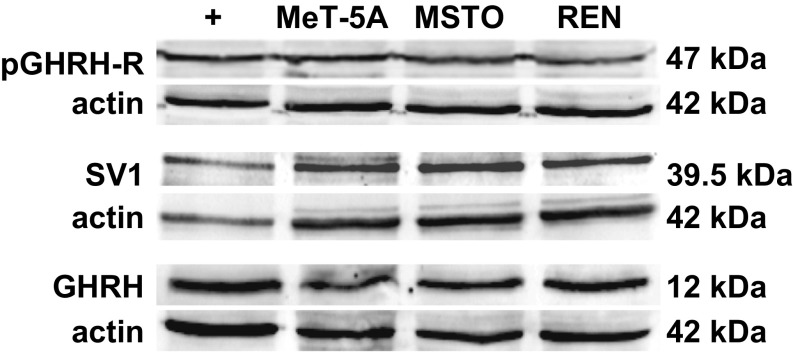
Results from Western blot analysis showed that both pGHRH-R and SV1 as well as GHRH proteins are expressed in pleural mesothelial cells (MeT-5A) and in pleural biphasic and epithelioid MPM cell lines (MSTO-211H and REN, respectively) (Fig. 1).
Fig. 1.
Protein expression for pGHRH-R (Upper), SV1 (Middle), and GHRH (Lower) in MeT-5A pleural mesothelial cells, and in MSTO-211H (MSTO) and REN human MPM cell lines, assessed by Western blot. LNCaP prostate cancer cells were used as positive control (+) for pGHRH-R and SV1, MCF-7 breast cancer cells were used as positive control for GHRH, and actin served as internal control.
GHRH Antagonists Inhibit Cell Survival and Proliferation, Promoting Apoptosis in MPM Cell Lines.
The inhibitory efficacy of MIA-602 and MIA-690 was initially determined in human MPM cell lines treated with the antagonists for 24 h at concentrations of 1 to 2,000 nM. We found a similar dose-dependent reduction in survival and proliferation of MSTO-211H and REN cells with both MIA-602 (SI Appendix, Fig. S1 A–D) and MIA-690 (SI Appendix, Fig. S1 E–H), particularly from 50 to 2,000 nM, compared with controls (IC50 for MIA-602: 3,925 nM in MSTO-211H and 2,350 nM in REN; IC50 for MIA-690: 2,779 nM in MSTO-211H and 2,431 nM in REN). Likewise, in cells treated for 48 h, the effect was observed from 50 nM for survival (SI Appendix, Fig. S2 A–D) and from 100 nM for proliferation (SI Appendix, Fig. S2 E–H) in both cell lines. Interestingly, in MeT-5A pleural mesothelial cells, both antagonists reduced survival only at the highest concentrations (SI Appendix, Fig. S3 A and B), while having no effect on proliferation (SI Appendix, Fig. S3 C and D). Because of the striking decrease with both antagonists in cell survival at 1,000 nM (1 μM) (MIA-602: 23% and MIA-690: 27.4% for MSTO-211H; MIA-602: 31.54% and MIA-690: 32.2% for REN) and proliferation (MIA-602: 23.63% and MIA-690: 24.93% for MSTO-211H; MIA-602: 25.59% and MIA-690: 27.34% for REN), 1 μM was chosen as concentration for subsequent studies.
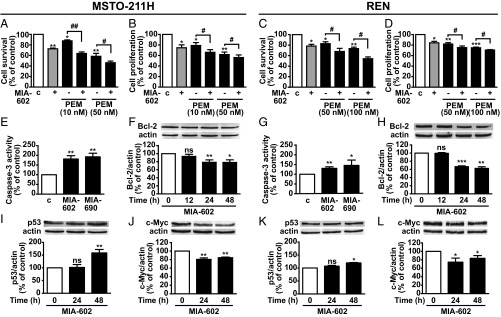
We next examined the possible combinatory cytotoxic activity produced by MIA-602 or MIA-690 with the anticancer drug PEM. MPM cells were treated for 72 h with each antagonist at 1 μM and with PEM at 10 or 50 nM in MSTO-211H cells or at 50 or 100 nM in REN cells, which are less sensitive to the cytotoxic effect of the drug. MIA-602 (Fig. 2 A–D) and MIA-690 (SI Appendix, Fig. S1 I–L) were synergistic in inhibiting cell survival in both MPM cell lines, with Combination Index (CI) values <1 at all of the concentrations of PEM tested. The proapoptotic effect of the antagonists was assessed at 12, 24, and 48 h in the MPM cell lines. MIA-602 and MIA-690 increased the activity of proapoptotic caspase-3 (Fig. 2 E and G) at 24 h in both MPM cell lines. Moreover, expression of the antiapoptotic protein Bcl-2 was reduced by MIA-602 in both MPM cell lines at 24 and 48 h (Fig. 2 F and H) and by MIA-690 at 48 h in MSTO-211H cells (SI Appendix, Fig. S1M) and at 12, 24, and 48 h in REN cells (SI Appendix, Fig. S1P). Accordingly, in both MPM cell lines, the tumor suppressor protein p53 was up-regulated by the antagonists at 48 h (Fig. 2 I and K and SI Appendix, Fig. S1 N and Q), whereas oncogenic c-Myc was reduced at 24 and 48 h (Fig. 2 J and L and SI Appendix, Fig. S1 O and R).
Fig. 2.
Inhibitory effects of GHRH antagonists in MPM cells. Cell survival (A and C) and proliferation (B and D) assessed by MTT and BrdU assays, respectively, in MSTO-211H and REN cells untreated or treated for 72 h in medium with 2.5% serum (c, control medium) with 1 μM MIA-602, alone or with PEM, at the concentrations indicated. Results, expressed as percent of control, are mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. c; #P < 0.05, ##P < 0.01; n = 4. Caspase-3 activity in MSTO-211H (E) and REN cells (G) treated for 24 h with 1 µM MIA-602 or MIA-690. Results, expressed as percent of control are mean ± SEM. *P < 0.05 and **P < 0.01 vs. control; n = 3. Representative Western blot for Bcl-2 (F and H), p53 (I and K), and c-Myc (J and L) in cells treated with 1 µM MIA-602 or MIA-690 for the indicated times. Results, normalized to actin and expressed as percent of control (Time 0), are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; n = 3. ns, not significant.
GHRH Antagonists Inhibit Growth and Migration of MPM Cell Lines.
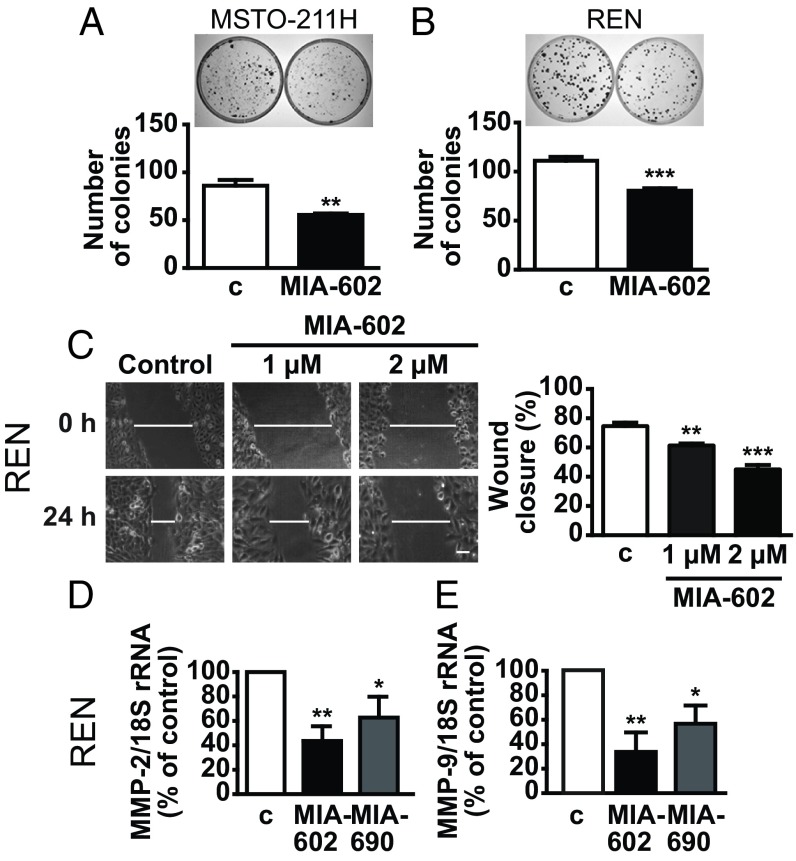
To further assess whether GHRH antagonists suppress the growth of MPM cells, we performed colony formation assay. Our data indicated a similar reduction in the ability to form colonies after 10 d in both MSTO-211H and REN cells treated with MIA-602 (Fig. 3 A and B) and MIA-690 (SI Appendix, Fig. S4 A and B), compared with untreated cells. The ability of MPM cells to migrate and invade the pleura is a major characteristic of tumor growth (4); thus, we next assessed cell migration by wound-healing assay. MIA-602 and MIA-690 strongly inhibited the wound closure in both MPM cell lines to a similar extent. In REN cells, the effect was detected at 1 μM and was even increased at 2 μM for both antagonists (Fig. 3C and SI Appendix, Fig. S4C). In MSTO-211H cells, only 1 μM was effective for both antagonists (SI Appendix, Fig. S4D). Moreover, in both cell lines, MIA-602 and MIA-690 equally reduced the mRNA levels of molecules implicated in cell migration and tumor growth [i.e., metalloproteinase (MMP)-2 and MMP-9] (Fig. 3 D and E and SI Appendix, Fig. S4 E and F).
Fig. 3.
Inhibitory effects of GHRH antagonists on cell growth and migration. Representative colony formation in MSTO-211H (A) and REN (B) cells untreated (c, control) or treated for 10 d with 1 μM MIA-602. Results are mean ± SEM. **P < 0.01 and ***P < 0.001 vs. c; n = 3. (C) Representative images of wound-healing assay in REN cells cultured in medium with 2.5% serum and treated for 24 h with MIA-602 at the indicated concentrations. (Scale bar: 20 μM.) Histogram (Right) shows the wound closure efficiency. Results, expressed as percent of control, are mean ± SEM. **P < 0.01 and ***P < 0.001 vs. c; n = 3. Real-time PCR for MMP-2 (D) and MMP-9 (E) mRNA expression normalized to 18S rRNA in REN cells treated for 24 h with 1 μM MIA-602 or MIA-690. Results, expressed as fold change of control, are mean ± SEM. *P < 0.05 and **P < 0.01 vs. c; n = 3.
MIA-602 and MIA-690 Induce Mitochondrial Damage.
Since mitochondria are central players in apoptosis (26), and we show here that GHRH antagonists promote apoptosis in MPM cells, we evaluated the role of mitochondria in the antitumor effects of MIA-602 and MIA-690. Mitochondrial membrane potential (ΔΨm), an indicator of mitochondrial activity, was dramatically reduced in MSTO-211H and REN cells treated with the antagonists for 48 h, as measured by flow cytometry analysis of the mitochondria-sensitive dye JC-1 (SI Appendix, Fig. S5 A and D). This result was accompanied by elevation of reactive oxygen species (ROS) (SI Appendix, Fig. S5 B and E), which causes impairment of ΔΨm, release of mitochondrial proteins, and activation of caspase cascades (27). Moreover, MIA-690, but not MIA-602, blunted the expression of superoxide dismutase 2 (SOD-2), a key mitochondrial antioxidant enzyme (SI Appendix, Fig. S5 C and F).
GHRH Antagonists Inhibit Cell Survival and Proliferation in Human Primary MPM Cells.
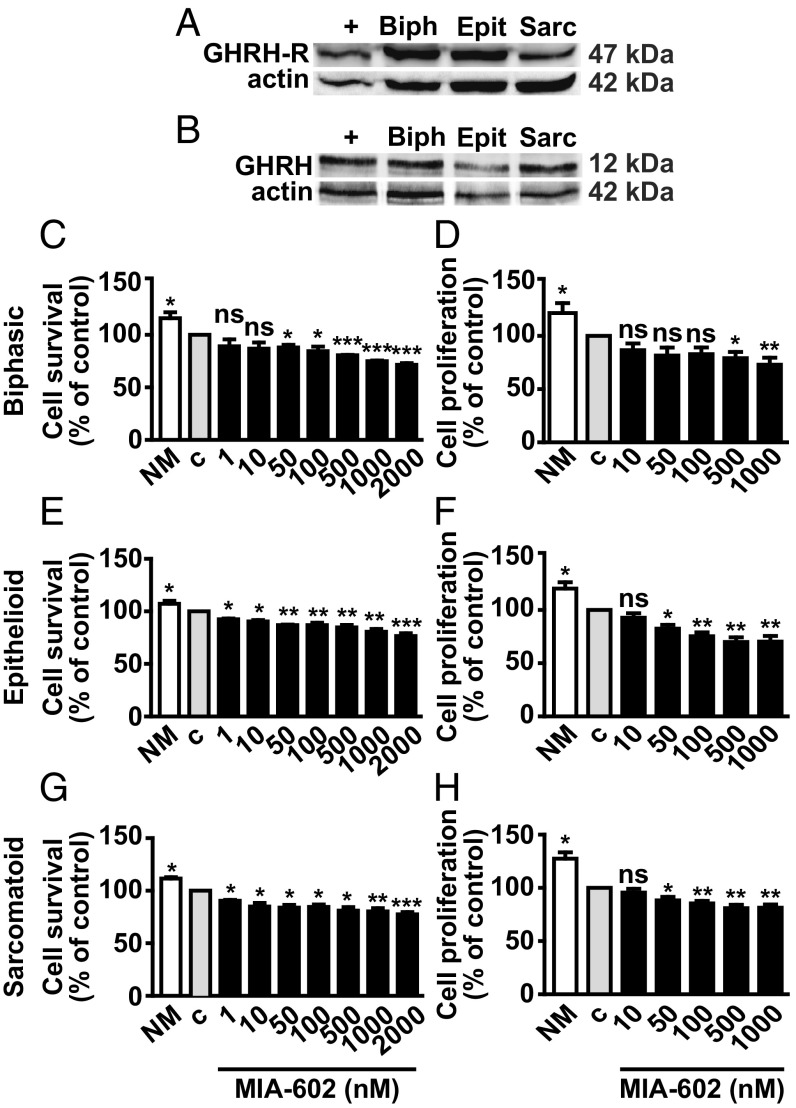
To next determine the effects of MIA-602 and MIA-690 in MPM cells obtained from pleural biopsy tissues of patients with MPM, we first analyzed the presence of GHRH-R and GHRH in biphasic, epithelioid, and sarcomatoid cells. Western blot and RT-PCR analysis showed expression of both GHRH-R and GHRH protein (Fig. 4 A and B) and mRNA (SI Appendix, Fig. S6 A and B) in all MPM types that were also positive for SV1 mRNA (SI Appendix, Fig. S6A). MIA-602 and MIA-690 displayed similar dose-dependent inhibitory activity on survival and proliferation in biphasic (Fig. 4 C and D and SI Appendix, Fig. S6 C and D), epithelioid (Fig. 4 E and F and SI Appendix, Fig. S6 E and F), and sarcomatoid cells (Fig. 4 G and H and SI Appendix, Fig. S6 G and H). MIA-602 was slightly more effective than MIA-690, being significant from 1 nM on survival, and from 50 nM on proliferation of epithelioid (Fig. 4 E and F) and sarcomatoid cells (Fig. 4 G and H). MIA-690, in turn, was more effective than MIA-602 in biphasic cells, starting from 1 nM for survival (SI Appendix, Fig. S6C) and from 100 nM for proliferation (SI Appendix, Fig. S6D).
Fig. 4.
Inhibitory effects of MIA-602 in primary MPM cells. Representative Western blot of GHRH-R (A) and GHRH protein (B) in biphasic (Biph), epithelioid (Epit), and sarcomatoid (Sarc) MPM cells. LNCaP prostate cancer cells and MCF-7 breast cancer cells were used as positive control (+) for GHRH-R and GHRH, respectively. Cell survival (MTT assay) and proliferation (BrdU assay) in biphasic (C and D), epithelioid (E and F), and sarcomatoid (G and H) cells cultured in either normal medium (NM) or serum-deprived medium (c, control) for 12 h, and then treated for 24 h with MIA-602 at the concentrations indicated. Results, expressed as percent of control, are mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. c; n = 3. ns, not significant.
GHRH Antagonists Inhibit the Growth of MPM in Vivo.
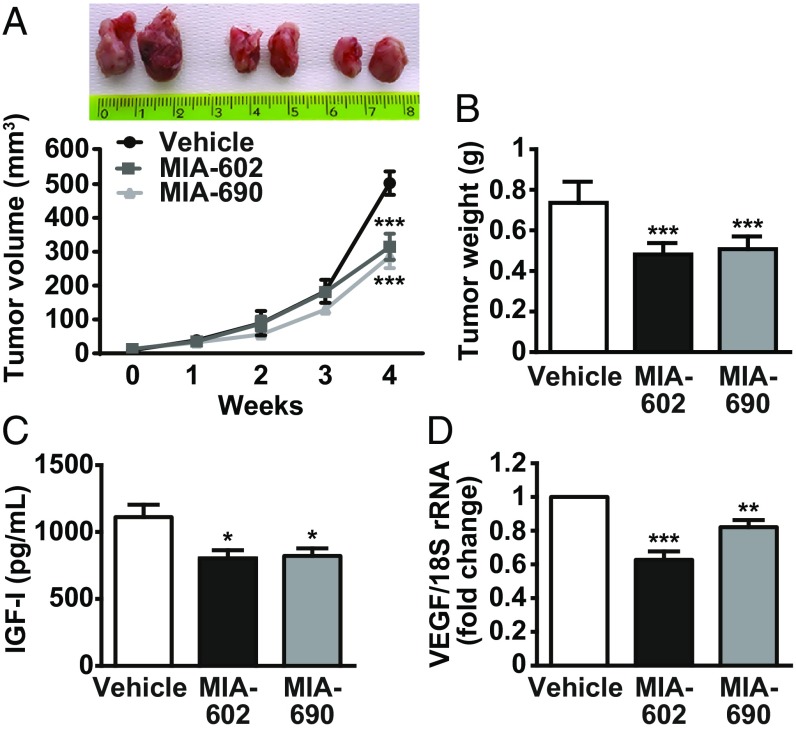
The therapeutic potential of MIA-602 and MIA-690 was evaluated in NOD/SCID/gamma chain−/− mice xenografted with MSTO-211H cells. When the tumors became palpable, mice were randomly assigned to receive a daily s.c. injection of MIA-602 or MIA-690 (5 μg/d) or vehicle for 4 wk. Both antagonists showed a remarkable inhibitory effect on tumor growth, as demonstrated by the reduction in tumor volume and weight (Fig. 5 A and B, P < 0.001). Moreover, in xenografts, the antagonists reduced the production of IGF-I protein (Fig. 5C) and blunted mRNA levels of VEGF (Fig. 5D). Histopathological examination of the tumors showed a uniform growth of neoplastic cells, morphologically consistent with biphasic MPM. More- or less-extensive areas of necrosis were observed in all cases, with a slight increase in treated animals (mean values 35% and 36% for treatments with MIA-602 and MIA-690, respectively, compared with 29% of untreated xenografts) (SI Appendix, Fig. S7).
Fig. 5.
Inhibitory effects of GHRH antagonists on the growth of MPM in vivo. (A) Representative MSTO-211H tumors, excised at the end of the experiments, from mice s.c. injected for 4 wk with either vehicle, MIA-602, or MIA-690 (5 μg/d) (Upper). The graph shows the tumor growth curves. Results are mean ± SEM. ***P < 0.001 vs. vehicle; n = 15 in each group. (B) Tumor weight in mice treated with either vehicle or GHRH antagonists. Results are mean ± SEM. ***P < 0.001 vs. vehicle; n = 15. (C) IGF-I levels measured in tumors by ELISA. Results are mean ± SEM. *P < 0.05 vs. vehicle; n = 6. (D) VEGF mRNA assessed by real-time PCR and normalized to 18S rRNA. Results, expressed as fold change of vehicle, are mean ± SEM. **P < 0.01 and ***P < 0.001 vs. vehicle; n = 10.
Discussion
MPM is an aggressive tumor with poor prognosis due to the unavailability of effective therapies. Even though MPM is a rare cancer, its incidence is expected to increase in the next two decades because of the worldwide exposure to asbestos over the past years (1).
MIA-602 and MIA-690 are part of the latest MIA series of GHRH antagonists with potent antitumor activity in different cancers, including lung cancer (16–25); however, their inhibitory effects in MPM remain to be investigated. This study shows that MIA-602 and MIA-690 can potently—and to a similar extent—inhibit the growth of human MPM cell lines and primary MPM cells in vitro and display antitumor effects in vivo in MPM xenografts.
GHRH-R and its splice variant SV1 have been implicated in the antitumor effects of GHRH antagonists (6, 14, 15). Importantly, the expression of nonhypothalamic GHRH, pGHRH-R, and SV1 has been demonstrated in different tumors and cancer cell lines, suggesting that locally produced GHRH might function as an autocrine/paracrine growth factor in various cancers. Interestingly, cancer cells transfected with SV1 exhibited increased cell proliferation, suggesting that blockade of ligand-independent activity of SV1 would lead to the development of anticancer therapies (28). Here, we demonstrate the presence of pGHRH-R, SV1 and GHRH in MPM cell lines and primary MPM cells, underpinning the potential inhibitory activities of GHRH antagonists in MPM.
The MPM cell lines examined in this study included epithelioid cells (the most common and with best prognosis for MPM patients) and biphasic cells (a mixture of epithelioid and sarcomatoid cells and with a prognosis depending on the percentage of the epithelioid component) (3). Primary epithelioid, sarcomatoid (with worst prognosis), and biphasic MPM cells were also analyzed. MIA-602 and MIA-690 similarly inhibited survival and proliferation in all of the cell types tested, indicating anticancer properties in the least-aggressive as well as the most-aggressive phenotypes. These effects were significant at both 24 and 48 h, even at very low concentrations, and were comparable with those previously observed for antagonists of MIA series in other cancer cells (16, 18–21).
Conversely, GHRH antagonists showed no effect in MeT-5A mesothelial cells, which expressed pGHRH-R, SV1, and GHRH. It is tempting to speculate that these cells, being nonmalignant, have a reduced autocrine/paracrine stimulatory loop produced by GHRH and/or IGF-I/II and are likely expressing lower levels of SV1 compared with MPM cells. Although attractive, these hypotheses require further investigation.
In addition to the inhibitory effects per se, GHRH antagonists acted synergistically with PEM to reduce MPM cell survival, suggesting the ability to sensitize the cells to chemotherapy-induced toxicity. Several factors may contribute to this synergism, including induction of proapoptotic signaling and inhibition of oncogenic and antiapoptotic molecules by GHRH antagonists. Moreover, different survival pathways are involved in resistance to chemotherapy in MPM, including PI3K/Akt, a downstream component of IGF-I receptor and a major antiapoptotic pathway and frequently overexpressed in cancer cells (29). Herein, MIA-602 and MIA-690 down-regulated IGF-I in MPM xenografts, providing, at least in part, a mechanistic explanation for the inhibitory effect on tumor growth. Thus, it will also be important to explore the role of Akt and its downstream effector proteins in the antitumor activities of GHRH antagonists in MPM. In fact, the mechanisms of action of GHRH antagonists in various cancers have been the subject of intense investigations (16, 19, 23, 30). Along with the endocrine effects on the GH/IGF-I axis, direct mechanisms include blockade of the autocrine/paracrine activity of GHRH and IGF-I/II production in tumors (14, 15). Suppression of tumor growth factors, like VEGF, has also been reported (24, 31, 32). Other emerging mechanisms comprise the up-regulation of molecules involved in cell cycle arrest and apoptosis, such as p53 and caspases, and the inhibition of oncogenic and antiapoptotic pathways like c-Myc and Bcl-2 (20, 25, 31, 33, 34). Accordingly, in MPM cells, MIA-602 and MIA-690 elevated p53 and reduced c-Myc levels, strongly increased caspase-3 activity, and inhibited Bcl-2 expression. Notably, p53 is mutated in ∼15% of MPMs, with hyperactivation of survival and antiapoptotic pathways and resistance to chemotherapy. Expression and activity of p53, in turn, can be dramatically increased by DNA damage, leading to cell cycle arrest, apoptosis, and inhibition of VEGF, which is highly expressed in MPM and implicated in tumor growth and angiogenesis (4, 31, 35, 36). It has been also shown that inhibition of c-Myc promotes apoptosis and sensitizes MPM cells to cytotoxicity by kinase inhibitors (37). Moreover, in line with recent findings (20, 25), MIA-602 and MIA-690 in the present study strongly inhibited cell motility and invasion of MPM cells and blunted the expression of MMP-2 and MMP-9, key regulators of tumor growth, metastasis, and angiogenesis (38).
It has been demonstrated that GHRH and its agonistic analogs, like MR-409 and MR-356, display extrapituitary activities, including cardioprotection, an increase in survival of pancreatic islets after transplantation, and acceleration of wound healing, suggesting their potential clinical use in the fields of cardiology, diabetes, and others (5–11, 13). GHRH, however, also functions as a stimulatory growth factor in cancer cells, thus raising serious concerns for its therapeutic use (14, 28, 31). Importantly, Schally et al. (39) recently reported that in vivo, MR-409 suppresses the growth of different human experimental cancers to an extent and at doses comparable to those observed for the antagonists MIA-602 and MIA-690. These effects were paralleled by the down-regulation of GHRH-R in pituitary and tumors and the reduction in serum levels of IGF-I. In line with these findings, a different study showed that MR-356 inhibited experimental stomach cancer in vivo and reduced serum IGF-I (40). Thus, along with the antineoplastic effects of GHRH antagonists, the abovementioned findings may provide indications for the broad therapeutic use of these agents and alleviate the concerns on tumor growth.
Bcl-2 family members are essential regulators of ΔΨm by maintaining the integrity of the mitochondrial outer membrane, whereas proapoptotic molecules disrupt the mitochondrial membrane with release of apoptotic factors (26). Here, both MIA-602 and MIA-690 caused a loss/alteration of ΔΨm, in line with the observed down-regulation of Bcl-2 and increase in p53 expression and caspase-3 activity. GHRH antagonists also elevated ROS production, which has been related to mitochondrial dysfunction and apoptosis in MPM (27, 41). Moreover, MIA-690, but not MIA-602, reduced the mRNA levels of SOD-2, encoding for a mitochondrial enzyme with antiapoptotic functions against oxidative stress (27). The involvement of mitochondria in the inhibitory activities of GHRH antagonists has been previously described, in line with our findings (42, 43). Interestingly, the results on the synergistic effect of GHRH antagonists and PEM are consistent with the apoptotic activity of PEM, which has been associated with mitochondrial dysfunction and generation of ROS (41).
The antitumor activities of MIA-602 and MIA-690 were further confirmed in primary cells from pleural biopsy tissues of patients with MPM, in which GHRH comparable inhibitory effects were observed in epithelioid, sarcomatoid, and biphasic cell types. Furthermore, in vivo, MIA-602 and MIA-690 strongly reduced tumor volume and weight in mice xenografted with MPM cells. Interestingly, GHRH antagonists reduced tumor IGF-I levels in these mice, suggesting that autocrine/paracrine mechanisms are implicated in suppression of tumor growth. Moreover, recent in vivo studies demonstrated that in the prostate, GHRH antagonists suppress IGF-I signaling, which can be ligand-independently activated by tumor-derived GHRH (25). Importantly, alterations in IGF-system components have been reported in MPM, suggesting implication in tumorigenesis (44). That VEGF expression is reduced in xenografts further strengthens the antitumor and antiangiogenic role of MIA-602 and MIA-690 in MPM, in agreement with studies in other cancer cell models, including non-small cell lung cancer (24, 32).
In conclusion, our results demonstrate in vitro and in vivo, the antitumor activity of MIA-602 and MIA-690 in MPM, suggesting that GHRH antagonists could be considered for use in future therapeutic strategies, alone or in combination with standard therapies.
Methods
Please see SI Appendix, Materials and Methods for more information.
Cell Lines and Reagents.
The MSTO-211H (human biphasic MPM) cell line and MeT-5A (human mesothelium) cells were obtained from the American Type Culture Collection and cultured following the manufacturer’s instructions. The REN (human epithelioid MPM) cell line was kindly provided by Giorgio Scagliotti, Department of Oncology, University of Turin, Orbassano, Italy and cultured as described previously (45). GHRH-R antagonists MIA-602 ([(PhAc-Ada)0-Tyr1, d-Arg2, Fpa56, Ala8, Har9, Tyr(Me)10, His11, Orn12, Abu15, His20, Orn21, Nle27, d-Arg28, Har29]hGH-RH(1-29)NH2) and MIA-690 ([(PhAc-Ada)0-Tyr1, d-Arg2, Cpa6, Ala8, Har9, Fpa510, His11, Orn12, Abu15, His20, Orn21, Nle27, d-Arg28, Har29]hGH-RH(1-29)NH2) were synthesized and purified in the A.V.S. laboratory as described previously (18).
Human Primary MPM Cells.
Human primary biphasic, epithelioid, and sarcomatoid MPM cells were isolated after diagnostic thoracoscopies of MPM patients and cultured as described previously (46). The study was approved by the ethical committees of the Biological Bank of Mesothelioma, the S.S. Antonio e Biagio General Hospital (Alessandria, Italy), and the San Luigi Gonzaga Hospital (Orbassano, Italy) (studies: #9/11/2011; #126/2016). MPM patients gave their informed consent for the use of primary cells obtained from thoracoscopies.
Cell Survival and Proliferation.
Cell survival and proliferation were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich) and 5-bromodeoxyuridine (BrdU) incorporation ELISA kit (Roche Diagnostic) as described previously (12).
Combination Studies.
Drug synergistic effect was performed according to the Chou-Talalay method of synergy quantitation using CompuSyn software (47). Using a cell survival assay and computerized software data, CI values were generated. A CI of 1 indicates an additive effect between two drugs; a CI greater than 1 indicates antagonism; and a CI less than 1 indicates a synergistic effect.
Caspase-3 Activity.
Caspase-3 activity was assessed by Caspase-3 Colorimetric Assay Kit (BioVision) in cell lysates, according to the manufacturer’s instruction.
Western Blot Analysis.
Western blotting was performed as described previously (9).
RT-PCR and Real-Time PCR.
RT-PCR and real-time PCR analyses were performed as described previously (9, 12).
Tumor Xenografts.
MSTO-211H cells (2 × 106) were resuspended in PBS 1×/matrigel (Matrigel; Corning) solution (ratio 1:1) and s.c. injected in the right flank of 6- to 8-wk-old NOD/SCID/gamma chain−/− male mice. When tumors became palpable, mice were injected with MIA-602 or MIA-690 (s.c., 5 µg/d) or vehicle, for 4 wk. All procedures were performed according to institutional guidelines in compliance with national (D.L. N.26, 04/03/2014) and international law and policies (new directive 2010/63/EU). All mice were bred at the Animal Facility of the Molecular Biotechnology Center (Turin, Italy), recognized and approved by the Italian Ministry of Health (protocol #52/2018-PR).
Supplementary Material
Acknowledgments
We thank Dr. Giorgio Scagliotti for helpful criticisms and the Neuroscience Institute of Turin. This work was supported by the University of Turin Grant Ex-60% 2017 (to R.G.), the Italian Ministry of Health Grant 2015ZKHFTA_008 (to E.G.), the Italian Association for Cancer Research Grant IG15232 (to C.R.), and an Institut Biochimique SA (IBSA) Foundation 2015 fellowship (I.G.). Work in the A.V.S. laboratory was supported by the Medical Research Service of the Department of Veterans Affairs and by the University of Miami Miller School of Medicine.
Footnotes
Conflict of interest statement: A.V.S. and R.C. are listed as co-inventors on the patent for GHRH agonists, assigned to the University of Miami, Miami, FL, and the Veterans Affairs Medical Center, Miami, FL. The remaining authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818865116/-/DCSupplemental.
References
- 1.Mutti L, et al. Scientific advances and new frontiers in mesothelioma therapeutics. J Thorac Oncol. 2018;13:1269–1283. doi: 10.1016/j.jtho.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibby AC, et al. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur Respir Rev. 2016;25:472–486. doi: 10.1183/16000617.0063-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Husain AN, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142:89–108. doi: 10.5858/arpa.2017-0124-RA. [DOI] [PubMed] [Google Scholar]
- 4.Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis. 2013;34:1413–1419. doi: 10.1093/carcin/bgt166. [DOI] [PubMed] [Google Scholar]
- 5.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22:311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Barabutis N, Schally AV. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9:4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 7.Granata R. Peripheral activities of growth hormone-releasing hormone. J Endocrinol Invest. 2016;39:721–727. doi: 10.1007/s40618-016-0440-x. [DOI] [PubMed] [Google Scholar]
- 8.Kanashiro-Takeuchi RM, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–2609. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gesmundo I, et al. Growth hormone-releasing hormone attenuates cardiac hypertrophy and improves heart function in pressure overload-induced heart failure. Proc Natl Acad Sci USA. 2017;114:12033–12038. doi: 10.1073/pnas.1712612114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, et al. Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice. Proc Natl Acad Sci USA. 2015;112:13651–13656. doi: 10.1073/pnas.1518540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui T, et al. Agonistic analogs of growth hormone releasing hormone (GHRH) promote wound healing by stimulating the proliferation and survival of human dermal fibroblasts through ERK and AKT pathways. Oncotarget. 2016;7:52661–52672. doi: 10.18632/oncotarget.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo D, et al. GH-releasing hormone promotes survival and prevents TNF-α-induced apoptosis and atrophy in C2C12 myotubes. Endocrinology. 2015;156:3239–3252. doi: 10.1210/EN.2015-1098. [DOI] [PubMed] [Google Scholar]
- 13.Granata R, et al. Growth hormone-releasing hormone promotes survival of cardiac myocytes in vitro and protects against ischaemia-reperfusion injury in rat heart. Cardiovasc Res. 2009;83:303–312. doi: 10.1093/cvr/cvp090. [DOI] [PubMed] [Google Scholar]
- 14.Kiaris H, Schally AV, Varga JL, Groot K, Armatis P. Growth hormone-releasing hormone: An autocrine growth factor for small cell lung carcinoma. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, et al. Inhibition of experimental small-cell and non-small-cell lung cancers by novel antagonists of growth hormone-releasing hormone. Int J Cancer. 2018;142:2394–2404. doi: 10.1002/ijc.31308. [DOI] [PubMed] [Google Scholar]
- 17.Szereday Z, et al. Antagonists of growth hormone-releasing hormone inhibit the proliferation of experimental non-small cell lung carcinoma. Cancer Res. 2003;63:7913–7919. [PubMed] [Google Scholar]
- 18.Zarandi M, et al. Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides. 2017;89:60–70. doi: 10.1016/j.peptides.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Gan J, et al. Growth hormone-releasing hormone receptor antagonists inhibit human gastric cancer through downregulation of PAK1-STAT3/NF-κB signaling. Proc Natl Acad Sci USA. 2016;113:14745–14750. doi: 10.1073/pnas.1618582114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Moreno L, Schally AV, Prieto JC, Carmena MJ, Bajo AM. Growth hormone-releasing hormone receptor antagonists modify molecular machinery in the progression of prostate cancer. Prostate. 2018;78:915–926. doi: 10.1002/pros.23648. [DOI] [PubMed] [Google Scholar]
- 21.Pópulo H, et al. Inhibitory effects of antagonists of growth hormone-releasing hormone (GHRH) in thyroid cancer. Horm Cancer. 2017;8:314–324. doi: 10.1007/s12672-017-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu WK, et al. Antagonists of growth hormone-releasing hormone receptor induce apoptosis specifically in retinoblastoma cells. Proc Natl Acad Sci USA. 2016;113:14396–14401. doi: 10.1073/pnas.1617427113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez JJ, et al. A new approach to the treatment of acute myeloid leukaemia targeting the receptor for growth hormone-releasing hormone. Br J Haematol. 2018;181:476–485. doi: 10.1111/bjh.15207. [DOI] [PubMed] [Google Scholar]
- 24.Klukovits A, et al. Novel antagonists of growth hormone-releasing hormone inhibit growth and vascularization of human experimental ovarian cancers. Cancer. 2012;118:670–680. doi: 10.1002/cncr.26291. [DOI] [PubMed] [Google Scholar]
- 25.Popovics P, Cai R, Sha W, Rick FG, Schally AV. Growth hormone-releasing hormone antagonists reduce prostatic enlargement and inflammation in carrageenan-induced chronic prostatitis. Prostate. 2018;78:970–980. doi: 10.1002/pros.23655. [DOI] [PubMed] [Google Scholar]
- 26.Simula L, Nazio F, Campello S. The mitochondrial dynamics in cancer and immune-surveillance. Semin Cancer Biol. 2017;47:29–42. doi: 10.1016/j.semcancer.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barabutis N, et al. Stimulation of proliferation of MCF-7 breast cancer cells by a transfected splice variant of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA. 2007;104:5575–5579. doi: 10.1073/pnas.0700407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cioce M, et al. Autocrine CSF-1R signaling drives mesothelioma chemoresistance via AKT activation. Cell Death Dis. 2014;5:e1167. doi: 10.1038/cddis.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rick FG, et al. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108:3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barabutis N, Schally AV, Siejka A. P53, GHRH, inflammation and cancer. EBioMedicine. 2018;37:557–562. doi: 10.1016/j.ebiom.2018.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siejka A, Barabutis N, Schally AV. GHRH antagonist inhibits focal adhesion kinase (FAK) and decreases expression of vascular endothelial growth factor (VEGF) in human lung cancer cells in vitro. Peptides. 2012;37:63–68. doi: 10.1016/j.peptides.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Wu HM, et al. Growth hormone-releasing hormone antagonist induces apoptosis of human endometrial cancer cells through PKCδ-mediated activation of p53/p21. Cancer Lett. 2010;298:16–25. doi: 10.1016/j.canlet.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Stangelberger A, et al. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate. 2012;72:555–565. doi: 10.1002/pros.21458. [DOI] [PubMed] [Google Scholar]
- 35.Sementino E, et al. Inactivation of Tp53 and Pten drives rapid development of pleural and peritoneal malignant mesotheliomas. J Cell Physiol. 2018;233:8952–8961. doi: 10.1002/jcp.26830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strizzi L, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 37.Tan Y, Sementino E, Chernoff J, Testa JR. Targeting MYC sensitizes malignant mesothelioma cells to PAK blockage-induced cytotoxicity. Am J Cancer Res. 2017;7:1724–1737. [PMC free article] [PubMed] [Google Scholar]
- 38.Alì G, et al. Differential expression of extracellular matrix constituents and cell adhesion molecules between malignant pleural mesothelioma and mesothelial hyperplasia. J Thorac Oncol. 2013;8:1389–1395. doi: 10.1097/JTO.0b013e3182a59f45. [DOI] [PubMed] [Google Scholar]
- 39.Schally AV, et al. Agonists of growth hormone-releasing hormone (GHRH) inhibit human experimental cancers in vivo by down-regulating receptors for GHRH. Proc Natl Acad Sci USA. 2018;115:12028–12033. doi: 10.1073/pnas.1813375115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui T, Schally AV. Growth hormone-releasing hormone (GHRH) and its agonists inhibit hepatic and tumoral secretion of IGF-1. Oncotarget. 2018;9:28745–28756. doi: 10.18632/oncotarget.25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang KE, et al. Pemetrexed induces apoptosis in malignant mesothelioma and lung cancer cells through activation of reactive oxygen species and inhibition of sirtuin 1. Oncol Rep. 2015;33:2411–2419. doi: 10.3892/or.2015.3830. [DOI] [PubMed] [Google Scholar]
- 42.Pozsgai E, et al. The effect of GHRH antagonists on human glioblastomas and their mechanism of action. Int J Cancer. 2010;127:2313–2322. doi: 10.1002/ijc.25259. [DOI] [PubMed] [Google Scholar]
- 43.Hohla F, et al. GHRH antagonist causes DNA damage leading to p21 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cell Cycle. 2009;8:3149–3156. doi: 10.4161/cc.8.19.9698. [DOI] [PubMed] [Google Scholar]
- 44.Hoang CD, et al. Selective activation of insulin receptor substrate-1 and -2 in pleural mesothelioma cells: Association with distinct malignant phenotypes. Cancer Res. 2004;64:7479–7485. doi: 10.1158/0008-5472.CAN-04-1898. [DOI] [PubMed] [Google Scholar]
- 45.Monica V, et al. Dasatinib modulates sensitivity to pemetrexed in malignant pleural mesothelioma cell lines. Oncotarget. 2016;7:76577–76589. doi: 10.18632/oncotarget.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kopecka J, et al. Loss of C/EBP-β LIP drives cisplatin resistance in malignant pleural mesothelioma. Lung Cancer. 2018;120:34–45. doi: 10.1016/j.lungcan.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.