Significance
CD8+ T lymphocytes, which are typically devoted to eliminate malignant and infected cells, have been described in the central nervous system (CNS) of patients and mice with amyotrophic lateral sclerosis (ALS). However, their role in ALS pathogenesis has yet to be unraveled. Here, we show that ablation of CD8+ T cells in ALS mice increased the number of surviving motoneurons. CD8+ T cells expressing the ALS-causing superoxide dismutase-1 mutant protein recognize and selectively kill motoneurons in vitro. To exert their cytotoxic function, mutant CD8+ T cells required presentation of the antigen-MHC-I complex at the surface of the motoneurons. Analysis of T cell receptor diversity supports the evidence that self-reactive CD8+ T lymphocytes infiltrate the CNS of ALS mice to exert cytotoxic function.
Keywords: amyotrophic lateral sclerosis, neuroimmunity, cytotoxic T lymphocytes, motoneuron, major histocompatibility complex I
Abstract
Adaptive immune response is part of the dynamic changes that accompany motoneuron loss in amyotrophic lateral sclerosis (ALS). CD4+ T cells that regulate a protective immunity during the neurodegenerative process have received the most attention. CD8+ T cells are also observed in the spinal cord of patients and ALS mice although their contribution to the disease still remains elusive. Here, we found that activated CD8+ T lymphocytes infiltrate the central nervous system (CNS) of a mouse model of ALS at the symptomatic stage. Selective ablation of CD8+ T cells in mice expressing the ALS-associated superoxide dismutase-1 (SOD1)G93A mutant decreased spinal motoneuron loss. Using motoneuron-CD8+ T cell coculture systems, we found that mutant SOD1-expressing CD8+ T lymphocytes selectively kill motoneurons. This cytotoxicity activity requires the recognition of the peptide-MHC-I complex (where MHC-I represents major histocompatibility complex class I). Measurement of interaction strength by atomic force microscopy-based single-cell force spectroscopy demonstrated a specific MHC-I-dependent interaction between motoneuron and SOD1G93A CD8+ T cells. Activated mutant SOD1 CD8+ T cells produce interferon-γ, which elicits the expression of the MHC-I complex in motoneurons and exerts their cytotoxic function through Fas and granzyme pathways. In addition, analysis of the clonal diversity of CD8+ T cells in the periphery and CNS of ALS mice identified an antigen-restricted repertoire of their T cell receptor in the CNS. Our results suggest that self-directed immune response takes place during the course of the disease, contributing to the selective elimination of a subset of motoneurons in ALS.
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disease that primarily affects upper and lower motoneurons. ALS has a complex multifactorial etiology as reflected by the large predominance of sporadic forms of the disease. Dominantly inherited mutations in the gene encoding superoxide dismutase-1 (SOD1) are among the most common genetic causes of hereditary ALS (1). Mice that express ALS-linked SOD1 mutations progressively develop a severe motoneuron disease that presents the main traits of human pathology. Those ALS mouse models have provided valuable clues to the cellular pathogenesis of the disease. Whereas the neurodegenerative process selectively affects motoneurons, non–cell-autonomous determinants that implicate glial cells also contribute to the pathogenic process (2). The neuroinflammatory environment resulting from functionally aberrant glial cells is additionally accompanied by the infiltration of blood-derived immune cells (3).
Infiltration of CD4+ and CD8+ T lymphocytes has been documented in the brain and spinal cord of ALS patients (4–6). In transgenic mice expressing mutant SOD1G93A, the number of CD4+ and CD8+ T cells infiltrating the spinal cord increases as the disease progresses (7, 8). CD4+ T lymphocytes have gained a certain interest due to their neuroprotective function in ALS. This was evidenced by the reduced number and suppressive abilities of T regulatory (Treg) lymphocytes, which are negatively correlated with the progression rate of the disease in ALS patients (9, 10), and by genetic ablation of CD4 or adoptive transfer of Treg on ALS pathogenesis in mice (7, 11, 12).
To typically mount an immune response, the T cell receptor (TCR) of CD8+ T cells interacts with antigens presented by heterodimeric MHC class I (MHC-I) molecules (13). MHC-I is expressed by motoneurons both under physiological conditions and during the asymptomatic disease stage, whereas the percentage of surviving motoneurons expressing MHC-I was found to be reduced in postmortem spinal cord samples of ALS patients as well as in the spinal cord of end-stage mutant SOD1 mice (14). The light chain of MHC-I β-2 microglobulin (β2m) is predominantly expressed by motoneurons, and expression increases during the disease progression (15). However, the contribution of a CD8+ cytotoxic T cell response to ALS pathogenesis still remains elusive.
Here, we observed that activated CD8+ T cells infiltrate the central nervous system (CNS) of symptomatic ALS mice. SOD1G93A mice depleted in CD8+ T cells exhibited an increased number of surviving motoneurons. We found that purified SOD1G93A-expressing CD8+ T cells selectively trigger the death of primary motoneurons in a MHC-I-dependent manner through granzyme and Fas death pathways. Atomic force microscopy- (AFM-) based single-cell force spectroscopy (AFM-SCFS) showed increased contact force between ALS cytotoxic CD8+ T cells and motoneurons which implicate MHC-I recognition. Finally, spectratyping analysis of the TCR repertoire showed a restricted usage of the TCR β-chain variable region (TRBV) by CD8+ T cells infiltrating the CNS confirming an antigen-specific CD8+ T cell response in ALS mice.
Results
Activated CD8+ T Cells Infiltrate the CNS of ALS Mice During the Symptomatic Stage.
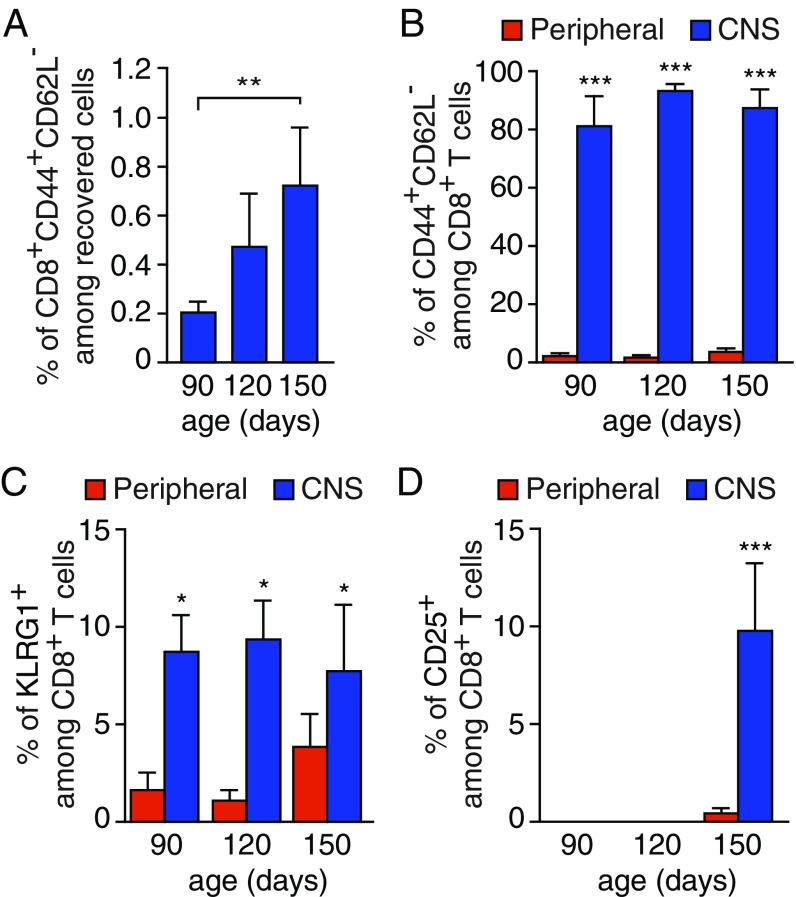
We first sought to determine the differentiation profile of CD8+ T cells infiltrating the CNS of SOD1G93A -expressing mice. We used a sequential gating strategy to accurately define CD8+ T cells among the CD45+Thy1.2+CD49b−CD3+ T lymphocyte lineages in the CNS of ALS mice by flow cytometry (SI Appendix, Fig. S1A). We first confirmed a significant accumulation of CD8+ T cells in the CNS of SOD1G93A mice at the symptomatic stage (150 d). Such an increase was not observed in the blood of age-matched SOD1 mutant mice (SI Appendix, Fig. S1 B and C). In situ hybridization using a Cd8a probe revealed a widespread distribution of CD8+ T cells in the gray matter of the SOD1G93A spinal cord (SI Appendix, Fig. S2). We next determined the differentiation profile of infiltrating SOD1G93A CD8+ T cells by using CD44 and CD62L markers whose levels distinguish between naive (CD44−CD62L+) and effector/effector memory (CD44+CD62L−) T cells. The frequency of CD44+CD62L− antigen-experienced T cells in the CNS of SOD1G93A mice increased with the disease progression (Fig. 1A). The majority of CD8+ T cells infiltrating the CNS being mainly effector/effector memory T cells (Fig. 1B). Markers of CD8+ T cell activation including killer cell lectinlike receptor subfamily G member 1 (KLRG1) and CD25 were found to be expressed by CD8+ T cells that accumulate in the CNS of ALS mice (Fig. 1 C and D).
Fig. 1.
Infiltration of activated CD8+ T cells in the CNS of SOD1G93A-expressing mice. (A) Analysis of CD44 and CD62L expression on CD8+ T cells isolated from the CNS of SOD1G93A mice at 90, 120, and 150 d of age (among viable, single event cells, SI Appendix, Fig. S1A). (B) Flow cytometry analysis indicating that the CD8+ T cells infiltrating the CNS are mainly CD44+CD62L− compared with circulating CD8+ T cells (peripheral). (C and D) Percentage of infiltrating CD8+ cells with expression of the KLRG1 (C) and CD25 (D) activation marker at indicated ages in the CNS and blood of SOD1G93A mice. Histograms show mean values ± scanning electron microscopy (SEM), n = 3 for each time point, *P < 0.05, **P < 0.01, ***P < 0.001, analysis of variance (ANOVA) with Tukey–Kramer’s post hoc test (A) or multiple t test (B–D).
Depletion of Cytotoxic CD8+ T Cells in SOD1 Mutant Mice Increases the Survival of Spinal Motoneurons.
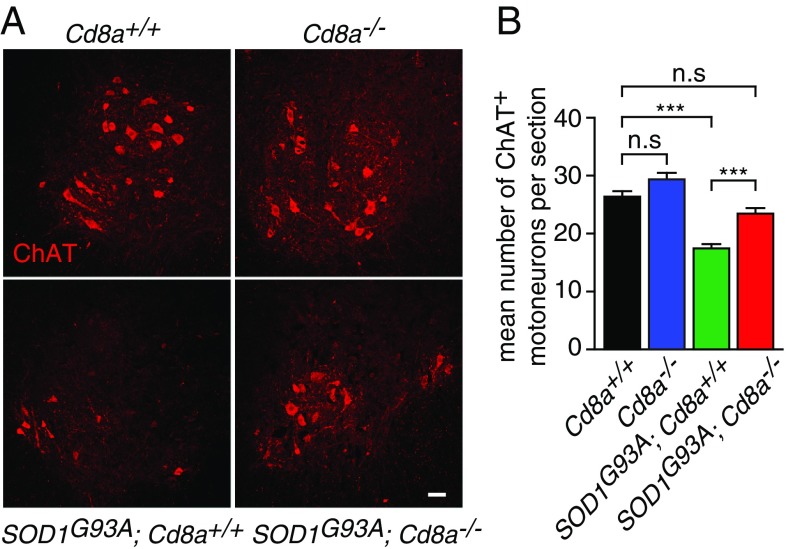
We next asked whether CD8+ T cells contribute to ALS pathogenesis. We bred SOD1G93A with Cd8a-deficient mice. Cd8a−/− mice are viable and fertile but fail to generate functional cytotoxic CD8+ T cells (16). We first ensured by flow cytometry analysis that the CD8+ T cell population was lost without the CD4+ T cell population being affected in the SOD1G93A;Cd8a−/− double mutant mice (SI Appendix, Fig. S3 A and B). We did not observe any effect at disease onset, motor performance, and life expectancy (SI Appendix, Fig. S4 A–D). However, we found that the loss of CD8+ T cell function significantly increased the number of surviving motoneurons in SOD1G93A mice (Fig. 2). To further confirm this observation, we repeatedly administrated a monoclonal anti-CD8 antibody to selectively deplete CD8+ T cells in mice (17). Treatment led to a marked and long-lasting reduction of blood-circulating CD8+ T cells without altering CD4+ T cells, CD19+ B cells, or CD11b+ macrophage populations (SI Appendix, Fig. S5 A–D). However, only 40% of CD8+ T cells were depleted in the CNS compared with 70% in the blood of SOD1G93A mice (SI Appendix, Fig. S5E). Although CD8 depletion did not ameliorate motor decline or extend the life span of SOD1G93A mice (SI Appendix, Fig. S6 A–C), a significant increased survival of motoneurons was observed (SI Appendix, Fig. S6D). Of note, the lower protective effect of CD8 depletion observed here might be explained by the partial depletion of CD8+ T cell in the CNS and the preferential action of the anti-CD8 antibody on the naive CD8+ T cell population (18).
Fig. 2.
Genetic depletion of CD8+ T lymphocytes increased the number of surviving motoneurons in ALS mice. (A) Representative images of lumbar spinal cord sections of 135-d-old mice of indicated genotype immunolabeled with choline acetyltransferase (ChAT) to visualize motoneurons. (Scale bar, 50 μm.) (B) Quantification of the number of ChAT+ motoneurons in 45 sections of the lumbar spinal cord of Cd8a+/+, Cd8a−/−, SOD1G93A;Cd8a+/+ and SOD1G93A;Cd8a−/− mice (n = 3). Values are means ± SEM; ***P < 0.001; n.s, nonsignificant, ANOVA with Tukey–Kramer’s post hoc test.
SOD1G93A-Expressing CD8+ T Cells Selectively Kill Primary Motoneurons.
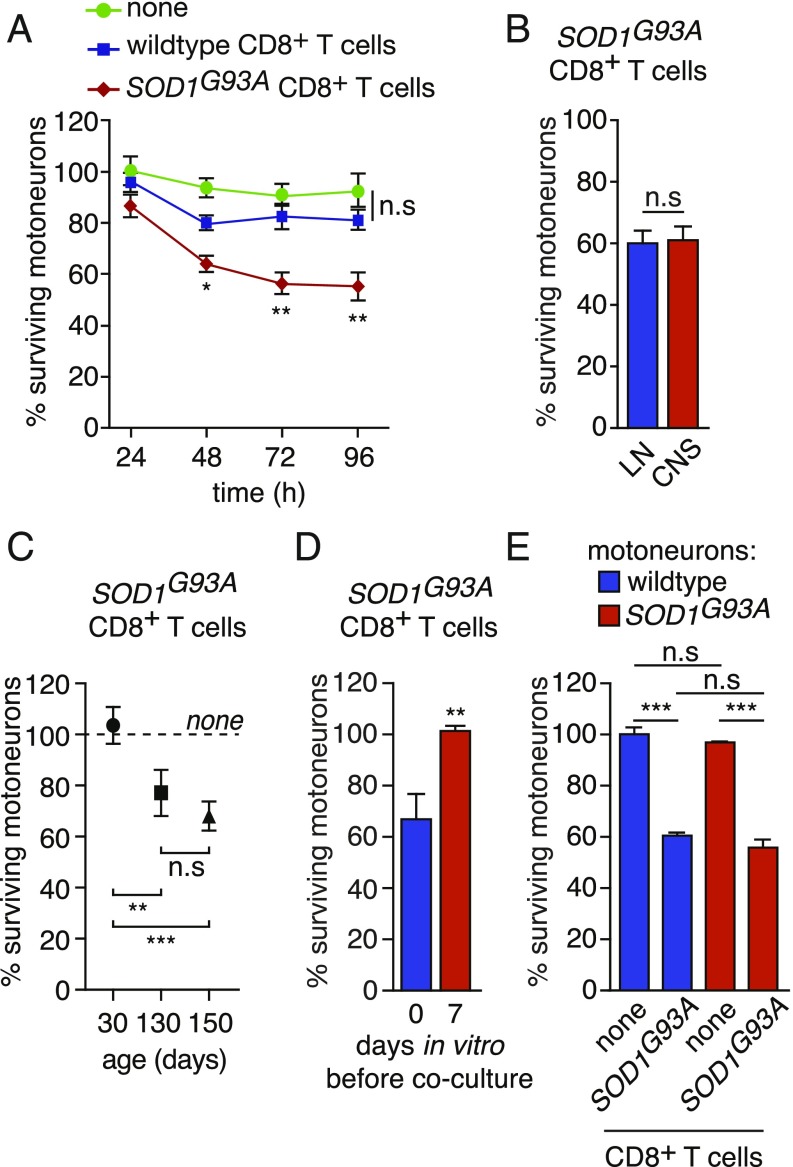
We cocultured mouse primary motoneurons and purified CD8+ T cells to investigate whether CD8+ T cells could directly mediate cytotoxicity toward motoneurons (SI Appendix, Fig. S7A). The presence of wild-type CD8+ T cells did not cause any loss of Hb9::GFP motoneurons that express GFP under the control of the motoneuron-selective Hb9 promoter to facilitate motoneuron identification (Fig. 3A) (19). When CD8+ T cells were isolated from the LNs of 150-d-old SOD1G93A mice, the percentage of surviving motoneurons was not significantly altered after 24 h of coculture but was significantly reduced by ∼40% after 48 h and was unchanged after 72 or 96 h (Fig. 3A). CD8+ T cells isolated from the LNs of SOD1 mutant mice have similar cytotoxicity toward motoneurons to that of CD8+ T cells isolated from the CNS (Fig. 3B). This neurotoxicity was only observed with CD8+ T cells isolated from symptomatic mice (130 and 150 d of age) but not from those isolated from asymptomatic 30-d-old SOD1G93A mice (Fig. 3C). When motoneurons were cultured for 7 d prior addition of SOD1G93A CD8+ T cells, we did not observe any effect on motoneuron survival (Fig. 3D). We then asked whether the expression of mutated SOD1 in motoneurons would render motoneurons more susceptible to SOD1G93A CD8+ T lymphocyte cytotoxicity. The survival of motoneurons expressing the SOD1G93A mutant was identical to that of wild-type motoneurons in the presence of mutant CD8+ T cells (Fig. 3E). The survival of SOD1G93A motoneurons was not modified by the presence of wild-type CD8+ T cells (SI Appendix, Fig. S7B). To determine whether mutant CD8+ T cell-induced death was specific to motoneurons, the survival of hippocampal, striatal, and cortical neurons was evaluated after 72 h of coculture. The survival of other neuronal types was not affected by the presence of lymphocytes isolated from wild-type mice or mice expressing mutated SOD1 (SI Appendix, Fig. S7 C–E).
Fig. 3.
Mutant SOD1-expressing CD8+ T cells selectively trigger the death of motoneurons in vitro. (A) Motoneurons were isolated from Hb9::GFP (where GFP represents green fluorescent protein) mice and cocultured for 24, 48, 72, and 96 h with CD8+ T cells immunopurified from the lymph nodes (LNs) of wild-type or SOD1G93A mice. Motoneuron survival was determined by direct counting of GFP+ motoneurons and expressed relative to survival in the absence of any T cells at 24 h. (B) CD8+ T cells were isolated either from the LNs or from the CNS of SOD1 mutant mice and cocultured with wild-type motoneurons. (C) CD8+ T cells were isolated from the LNs of SOD1G93A mice at the indicated age and cocultured with wild-type motoneurons. (D) CD8+ T cells were isolated from SOD1G93A mice at 150 d of age and added to motoneurons at the time of seeding (0) or 7 d later. (B–D) Survival was determined 72 h later and expressed relative to the survival in the absence of T cells. (E) Motoneurons were isolated from either wild-type or SOD1G93A mice and cocultured with SOD1 mutant CD8+ T cells. Motoneuron survival was determined after 72 h and expressed relative to wild-type motoneurons cultured in the absence of T cells. The results shown are the mean values ± standard deviation (SD) of, at least, three independent experiments performed in triplicate. (B and D) Unpaired two-tailed t test, (A, C, and E) ANOVA with Tukey–Kramer’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001; n.s, nonsignificant.
ALS CD8+ T Cells Recognize and Induce the Death of Motoneurons in a MCH-I-Dependent Manner.
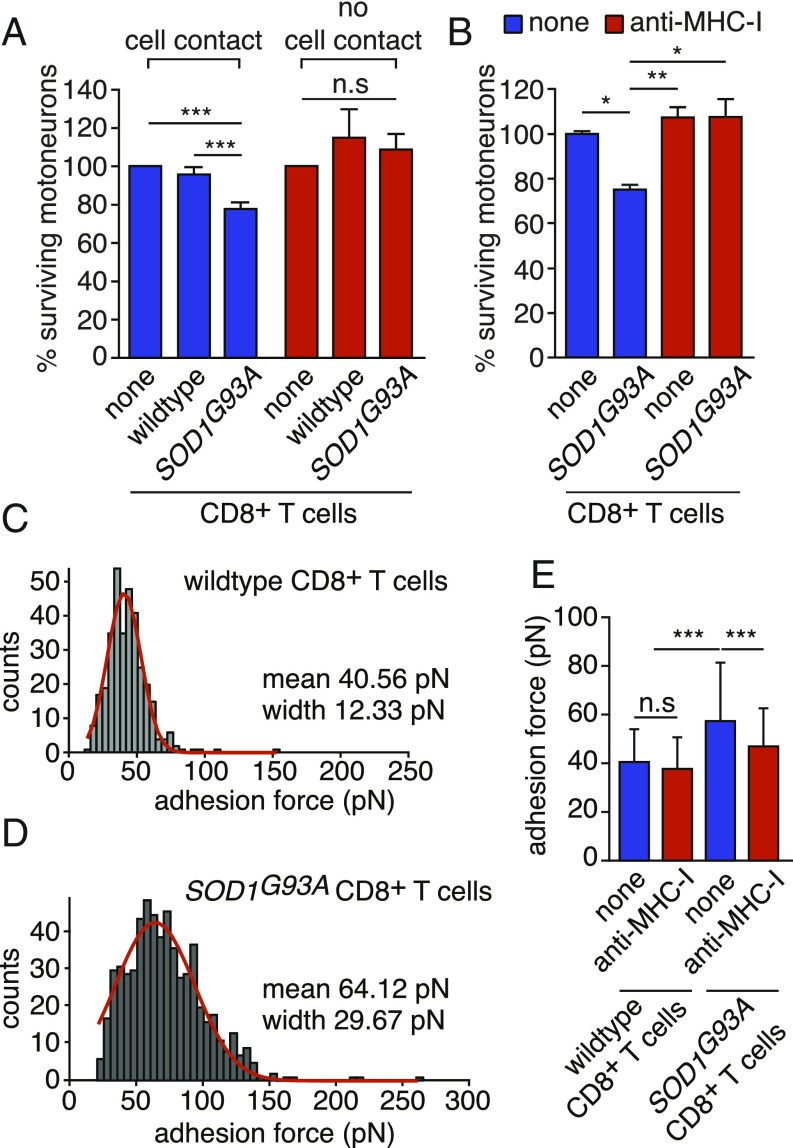
We evaluated whether CD8+ T cells expressing mutated SOD1 required cell-cell contact to trigger the death of motoneurons. We used a coculture system with a transwell insert and showed that SOD1 mutant cytotoxic CD8+ T lymphocytes require cell contact to trigger the death of motoneurons (Fig. 4A). To determine the requirement of antigen-MHCI-I recognition by CD8+ T cells, we used a function-blocking anti-MHC-I H2-Db antibody and observed that motoneurons were saved from CD8+ T cell cytotoxicity (Fig. 4B). To accurately quantify the strength of CD8+ T lymphocyte-motoneuron interaction, we used AFM-SCFS (SI Appendix, Fig. S8 A–D). Beginning with a dwelling time of 1 s, the mean adhesion force between motoneurons and SOD1G93A CD8+ T cells was higher than that obtained with wild-type lymphocytes (Fig. 4 C and D). By increasing the dwelling time to 5 s, the magnitude of the binding strength of mutant CD8+ T cells with motoneurons was increased, whereas the adhesion force between wild-type CD8+ T cells and motoneurons remains stable, suggesting nonspecific interaction (SI Appendix, Fig. S8 E and F). Blocking TCR/MHC-I interaction, the anti-MHC-I antibody significantly decreased adhesion force (Fig. 4E and SI Appendix, Fig. S8G).
Fig. 4.
Mutant cytotoxic CD8+ T cells mediate the death of motoneurons in a cell contact-, MHCI-dependent manner. (A) Wild-type and SOD1G93A CD8+ T cells were seeded with motoneurons for direct coculture (cell contact) or seeded into the upper transwell chamber (no cell contact). (B) Function-blocking anti-MHC-I antibody (1 µg/mL) was added to motoneurons cocultured with mutant SOD1 CD8+ T cells. Motoneuron survival was determined after 72 h of coculture and expressed relative to the absence of T cells (none). (C and D) Adhesion force histograms obtained by recording force curves of wild-type (C) or SOD1G93A (D) CD8+ T cells with wild-type motoneurons with a dwelling time of 1 s. (C) The results represent seven cell pairs from two different cell cultures. (D) The results represent 14 cell pairs from four different cell cultures. (E) Mean adhesion force between wild-type or SOD1G93A CD8+ T cells and wild-type motoneurons in the presence of an anti-MHC-I antibody with a dwelling time of 1 s. The values are Gaussian fit means ± SD, ANOVA with repeated measures, Newman–Keuls’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, n.s, nonsignificant.
ALS Mutant Cytotoxic T Cell-Mediated Death of Motoneurons Involves Granzyme and Fas.
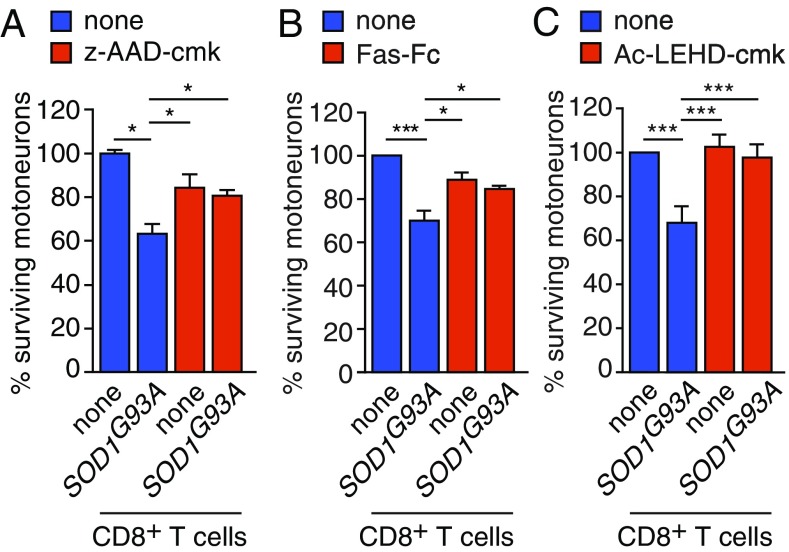
CD8+ cytotoxic T cells eliminate target cells by two major pathways: the perforin-mediated delivery of granzyme serine proteases in the cytoplasm of target cells, resulting in effector caspase activation, and the commitment of the Fas death pathway (20). We first used the granzyme B inhibitor z-AAD-cmk at the maximum concentration that motoneurons can tolerate and found that it partly rescued motoneurons from cytotoxicity mediated by SOD1G93A CD8+ T cells (Fig. 5A). When we then blocked Fas-FasL interaction by Fas-Fc, the motoneurons were partly saved from mutant CD8+ T cell-induced death (Fig. 5B). Granzyme B as well as Fas converge both at the mitochondrial caspase-9 pathway (21, 22). Consistently, treatment with the caspase-9 inhibitor Ac-LEHD-cmk completely rescued the motoneurons from death induced by SOD1G93A CD8+ T cells (Fig. 5C).
Fig. 5.
SOD1G93A-expressing CD8+ T lymphocytes kill motoneurons through both granzyme and Fas pathway. (A) The z-AAD-CMK granzyme B inhibitor (1 µg/mL) was added or not to motoneurons and CD8+ T cell cocultures. (B) Motoneuron-cytotoxic CD8+ lymphocyte cocultures were challenged with the Fas-Fc chimera (1 µg/mL). (C) The selective inhibitor of the central executioner caspase-9 in motoneurons Ac-LEHD-cmk (0.5 µM) was added to Hb9::GFP motoneurons cocultured or not with ALS mutant CD8+ T cells. In A–C, the percentage of surviving GFP+ motoneurons was determined 72 h later and expressed relative to the nontreated condition without the presence of CD8+ T cells. The values are means ± SD of, at least, three independent experiments performed in triplicate, ANOVA with Tukey—Kramer’s post hoc test. *P < 0.05, ***P < 0.001.
Activation of CD8+ T cells leads to interferon-γ (IFNγ) production, which can contribute to cell killing through the up-regulation of MHC-I in target cells (23). Intracellular staining followed by flow cytometry showed that IFNγ is expressed at higher levels in ALS mutant CD8+ T cells that are recruited to the CNS compared with wild-type T cells in the blood and those who patrol the CNS under physiological conditions (SI Appendix, Fig. S9A) (24). Levels of IFNγ in the spinal cord of SOD1G93A mice significantly increase with disease progression (19). We asked whether increased levels of IFNγ might be associated with increased MHC-I and β2m levels in the spinal cord. We observed a significant increase in H2-Db and β2m transcript levels in the spinal cord of SOD1G93A mice at 150 d of age compared with age-matched wild-type and 90-d-old SOD1 mutant mice (SI Appendix, Fig. S9 B and C). Motoneurons exposed to a sublethal dose of recombinant IFNγ (19) significantly increased the somatic expression of MHC-I (SI Appendix, Fig. S9 D and E) and β2m that is required for transport and stabilization of MHC-I at the cell surface (SI Appendix, Fig. S9 F and G). The addition of a neutralizing anti-IFNγ antibody to the culture medium saved motoneurons from death induced by SOD1G93A CD8+ T lymphocytes (SI Appendix, Fig. S9H). Of note, wild-type motoneurons exposed to IFNγ do not become susceptible to wild-type CD8+ T cells (SI Appendix, Fig. S9I). We previously demonstrated that IFNγ triggers the death of motoneurons through the activation of the lymphotoxin β receptor (LT-βR) by tumor necrosis factor superfamily member 14 (19). We observed that the decoy receptor LT-βR-Fc did not rescue motoneurons from SOD1G93A CD8+ T cell-induced cytotoxicity (SI Appendix, Fig. S9J). These results show that activated IFNγ-producing SOD1G93A CD8+ T cells induce the death of motoneurons through Fas and granzyme pathways, whereas IFNγ up-regulates the expression of the MHC-I/β2m complex in motoneurons.
ALS CD8+ T Cells That Infiltrate the CNS Show a Restricted TCR Repertoire.
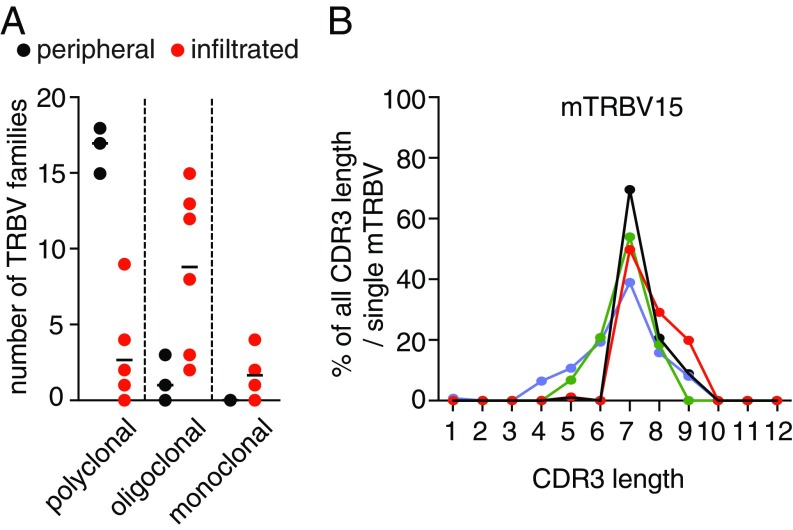
To confirm that autoreactive CD8+ T cells are selectively recruited to the CNS of ALS mice, we analyzed the TCR Vβ repertoire of infiltrated and peripheral CD8+ T cells by spectratyping of the complementary determining region 3 (CDR3) of TRBV genes (25). We first determined the TRBV CDR3 length distribution (CDR3-LD) of peripheral CD8+ T cells isolated from wild-type and SOD1G93A mice. The spectratyping of 18 TRBV showed a normal distribution of CDR3 lengths in TRBV families between wild-type and ALS mice (SI Appendix, Fig. S10 A and B). Interestingly, when we compared the CDR3-LD from paired samples of peripheral and infiltrated CD8+ T cells in SOD1 mutant mice, we observed a shift from polyclonal to oligoclonal and monoclonal TRBV gene usage in the CNS (Fig. 6A). To quantify the similarity of the TRBV repertoire between peripheral and infiltrated CD8+ T cells, two metrics were used, linear correlation and distance score (25). We demonstrated that the TRBV repertoire of infiltrated CNS CD8+ T cells differ from those of peripheral CD8+ T cells (SI Appendix, Fig. S11 A and B). We then analyzed the CDR3 length of the 18 TRBV individually to determine the relative use of each in the CNS of SOD1G93A mice and observed a specific selection of mTRBV15 (Fig. 6B and SI Appendix, Fig. S12). Consistently, a targeted analysis of mTRBV15 CDR3-LD showed that CNS-infiltrating mTRBV15 CD8+ T cells are also selectively detected in the LNs (SI Appendix, Fig. S13).
Fig. 6.
CD8+ T cells infiltrating the spinal cord of ALS mice show a restricted T cell receptor repertoire. (A) Comparison of CDR3-LD (polyclonal, oligoclonal, or monoclonal distribution) of TRBV families in CD8+ T cells isolated from LNs (peripheral) and CNS (infiltrated) of 150-d-old SOD1G93A mice (n = 6). (B) Distribution of the mTRBV15 CDR3 length across the CD8+ T cell infiltrating the CNS shared among four mice (each color corresponding to one mouse).
Discussion
CD8+ T cell-mediated cytotoxic immune response plays a determinant role in the elimination of virally infected or tumor cells. Here, we provide evidence that SOD1G93A CD8+ effector T cells recognize the self-peptide-MHC-I complex on motoneurons, independent of the expression of human SOD1G93A by motoneurons that could have generated antigenic peptides. This might imply that motoneuron-derived antigens have to be internalized by professional antigen-presenting cells (APC) in the secondary lymphoid organs and presented in the context of MHC-I, a process termed cross presentation. Consistently, we found a mTRBV15 restricted clonal diversity within the LNs and CNS of SOD1G93A mice. It is therefore possible that motoneuron-specific antigens released during the degenerative process may be accessible in the periphery to mount a motoneuron-targeted immune response. Peripheral capture of a self-antigen by cross-presenting APC and priming of naive T cells in LNs will then also be determinant in defining the homing phenotype of activated CD8+ T cells (26).
Our observations pose the puzzle of the contribution of the MHC-I/β2m complex in ALS pathogenesis. Indeed, as previously reported, the surviving motoneurons at the end stage of the disease show reduced levels of MHC-I and viral-mediated overexpression of MHC-I heavy chain variants in the spinal cord extended the lifespan of SOD1G93A mice (14). The reduced levels of MHC-I on the surviving motoneurons observed (14) do not necessarily exclude MHC-I-dependent killing of some motoneuron populations by infiltrating CD8+ T cells. Either the proportion of motoneurons that are targeted by cytotoxic T cells have already been eliminated, those remaining that might be eliminated by a MHC-I-independent mechanism (14), or the low MHC-I expression levels are yet effective to promote recognition and cytotoxicity by CD8+ T cells. Two studies have explored the contribution of β2m in ALS pathogenesis. The first observed that the genetic deletion of β2m in SOD1G93A mice does not influence the disease onset but significantly reduces the life span of mice (15), whereas the second found that the deletion of β2m in SOD1G93A mice accelerates the disease onset and prolongs the survival of mice (27). Despite the contradictory character of these two observations, it is important to stress that, with regard to our concerns, cells from β2m−/− are not completely devoid of MHC-I cell surface expression and that cytotoxic CD8+ T lymphocytes can still be generated and are able to trigger the death of β2m−/− target cells in a MHC-I-restricted manner (28, 29). We cannot exclude that subnormal levels of MHC-I are present at the surface of β2m−/− motoneurons in the spinal cord, thus recognized and eliminated by peptide-specific cytotoxic CD8+ T cells. Moreover, the functions of β2m are not exclusively limited to classical MHC-I molecules as illustrated by the phenotypic defects observed in β2m-deficient mice with immunoglobulin (Ig) and albumin hypercatabolism reduced IFNγ production or iron overload (30).
The role of MHC-I in neuronal differentiation, synapse formation and function, and plasticity has been documented (31). MHC-I is also involved in the stabilization of inhibitory synapses on motoneurons and regeneration following nerve lesion (32). Forced expression of MHC-I negatively regulates glutamatergic and γ-aminobutyric acidergic synaptic transmission. The effect of MHC-I on synaptic density is independent of bound β2m (33). MHC-I can also bind to the paired Ig-like receptor B and restrict synaptic plasticity as well as functional recovery following ischemic damage (34). In addition, β2m can associate with CD1 family members, Qa, the MHC-related-1 protein MR1, the neonatale Fc receptor FcRn, and human hemochromatosis protein (30), whose functions in the CNS remain elusive. The study of these additional immune-independent mechanisms might be considered to gain further insight into ALS pathogenic mechanisms.
Our paper raises questions concerning the functional characteristics of motoneurons whose death is induced by CD8+ T cells in vivo. Indeed, we observed a significant increase in the number of spinal motoneurons following the depletion of CD8+ T cells in ALS mice without any change in motor decline or life expectancy. These findings suggest that nonfunctional motoneurons might be eliminated by an orchestrated cell death program triggered by CD8+ T lymphocytes for proper removal. Alternatively, aberrant motoneuron electrical activity or those committed to die by the dying-back process induce changes in gene expression that might generate new autoantigens and killing by cytotoxic CD8+ T cells. The latter have yet to be identified. We observed that IFNγ can elicit MHC-I expression on mouse primary motoneurons as previously observed with rat primary motoneurons (35). IFNγ can be produced by activated CD8+ T cells as well as ALS astrocytes (19) to elicit and/or maintain sufficient MHC-I expression levels on motoneurons allowing them to be recognized by self-reactive cytotoxic CD8+ T cells. It is noteworthy that somatic expression of MHC-I occurred in the presence of IFNγ only in electrically silent neurons (36). Interestingly, the cytotoxicity of mutant CD8+ lymphocytes is observed on electrically immature motoneurons but not on those that after 7 d in vitro become electrically mature as we showed previously (37, 38). Cytotoxic CD8+ T cells could thus contribute to the elimination of nonfunctional motoneurons during the disease.
Together, these results suggest that an autoimmune T cell response contributes to ALS pathogenesis. The presence of autoantibodies in the cerebrospinal fluid (CSF) or serum of patients, the cytotoxicity of the CSF from ALS patients toward neurons in vitro signs of systemic immune activation in the serum, and the CSF of ALS patients and the infiltration of T cells have suggested that autoimmunity might contribute to ALS etiology and pathogenesis (3, 39). Early clinical interventions targeting autoimmunity through the administration of cyclophosphamide (40, 41); plasmapheresis combined with immunosuppression (42); or treatment with azathioprine and prednisone (43) led to disappointing results in patients. Despite all that, the state of our current knowledge about the complexity of the immune response with respect to the functional identity of lymphocyte subpopulations and the dynamics of this response during the course of the disease as well as our present findings prompt us to critically reconsider these early clinical data. The use of drugs with an unfocused spectrum of action (cyclophosphamide and azathioprine inhibit DNA replication and cell proliferation, and prednisone is an antiinflammatory and immunosuppressant synthetic glucocorticoid) does not afford relevant insight into the selective contribution of autoimmunity in ALS. Overall, this paper provides evidence of autoreactive CD8+ T cells that directly interact with and trigger the death of motoneurons. The inherent challenge is now to identify autoantigens that are recognized by those cytotoxic T cells and to define pertinent combinatorial therapeutic approaches embracing the complexity of the immune response in ALS.
Materials and Methods
Detailed information for animal experimentation, CD8+ T cell isolation, fluorescence-activated cell sorting, in situ hybridization, primary neuron cultures, neuron-CD8+ T cell cocultures, immunostaining, CD8+ T cell depletion, atomic force microscopy-based single-cell force spectroscopy, the reverse transcription quantitative polymerase chain reaction, TCR repertoire analysis, and statistical analysis is provided in SI Appendix, Materials and Methods.
All animal experiments were approved by the national ethics committee on animal experimentation, and were performed in compliance with the European community and national directives for the care and use of laboratory animals.
Supplementary Material
Acknowledgments
We thank all members of the team; Solange Desagher, Pierre-Henri Puech, and Luc Dupuis for their helpful comments throughout the work; Paul Walker for his technical help and advice; the personnel of the Montpellier Réunion Inter Organisme (RIO) imaging platform, and the Neuroscience Institute of Montpellier animal facility for their services. This work was supported by grants from the National Institute for Health and Medical Research, the Association Française Pour la Recherche sur la SLA, ANR E-RARE FaSMALS, and ANR GliALS. E.C. was a recipient of an Association Française Contre les Myopathies PhD fellowship. The TCR repertoire facility was developed in the context of the IHU-Cesti project supported by Grant ANR-10-IBHU-005, Nantes Metropole and the Pays de la Loire Region.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815961116/-/DCSupplemental.
References
- 1.Leblond CS, Kaneb HM, Dion PA, Rouleau GA. Dissection of genetic factors associated with amyotrophic lateral sclerosis. Exp Neurol. 2014;262:91–101. doi: 10.1016/j.expneurol.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Valori CF, Brambilla L, Martorana F, Rossi D. The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci. 2014;71:287–297. doi: 10.1007/s00018-013-1429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowerman M, et al. Neuroimmunity dynamics and the development of therapeutic strategies for amyotrophic lateral sclerosis. Front Cell Neurosci. 2013;7:214. doi: 10.3389/fncel.2013.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50:30–36. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- 5.Fiala M, et al. IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. J Neuroinflammation. 2010;7:76. doi: 10.1186/1742-2094-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamata T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- 7.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu IM, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci USA. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beers DR, et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight. 2017;2:e89530. doi: 10.1172/jci.insight.89530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henkel JS, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5:64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee R, et al. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beers DR, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134:1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossjohn J, et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. 2015;33:169–200. doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- 14.Song S, et al. Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat Med. 2016;22:397–403. doi: 10.1038/nm.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staats KA, et al. Beta-2 microglobulin is important for disease progression in a murine model for amyotrophic lateral sclerosis. Front Cell Neurosci. 2013;7:249. doi: 10.3389/fncel.2013.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung-Leung WP, et al. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 17.Cobbold SP, Martin G, Waldmann H. The induction of skin graft tolerance in major histocompatibility complex-mismatched or primed recipients: Primed T cells can be tolerized in the periphery with anti-CD4 and anti-CD8 antibodies. Eur J Immunol. 1990;20:2747–2755. doi: 10.1002/eji.1830201232. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeois C, Stockinger B. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J Immunol. 2006;177:4558–4566. doi: 10.4049/jimmunol.177.7.4558. [DOI] [PubMed] [Google Scholar]
- 19.Aebischer J, et al. IFNγ triggers a LIGHT-dependent selective death of motoneurons contributing to the non-cell-autonomous effects of mutant SOD1. Cell Death Differ. 2011;18:754–768. doi: 10.1038/cdd.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golstein P, Griffiths GM. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol. 2018;18:527–535. doi: 10.1038/s41577-018-0009-3. [DOI] [PubMed] [Google Scholar]
- 21.Froelich CJ, Metkar SS, Raja SM. Granzyme B-mediated apoptosis–The elephant and the blind men? Cell Death Differ. 2004;11:369–371. doi: 10.1038/sj.cdd.4401381. [DOI] [PubMed] [Google Scholar]
- 22.Raoul C, et al. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 23.Cohen ES, Bodmer HC. Cytotoxic T lymphocytes recognize and lyse chondrocytes under inflammatory, but not non-inflammatory conditions. Immunology. 2003;109:8–14. doi: 10.1046/j.1365-2567.2003.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritzel RM, et al. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J Immunol. 2016;196:3318–3330. doi: 10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salou M, et al. Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann Clin Transl Neurol. 2015;2:609–622. doi: 10.1002/acn3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calzascia T, et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Nardo G, et al. Counteracting roles of MHCI and CD8+ T cells in the peripheral and central nervous system of ALS SOD1G93A mice. Mol Neurodegener. 2018;13:42. doi: 10.1186/s13024-018-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glas R, et al. Major histocompatibility complex class I-specific and -restricted killing of beta 2-microglobulin-deficient cells by CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1992;89:11381–11385. doi: 10.1073/pnas.89.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamousé-Smith E, Clements VK, Ostrand-Rosenberg S. Beta 2M-/- knockout mice contain low levels of CD8+ cytotoxic T lymphocyte that mediate specific tumor rejection. J Immunol. 1993;151:6283–6290. [PubMed] [Google Scholar]
- 30.Argyropoulos CP, et al. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med (Lausanne) 2017;4:73. doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmer BM, McAllister AK. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 2012;35:660–670. doi: 10.1016/j.tins.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira AL, et al. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci USA. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn MW, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442–451. doi: 10.1038/nn.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adelson JD, et al. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73:1100–1107. doi: 10.1016/j.neuron.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindå H, et al. Expression of MHC class I and beta2-microglobulin in rat spinal motoneurons: Regulatory influences by IFN-gamma and axotomy. Exp Neurol. 1998;150:282–295. doi: 10.1006/exnr.1997.6768. [DOI] [PubMed] [Google Scholar]
- 36.Neumann H, Cavalié A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 37.Camu W, et al. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35:1198–1205. doi: 10.1016/j.neurobiolaging.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Bowerman M, et al. KCC3 loss-of-function contributes to Andermann syndrome by inducing activity-dependent neuromuscular junction defects. Neurobiol Dis. 2017;106:35–48. doi: 10.1016/j.nbd.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Couratier P, Hugon J, Sindou P, Vallat JM, Dumas M. Cell culture evidence for neuronal degeneration in amyotrophic lateral sclerosis being linked to glutamate AMPA/kainate receptors. Lancet. 1993;341:265–268. doi: 10.1016/0140-6736(93)92615-z. [DOI] [PubMed] [Google Scholar]
- 40.Smith SA, Miller RG, Murphy JR, Ringel SP. Treatment of ALS with high dose pulse cyclophosphamide. J Neurol Sci. 1994;124:84–87. doi: 10.1016/0022-510x(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 41.Brown RH, Jr, Hauser SL, Harrington H, Weiner HL. Failure of immunosuppression with a ten- to 14-day course of high-dose intravenous cyclophosphamide to alter the progression of amyotrophic lateral sclerosis. Arch Neurol. 1986;43:383–384. doi: 10.1001/archneur.1986.00520040063021. [DOI] [PubMed] [Google Scholar]
- 42.Kelemen J, Hedlund W, Orlin JB, Berkman EM, Munsat TL. Plasmapheresis with immunosuppression in amyotrophic lateral sclerosis. Arch Neurol. 1983;40:752–753. doi: 10.1001/archneur.1983.04050110070012. [DOI] [PubMed] [Google Scholar]
- 43.Werdelin L, Boysen G, Jensen TS, Mogensen P. Immunosuppressive treatment of patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 1990;82:132–134. doi: 10.1111/j.1600-0404.1990.tb01602.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.