Abstract
Insulin autoimmune syndrome (IAS) is a rare cause of nondiabetic hypoglycemia characterized by hyperinsulinemia and autoantibodies to endogenous insulin without prior exposure to exogenous insulin. We report a drug-induced case of IAS in a 59-year-old Nepalese female. She had been taking carbimazole for Graves’ disease and later presented with recurrent episodes of hypoglycemia, with laboratory findings of low blood glucose, increased molar ratio of insulin to C-peptide, and elevated autoantibodies to insulin. IAS should be considered while evaluating hypoglycemia to prevent unwarranted invasive procedures and surgical interventions.
Keywords: insulin autoimmune syndrome, hypoglycemia, carbimazole, Hirata disease
Introduction
Insulin autoimmune syndrome (IAS), a rare cause of reversible autoimmune hypoglycemia also known as Hirata’s disease, was first described by Hirata in Japan in 1970.1 IAS has been reported from different parts of the world since then. Usual presenting features are multiple episodes of spontaneous hypoglycemia and appearance of insulin autoantibodies without prior history of administration of exogenous insulin.2
Affected persons are usually adults presenting with neuroglycopenic symptoms.3 The mechanism of IAS is not clearly understood but interaction of disulfide bond in the insulin molecule with sulfhydryl group drugs such as methimazole, carbimazole, captopril, isoniazid, hydralazine, imipenem, and also with lipoic acid has been suggested.4–6 Drug-induced autoimmunization is evidenced by insulin autoantibodies appearing a few weeks after the intake of drug containing the sulfhydryl group. IAS has a significant genetic predisposition as its association with specific HLA class has been observed.
Following a meal, glucose concentration in the bloodstream rises, providing a stimulus for insulin secretion. Autoantibodies bind to these insulin molecules, rendering them unable to exert their effects. The resultant hyperglycemia promotes further insulin release. As glucose concentration eventually falls, insulin secretion also subsides, and the total insulin level decreases. Now insulin molecules spontaneously dissociate from the autoantibodies, giving rise to a raised free insulin level inappropriate for the glucose concentration, causing hypoglycemia.3
In IAS, the insulin level is significantly high, usually up to 100 mIU/L, C-peptide level is markedly elevated, and insulin antibodies are positive. The best known treatment is recommending frequent, small meals and to avoid simple sugars. Sulfhydryl group-containing drugs should be avoided and steroids can be used in resistant cases.7
Case presentation
A 59-year-old female from eastern Nepal, presented with a history of multiple episodes of restlessness, sweating, palpitation, anxiety, and tremors a few hours after meals for 2 weeks. Symptoms were relieved temporarily on ingestion of carbohydrate-rich foods. She was non-hypertensive, non-diabetic.
There was no history of any loss of consciousness, trauma, and major surgery. She was known to have hyperthyroidism (Graves’ disease) for which she was taking carbimazole. No prior administration of insulin or intake of any hypoglycemic agents was noted. Family history was negative for any endocrine tumors. She was a non-smoker and did not consume alcohol.
Her general and systemic examination was normal and vitals were stable. Laboratory investigations like complete blood count, organ function test, HbA1c, and lipid profile were within normal limits. Adrenocorticotropic hormone stimulation test indicated an adequate cortisol response.
On the second day of admission, an abdominal computed tomography (CT) scan was done which was normal. Exogenous administration of hypoglycemic agents was ruled out during hospitalization. During hospitalization, 24 hours of fasting was ordered. She did not develop features of hypoglycemia like loss of consciousness or sweating during this fast though she was hungry. Her recorded blood glucose was 4.6 mmol/L. This biochemical finding along with normal CT scan of the abdomen ruled out the possibility of insulinoma. After fasting, a mixed meal was provided and a few hours later she developed features of hypoglycemia and the recorded blood glucose was 1.88 mmol/L.
On further work-up, serum c-peptide and insulin level was measured, which was very high (Table 1). The measurement of serum insulin and c-peptide was done during hypoglycemic event. A high level of insulin can also occur due to immunoassay interference in a clinical biochemistry laboratory. This interference was ruled out by obtaining a high level of serum insulin in a different assay system and use of heterophile antibody blocking tube. Serum anti-insulin antibody was also measured, which was high (Table 1).
Table 1.
Laboratory investigations performed during hypoglycemic event
| Parameters | Result | Reference range in units |
|---|---|---|
| Serum glucose | 1.88 mmol/L | 3.5–6.1 mmol//L |
| Serum beta- hydroxybutyrate | <0.30 mmol/L | <0.30 mmol/L |
| Serum insulin | 78,140.5 pmol/L | <10 pmol/L if blood glucose is <2.7 mmol/L |
| Serum c-peptide | 19.38 ng/mL (6,416 pmol/L) | 0.81–3.85 ng/mL |
| Insulin: c-peptide molar ratio | 12.17 | <1 |
| Serum anti insulin antibody | >300 U/mL | <12 U/mL |
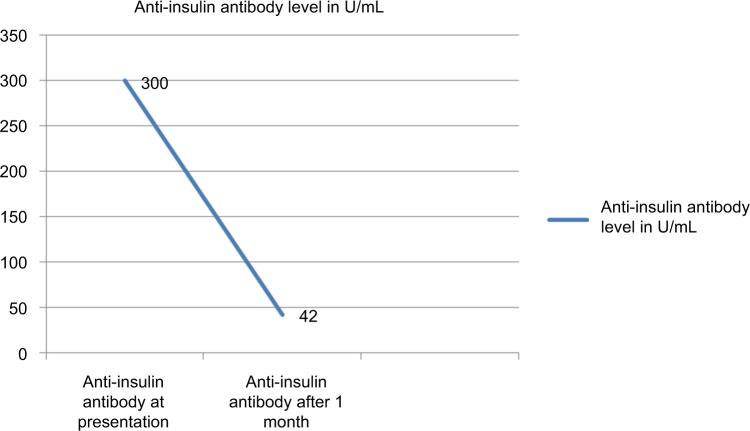
Serological tests for thyroid peroxidase antibody, rheumatoid antibody, and antinuclear antibody were negative. Then the diagnosis of IAS secondary to carbimazole was made. The patient was admitted under continuous dextrose drip and electrolyte level was monitored. Carbimazole was stopped followed by immediate radio ablation iodine therapy for Graves’ disease. The patient improved symptomatically and was discharged. Her follow-up anti-insulin antibody was significantly decreased, as shown in Figure 1. Written consent was obtained from the patient for publication of this case report. Ethical approval was obtained from the Institutional Research Board of the Institute of Medicine.
Figure 1.
Plot showing serum level of anti-insulin antibody before and after treatment.
Discussion
Here we present a case of IAS or Hirata’s disease, a rare cause of hyperinsulinemic hypoglycemia, usually seen in adult patients older than 40 years of age without any sex predilection. It is characterized by autoimmune antibodies to endogenous insulin in individuals without prior exposure to exogenous insulin. Autoimmune hypoglycemia is usually associated with autoimmune disorders or medication-induced. In our case, the patient had been taking carbimazole for 2 months for the treatment of Graves’ disease.
Our patient had multiple episodes of neuroglycopenic symptoms a few minutes after meals, which again improved after consumption of food or sugary drinks. Initially we suspected insulinoma as differential diagnosis. Normal abdominal CT scan along with normal blood glucose after fast test excluded insulinoma. During investigation, the patient had hypoketotic hypoglycemia which can occur in fatty acid oxidation disorder. Due to the postprandial nature of hypoglycemia, fatty acid oxidation disorder was excluded. Hypoglycemia due to metabolic error like metabolic storage diseases was ruled out by demonstrating normal ketone bodies in serum and there was no organomegaly either. Exogenous insulin administration was ruled out by strict monitoring during hospitalization as well as by demonstrating high level of serum c-peptide.
Discrepancies between an unusually high insulin concentration and moderately raised c-peptide with history of onset of illness after intake of carbimazole suggested IAS in this case.
Insulin and c-peptide are secreted at equimolar concentration from pancreatic beta cells but due to difference in half-life, the insulin to c-peptide molar ratio is below one. This ratio is more than one in either exogenous insulin administration or IAS. In this case, this molar ratio was very high. According to the Endocrine Society Guidelines, if pro-insulin levels are >5 pmol/L, endogenous insulin production should be suspected and testing for insulin antibody in the presence of documented endogenous hypoglycemia is suggested.8 But in our case, we could not evaluate proinsulin level due to unavailability of the test. The most widely accepted hypothesis is that sulfhydryl group drugs may cleave the disulfide bond of insulin molecule in vivo and enhance its immunogenicity. Since insulin is the only beta cell-specific autoantigen, a high level of anti-insulin antibody in this case is consistent with the diagnosis of IAS.
There is also a possibility of presence of anti-insulin antibody that is directed against cell surface insulin receptor. This has a stimulatory effect and is associated with an inappropriately high level of insulin with postprandial hypoglycemia or fasting hypoglycemia or both.6,9 Testing for presence of insulin receptor autoantibody was not done in our case since this entity presents with many other features of insulin resistance that were not present in our case.
Food with a low glycemic index remains the first line of treatment as this avoids postprandial hyperglycemia, and thereby the stimulus for insulin secretion. Steroid use may be useful as an adjunct therapy.7 Any potentially incriminating medication should be discontinued. Our patient improved after stopping carbimazole. Reporting of this case is beneficial for physicians as it avoids inappropriate investigation and therapeutic intervention.
Conclusion
The autoimmune form of hypoglycemia is rare but should be taken into consideration in patients taking carbimazole. Early laboratory testing for insulin autoantibody in patients with hypoglycemia without diabetes mellitus is highly recommended.
Consent
Written informed consent was obtained from the patient for publication of this case report.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hirata Y. Insulin autoimmunity in a case of spontaneous hypoglycemia. J Jpn Diabet Soc. 1970;13:312–319. [Google Scholar]
- 2.Uchigata Y. Insulin autoimmune syndrome (IAS, Hirata disease) Ann Med Interne (Paris) 1999;150:245–253. [PubMed] [Google Scholar]
- 3.Redmon JB, Nuttall FQ. Autoimmune hypoglycemia. Endocrinol Metab Clin North Am. 1999;28(3):603–618. doi: 10.1016/s0889-8529(05)70090-6. [DOI] [PubMed] [Google Scholar]
- 4.Yamada T, Imai J, Ishigaki Y, Hinokio Y, Oka Y, Katagiri H. Possible relevance of HLA-DRB1*0403 haplotype in insulin autoimmune syndrome induced by alpha-lipoic acid, used as a dietary supplement. Diabetes Care. 2007;30(12):e131–e131. doi: 10.2337/dc07-1636. [DOI] [PubMed] [Google Scholar]
- 5.Uchigata Y, Hirata Y, Iwamoto Y. Drug-induced insulin autoimmune syndrome. Diabetes Res Clin Pract. 2009;83(1):e19–e20. doi: 10.1016/j.diabres.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore) 2009;88(3):141–153. doi: 10.1097/MD.0b013e3181a5b42e. [DOI] [PubMed] [Google Scholar]
- 7.Ismail AA. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: an update on the pathophysiology, biochemical investigations and diagnosis. Clin Chem Lab Med. 2016;54(11):1715–1724. doi: 10.1515/cclm-2015-1255. [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 9.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81(2):87–100. doi: 10.1097/00005792-200203000-00001. [DOI] [PubMed] [Google Scholar]