Abstract
Background
Recent studies have highlighted that Takotsubo syndrome (TTS), mimicking acute coronary syndrome (ACS), is associated with poor clinical outcome. TTS is associated with different repolarization disorders including ST-segment elevation. ST elevation myocardial infarction (STEMI) in ACS is associated with declined prognosis. However, the clinical and prognostic impact of ST-segment elevation on TTS remains lacking.
Aim
The aim of this study was to determine the short- and long-term prognostic impact of ST-segment elevation on TTS patients as compared with STEMI patients.
Patients and methods
Our institutional database constituted a consecutive cohort of 138 TTS patients and 138 ACS patients matched for age and sex. TTS patients (n=41) with ST-segment elevation were compared with ACS patients with ST-segment elevation (n=64).
Results
Chest pain was significantly more documented in STEMI patients as compared with TTS patients (48.8% vs 78.1%; P<0.01). Cardiovascular risk factors such as diabetes mellitus (12.2% vs 29.7%; P=0.02) were significantly more presented in STEMI patients. Although the initial left ventricular ejection fraction (LVEF) was more declined in TTS patients (39%±9% vs 45%±16%; P<0.01), the LVEF was more declined in STEMI patients at follow-up (54%±10% vs 45%±16%; P=0.04). Inhospital complications such as respiratory failure were significantly more presented in TTS patients (68.3% vs 20.3%; P<0.01). The short-term as well as the long-term morality was similar in both groups. In univariate analysis, male sex, ejection fraction (EF) <35%, glomerular filtration rate (GFR) <60 mL/min, cardiogenic shock, inotropic drugs, and history of cancer were predictors of 5-year mortality.
Conclusion
Rates of the long-term mortality in TTS patients with ST elevations are comparable with STEMI patients.
Keywords: Takotsubo syndrome, coronary artery syndrome, prognosis, ST elevation
Introduction
Takotsubo syndrome (TTS) is a transient disorder of left ventricular (LV) wall dysfunction characterized by a range of wall motion abnormalities and clinically representative of an acute heart failure syndrome with substantial risk of adverse events.1–3 Patients present with symptoms such as chest pain and dyspnea, mimicking acute coronary syndrome (ACS). TTS can be associated with critical complications such as heart failure, life-threatening arrhythmias, atrial fibrillation, QT prolongation, thromboembolic events, recurrence of TTS, LV outflow obstruction, mitral valve regurgitation, and cardiac rupture.6–9 It has been reported that TTS is associated with cardiac fibrosis and inflammation.10 Recently published data confirmed that TTS is not associated with a favorable prognosis.4 However, it has been reported that the prognosis of TTS might be worse than the prognosis of ST elevation myocardial infarction (STEMI) patients.12 ST elevation is known as a predictor of the prognostic impact of an ACS.13 There is a lack of data describing the impact on TTS patients compared with STEMI patients. Therefore, the present study was performed to determine the prognostic impact of ST elevation on TTS patients compared with STEMI patients.
Patients and methods
Consecutive patients with the diagnosis of TTS (n=138) during the years 2003–2017 were included in the TTS database of our institution, of whom 41 (29.9%) presented with ST elevations. Patients were diagnosed using the Mayo Clinic criteria, which outlines the clinical features associated with TTS.14
The first criterion describes the transient wall motion abnormality in the LV mid-segments with or without apical involvement, regional wall motion abnormalities that extend beyond a single epicardial vascular distribution, and frequently, but not always in the event of a stressful trigger. The second criterion stipulates the absence of obstructive coronary disease. The third criterion outlines the appearance of new electrocardiogram (ECG) pathologies that mimic ACS or modest elevations of cardiac troponin levels. The final criterion is the absence of pheochromocytoma and myocarditis in the patient.
Furthermore, we consecutively collected the data of 532 ACS patients, who were admitted to our institution in the years 2007 and 2008. Two hundred and twenty-six of these patients were diagnosed with STEMI, and 59 out of the 226 patients were female. TTS or ACS patients (n=138 per group) were matched for age and sex and assessed and divided into two groups: TTS with ST-segment elevation (n=41) and STEMI (n=64).
The diagnosis of ACS has been defined in accordance with the joint American Heart Association/European Society of Cardiology (ESC) definition, including elevation of cardiac troponin, significant coronary arterial stenosis, and occurrence of typical ECG pathologies such as ST-segment elevation or T inversion.
The diagnosis of STEMI has been defined in accordance with the ESC guidelines, as a new ST elevation at the J point in two contiguous leads of >0.1 mV in all leads other than leads V2–V3. For leads V2–V3, the following cut points apply: ≥0.2 mV in men ≥40 years, ≥0.25 mV in men <40 years, or ≥0.15 mV in women.
The angiograms, echocardiograms, and ECGs were independently reviewed by two senior cardiologists to evaluate the diagnosis of TTS. Patients were subsequently treated with percutaneous coronary intervention (PCI) and stent implantation (non-ST elevation myocardial infarction [NSTEMI] or STEMI). The study was conducted in compliance with the Declaration of Helsinki with regard to investigations in human subjects, and the study protocol was approved by the ethics committee of the Medical Faculty Mannheim. All participants provided written informed consent.
Life-threatening arrhythmias (including ventricular tachycardia [VT], hemodynamically relevant nonsustained ventricular tachycardia [NSVT], and asystolia), thromboembolic events, major stroke, pulmonary congestion with the use of noninvasive, positive pressure ventilation, intubation, use of a temporary pacemaker, use of inotropic agents, cardiogenic shock, and death were assessed at index event of TTS/STEMI and during the follow-up of 1,682±1,332 days (median 1,751 days) for TTS and 1,181±1,312 days (median 632 days) for STEMI based on chart review. The primary end point was all-cause mortality rate as assessed by chart review and/or telephone review. Missing data on the cause of death were labeled as death due to unknown cause.
Statistical analyses
Data are presented as mean ± SD for continuous variables with a normal distribution, median (IQR) for continuous variables with a non-normal distribution, and as frequency (%) for categorical variables. The Kolmogorov–Smirnov test was used to assess normal distribution. Student’s t-test and Mann–Whitney U-test were used to compare continuous variables with normal and non-normal distributions, respectively. Chi-squared test or Fisher’s exact test was used to compare categorical variables. The log-rank test was used to compare the survival curves between the TTS group with ST elevation and the STEMI group. Statistical analysis was performed with SPSS statistics 23.0 (IBM Corporation, Armonk, NY, USA) in all analyses; P≤0.05 (two-tailed) was used to indicate statistical significance.
Results
Baseline demographics
We studied clinical and echocardiographic data of 41 TTS patients showing ST elevation with a mean follow-up of 1,682±1,332 days (median 1,751 days) and 64 STEMI patients with a mean follow-up of 1,181±1,312 days (median 632 days), P=0.44. Baseline demographics with a predominance of postmenopausal females in the TTS group are presented in Table 1. Compared with the TTS group, the incidence of chest pain (48.8% vs 78.1%; P<0.01) as well as diabetes mellitus (12.2% vs 29.7%; P=0.02) was higher in the STEMI group. The incidence of COPD was higher in TTS patients (22.0 vs 6.3; P=0.04). TTS patients had higher heart rates (99±20 vs 80±18; P<0.01) and were more frequently treated with angiotensin-converting enzyme (ACE) inhibitors (41.5% vs 21.9%; P<0.03) and aspirin at admission (35.0% vs 17.2%; P=0.04) compared with the STEMI group. However, STEMI patients were treated more frequently with aspirin (41.5% vs 76.6%; P<0.01) and statins at discharge (41.5% vs 71.8%; P<0.01), while TTS patients received more therapeutic anticoagulation (24.4% vs 7.8%; P=0.02). Similarly, echocardiographic data showed higher prevalence of valvular heart disease in the TTS group. There was a significant difference with relation to LV ejection fraction (LVEF; TTS 39%±9% vs STEMI 45%±16%; P=0.01).
Table 1.
Baseline characteristics of 41 TTS patients with ST elevation and 64 STEMI patients
| Variables | TTS (n=138) | Matched ACS (n=138) | TTS with ST elevation (n=41) | STEMI (n=64) | P-valuea |
|---|---|---|---|---|---|
|
| |||||
| Demographics | |||||
| Age, mean ± SD (years) | 67±11 | 66±15 | 68±12 | 68±16 | 1.00 |
| Female, n (%) | 117 (84.8) | 117 (84.8) | 38 (92.7) | 59 (92.2) | 0.93 |
| Symptoms, n (%) | |||||
| Dyspnea | 54 (39.1) | 44 (31.9) | 13 (31.7) | 17 (26.6) | 0.57 |
| Chest pain | 69 (50.4) | 107 (77.5) | 20 (48.8) | 50 (78.1) | <0.01 |
| Clinical parameter | |||||
| SBP, mmHg, IQR | 141 (62–240) | 137 (60–240) | 135 (80–220) | 130 (60–210) | 0.49 |
| DBP, mmHg, IQR | 79 (40–151) | 76 (7–120) | 81 (50–111) | 74 (7–120) | 0.44 |
| Heart rate, bpm, IQR | 99±26 | 81±19 | 99±20 | 80±18 | <0.01 |
| ECG data | |||||
| ST-segment elevation, n (%) | 41 (29.9) | 64 (46.4) | 41 (100.0) | 64 (100.0) | 1.00 |
| Inversed T-waves, n (%) | 123 (93.2) | 69 (50.0) | 36 (92.3) | 21 (32.8) | <0.01 |
| PQ interval, mean ± SD | 159±28 | 166±31 | 157±28 | 169±33 | 0.09 |
| QTc (ms), mean ± SD | 475 (62–604) | 457 (358–614) | 475 (130–604) | 456 (373–614) | 0.14 |
| Laboratory values, mean ± SD | |||||
| Troponin I (U/L; IQR) | 63.15 (0.01–2,738.00) | 20.02 (0.03–233.88) | 77.44 (0.06–2,631) | 29.10 (0.03–233.88) | 0.37 |
| Creatine phosphokinase (U/L; IQR) | 587 (39–26,600) | 1,028 (35–10,250) | 475 (48–939) | 1,353 (53–6,260) | 0.56 |
| CKMB (U/L; IQR) | 35 (1–415) | 79 (0–741) | 77 (4–167) | 108 (0–589) | 0.49 |
| C-reactive protein (mg/L; IQR) | 48.2 (0.4–467.1) | 45.2 (1.0–594.0) | 29.8 (1.4–467.1) | 45.2 (1.0–323.7) | 0.65 |
| Hemoglobin, mean ± SD | 12.2±2.0 | 12.8±1.9 | 12.4±2.4 | 12.8±2.2 | 0.32 |
| Creatinine (mg/dL; IQR) | 1.12 (0.40–5.56) | 1.10 (0.43–6.44) | 0.97 (0.40–1.85) | 0.98 (0.43–1.87) | 0.82 |
| Echocardiography data, n (%) | |||||
| LVEF%, mean ± SD | 39±10 | 49±15 | 39±9 | 45±16 | 0.01 |
| LVEF% follow-up, mean ± SD | 52±11 | 49±15 | 54±10 | 45±16 | <0.01 |
| Mitral regurgitation | 66 (47.8) | 39 (28.3) | 19 (46.3) | 14 (21.9) | <0.01 |
| Tricuspid regurgitation | 54 (39.1) | 20 (14.5) | 14 (34.1) | 9 (14.1) | <0.01 |
| Medical history, n (%) | |||||
| Smoking | 41 (29.7) | 45 (32.6) | 10 (24.4) | 19 (29.7) | 0.38 |
| Diabetes mellitus | 31 (22.5) | 49 (35.5) | 5 (12.2) | 19 (29.7) | 0.02 |
| BMI >25 kg/m2 | 36 (31.3) | 74 (53.6) | 10 (24.4) | 29 (45.3) | 0.11 |
| Hypertension | 82 (59.4) | 98 (71.0) | 25 (61.0) | 39 (60.9) | 0.80 |
| COPD | 28 (20.3) | 12 (8.7) | 9 (22.0) | 4 (6.3) | 0.04 |
| Atrial fibrillation | 26 (18.8) | 19 (13.8) | 5 (12.2) | 8 (12.5) | 0.96 |
| History of malignancy | 17 (12.3) | 8 (5.8) | 2 (5.1) | 3 (4.7) | 1.00 |
| Drugs on admission, n (%) | |||||
| Beta-blocker | 46 (35.4) | 32 (23.7) | 14 (35.0) | 13 (20.6) | 0.11 |
| ACE inhibitor | 51 (39.2) | 31 (22.6) | 17 (41.5) | 14 (21.9) | 0.03 |
| ARB | 12 (9.3) | 8 (5.8) | 4 (10.2) | 4 (6.3) | 0.49 |
| Aldosterone inhibitor | 1 (0.8) | 2 (1.5) | 1 (2.5) | 0 (0.0) | 0.39 |
| Aspirin | 36 (27.7) | 31 (22.6) | 14 (35.0) | 11 (17.2) | 0.04 |
| Statin | 26 (20.0) | 17 (12.4) | 10 (25.0) | 10 (15.6) | 0.24 |
| OAD | 6 (4.6) | 11 (8.0) | 1 (2.5) | 5 (7.8) | 0.25 |
| Insulin | 12 (9.3) | 17 (12.4) | 2 (5.1) | 5 (7.8) | 0.59 |
| Therapeutic anticoagulation | 12 (9.3) | 9 (6.5) | 4 (10.2) | 4 (6.3) | 0.47 |
| Heparin | 6 (4.6) | 3 (2.2) | 3 (7.7) | 1 (1.6) | 0.16 |
| Drugs on discharge, n (%) | |||||
| Beta-blocker | 103 (74.6) | 110 (79.7) | 32 (78.0) | 48 (75.0) | 0.94 |
| ACE inhibitor | 82 (59.4) | 85 (61.6) | 24 (58.5) | 32 (50.0) | 0.54 |
| ARB | 10 (7.2) | 20 (14.5) | 5 (12.2) | 10 (15.6) | 0.62 |
| Aldosterone inhibitor | 2 (1.4) | 2 (1.5) | 0 (0.0) | 1 (1.6) | 1.00 |
| Aspirin | 53 (38.4) | 113 (81.9) | 17 (41.5) | 49 (76.6) | <0.01 |
| Statin | 48 (34.8) | 110 (79.7) | 17 (41.5) | 46 (71.8) | <0.01 |
| OAD | 4 (2.9) | 8 (5.8) | 0 (0.0) | 4 (6.3) | 0.32 |
| Insulin | 14 (10.1) | 18 (13.0) | 3 (7.7) | 6 (9.4) | 0.78 |
| Therapeutic anticoagulation | 33 (23.9) | 10 (7.2) | 10 (24.4) | 5 (7.8) | 0.02 |
| Heparin | 18 (13.0) | 8 (5.8) | 7 (17.1) | 4 (6.3) | 0.18 |
Notes:
P-values for the comparison between TTS in the presence of ST elevation as compared with STEMI. Statically significant P-value (P<0.05) shown in bold.
Abbreviations: ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; CKMB, creatin kinase muscle/brain; ECG, electrocardiogram; LVEF, left ventricular ejection fraction; OAD, oral antidiabetic drugs; STEMI, ST elevation myocardial infarction; TTS, Takotsubo syndrome.
In hospital events
The TTS group needed significantly more often intubation due to pulmonary congestion than the STEMI group (68.3% vs 20.3%, P<0.01; Table 2). Furthermore, thromboembolic events were more frequently documented in TTS patients (22.0% vs 4.7%; P=0.02).
Table 2.
Inhospital events and treatment strategy in TTS patients with ST elevation and STEMI patients
| Variables | TTS (n=138) | Matched ACS (n=138) | TTS with ST elevation (n=41) | STEMI (n=64) | P-valuea |
|---|---|---|---|---|---|
|
| |||||
| Life-threatening arrhythmia | 12 (8.8) | 16 (11.6) | 5 (12.2) | 11 (17.2) | 0.59 |
| NPPV and/or intubation | 80 (58.0) | 16 (11.6) | 28 (68.3) | 13 (20.3) | <0.01 |
| Inotropic agents | 23 (16.7) | 18 (13.0) | 8 (19.5) | 14 (21.9) | 0.77 |
| Resuscitation | 4 (12.5) | 17 (12.3) | 3 (7.3) | 11 (17.2) | 0.15 |
| Cardiac electronic device implantation | 4 (2.9) | 9 (6.5) | 1 (2.4) | 5 (7.8) | 0.25 |
| Admission to ICU, length of stay, days (IQR) | 5 (0–52) | 3 (0–14) | 4 (0–20) | 3 (0–11) | 0.43 |
| Inhospital death | 10 (7.2) | 17 (12.3) | 3 (7.3) | 13 (20.3) | 0.10 |
| Cardiogenic shock | 25 (18.5) | 16 (11.6) | 6 (15.4) | 13 (20.3) | 0.51 |
| Thromboembolic events | 18 (13.0) | 3 (2.2) | 9 (22.0) | 3 (4.7) | 0.02 |
Notes:
P-values for the comparison between TTS in the presence of ST elevation as compared with STEMI. Data presented as n (%). Statically significant P-value (P<0.05) shown in bold.
Abbreviations: ACS, acute coronary syndrome; ICU, intermediate care unit; NPPV, noninvasive positive pressure ventilation; STEMI, ST elevation myocardial infarction; TTS, Takotsubo syndrome.
Short- and long-term outcome
After a slightly higher mortality rate in STEMI patients at the index event (7.3% vs 20.3%; P=0.10), there were more events in the TTS group during follow-up, which would lead to a higher long-term mortality (26.8% vs 21.9%) after 5 years (Table 3 and Figure 1). However, there was no significant difference in mortality between TTS patients with ST-segment elevation and STEMI patients. The causes of death in TTS and STEMI patients are listed in Table 4.
Table 3.
Outcome in 41 TTS patients with ST elevation and 64 STEMI patients
| Variables | TTS (n=138) | Matched ACS (n=138) | TTS with ST elevation (n=41) | STEMI (n=64) | RR (95% CI) | P-valuea |
|---|---|---|---|---|---|---|
|
| ||||||
| Inhospital mortality | 10 (7.2) | 17 (12.3) | 3 (7.3) | 13 (20.3) | 0.4 (0.1–1.2) | 0.10 |
| 30-day mortality | 10 (7.2) | 17 (12.3) | 4 (9.8) | 13 (20.3) | 0.5 (0.7–1.4) | 0.18 |
| 1-year mortality | 14 (10.1) | 18 (13.0) | 4 (9.8) | 13 (20.3) | 0.5 (0.7–1.4) | 0.18 |
| 2-year mortality | 21 (15.2) | 19 (13.8) | 5 (12.2) | 14 (21.9) | 0.6 (0.2–1.4) | 0.30 |
| 3-year mortality | 23 (16.7) | 19 (13.8) | 7 (17.1) | 14 (21.9) | 0.8 (0.3–1.8) | 0.55 |
| 4-year mortality | 29 (21.0) | 19 (13.8) | 8 (19.5) | 14 (21.9) | 0.9 (0.4–1.9) | 0.77 |
| Long-term mortality | 35 (25.4) | 22 (15.9) | 11 (26.8) | 14 (21.9) | 1.2 (0.6–2.4) | 0.56 |
| Cardiovascular cause of death | 11 (8.0) | 17 (12.3) | 6 (14.6) | 11 (17.2) | 0.9 (0.3–2.1) | 0.73 |
| Non-cardiovascular cause of death | 15 (10.9) | 5 (3.6) | 2 (4.9) | 3 (4.7) | 1.0 (0.2–6.0) | 1.00 |
| Unknown cause of death | 9 (6.5) | 0 (0.0) | 3 (7.3) | 0 (0.0) | 0.06 | |
| 30-day stroke | 4 (2.9) | 1 (0.7) | 2 (4.9) | 0 (0.0) | 0.15 | |
| 1-year stroke | 5 (3.6) | 2 (1.4) | 2 (4.9) | 0 (0.0) | 0.15 | |
| 2-year stroke | 7 (5.1) | 2 (1.4) | 3 (7.3) | 0 (0.0) | 0.06 | |
| 3-year stroke | 7 (5.1) | 3 (2.2) | 3 (7.3) | 1 (1.6) | 4.7 (0.5–43.5) | 0.30 |
| 4-year stroke | 8 (5.8) | 4 (2.9) | 3 (7.3) | 1 (1.6) | 4.7 (0.5–43.5) | 0.30 |
| Long-term stroke | 9 (6.5) | 6 (4.3) | 3 (7.3) | 3 (4.7) | 1.6 (0.3–7.4) | 0.68 |
| 30-day life-threatening arrhythmia | 12 (8.7) | 16 (11.6) | 5 (12.2) | 11 (17.2) | 0.7 (0.3–1.9) | 0.59 |
| 1-year life-threatening arrhythmia | 13 (9.4) | 16 (11.6) | 5 (12.2) | 11 (17.2) | 0.7 (0.3–1.9) | 0.59 |
| 2-year life-threatening arrhythmia | 14 (10.1) | 16 (11.6) | 5 (12.2) | 11 (17.2) | 0.7 (0.3–1.9) | 0.59 |
| 3-year life-threatening arrhythmia | 14 (10.1) | 16 (11.6) | 5 (12.2) | 11 (17.2) | 0.7 (0.3–1.9) | 0.59 |
| 4-year life-threatening arrhythmia | 14 (10.1) | 17 (12.3) | 5 (12.2) | 11 (17.2) | 0.7 (0.3–1.9) | 0.59 |
| Long-term life-threatening arrhythmia | 14 (10.1) | 17 (12.3) | 5 (12.2) | 11 (17.2) | 0.7 (0.3–1.9) | 0.59 |
| 30-day heart failure | 3 (2.2) | 8 (5.8) | 2 (4.9) | 7 (10.9) | 0.4 (0.1–1.1) | 0.48 |
| 1-year heart failure | 4 (2.9) | 14 (10.1) | 2 (4.9) | 8 (12.5) | 0.4 (0.1–1.7) | 0.31 |
| 2-year heart failure | 5 (3.6) | 15 (10.9) | 2 (4.9) | 8 (12.5) | 0.4 (0.1–1.7) | 0.31 |
| 3-year heart failure | 5 (3.6) | 18 (13.0) | 2 (4.9) | 10 (15.6) | 0.3 (0.1–0.7) | 0.12 |
| 4-year heart failure | 5 (3.6) | 19 (13.8) | 2 (4.9) | 10 (15.6) | 0.3 (0.1–0.7) | 0.12 |
| Long-term heart failure | 5 (3.6) | 19 (13.8) | 2 (4.9) | 10 (15.6) | 0.3 (0.1–0.7) | 0.12 |
| 30-day recurrence | 0 (0.0) | 3 (2.2) | 0 (0.0) | 0 (0.0) | ||
| 1-year recurrence | 1 (0.7) | 7 (5.1) | 1 (2.4) | 2 (3.1) | 0.8 (0.1–8.3) | 1.00 |
| 2-year recurrence | 5 (3.6) | 10 (7.2) | 2 (4.9) | 3 (4.7) | 1.0 (0.2–6.0) | 1.00 |
| 3-year recurrence | 7 (5.1) | 11 (8.0) | 3 (7.3) | 3 (4.7) | 1.6 (0.3–7.4) | 0.68 |
| 4-year recurrence | 7 (5.1) | 15 (10.9) | 3 (7.3) | 5 (7.8) | 0.9 (0.2–3.7) | 1.00 |
| Long-term recurrence | 7 (5.1) | 15 (10.9) | 3 (7.3) | 5 (7.8) | 0.9 (0.2–3.7) | 1.00 |
| 30-day thromboembolic events | 18 (13.0) | 5 (3.6) | 9 (22.0) | 3 (4.7) | 4.2 (1.2–14.8) | 0.02 |
| 1-year thromboembolic events | 18 (13.0) | 6 (4.3) | 9 (22.0) | 3 (4.7) | 4.2 (1.2–14.8) | 0.02 |
| 2-year thromboembolic events | 19 (13.8) | 6 (4.3) | 9 (22.0) | 3 (4.7) | 4.2 (1.2–14.8) | 0.02 |
| 3-year thromboembolic events | 24 (17.4) | 8 (5.8) | 13 (31.7) | 4 (6.3) | 4.7 (1.6–13.5) | <0.01 |
| 4-year thromboembolic events | 26 (18.8) | 10 (7.2) | 14 (34.1) | 5 (7.8) | 4.1 (1.6–10.5) | <0.01 |
| Long-term thromboembolic events | 29 (21.0) | 12 (8.7) | 15 (36.6) | 6 (9.4) | 3.6 (1.5–8.7) | <0.01 |
Notes:
P-values for the comparison between TTS in the presence of ST elevation with compared with STEMI. Data presented as n (%) unless stated otherwise. Statically significant P-value (P<0.05) shown in bold.
Abbreviations: ACS, acute coronary syndrome; STEMI, ST elevation myocardial infarction; TTS, Takotsubo syndrome.
Figure 1.
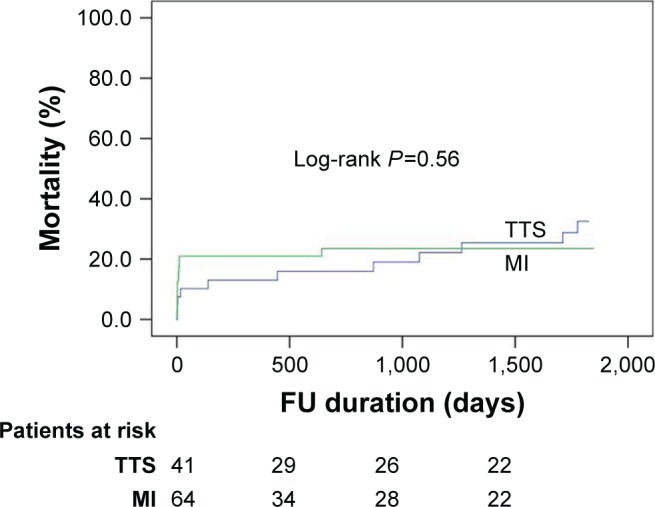
Short- and long-term mortality rate in TTS patients with ST-segment elevation as compared with STEMI patients over mean follow-up of 5 years.
Notes: Expansion of FU in TTS patients: 0–3893. Expansion of FU in MI patients: 0–3934 days.
Abbreviations: FU, follow-up; MI, myocardial infarction; STEMI, ST elevation myocardial infarction; TTS, Takotsubo syndrome.
Table 4.
Causes of death in TTS patients with ST elevation and STEMI patients
| Variables | TTS (n=41) | STEMI (n=64) |
|---|---|---|
|
| ||
| Sudden cardiac death | 2 | 1 |
| Cardiac decompensation | 2 | 1 |
| Cardiogenic shock | 2 | 7 |
| Ventricular fibrillation | 0 | 1 |
| Septic shock | 0 | 1 |
| Anal carcinoma | 1 | 0 |
| COPD | 1 | 0 |
| Lung edema | 0 | 1 |
| Pulmonary embolism | 0 | 1 |
| Myocardial perforation | 0 | 1 |
| Unknown cause of death | 3 | 0 |
Abbreviations: STEMI, ST elevation myocardial infarction; TTS, Takotsubo syndrome.
Over long-term follow-up, there was a significantly higher rate of thromboembolic events in TTS patients as compared with STEMI patients (36.6% vs 9.4%; P<0.01). There were no significant differences in life-threatening arrhythmia, stroke, recurrence, and rehospitalization due to heart failure. In univariate analysis, male sex, ejection fraction (EF) <35%, glomerular filtration rate (GFR) <60 mL/min, cardiogenic shock, inotropic drugs, and history of cancer were predictors of 5-year mortality. ST-segment elevation in TTS patients did not impact the outcome. The univariate analysis is presented in Table 5.
Table 5.
Univariate analysis for the end point in TTS patients with ST-segment elevation
| Univariate analysis
|
|||
|---|---|---|---|
| HR | 95% CI | P-value | |
|
| |||
| Male | 2.2 | 1.0–5.0 | 0.04 |
| Age (years) | 1.0 | 0.9–1.0 | 0.45 |
| EF <35% | 2.1 | 1.1–4.3 | 0.02 |
| COPD | 1.1 | 0.4–2.4 | 0.85 |
| ST-segment elevation | 0.9 | 0.4–1.9 | 0.81 |
| GFR <60 mL/min | 2.4 | 1.2–4.9 | 0.01 |
| Cardiogenic shock | 4.6 | 2.2–9.3 | <0.01 |
| Inotropic drugs | 3.9 | 1.9–7.8 | <0.01 |
| DM type II | 1.0 | 0.4–2.2 | 0.97 |
| Hypertension | 0.7 | 0.3–1.5 | 0.41 |
| Apical ballooning | 1.8 | 0.7–4.3 | 0.18 |
| History of cancer | 2.8 | 1.3–6.4 | <0.01 |
| Smoking | 0.8 | 0.3–1.7 | 0.64 |
Note: Statically significant P-value (P<0.05) shown in bold.
Abbreviations: DM, diabetes mellitus; GFR, glomerular filtration rate; EF, ejection fraction; TTS, Takotsubo syndrome.
Discussion
We performed a retrospective clinical investigation in 138 consecutive TTS patients, of whom 41 showed ST-segment elevations. These patients were compared with an age- and sex-matched group of 64 STEMI patients. We demonstrated that 1) ST elevation in TTS is not uncommon; 2) those patients have similar short-term prognosis as compared with STEMI patients, and however, with a higher complication rate 3) the long-term prognosis was similar in TTS patients with ST-segment elevation as compared with STEMI patients.
Similar to other studies, the incidence of ST elevation in our TTS patient population is about one-third (29.9%).15 Several studies showed that STEMI patients are suffering from a higher mortality rate as compared with NSTEMI patients. In the present study, we thought to study the impact of ST-segment elevation in TTS patients as compared with STEMI patients. To date, TTS has been described as a benign disease with favorable prognosis. Recently, this has been disputed.11,14,16–18
Inhospital mortality rate among TTS patients ranges between 1% and 8%.5,19,20 In our study, the inhospital mortality rate was 7.3% in TTS patients with ST elevation and 20.3% in STEMI patients. This did not differ significantly between both groups. The long-term mortality rate was similar in TTS as compared with STEMI patients. However, clinical complications such as thromboembolic events were more frequent in TTS patients during follow-up.
Studies indicated that thromboembolism is a common complication, especially in the acute phase of TTS.21,22 Furthermore, it has been reported that a thromboembolic event, such as pulmonary artery embolism, is associated with ST-segment elevation. However, there are also studies estimating the prevalence of LV thrombi in TTS patients between 2% and 8%. The gold standard in diagnosing LV thrombi is the two-dimensional echocardiogram and transesophageal echocardiogram. However, there are certain limitations, as the number of echocardiograms performed per patient, time point, operator skills, and the use of contrast agents may all influence the sensitivity and specificity of this tool in thrombus detection. Although most thrombi occur in the LV apex, the area adjacent to the papillary muscle should be carefully examined. However, other sites may also be affected for developing a thrombus.7,23 In rare cases, right ventricular involvement with concomitant thrombus formation might also be seen. Another difficulty is the differentiation of thrombus formation from a tumor, with also the possibility of overlooking smaller thrombi in echocardiography.24–26 According to some study groups, patients presenting with symptoms of stroke with a supposed cardiac embolic origin should receive cardiac magnetic resonance (CMR) in addition to computer tomography (CT) to rule out an intraventricular thrombus.27,28 If patients have been mechanically ventilated, the presenting signs and symptoms of an LV thrombus could be masked, which leads to underdiagnosing. Therefore, a high degree of clinical skill and interpretation is necessary in this scenario.
In our patient population, the rate of inhospital thromboembolic events (22.0% vs 4.7%) as well as the incidence of thromboembolic events during follow-up (36.6% vs 9.4%) was significantly higher in TTS patients compared with STEMI patients.
One possible explication may be the low blood flow in the ventricle during the initial phase of TTS, which promotes the development of ventricular thrombus.22 Another study revealed that TTS patients who experience thromboembolic events have higher C-reactive protein (CRP) levels. This indicates a potential pathogenic role of inflammation in the development of thromboembolic events.29
Due to the misapprehension that TTS is thought to be benign, there is a lack of close follow-up and treatment. This might result in underdiagnosing of several clinical complications. For example, it has been shown that there is no protective effect of a treatment with ß-antagonists in TTS patients.4
There is evidence that ACE inhibitors and in some cases oral anticoagulation are associated with a better clinical outcome as compared with conservative wait-and-watch management.4,30–33 Especially, high-risk TTS patients might benefit from anticoagulation therapy to prevent thromboembolic events.
There seems to be an association between male sex and a poor outcome. Emotional stress, depressive symptoms, and estrogen decrease may explain the sex differences, but are not clearly identified yet.34 Some studies point out an association between TTS and the occurrence of malignancies, which leads to a poorer outcome.35 In our study, univariate analysis showed the history of cancer as a predictor of 5-year mortality.
Limitations
Our study has several limitations, one being it was a single-center, retrospective observational study including patients admitted over the period of 15 years. ST elevation was diagnosed based on ECG findings. Therefore, the prevalence of ST elevations may have been underestimated. In addition, we did not routinely perform magnetic resonance imaging. The impact of remnant edema and inflammation, on long-term prognosis, therefore remains undefined.
Conclusion
ST elevation in TTS patients is not uncommon. The mortality rates in this patient population were similar as compared with those suffering from STEMI. Clinical complications such as thromboembolic events were more frequent in TTS patients with ST-segment elevation as compared with STEMI patients. Therefore, short-term as well as long-term follow-up is required in TTS patients as compared with ACS patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21(2):203–214. [PubMed] [Google Scholar]
- 2.Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome: Correlate of myocardial injury. Am Heart J. 2006;152(3):469.e9–46469. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Haghi D, Papavassiliu T, Fluchter S, Kaden JJ, Porner T, Borggrefe M. Variant form of the acute apical ballooning syndrome (takotsubo cardio-myopathy): observations on a novel entity. Heart. 2006;92(3):392–394. doi: 10.1136/hrt.2005.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchihashi K, Ueshima K, Uchida T, Oh-Mura N, Kimura K, Metal O. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina pectoris-myocardial infarction investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher J, Wilcox I. Basal stress cardiomyopathy induced by exogenous catecholamines in younger adults. Int J Cardiol. 2013;168(6):e158–e160. doi: 10.1016/j.ijcard.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 7.Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in takotsubo cardiomyopathy assessed with echocardiography. QJM. 2008;101(5):381–386. doi: 10.1093/qjmed/hcn017. [DOI] [PubMed] [Google Scholar]
- 8.Schneider B, Athanasiadis A, Schwab J, et al. Complications in the clinical course of takotsubo cardiomyopathy. Int J Cardiol. 2014;176(1):199–205. doi: 10.1016/j.ijcard.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 9.El-Battrawy I, Lang S, Ansari U, et al. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. EP Europace. 2017;20(5):843–850. doi: 10.1093/europace/eux073. [DOI] [PubMed] [Google Scholar]
- 10.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306(3):277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 11.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 12.Stiermaier T, Moeller C, Oehler K, et al. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18(6):650–656. doi: 10.1002/ejhf.494. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Pearson M, Sterry H, et al. The entry ECG in the early diagnosis and prognostic stratification of patients with suspected acute myocardial infarction. Eur Heart J. 1984;5(9):690–696. doi: 10.1093/oxfordjournals.eurheartj.a061728. [DOI] [PubMed] [Google Scholar]
- 14.Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Takotsubo cardiomyopathy and long-term prognosis. Herz. 2010;35(4):240–244. doi: 10.1007/s00059-010-3339-x. [DOI] [PubMed] [Google Scholar]
- 15.Dib C, Asirvatham S, Elesber A, Rihal C, Friedman P, Prasad A. Clinical correlates and prognostic significance of electrocardiographic abnormalities in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy) Am Heart J. 2009;157(5):933–938. doi: 10.1016/j.ahj.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro VF, Vasconcelos M, Melão F, Ferreira E, Malangatana G, Maciel MJ, Short MMJ. Short and long-term outcome of stress-induced cardiomyopathy: what can we expect? Arq Bras Cardiol. 2014;102(1):80–85. doi: 10.5935/abc.20130228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redfors B, Vedad R, Angerås O, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction – A report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 18.Wagdy K, Elmaghawry M. Takotsubo cardiomyopathy: A potentially serious trap (Data from the International Takotsubo Cardiomyopathy Registry) Glob Cardiol Sci Pract. 2015;2015(4):55. doi: 10.5339/gcsp.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vriz O, Brosolo G, Martina S, et al. In-hospital and long-term mortality in Takotsubo cardiomyopathy: a community hospital experience. J Community Hosp Intern Med Perspect. 2016;6(3):31082. doi: 10.3402/jchimp.v6.31082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;10(4):311–316. doi: 10.1007/s10741-005-8555-8. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuma W, Kodama M, Ito M, et al. Thromboembolism in Takotsubo cardiomyopathy. Int J Cardiol. 2010;139(1):98–100. doi: 10.1016/j.ijcard.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 22.El-Battrawy I, Behnes M, Hillenbrand D, et al. Prevalence, clinical characteristics, and predictors of patients with thromboembolic events in takotsubo cardiomyopathy. Clinical Medicine Insights: Cardiology. 2016;10:117–122. doi: 10.4137/CMC.S38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol. 2008;131(1):18–24. doi: 10.1016/j.ijcard.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi K, Nakamura F, Ikutomi M, et al. Usefulness of contrast computed tomography to detect left ventricular apical thrombus associated with takotsubo cardiomyopathy. Heart Vessels. 2016;31(5):822–827. doi: 10.1007/s00380-015-0637-5. [DOI] [PubMed] [Google Scholar]
- 25.Rinuncini M, Zuin M, Scaranello F, et al. Differentiation of cardiac thrombus from cardiac tumor combining cardiac MRI and 18F-FDG-PET/CT Imaging. Int J Cardiol. 2016;212:94–96. doi: 10.1016/j.ijcard.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 26.Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging. 2011;4(7):702–712. doi: 10.1016/j.jcmg.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabowski A, Kilian J, Strank C, Cieslinski G, Meyding-Lamadé U. Takotsubo cardiomyopathy – a rare cause of cardioembolic stroke. Cerebrovasc Dis. 2007;24(1):146–148. doi: 10.1159/000103620. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzono K, Ikeda Y, Deguchi S, et al. Cerebral embolic stroke after disappearing takotsubo cardiomyopathy. J Stroke Cerebrovasc Dis. 2013;22(8):e682–e683. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Bakalli A, Georgievska-Ismail L, Koçinaj D, Musliu N, Krasniqi A, Pllana E. Prevalence of left chamber cardiac thrombi in patients with dilated left ventricle at sinus rhythm: the role of transesophageal echocardiography. J Clin Ultrasound. 2013;41(1):38–45. doi: 10.1002/jcu.21953. [DOI] [PubMed] [Google Scholar]
- 30.Abanador-Kamper N, Kamper L, Wolfertz J, Pomjanski W, Wolf-Pütz A, Seyfarth M. Evaluation of therapy management and outcome in Takotsubo syndrome. BMC Cardiovascular Disorders. 2017;17(1):225. doi: 10.1186/s12872-017-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the heart failure association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(1):8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 32.de Gregorio C. Cardioembolic outcomes in stress-related cardiomyopathy complicated by ventricular thrombus: a systematic review of 26 clinical studies. Int J Cardiol. 2010;141(1):11–17. doi: 10.1016/j.ijcard.2009.09.468. [DOI] [PubMed] [Google Scholar]
- 33.Mejía-Rentería HD, Núñez-Gil IJ. Takotsubo syndrome: Advances in the understanding and management of an enigmatic stress cardiomyopathy. World J Cardiol. 2016;8(7):413–424. doi: 10.4330/wjc.v8.i7.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111(4):472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 35.Sattler K, El-Battrawy I, Lang S, et al. Prevalence of cancer in Takotsubo cardiomyopathy: Short and long-term outcome. Int J Cardiol. 2017;238:159–165. doi: 10.1016/j.ijcard.2017.02.093. [DOI] [PubMed] [Google Scholar]


