Significance
The biomedical applications of fluorescent anticancer agents in the first near-IR region (NIR-I) have been hampered by problems of photostability, autofluorescence, and limited penetration depth in biological tissues. To shift the fluorescence theranostic region from the NIR-I to the longer-wavelength region for accurate cancer diagnosis and treatment, Pt(II) metallacycle-based fluorescent nanoprobe 1 with fluorescence emission in the NIR-II region is designed. In vitro and in vivo studies demonstrate that 1 has good photostability and the ability to efficiently inhibit the growth of tumor with minimal side effects. It also improves the fluorescent imaging quality and signal-to-noise ratio for cancer theranostics. This study provides an opportunity for further design of NIR-II theranostic anticancer agents for future biomedical applications.
Keywords: supramolecular coordination complexes, metallacycle, second near-infrared region, image-guided therapy, theranostic
Abstract
Fluorescent theranostics probes at the second near-IR region (NIR-II; 1.0–1.7 µm) are in high demand for precise theranostics that minimize autofluorescence, reduce photon scattering, and improve the penetration depth. Herein, we designed and synthesized an NIR-II theranostic nanoprobe 1 that incorporates a Pt(II) metallacycle 2 and an organic molecular dye 3 into DSPE-mPEG5000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000]). This design endows 1 with good photostability and passive targeting ability. Our studies show that 1 accurately diagnoses cancer with high resolution and selectively delivers the Pt(II) metallacycle to tumor regions via an enhanced permeability and retention effect. In vivo studies reveal that 1 efficiently inhibits the growth of tumor with minimal side effects. At the same time, improved fluorescent imaging quality and signal-to-noise ratios are shown due to the long emission wavelengths. These studies demonstrate that 1 is a potential theranostic platform for tumor diagnosis and treatment in the NIR-II region.
Over the past several decades, coordination-driven self-assembly via spontaneous formation of metal−ligand bonds has become a widespread strategy for preparing discrete supramolecular coordination complexes (SCCs). The SCCs technique has recently attracted attention not only for aesthetic reasons but also, for its wide application in the medical sciences (1–8). The macrocyclic structure of SCCs can enhance their cellular uptake compared with their corresponding small molecule precursors as well as the binding with biomolecules with high affinity. Our group and others reported that Pt(II) metallacycles-based SCCs can act as anticancer agents or selective sensors for biologically important analytes as well as interact with DNA and proteins (9–14). However, the poor photostability, low tumor uptake, and limited penetration depth of Pt-based SCCs are challenges to overcome.
Fluorescent imaging in the second near-IR region (NIR-II; 1.0–1.7 µm) has recently been highlighted for biomedical applications due to its remarkable advantages over the traditional visible and NIR-I region (0.4–0.9 µm), such as minimized scattering, improved photon penetration depth, and negligible background autofluorescence (15–18). Considering these benefits from the NIR-II region for bioimaging, a series of materials, such as carbon nanotubes, quantum dots, small molecules, and rare earth nanoparticles, has been used for this fluorescent modality (19–24). Recent breakthroughs in the NIR-II region have a wide range of biomedical applications (25–27). This powerful imaging technique combined with high-performance NIR-II probes will open numerous possibilities for image-guided therapeutic procedures in vivo.
Herein, we designed and synthesized a Pt(II) metallacycle-based theranostic agent that incorporates a rhomboid 2 and an NIR-II molecular dye 3 into PEGylated surfactant DSPE-mPEG5000, forming complex 1 (Fig. 1). The incorporation of an NIR-II molecular dye in a theranostic agent may improve the photophysical properties for biomedical applications, such as excellent photostability and long-wavelength emission (15). Furthermore, due to the long-wavelength emission in the NIR-II region (1.0–1.7 µm), improvements in tissue penetration, signal-to-noise (S/N) ratio, and micrometer-scale resolution of the theranostic probe can be anticipated over the traditional visible or NIR-I regions. Therefore, more precise diagnosis of cancer, monitoring of tumor development, and improvement in the efficacy of treatment in vivo may be achieved (15, 28). Moreover, the utilization of DSPE-mPEG5000 not only enhances the stability and biocompatibility of the theranostic agent in vivo but also, selectively delivers rhomboidal metallacycle 2 and the organic NIR-II dye 3 to the tumor regions via the enhanced permeability and retention (EPR) effect, resulting in more precise cancer detection and treatment. Notably, 1 shows good photostability as well as stability in vitro. In vivo NIR-II fluorescence images indicate that complex 1 has high nonspecific tumor uptake along with superior S/N ratios, which can guide future therapy and evaluate the efficacy of treatment. Compared with Food and Drug Administration (FDA)-approved cisplatin, the presence of rhomboid 2 in 1 has better antitumor activity and much lower side effects in the HepG2 tumor-bearing nude mice models, which may be attributed to the enhanced permeability and retention effect.
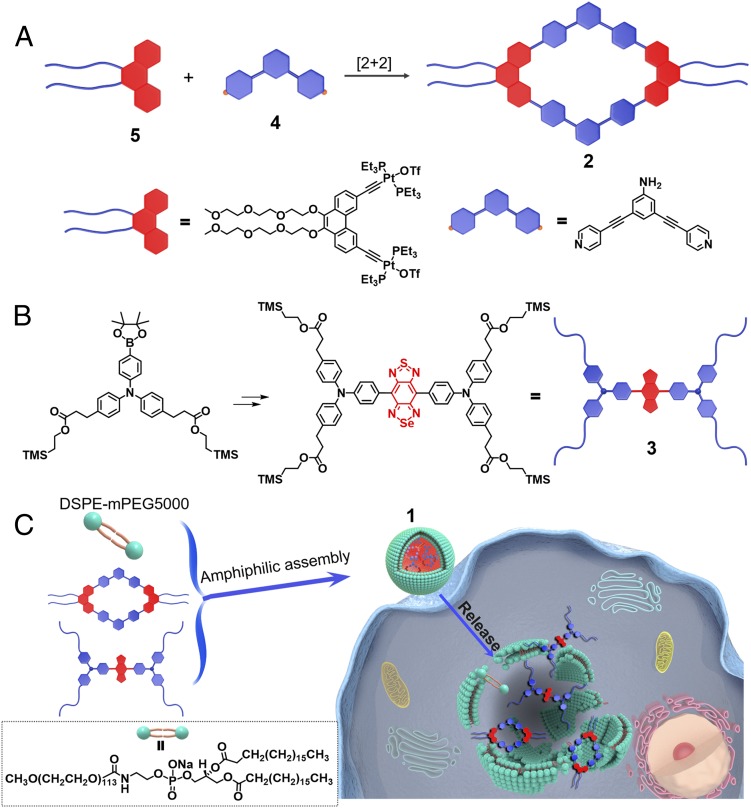
Fig. 1.
(A) Self-assembly of dipyridyl donor 4 and diplatinum(II) acceptor 5 to form the rhomboid 2. (B) The synthesis of the NIR-II molecular dye 3. (C) Cartoon illustration of the cellular uptake of rhomboid 2 from nanoprobe 1.
Results and Discussion
Synthesis, Characterization, and Photophysical Properties of 1.
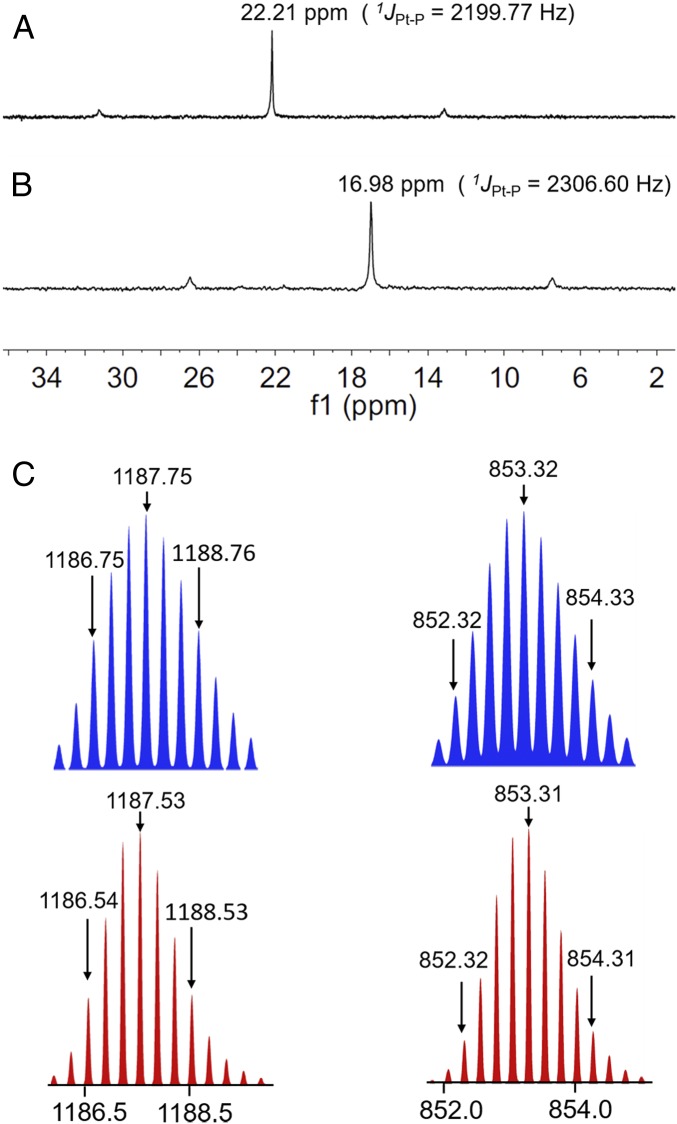
According to the principle of directional bonding, rhomboid 2 can be synthesized in high yield using the combination of 120° precursor 4 and a 60° Pt(II) precursor 5 (Fig. 1A) (1). We used multinuclear NMR analysis to verify the structure of rhomboid 2. Compared with the relevant 31P{1H} NMR spectra of the starting Pt(II) acceptor 5, rhomboid 2 exhibited an upfield shift of ∼5.23 ppm and has a sharp singlet peak at ca. 16.98 ppm, with concomitant 195Pt satellites corresponding to a single-phosphorus environment (Fig. 2A). In the 1H NMR spectrum, the protons of the pyridine Ha and Hb in the rhomboid 2 were shifted downfield compared with those of the free dipyridyl ligand 4 due to the formation of the Pt–N bond (SI Appendix, Fig. S18). Electrospray ionization (ESI)-TOF-MS confirmed the stoichiometry of the rhomboid 2, namely m/z = 1,187.53 for [M–3OTf]3+ and m/z = 853.31 for [M–4OTf]4+. These peaks are in good agreement with their calculated theoretical distributions, further indicating the formation of the metallacycle (Fig. 2C). The NIR-II molecular dye 3 was synthesized from commercially available chemicals, and it was characterized by 1H NMR, 13C NMR, and MALDI-TOF-MS (SI Appendix, Figs. S23–S28). We also calculated the structure of rhomboid 2 (SI Appendix, Fig. S22).
Fig. 2.
Partial 31P{1H} NMR[CH2Cl2] spectra of (A) diplatinum(II) acceptor 5 and (B) rhomboid 2. (C) Experimental (red) and calculated (blue): ESI-TOF-MS spectra of discrete rhomboid 2 [M–3OTf]3+, [M−4OTf]4+.
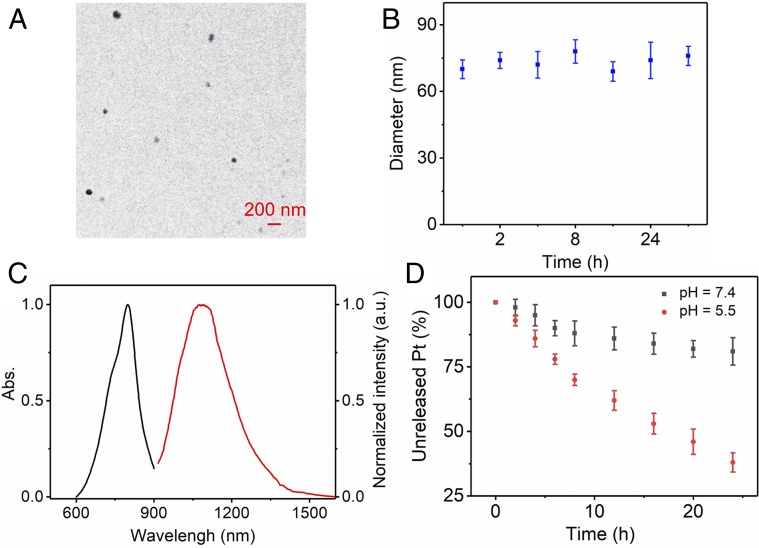
The nanoprobe, 1, was synthesized by mixing 2, 3, and DSPE-PEG5000 in a 1:1:8 weight ratio (Fig. 1C and SI Appendix, section A). Transmission electron microscopy (TEM) studies indicated that the complex 1 displays a high monodispersity in solution (∼60 nm) (Fig. 3A). The hydrodynamic diameter of 1 is ∼70 nm as determined by dynamic light scattering (DLS) (SI Appendix, Fig. S29). The stability of 1 in vitro is established in PBS containing 10% (vol/vol) FBS at 37 °C. There was no observable size variation over 48 h (Fig. 3B). The fluorescence emission wavelength of 1 is ∼1.1 µm, demonstrating a bright fluorescence in the NIR-II region (Fig. 3C). The release of rhomboid 2 from 1 in vitro was also investigated using the dialysis bag diffusion method and quantified by inductively coupled plasma mass spectrometry (ICP-MS) measurements. The results indicate that ∼70% of the rhomboid 2 was released from 1 over 24 h under the acidic conditions of a tumor (pH 5.5) (Fig. 3D). Due to the advantage of NIR-II fluorescence imaging, complex 1 showed good photostability and minimal decay in different mediums with continuous 808-nm excitation for 1 h (SI Appendix, Fig. S30). In contrast, the FDA-approved NIR-I theranostic ICG (indocyanine green, control experiment) decayed ∼50% under the same conditions (SI Appendix, Fig. S31). The quantum yield (QY) of 1 in water was ∼0.03% (IR-26 as a reference fluorophore; QY = 0.05%) (29). The amount of 3 encapsulated in 1 was measured using a UV/Vis spectrophotometer. The dye 3 encapsulation efficiency of 1 was calculated to be 82.5 ± 1.3% (SI Appendix, Fig. S32).
Fig. 3.
(A) The TEM image of 1. (B) The average diameters of 1 were determined by DLS measurements in PBS containing 10% FBS after various incubation times. (C) UV absorbance and NIR-II fluorescence emission of 1. (D) Release of rhomboid 2 from 1 at various pH values.
In Vitro Cell Imaging and Cell Study.
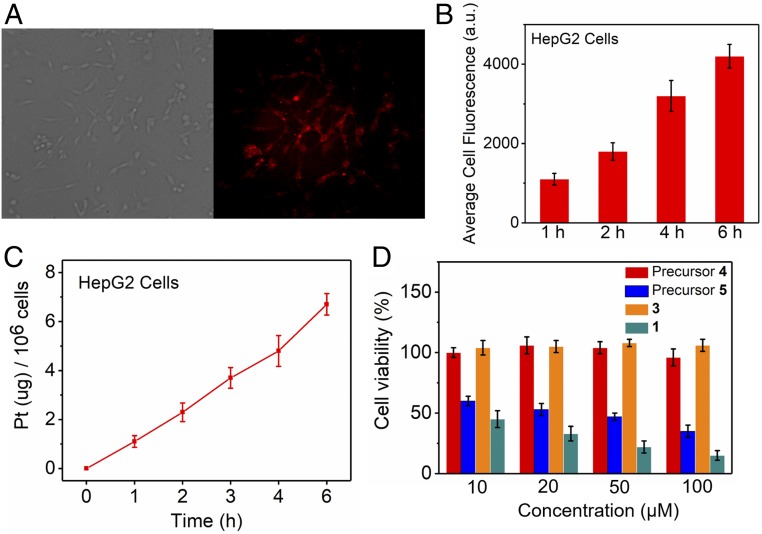
Complex 1 was investigated as a fluorescent probe for the imaging of cancer cells via its endocytosis behavior. The HepG2 cells showed strong NIR-II fluorescence after incubation with 1 for 1 h (Fig. 4A), and the fluorescence intensity became much stronger when the incubation time was extended to 6 h (Fig. 4B). The cellular uptake and biodistribution of the rhomboid 2 from 1 were further quantified using ICP-MS analysis. The HepG2 cells were incubated with 1 for 1, 2, 3, 4, and 6 h. As shown in Fig. 4C, the results of the ICP-MS analysis indicate that the cellular uptake of 1 gradually increased with incubation time, in agreement with the observed increase in fluorescence intensity. To determine the uptake and distribution behavior of complex 1 in the normal cell, we choose L929 as a model. We evaluated the HepG2 and L929 cellular uptake of platinum from complex 1 by ICP-MS and normalized the uptake vs. protein content (values are in per milligram of protein) following established procedure (30). Nearly 97.5% of the rhomboid 2 content was within the nucleus (172 ± 18.2 ng Pt per 1 mg protein), while a smaller fraction 2 was found in the cytoplasm (4.28 ± 0.353 ng Pt per 1 mg protein) (SI Appendix, Table S1). This may be due to the structure of complex 1 being destroyed in the endolysosomes at the relatively low pH so that, after internalization, the released rhomboid 2 entered the cell nuclei and coordinated with DNA (31, 32). Compared with the cancer cell HepG2, complex 1 has a much lower uptake in both the nucleus and cytoplasm of L929. The cytotoxicity of the individual building blocks, 3, as well as 1 over a range of 10–100 µM was evaluated using a 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide assay. The cellular toxicity assay showed no observable toxicity for precursor 4 or the NIR-II dye 3 even at a relatively high dose of up to 100 µM (Fig. 4D). Precursor 5 also showed good anticancer activity toward HepG2 cells, which may be due to their ability to form intra- and interstrand cross-links on DNA via coordination of the N7 atoms of purine bases to the Pt centers (33, 34). However, complex 1 exhibited better anticancer activity as well as antiproliferative activity against HepG2 cells that increases with dose (Fig. 4D).
Fig. 4.
(A) NIR-II fluorescence images of HepG2 cells incubated with 1 for 4 h at 37 °C. NIR-II images were taken with exposure for 100 ms and 808-nm excitation wavelength using the NIR-II fluorescence microscope at 100× magnification. (B) Average cell fluorescence of HepG2 cells is obtained by the average fluorescence intensity from 20 cells in each NIR-II fluorescence image at different times. (C) Time-dependent quantitative analysis of the amount of platinum in the HepG2 cells after incubation. (D) Cellular toxicity of precursor 4, precursor 5, NIR-II molecular dye 3, and nanoprobe 1 on HepG2 cells.
In Vivo Fluorescent Imaging in the NIR-II Region.
We also investigated the NIR-II fluorescent imaging of 1 in vivo. Accurately visualizing the lymphatic system, including the lymphatic vessels and lymph nodes, is an important application of fluorescent imaging for the diagnosis of cancer metastasis (35). To demonstrate the advantages of the bright fluorescence and nanoparticle features of 1 for lymphatic system NIR-II imaging, an intradermal injection of nanoprobe 1 was administered at the rear paw of C57BL/6 mice. The lymphatic vessels visualized in the NIR-II region showed a higher SBR (signal-to-background ratio) and enhanced feature sharpness using 1 (∼12.89 and 230 µm, respectively) (SI Appendix, Fig. S33), thus improving the real-time monitoring of lymphatic drainage in living subjects. We then performed in vivo brain vessel network imaging in C57BL/6 mice. Visualization through the intact scalp and skull was done in a noninvasive manner via injection of 1. Due to the bright fluorescence of 1, it was possible to carry out imaging and tracking of the vessel network in the mouse brains with high SBR as well as sharp images (∼7.53 and 187 µm, respectively) (SI Appendix, Fig. S33). Moreover, a high-resolution imaging of blood vessels was obtained immediately after injection of HepG2 tumor-bearing nude mice with 1. These results confirmed the advantages of 1 over traditional visible and NIR-I theranostic agents.
To investigate the in vivo pharmacokinetics of 1, C57BL/6 mice (n = 3) were injected with 1 for NIR-II imaging, and the Pt content in the plasma was analyzed using ICP-MS at various times. A very strong fluorescent signal was observed from 1 in the liver, suggesting that the clearance route for 1 is predominantly through the hepatobiliary system (SI Appendix, Fig. S34). Furthermore, the results of the ICP-MS analysis indicate that 1 has a longer blood circulation time than cisplatin (SI Appendix, Fig. S35), thus resulting in enhanced efficacy over traditional cisplatin.
Theranostic Functions of 1 in Vivo.
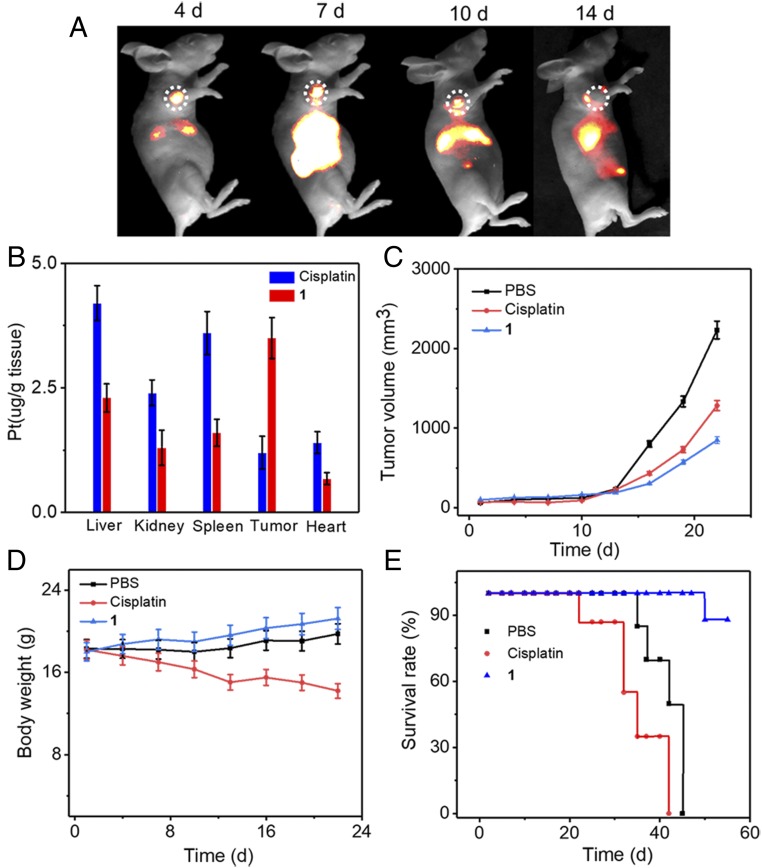
Fluorescence imaging facilitates the noninvasive characterization and measurement of liver cancer and the monitoring of the therapeutic response in intact tissue (36, 37). HepG2 tumor mice models were used to evaluate the theranostic utility of 1, while cisplatin was used as the control. From NIR-II imaging, the HepG2 tumor could be clearly visualized during a 4- to 48-h postinjection period, and the NIR-II fluorescence signal at the tumor region gradually increased with time and reached a maximum at 24 h with good contrast (SI Appendix, Fig. S36). A biodistribution study showed accumulation of 1 in the liver, spleen, and tumor (SI Appendix, Fig. S37). By taking advantage of the long emission wavelength, it was possible to carry out dynamic imaging and monitoring of the therapeutic response for a much longer time. As shown in Fig. 5A, the fluorescence signal at the tumor region was clearly observed even after 2 weeks. To quantitatively analyze the distributions of Pt content in tissues, 1 or cisplatin was injected into HepG2 tumor model animals (n = 3 per group). After 12 h postinjection, the amounts of rhomboid 2 in the tumor and normal tissues were analyzed by ICP-MS to evaluate the accumulation of 1. Compared with cisplatin, a significant accumulation of Pt in the tumor from 1 was clearly observed, which can be attributed to the enhanced permeability and retention effect and the prolonged circulation time via PEGylation. Furthermore, 1 displayed a lower Pt uptake in the normal organs, which suggests that 1 could reduce the systematic toxicity of rhomboid 2 for healthy tissues (Fig. 5B).
Fig. 5.
(A) NIR-II images of monitoring the 1 therapeutic response in HepG2 tumors. (B) The distributions of Pt in the main organs at 12 h after the injection of 1. (C) HepG2 tumor growth inhibition curves for PBS, cisplatin, and 1. (D) Average body weights were analyzed. (E) Survival rate of the mice bearing HepG2 tumors after different treatments.
To evaluate the selective delivery and the ability to inhibit tumor growth, HepG2 tumor-bearing mice were i.v. injected with 1, cisplatin, or PBS (dose: 2 mg Pt/kg body weight). The PBS-treated group did not show any obvious tumor inhibition (Fig. 5C). Cisplatin demonstrated moderate tumor growth inhibition, and 1 exhibited the highest antitumor efficiency. Due to the enhanced permeability and retention effect of 1, the targeted delivery of the Pt(II)-based drug in vivo was more effective in the therapy compared with traditional cisplatin treatment. Moreover, loss of body weight was observed during the experimental period in the mice treated with cisplatin, whereas the 1-treated group did not exhibit any obvious body weight loss, indicating that 1 has a better in vivo biocompatibility as well as reduced systemic toxicity (Fig. 5D). The Kaplan–Meier survival plots are shown in Fig. 5E. All mice treated with cisplatin were dead at day 42 due to the damage suffered by the primary organs from the free Pt(II) drug. In contrast, 1 significantly prolonged the survival of the mice, suggesting that 1 has low systemic toxicity. H&E staining, Ki67-positive immunohistochemical staining, and the TUNEL staining assay were performed to examine the therapeutic efficacy of complex 1 (SI Appendix, Fig. S38). For H&E staining, compared with the PBS group, the tumor tissues of complex 1 exhibit the highest death of tumor cells, suggesting that complex 1 has high antitumor activity. Ki67-positive immunohistochemical staining was also performed. The brown signals shown in Ki67-positive immunohistochemical staining indicate tumor cell proliferation sites. The number of Ki67-positive cells in the tumor tissue of the PBS group is higher than that in cisplatin and complex 1. The TUNEL staining was used to detect tumor cell apoptosis, and the brown signals shown in the TUNEL staining indicated apoptotic tumor cells. The results indicate that the number of apoptotic tumor cells in complex 1 was higher than that in the PBS and cisplatin. Overall, these experimental data suggest that 1 has an enhanced efficiency against proliferation and induces the apoptosis of tumor cells.
Conclusion
In conclusion, we designed a Pt(II)-based theranostic agent that incorporates both a Pt(II) metallacycle and an organic molecular dye into DSPE-mPEG5000 (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000]) to form the theranostic nanoprobe, 1, that has a long emission wavelength. This design endows 1 with good photostability and passive targeting ability, and therefore, it can provide precise diagnosis of cancer with high resolution and selective delivery of the Pt(II) metallacycle to the tumor region via the enhanced permeability and retention effect. In vitro and in vivo results further indicated that 1 has higher antitumor efficacy and lower side effects than cisplatin. These properties of 1 suggest the potential as a theranostic platform for the development of noble metal-based targeted theranostic probes in the NIR-II region.
Materials and Methods
The 1H and 13C NMR spectra were acquired on a Bruker 400-MHz magnetic resonance spectrometer. MALDI-MS spectrometric analyses were performed on an Applied Biosystems 4700 MALDI TOF mass spectrometer. UV/Vis absorbance of the probe was recorded on a PekinElmer Lambda 25 UV-Vis spectrophotometer. The NIR-II fluorescence microscope and NIR-II in vivo imaging system were purchased from Suzhou NIR-Optics Technologies Co., Ltd. Hydrodynamic diameter was measured using a Malvern Zetasizer Nano ZS. HPLC was performed on a Dionex HPLC System (Dionex Corporation), and a reversed-phase C18 column was used for analysis (5 μm; 4.6 × 250 mm; Phenomenax) and semipreparation (5 μm; 10 × 250 mm; Agilent). TEM images were recorded on a Hitachi TEM system, and the measurements were conducted with TEM operated at 200 kV. All animal experimentation was performed under the approval of the Central China Normal University Administrative Panel on Laboratory Animal Care.
Supplementary Material
Acknowledgments
We thank Suzhou NIR-Optics Technologies Co., Ltd. for their technical support. This work was supported by National Key R&D Program 2017YFA0505203 (to G.Y.). Y.S. was supported by National Natural Science Foundation of China Grant 21708012 and 111 Project B17019; the self-determined research funds of Central China Normal University from the colleges; Wuhan Morning Light Plan of Youth Science and Technology Grant 201705304010321; and the Open Research Fund of Jiangsu Key Laboratory of Medical Optics Grant JKLMO201803. This work was also supported by National Natural Science Foundation of China Grants 21572076, 21372092 (to H.L.), 81371840 (to Y.Z.), and 51402099 (to X.L.); and NIH Grant R01 CA215157 (to P.J.S.).
Footnotes
Conflict of interest statement: C.A.M. is an Associate Editor of the Journal of the American Chemical Society, and P.J.S. is the Editor-in-Chief of the Journal of the American Chemical Society.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817021116/-/DCSupplemental.
References
- 1.Cook TR, Stang PJ. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem Rev. 2015;115:7001–7045. doi: 10.1021/cr5005666. [DOI] [PubMed] [Google Scholar]
- 2.Saha ML, Yan X, Stang PJ. Photophysical properties of organoplatinum(II) compounds and derived self-assembled metallacycles and metallacages: Fluorescence and its applications. Acc Chem Res. 2016;49:2527–2539. doi: 10.1021/acs.accounts.6b00416. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, et al. Alanine-based chiral metallogels via supramolecular coordination complex platforms: Metallogelation induced chirality transfer. J Am Chem Soc. 2018;140:3257–3263. doi: 10.1021/jacs.7b10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook TR, Vajpayee V, Lee MH, Stang PJ, Chi KW. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc Chem Res. 2013;46:2464–2474. doi: 10.1021/ar400010v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConnell AJ, Wood CS, Neelakandan PP, Nitschke JR. Stimuli-responsive metal-ligand assemblies. Chem Rev. 2015;115:7729–7793. doi: 10.1021/cr500632f. [DOI] [PubMed] [Google Scholar]
- 6.Zeng L, et al. The development of anticancer ruthenium(ii) complexes: From single molecule compounds to nanomaterials. Chem Soc Rev. 2017;46:5771–5804. doi: 10.1039/c7cs00195a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell NP. Multi-platinum anti-cancer agents. Substitution-inert compounds for tumor selectivity and new targets. Chem Soc Rev. 2015;44:8773–8785. doi: 10.1039/c5cs00201j. [DOI] [PubMed] [Google Scholar]
- 8.Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev. 2016;116:3436–3486. doi: 10.1021/acs.chemrev.5b00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieltyka R, et al. A platinum supramolecular square as an effective G-quadruplex binder and telomerase inhibitor. J Am Chem Soc. 2008;130:10040–10041. doi: 10.1021/ja8014023. [DOI] [PubMed] [Google Scholar]
- 10.Grishagin IV, et al. In vivo anticancer activity of rhomboidal Pt(II) metallacycles. Proc Natl Acad Sci USA. 2014;111:18448–18453. doi: 10.1073/pnas.1418712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JS, Zhao GJ, Cook TR, Han KL, Stang PJ. Photophysical properties of self-assembled multinuclear platinum metallacycles with different conformational geometries. J Am Chem Soc. 2013;135:6694–6702. doi: 10.1021/ja402421w. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, et al. Heterometallic Ru-Pt metallacycle for two-photon photodynamic therapy. Proc Natl Acad Sci USA. 2018;115:5664–5669. doi: 10.1073/pnas.1802012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu G, et al. Antitumor activity of a unique polymer that incorporates a fluorescent self-assembled metallacycle. J Am Chem Soc. 2017;139:15940–15949. doi: 10.1021/jacs.7b09224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu G, et al. Tetraphenylethene-based highly emissive metallacage as a component of theranostic supramolecular nanoparticles. Proc Natl Acad Sci USA. 2016;113:13720–13725. doi: 10.1073/pnas.1616836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong GS, Antaris AL, Dai HJ. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng. 2017;1:0010. [Google Scholar]
- 16.Ding F, Zhan Y, Lu X, Sun Y. Recent advances in near-infrared II fluorophores for multifunctional biomedical imaging. Chem Sci (Camb) 2018;9:4370–4380. doi: 10.1039/c8sc01153b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, et al. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat Commun. 2018;9:2898. doi: 10.1038/s41467-018-05113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S, et al. Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc Natl Acad Sci USA. 2017;114:962–967. doi: 10.1073/pnas.1617990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Li J, Zhen X, Xie C, Pu K. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: A comparative study. Adv Mater. 2018;30:1705980. doi: 10.1002/adma.201705980. [DOI] [PubMed] [Google Scholar]
- 20.Ding F, et al. PEGylation regulates self-assembled small-molecule dye-based probes from single molecule to nanoparticle size for multifunctional NIR-II bioimaging. Adv Healthc Mater. 2018;7:1800973. doi: 10.1002/adhm.201800973. [DOI] [PubMed] [Google Scholar]
- 21.Antaris AL, et al. A small-molecule dye for NIR-II imaging. Nat Mater. 2016;15:235–242. doi: 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- 22.He S, Song J, Qu J, Cheng Z. Crucial breakthrough of second near-infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem Soc Rev. 2018;47:4258–4278. doi: 10.1039/c8cs00234g. [DOI] [PubMed] [Google Scholar]
- 23.Bruns OT, et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat Biomed Eng. 2012;1:0056. doi: 10.1038/s41551-017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Lu L, Zhao M, Lei Z, Zhang F. An efficient 1064 nm NIR-II excitation fluorescent molecular dye for deep-tissue high-resolution dynamic bioimaging. Angew Chem Int Ed Engl. 2018;57:7483–7487. doi: 10.1002/anie.201801226. [DOI] [PubMed] [Google Scholar]
- 25.Xu G, et al. Imaging of colorectal cancers using activatable nanoprobes with second near-infrared window emission. Angew Chem Int Ed Engl. 2018;57:3626–3630. doi: 10.1002/anie.201712528. [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat Nanotechnol. 2018;13:941–946. doi: 10.1038/s41565-018-0221-0. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, et al. Novel dual-function near-infrared II fluorescence and PET probe for tumor delineation and image-guided surgery. Chem Sci (Camb) 2018;9:2092–2097. doi: 10.1039/c7sc04774f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, et al. Compact plasmonic blackbody for cancer theranosis in the near-infrared II window. ACS Nano. 2018;12:2643–2651. doi: 10.1021/acsnano.7b08725. [DOI] [PubMed] [Google Scholar]
- 29.Semonin QE, et al. Absolute photoluminescence quantum yields of IR-26 dye, PbS, PbSe quantum. J Phys Chem Lett. 2010;1:2445–2450. [Google Scholar]
- 30.Naik A, Rubbiani R, Gasser G, Spingler B. Visible-light-induced annihilation of tumor cells with platinum-porphyrin conjugates. Angew Chem Int Ed Engl. 2014;53:6938–6941. doi: 10.1002/anie.201400533. [DOI] [PubMed] [Google Scholar]
- 31.Zheng YR, Suntharalingam K, Johnstone TC, Lippard SJ. Encapsulation of Pt(IV) prodrugs within a Pt(II) cage for drug delivery. Chem Sci (Camb) 2015;6:1189–1193. doi: 10.1039/c4sc01892c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JJ, Lippard SJ. Synthetic methods for the preparation of platinum anticancer complexes. Chem Rev. 2014;114:4470–4495. doi: 10.1021/cr4004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnstone TC, Wilson JJ, Lippard SJ. Monofunctional and higher-valent platinum anticancer agents. Inorg Chem. 2013;52:12234–12249. doi: 10.1021/ic400538c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizarro AM, et al. Cellular accumulation, lipophilicity and photocytotoxicity of diazido platinum(IV) anticancer complexes. ChemMedChem. 2014;9:1169–1175. doi: 10.1002/cmdc.201402066. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, et al. Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery. Chem Sci (Camb) 2017;8:3489–3493. doi: 10.1039/c7sc00251c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003;2:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, et al. Strained cyclooctyne as a molecular platform for construction of multimodal imaging probes. Angew Chem Int Ed Engl. 2015;54:5981–5984. doi: 10.1002/anie.201500941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.