Abstract
Cutaneous T-cell lymphoma (CTCL) poses unique treatment challenges, given its range of presentations and numerous systemic therapy options. These options often lack comparative evidence or are characterized by low response rates and short remission duration in relapsed/refractory disease. The approval of mogamulizumab, a humanized, glycoengineered IgG1κ monoclonal antibody targeting the chemokine receptor type 4 (CCR4) chemokine receptor, brings a novel tool into the spectrum of treatment options for advanced CTCL and adult T-cell leukemia/lymphoma (ATLL). CCR4 is expressed in almost all cases of ATLL, and in a majority of CTCLs, particularly when blood involvement is present. In a Phase III randomized trial, mogamulizumab was associated with 28% overall response rate among patients with relapsed CTCL, median progression-free survival of 7.7 months, and median duration of remission of 14.1 months. Responses are more frequent among patients with Sézary syndrome and within the blood compartment. Common adverse effects include rash and infusion reactions, which are usually low grade. Sentinel reports indicate that exposure to mogamulizumab may result in severe or refractory graft vs host disease after allogeneic bone marrow transplantation, highlighting the need for vigilance and expert management. Further research may establish incremental efficacy of combining mogamulizumab with cytotoxic or immunomodulatory agents in CTCL, ATLL, and possibly other lymphomas and even solid tumors.
Keywords: cutaneous T-cell lymphoma, mogamulizumab, CCR4, adult T-cell leukemia, lymphoma, Sezary syndrome, mycosis fungoides
Introduction
The conventional approach to treatment of T-cell lymphomas is often to use regimens active in B-cell lymphomas, but this approach has suffered from lack of an effective T-cell-directed monoclonal antibody to use in place of rituximab. The recent Food and Drug Administration (FDA) approval of mogamulizumab in adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) after at least one prior systemic therapy provides a new clinical tool that may fill in this historical gap. Cutaneous T-cell lymphoma (CTCL) is a rare non-Hodgkin lymphoma of skin-homing T-cells with a wide range of presentation and prognosis. In the USA, it has an overall incidence of 7.5 per million with the two most prevalent forms, MF and SS, accounting for two thirds of cases.1,2 Less common types of CTCL include primary cutaneous CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma [PCALCL]), primary cutaneous gamma/delta T-cell lymphoma, CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma, acral CD8-positive T-cell lymphoma, and CD4+ small/medium T-cell lymphoproliferative disorder.3 Furthermore, skin involvement may occur in other T-cell lymphomas, including subcutaneous panniculitis-like T-cell lymphoma, extranodal natural killer (NK)/T-cell lymphoma, nasal type, or peripheral T-cell lymphoma (PTCL), not otherwise specified.4 Conventional thought had been that MF and SS represent different phases of disease progression, although molecular analysis suggests that the two forms of CTCL may in fact be distinct diseases.5 MF typically presents with plaques and patches, and despite low associated mortality, the lesions can cause significant pruritus affecting the quality of life. They may also cause pain and disfigurement.6 Making the diagnosis of early-stage MF can be difficult as it resembles many benign dermatologic conditions.7,8 A recent large international study found a median time to diagnosis from first symptom of 36 months.9 Advanced MF involves lymph nodes, blood, and organs outside the skin. In contrast, SS presents with diffuse erythroderma and blood involvement, with or without lymphadenopathy, and is characterized by a more aggressive course with 5-year overall survival (OS) of only about 26%.4,10,11 In advanced MF or SS, treatment is multidisciplinary and overall palliative. Relapses usually occur upon cessation of therapy, necessitating chronic management. Outside of allogeneic bone marrow transplantation, neither form of CTCL is curable.5
The majority of patients with CTCL are diagnosed as early-stage MF (stage IA–II according to the tumor-node-metastasis-blood (TNBM) classification), and observation or local therapy alone are reasonable first steps.12 Local therapy includes topical steroids, topical chemotherapy (mechlorethamine), immunomodulators (imiquimod), radiation, or phototherapy, usually at the direction of a dermatologist.10 The 5-year disease-specific survival in early-stage MF approaches 90% compared with 30%–50% for advanced disease (stage IIB–IVB).12–14 In fact, in a large single-center series of patients with CTCL, only 11.6% progressed to a higher stage, and for T1 disease, median OS and disease-specific survival were not reached.15 In SS, patients with <1,000 Sézary cells/μL have median OS of 7.6 years, yet with ≥10,000 cells/μL, median survival plummets to 2.1 years.16 MF/SS patients often proceed through many lines of systemic treatment through their lifetime. Standard therapies include extracorporeal photopheresis, retinoids (bexarotene), histone deacetylase (HDAC) inhibitors (vorinostat and romidepsin), interferon α, methotrexate, pralatrexate, alemtuzumab, brentuximab vedotin, and now, mogamulizumab (Table 1).4,10,17–29 All have relatively low response rates ranging from 14% to 60% (mostly 20%–30%) and median duration of response rarely exceeding 1 year.10,30
Table 1.
Comparison of mogamulizumab with other systemic treatment options for CTCL
| Drugs | Study/reference | Disease | N | Phase | Median prior lines | ORR (%) | PFS (months) | DOR (months) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mogamulizumab | Ogura et al (2014)50 | PTCL, CTCL | 37 | 2 | 2 | 38a | 3.0 | NR |
| Mogamulizumab | Duvic et al (2015)55 | CTCL | 42 | 1/2 | 3 | 37 | 11.4 | 10.4 |
| Mogamulizumab | Kim et al (2018)36 | CTCL | 186 | 3 | 3 | 28 | 7.7 | 14.1 |
| Alemtuzumab | Lundin et al (2003)31 | CTCL | 22 | 2 | 3 | 55 | NR | 12.0 |
| Belinostat | Foss et al (2015)19 | PTCL, CTCL | 53 | 2 | 4 | 14a | 1.4a,b | 2.7a |
| Bendamustine | Damaj et al (2013)20 | PTCL, CTCL | 60 | 2 | 1 | 50 | 3.6 | 3.5 |
| Bexarotene | Duvic et al (2001)21 | CTCL | 94 | 2 | 5 | 45, 55c | NR | 9.8, 12.6c |
| Bexarotene (59%) or methotrexate (41%)b | Prince et al (2017)23 | CD30+ CTCL | 64 | 3 | 4 | 13 | 3.5 | 18.3 |
| Brentuximab vedotin | Prince et al (2017)23 | CD30+ CTCL | 66 | 3 | 4 | 56 | 16.7 | 15.1 |
| Denileukin diftitox | Prince et al (2010)22 | CD25+ CTCL | 144 | 3 | 2 | 44 | 26.0 | 7.8 |
| Lenalidomide | Querfeld et al (2014)27 | CTCL | 32 | 2 | 6 | 28 | 8.0 | 10.0 |
| Pembrolizumab | Khodadoust et al (2016)26 | CTCL | 24 | 2 | 4 | 38 | NR | NR |
| Pralatrexate | Horwitz et al (2012)25 | CTCL | 54 | 1/2 | 4 | 41 | 12.7 | Not reached |
| Pralatrexate and bexarotene | Duvic et al (2017)24 | CTCL | 34 | 1/2 | 3.5 | 60 | 12.8 | >29 |
| Romidepsin | Whittaker et al (2010)29 | CTCL | 96 | 2 | 4 | 34 | 8.0d | 15.0 |
| Vorinostat | Olsen et al (2007)28 | CTCL | 74 | 2c | 3 | 30 | 1.8d | >6.1 |
| Vorinostat | Kim et al (2018)36 | CTCL | 186 | 3 | 3 | 5 | 3.1 | 9.1 |
Note:
CTCL patients only,
control arm (physician’s choice) of the ALCANZA trial,
bexarotene dose 300 mg/m2/d and >300 mg/m2/d, respectively,
time to progression reported rather than PFS.
Abbreviations: CTCL, cutaneous T-cell lymphoma; DOR, duration of remission; NR, not reported; ORR, overall response rate; PFS, progression-free survival; PTCL, peripheral T-cell lymphoma.
Given that monoclonal antibodies have revolutionized treatment for B-cell lymphoma, with survival outcomes often markedly differing between the eras before and after introduction of rituximab, the elusive goal in T-cell lymphoma has been to develop an equally safe and effective targeted antibody. Alemtuzumab (a monoclonal antibody against CD52) has shown efficacy, but with unacceptably high risk of infectious complications due to profound T- and B-cell depletion.31 While a naked anti-CD30 monoclonal antibody SGN-30 had only modest activity,32 brentuximab vedotin, an anti-CD30 antibody-drug conjugate, has demonstrated high efficacy in CD30+ CTCL and has been approved by FDA for PCALCL and for CD30-expressing MF.23,33 However, there is an ongoing need to identify other monoclonal antibodies that could selectively target T-cell-specific antigens with clinical efficacy and acceptable toxicity and that could be potentially combined with cytotoxic agents.34,35 Recently, mogamulizumab (Poteligeo®, Kyowa Kirin, Tokyo, Japan), a fully humanized, defucosylated IgG1 antibody against the C–C chemokine receptor type 4 (CCR4), has been granted FDA approval in CTCL based on improved outcomes demonstrated in the Phase III MAVORIC study (Figure 1).36
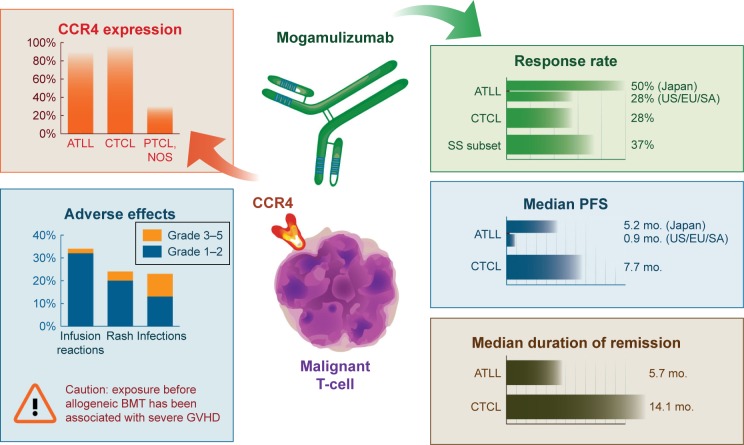
Figure 1.
A synthesis of clinical data on mogamulizumab in ATLL and CTCL.
Abbreviations: BMT, bone marrow transplantation; CCR4, chemokine receptor type 4; GVHD, graft-vs-host disease; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma; SS, Sézary syndrome; US/EU/SA, United States, Europe, and South America; ATLL, adult T-cell leukemia/lymphoma; CTCL, cutaneous T-cell lymphoma; Mo., months.
CCR4 expression and genomic profiling in T-cell lymphomas
CCR4 is a transmembrane chemokine receptor, which plays an important role in T-cell’s ability for homing and migration to the skin.37,38 While it has a particularly high expression on malignant cells, CCR4 is normally expressed on Treg cells and serves as the dominant chemokine receptor on Th2 and cutaneous lymphocyte antigen-expressing skin-homing T-cells.37,39 CCR4 is a receptor for the C–C chemokine ligand 17 (CCL17, originally termed thymus- and activation-regulated chemokine) and for CCL22, also known as macrophage-derived chemokine.37,40,41 It is one of the 18 known human chemokine receptors, whose overall job is to coordinate cell migration.37
CCR4 expression by lymphoma cells varies by T-cell subtype and between studies, which use different qualitative and quantitative approaches to determine positive status, as well as various methods of assessment: immunohistochemistry of the skin biopsies (with cutoffs of “any,” 5%, or 10% positive cells), flow cytometry analysis of the blood, or reverse transcription polymerase chain reaction (Table 2).42–51 In adult T-cell leukemia/lymphoma (ATLL), CCR4 expression is nearly universal, in contrast to CTCL and PTCL, where expression varies greatly but appears to correlate overall with advanced or relapsed/refractory disease, particularly with blood involvement.49,52–54 Some T-cell lymphomas are Th1-polarized with high CXCR3 expression and no or minimal CCR4 expression; these include many cases of PTCL and angioimmunoblastic T-cell lymphoma.49 Others, like the ALK-positive anaplastic large cell lymphoma and about a third of PTCLs, express CCR4. While immunohistochemical expression in the skin of CTCL patients ranges from 14% to 97%, the proportion of positive cases is significantly larger (90%–100%) in clinical trials enrolling patients with relapsed disease.36,50,55 CCR4 is detectable using flow cytometry in almost all cases of CTCL involving the blood, with percentage of positive cells that vary from 31% to 97%, significantly higher than among healthy individuals (27%, on average).39,48 Furthermore, CCR4 expression is higher on circulating CD4+ CD26− lymphocytes in SS (59%) compared with inflammatory erythroderma (11%) or healthy controls (4%), although the difference is not evident in erythrodermic skin biopsies.56 In the pivotal MAVORIC trial, out of 290 studied samples of relapsed/refractory MF/SS, 280 (97%) demonstrated CCR4 expression, with median percentage of positive cells of 80% (range 1%–100%), indicating that CCR4 expression in CTCL may actually be on par with ATLL.36 Of note, the percentage of CCR4-positive cells did not correlate with response to mogamulizumab in any of the clinical trials conducted to date.36,50,55
Table 2.
CCR4 expression in subtypes of non-Hodgkin lymphoma
| Histology | Study/reference | CCR4+ (%) | CCR4+ (N/N total) | Method |
|---|---|---|---|---|
|
| ||||
| MF | Jones et al (2000)49 | 14 | 1/7 | IHC |
| MF | Kallinich et al (2003)42 | 92 | 11/12 | IHC |
| MF | Yagi et al (2006)43 | 54 | 14/26 | IHC |
| Transformed MF | Jones et al (2000)49 | 100 | 5/5 | IHC |
| Transformed MF | Ishida et al (2004)44 | 41 | 7/17 | IHC |
| MF and SS | Ferenczi et al (2002)48 | 100 | 11/11 | FC |
| MF and SS | Sugaya et al (2015)45 | 57 | 13/23 | IHC |
| MF and SS | Duvic et al (2012, 2015)46,55 | 89 | 31/35 | FC and IHC |
| MF and SS | Kim et al (2018)36 | 97 | 280/290 | IHC |
| SS | Narducci et al (2006)39 | 100 | 12/12 | FC |
| SS | Yagi et al (2006)43 | 100 | 5/5 | IHC |
| MF, SS, or PTCL | Ogura et al (2014)50 | 78 | 50/64 | IHC |
| ATLL | Yoshie et al (2002)52 | 92 | 22/24 | RT-PCR |
| ATLL | Ishida et al (2004)53 | 88 | 91/103 | FC |
| ATLL | Phillips et al (2016)51 | 91 | 65/71 | FC and IHC |
| ALCL | Jones et al (2000)49 | 73 | 8/11 | IHC |
| ALCL | Yagi et al (2006)43 | 100 | 5/5 | IHC |
| PTCL, NOS | Ishida et al (2004)44 | 38 | 19/50 | IHC |
| PTCL, NOS | Jones et al (2000)49 | 27 | 4/15 | IHC |
| AITL | Ishida et al (2004)44 | 35 | 8/23 | IHC |
| DLBCL | Nakayama et al (2013)47 | 13 | 10/80 | IHC |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; CCR4, chemokine receptor type 4; DLBCL, diffuse large B-cell lymphoma; FC, flow cytometry; IHC, immunohistochemistry; MF, mycosis fungoides; NOS, not otherwise specified; PTCL, peripheral T-cell lymphoma; SS, Sézary syndrome.
Genomic profiling studies have further evaluated the role of CCR4 gene alterations in T-cell lymphomas. Activating (gain of function) CCR4 mutations were first identified in 26%–33% of ATLL cases,57–59 and then in 7% of patients with SS.60 Mutations in CCR4, along with TP53, have been described as potential cancer driver mutations in SS.60 CCR4 upregulation can also be used along with other identified genes in making a diagnosis of CTCL over similar appearing dermatoses, and it may be prognostic for progression and survival along with other genes.61–63 At least in ATLL, a small series suggests that CCR4 gain of function mutations are predictive of a better response to mogamulizumab without a difference in response to other treatments.59
Emerging evidence suggests also that CCR4 expression may be regulated by class I HDAC, specifically HDAC2.64 In an elegant study, Kitadate et al assessed CCR4 expression before and after vorinostat therapy and found expression that ranged from 5% to 95% dropped to 5% to 20%.64 The authors suggested that their findings may influence the order of treatments, as therapy with vorinostat might lower the amount of the target molecule for mogamulizumab. So far, this effect has not been observed in clinical experience, as the responses to mogamulizumab in CTCL were similar among patients crossing over from vorinostat (30%) compared with experimental arm (28%).65
In addition to mogamulizumab, CCR4 may serve as a target for other therapeutic modalities. Earlier attempts at targeting CCR4 were through “chemotoxins,” which fused CCL17 (CCR4 ligand) with neurotoxins or truncated Pseudomonas exotoxin released into the cytosol upon binding.66 More recently, CCR4 has been trialed in vivo as a target for chimeric antigen receptor T-cells.67
Development of mogamulizumab and its role in ATLL
First approved in Japan for ATLL in 2012, mogamulizumab (KW-0761) is a defucosylated humanized IgG1κ monoclonal antibody.37 Its approval in Japan was expanded to PTCL and CTCL in 2014, and it gained FDA approval for MF and SS in 2018. Mogamulizumab, like its chimeric predecessor KM2760, binds to the N-terminal domain of CCR4 causing antibody-dependent cellular cytotoxicity (ADCC) rather than complement-mediated killing or direct cytotoxicity.68,69 ADCC depends on effector immune cells including macrophages, monocytes, and especially NK cells.70 Mogamulizumab binds to NK cell Fcγ receptor IIIa.71 Enhanced ADCC by monoclonal antibodies has been achieved by modifying the oligosaccharides in human IgG, particularly fucose.71–73 Defucosylation also allows for improved efficacy with drastically smaller doses of the drug compared with other antibodies.74
In vitro and murine studies have demonstrated the efficacy of KM2760 in models of ATLL and CTCL.69,71 In vivo, KM2760 caused ADCC (executed by peripheral blood mononuclear cells from healthy donors) on both established CTCL lines and tumor cells from patients with aggressive MF and SS.69 In a murine model, mice inoculated with a human CTCL cell line quickly developed large tumors and died within 3 months, while those treated with KM2760 lived longer without any obvious toxicity from the drug. These findings led to the development of a glycoengineered, fully defucosylated antibody KW-0761 (mogamulizumab), in a process similar to the one used to generate obinutuzumab.75,76
Mogamulizumab was first studied in a Phase I clinical trial (NCT00355472) enrolling 16 patients with ATLL (N=13), PTCL-NOS (N=2), and MF (N=1), which established the recommended dose of 1 mg/kg weekly for 4 weeks.75 No dose-limiting toxicities were observed in the dose escalation phase, and only one patient experienced a dose-limiting toxicity (grade 3 rash and febrile neutropenia) in the expansion cohort. Frequent (44%), although manageable infusion reactions, as well as rare reactivations of viral hepatitis and varicella-zoster virus infection were observed. In a subsequent multicenter Phase II trial (NCT00920790) in 27 subjects with relapsed ATLL, mogamulizumab showed 50% overall response rate with median progression-free survival (PFS) of 5.2 months and OS of 13.7 months.77 In that experience, infusion reactions were common (89%), but almost entirely grade ≤2. Rash was an additional frequent adverse effect observed in 63% of patients (19% grade 3). In a further follow-up of these Phase I and II trials, long-term survivors were observed, which was very encouraging for a disease associated with poor prognosis like the ATLL.78 In NCT00355472, four patients have survived over 3 years, and in NCT00920790, six (26%) had PFS exceeding a year, with estimated OS at 3 years of 23%.78
The efficacy of mogamulizumab in relapsed/refractory ATLL was confirmed in an international randomized trial (NCT01626664), which enrolled 71 subjects receiving (in a 2:1 ratio) the monoclonal antibody or investigator choice.51 Overall response rate favored mogamulizumab (28% vs 8% in the control arm), with a median duration of response of 5.7 months, 47% rate of infusion reactions, 43% rate of rash, and 51% rate of infections. No significant differences in PFS (0.9 months in both arms) or OS (4.9 vs 6.9 months, including crossover of 75% of control arm to mogamulizumab) were seen between the study arms, although the trial was not powered to analyze these endpoints.79 The marked difference in PFS observed in this trial and in the prior Phase II trial may reflect a different mix of ATLL subtypes (acute, lymphomatous, and chronic) but may also suggest a different clinical profile of ATLL patients enrolled in USA, Europe, and Latin America compared with Japan.
Clinical experience with mogamulizumab in CTCL
Given the efficacy of mogamulizumab in ATLL and high CCR4 expression in MF and SS, mogamulizumab was naturally well-suited for a trial in CTCL. Assessment of drug efficacy in CTCL demonstrates unique challenges. While OS and PFS are valid endpoints, they fail to capture the benefit of symptom management, particularly pruritus, pain, and erythroderma, which have significant impact on patients’ quality of life. A standardized response assessment for use in clinical trials was proposed in 2011.80 Skin burden is assessed using the Severity Weighted Assessment Tool in original or modified form, SWAT or mSWAT, respectively, which uses body surface area and type of lesion (patch, plaque, or tumor), to calculate a score.28,80,81 Complete response (CR) in the skin is defined as clearance of disease in all areas, partial response as >50% regression, and stable disease as between 50% decrease and 25% increase according to mSWAT assessment.80 A Global Response Score, which incorporates responses within every disease compartment: skin, nodes, viscera, and blood, has been adopted by many studies in CTCL to report the overall response rate.
The first published Phase II study of mogamulizumab in CTCL was a multicenter study in Japan (NCT01192984), in which 38 patients with CTCL and PTCL, selected based on CCR4-positive status (after screening 65 candidates), received mogamulizumab 1 mg/kg weekly for eight treatments only.50 Of the 37 treated subjects, 13 (35%) responded and five (14%) achieved a CR according to International Working Group criteria suitable more for PTCL. Median PFS was relatively brief at 3.0 months (95% CI, 1.6–4.9). In the subset of patients with CTCL, the response rate was 50% (4 out of 8) using the standardized CTCL Global Response Score. The authors also noted a pronounced and prolonged decrease in Treg cells. Like in other studies, percentage of CCR4-positive cells had no correlation with response. Adverse events included lymphopenia (81%, 73% grade 3/4), neutropenia (38%, 19% grade 3/4), infusion reactions (24%, no grade 3/4 events), and skin disorders (51%, 11% grade 3/4). Additionally, one patient developed grade 3 polymyositis, two cytomegalovirus retinitis, and one second primary malignancy. The response rate in CTCL was considered encouraging, particularly considering selection by CCR4 expression, which may correlate with more advanced disease.
In another Phase I/II study (NCT00888927), Duvic et al also demonstrated promising safety and efficacy for mogamulizumab in CTCL, including 22 subjects with MF and 19 with SS.55 Median age was 66 years (35–85 years), median number of prior systemic therapies was 3, and 63% of patients had stage IV disease. Similar to the ATLL experience, the researchers observed no dose-limiting toxicities, and in the Phase II they used 1.0 mg/kg weekly for 4 weeks, followed by every-2-week dosing until progression. The reported global response rate was 37% with more responders in the SS subgroup (47%) than in MF (29%). Skin-based responses occurred in 42% of patients, whereas responses in lymph nodes occurred in 25%. Furthermore, among patients with blood involvement by the lymphoma, the response rate in the blood was 95% (18 out of 19), with 58% (11) achieving a CR in that compartment. Three patients had a global CR. Median PFS was 11.4 months, impressive for relapsed CTCL, and median duration of response was 10.4 months. Most adverse events were grade 1/2, including nausea (31%), infusion reactions (21%), chills (24%), headache (21%), fever (19%), fatigue (17%), and rash (17%). There were no grade 4 events in the study, but the overall rate of serious adverse effects was 24%. Lymphopenia occurred in 41% and was considered an expected on-target effect.
Just over 10 years since the early in vivo studies of mogamulizumab in CTCL, the drug has received FDA approval for the treatment of MF or SS relapsing after ≥1 line of therapy. The approval followed a release of results of the largest randomized trial performed in CTCL, the Phase III international MAVORIC trial (NCT01728805).36 In MAVORIC, 372 patients were randomized to either mogamulizumab (four weekly doses at 1.0 mg/kg, followed by every-2-week dosing until progression) or vorinostat 400 mg daily – an FDA-approved oral HDAC inhibitor. Patient characteristics were well balanced between groups, with stage of disease ranging from IB to IVB, and slightly more MF (55%) than SS. All patients had received ≥1 prior line of systemic therapy (median 3) and had performance status of 1 or less on the Eastern Cooperative Oncology Group scale. The primary endpoint was PFS. As neither patients nor clinicians were blinded to treatment, the investigators used a blinded independent review to assess response and progression (including mSWAT evaluations and radiology scans), which were determined according to the global composite response score. Crossover was allowed from vorinostat to mogamulizumab and 136 of 186 subjects in the vorinostat arm crossed over (109 for progression, 27 for toxicity). The overall response rate for mogamulizumab was 28% compared with 5% for vorinostat, which in earlier Phase II study had a response rate of 30%.28 Stage IV MF or SS patients had again a higher response rate with mogamulizumab at 36% and 37%, respectively. However, only five patients achieved a global CR. PFS was superior in the mogamulizumab arm with median of 7.7 months (95% CI, 5.7–10.3) vs 3.1 months (95% CI, 2.9–4.1) for vorinostat (hazard ratio, 0.53; 95% CI, 0.41–0.69), sustained on independent review.36 Considering crossover, no significant difference in OS was observed (not reached for mogamulizumab vs 43.9 months for vorinostat, P=0.94). The PFS advantage for mogamulizumab persisted in all predefined subsets with the exception of stage I/IIB disease, where there was no difference (hazard ratio, 0.88; 95% CI, 0.58–1.35). Median time to response with mogamulizumab was 3.3 months, and median duration of response was 14.1 months. Responses were higher in the blood compartment (68%) than in the skin (42%), lymph nodes (17%), or viscera (0%). Among patients from the control arm who crossed over to receive mogamulizumab upon progression, response rate was 31% and median PFS was 8.9 months. Uniquely, MAVORIC researchers have also shown improvement in some aspects of quality of life, including skin pain and fatigue, among patients treated with mogamulizumab.82
In the Phase III trial, as in prior experience, infusion-related reactions (35%) and rash (24%) were the most common adverse events, together with diarrhea (24%) and fatigue (24%). Most adverse events were grade 2 or lower, with rare (1%–2%) rates of higher grade constipation, nausea, diarrhea, fatigue, fever, cellulitis, pneumonia, sepsis, infusion reaction, hepatitis, weight loss, anorexia, hypertension, and rash. The rate of grade 3/4 events was 41%, equal in both arms, and rates of serious adverse events were 20% for mogamulizumab and 16% for vorinostat. Nineteen percent of patients discontinued mogamulizumab because of toxicity. Two treatment-related deaths occurred in the mogamulizumab arm: one related to sepsis and one related to polymyositis.
While mogamulizumab has been consistently relatively safe in clinical trials, serious rare adverse events have been noted in clinical experience. One rare, yet potentially fatal toxicity is Steven–Johnson syndrome or toxic epidermal necrolysis so far reported in less than ten cases, all among patients with ATLL.83 Another important risk results from depletion of CCR4-expressing nonmalignant Treg lymphocytes in patients who subsequently undergo allogeneic bone marrow transplantation, putting them at increased risk of graft-vs-host disease (GVHD).84–88 ATLL patients exposed to mogamulizumab have 1.8 times increased risk of grade 3/4 acute GVHD and 2.1 times increased risk of steroid-refractory GVHD, resulting in 44% nonrelapse mortality at 1 year.85 In a preliminary report, one of eight CTCL patients undergoing allogeneic bone marrow transplantation developed a severe acute GVHD.89 For any patient treated with mogamulizumab, transplantation should be delayed for at least 50 days from the last dose, or longer, and Treg counts may be assessed prior to transplant.84,85,87
Conclusion
Mogamulizumab is a useful novel tool in the management of CTCL, and considering a favorable risk/benefit ratio, it is likely to be widely used for patients with relapsed MF/SS in USA. Unfortunately, its efficacy as a single agent remains modest, particularly in comparison with highly active monoclonal antibodies used in B-cell lymphomas. Mogamulizumab offers some unique benefits over alternative therapies in CTCL: the relatively long duration of remission confirmed in a large trial, high response rates within the blood compartment, and in SS. It may also prove useful in combination with other systemic agents, both as a direct antineoplastic agent and as immune modulator. For example, several ongoing trials combine mogamulizumab with checkpoint inhibitors (NCT03309878, NCT02476123) as a means of depleting undesirable Treg cells and enhancing their immune effect. Disappointingly, the first trial of combined chemotherapy and mogamulizumab in ATLL did not show improved rates of response or survival over chemotherapy alone.90 Clinicians using mogamulizumab for CTCL and ATLL should be aware of the associated risks, particularly infusion reactions and rash (which rarely may become severe or even fatal), as well as the increased risk of GVHD, given that allogeneic bone marrow transplantation remains an important curative modality for both advanced CTCL and ATLL.
Footnotes
Disclosure
AJO reports research funding from Spectrum Pharmaceuticals, TG Therapeutics, and Genentech, and consulting from Spectrum Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- 1.Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149(11):1295–1299. doi: 10.1001/jamadermatol.2013.5526. [DOI] [PubMed] [Google Scholar]
- 2.Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch Dermatol. 2007;143(7):854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, World Health Organization, International Agency for Research on Cancer . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2017. Revised 4th ed. [Google Scholar]
- 4.Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M, ESMO Guidelines Committee Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl_4):iv30–iv40. doi: 10.1093/annonc/mdy133. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116(5):767–771. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demierre MF, Gan S, Jones J, Miller DR. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer. 2006;107(10):2504–2511. doi: 10.1002/cncr.22252. [DOI] [PubMed] [Google Scholar]
- 7.Kirsch IR, Watanabe R, O’Malley JT, et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med. 2015;7(308):308ra158. doi: 10.1126/scitranslmed.aaa9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Doorn R, van Haselen CW, van Voorst Vader PC, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol. 2000;136(4):504–510. doi: 10.1001/archderm.136.4.504. [DOI] [PubMed] [Google Scholar]
- 9.Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2018 Sep 29; doi: 10.1111/bjd.17258. Epub. [DOI] [PubMed] [Google Scholar]
- 10.Whittaker S, Hoppe R, Prince HM. How I treat mycosis fungoides and Sezary syndrome. Blood. 2016;127(25):3142–3153. doi: 10.1182/blood-2015-12-611830. [DOI] [PubMed] [Google Scholar]
- 11.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox RA. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92(10):1085–1102. doi: 10.1002/ajh.24876. [DOI] [PubMed] [Google Scholar]
- 13.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110(6):1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 14.Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous Lymphoma International Consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33(32):3766–3773. doi: 10.1200/JCO.2015.61.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talpur R, Singh L, Daulat S, et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sezary syndrome from 1982 to 2009. Clin Canc Res. 2012;18(18):5051–5060. doi: 10.1158/1078-0432.CCR-12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidulich KA, Talpur R, Bassett RL, Duvic M. Overall survival in erythrodermic cutaneous T-cell lymphoma: an analysis of prognostic factors in a cohort of patients with erythrodermic cutaneous T-cell lymphoma. Int J Dermatol. 2009;48(3):243–252. doi: 10.1111/j.1365-4632.2009.03771.x. [DOI] [PubMed] [Google Scholar]
- 17.Olsen EA, Rook AH, Zic J, et al. Sézary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC) J Am Acad Dermatol. 2011;64(2):352–404. doi: 10.1016/j.jaad.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Duvic M. Choosing a systemic treatment for advanced stage cutaneous T-cell lymphoma: mycosis fungoides and Sézary syndrome. Hematology Am Soc Hematol Educ Program. 2015;2015(1):529–544. doi: 10.1182/asheducation-2015.1.529. [DOI] [PubMed] [Google Scholar]
- 19.Foss F, Advani R, Duvic M, et al. A phase II trial of belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168(6):811–819. doi: 10.1111/bjh.13222. [DOI] [PubMed] [Google Scholar]
- 20.Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013;31(1):104–110. doi: 10.1200/JCO.2012.43.7285. [DOI] [PubMed] [Google Scholar]
- 21.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol. 2001;19(9):2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 22.Prince HM, Duvic M, Martin A, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(11):1870–1877. doi: 10.1200/JCO.2009.26.2386. [DOI] [PubMed] [Google Scholar]
- 23.Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390(10094):555–566. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 24.Duvic M, Kim YH, Zinzani PL, Horwitz SM. Results from a phase I/II open-label, dose-finding study of pralatrexate and oral bexarotene in patients with relapsed/refractory cutaneous T-cell lymphoma. Clin Canc Res. 2017;23(14):3552–3556. doi: 10.1158/1078-0432.CCR-16-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2012;119(18):4115–4122. doi: 10.1182/blood-2011-11-390211. [DOI] [PubMed] [Google Scholar]
- 26.Khodadoust M, Rook A, Porcu P, et al. Pembrolizumab for treatment of relapsed/refractory mycosis fungoides and Sezary syndrome: clinical efficacy in a CITN multicenter phase 2 study. Blood. 2016;128(22):181. [Google Scholar]
- 27.Querfeld C, Rosen ST, Guitart J, et al. Results of an open-label multi-center phase 2 trial of lenalidomide monotherapy in refractory mycosis fungoides and Sezary syndrome. Blood. 2014;123(8):1159–1166. doi: 10.1182/blood-2013-09-525915. [DOI] [PubMed] [Google Scholar]
- 28.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(21):3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker SJ, Demierre M-F, Kim EJ, et al. Final results from a multi-center, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(29):4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 30.Photiou L, van der Weyden C, McCormack C, Miles Prince H. Systemic treatment options for advanced-stage mycosis fungoides and Sézary syndrome. Curr Oncol Rep. 2018;20(4):32. doi: 10.1007/s11912-018-0678-x. [DOI] [PubMed] [Google Scholar]
- 31.Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood. 2003;101(11):4267–4272. doi: 10.1182/blood-2002-09-2802. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett NL, Younes A, Carabasi MH, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111(4):1848–1854. doi: 10.1182/blood-2008-01-127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai A, Telang GH, Olszewski AJ. Remission of primary cutaneous anaplastic large cell lymphoma after a brief course of brentuximab vedotin. Ann Hematol. 2013;92(4):567–568. doi: 10.1007/s00277-012-1610-3. [DOI] [PubMed] [Google Scholar]
- 34.Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–6767. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 35.Casulo C, O’Connor O, Shustov A, et al. T-cell lymphoma: recent advances in characterization and new opportunities for treatment. J Natl Cancer Inst. 2017;109(2) doi: 10.1093/jnci/djw248. djw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 37.Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 38.Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400(6746):776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 39.Narducci MG, Scala E, Bresin A, et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107(3):1108–1115. doi: 10.1182/blood-2005-04-1492. [DOI] [PubMed] [Google Scholar]
- 40.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 41.Wilcox RA. Mogamulizumab: 2 birds, 1 stone. Blood. 2015;125(12):1847–1848. doi: 10.1182/blood-2015-02-625251. [DOI] [PubMed] [Google Scholar]
- 42.Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. J Invest Dermatol. 2003;121(5):1045–1052. doi: 10.1046/j.1523-1747.2003.12555.x. [DOI] [PubMed] [Google Scholar]
- 43.Yagi H, Seo N, Ohshima A, et al. Chemokine receptor expression in cutaneous T cell and NK/T-cell lymphomas: immunohistochemical staining and in vitro chemotactic assay. Am J Sur Pathol. 2006;30(9):1111–1119. doi: 10.1097/01.pas.0000213267.92349.59. [DOI] [PubMed] [Google Scholar]
- 44.Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Canc Res. 2004;10(16):5494–5500. doi: 10.1158/1078-0432.CCR-04-0371. [DOI] [PubMed] [Google Scholar]
- 45.Sugaya M, Morimura S, Suga H, et al. CCR4 is expressed on infiltrating cells in lesional skin of early mycosis fungoides and atopic dermatitis. J Dermatol. 2015;42(6):613–615. doi: 10.1111/1346-8138.12852. [DOI] [PubMed] [Google Scholar]
- 46.Duvic M, Pinter-Brown L, Foss F, et al. Correlation of target molecule expression and overall response in refractory cutaneous T-cell lymphoma patients dosed with mogamulizumab (KW-0761), a monoclonal antibody directed against CC chemokine receptor type 4 (CCR4) Blood. 2012;120(21):3697. [Google Scholar]
- 47.Nakayama S, Yokote T, Hirata Y, et al. Aberrant expression of CCR4 in diffuse large B-cell lymphoma, not otherwise specified. Leukemia. 2013;27(12):2382–2385. doi: 10.1038/leu.2013.128. [DOI] [PubMed] [Google Scholar]
- 48.Ferenczi K, Fuhlbrigge RC, Kupper TS, Pinkus JL, Pinkus GS. Increased CCR4 expression in cutaneous T cell lymphoma. J Investig Dermatol. 2002;119(6):1405–1410. doi: 10.1046/j.1523-1747.2002.19610.x. [DOI] [PubMed] [Google Scholar]
- 49.Jones D, O’Hara C, Kraus MD, et al. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000;96(2):685–690. [PubMed] [Google Scholar]
- 50.Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- 51.Phillips AA, Fields P, Hermine O, et al. A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (MogA) vs investigator’s choice (IC) in the treatment of patients (PTS) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL) J Clin Oncol. 2016;34(15_suppl):7501. [Google Scholar]
- 52.Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99(5):1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- 53.Ishida T, Iida S, Akatsuka Y, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-cell leukemia/lymphoma. Clin Canc Res. 2004;10(22):7529–7539. doi: 10.1158/1078-0432.CCR-04-0983. [DOI] [PubMed] [Google Scholar]
- 54.Sokolowska-Wojdylo M, Wenzel J, Gaffal E, et al. Circulating clonal CLA+ and CD4+ T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol. 2005;152(2):258–264. doi: 10.1111/j.1365-2133.2004.06325.x. [DOI] [PubMed] [Google Scholar]
- 55.Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883–1889. doi: 10.1182/blood-2014-09-600924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fierro MT, Comessatti A, Quaglino P, et al. Expression pattern of chemokine receptors and chemokine release in inflammatory erythroderma and Sézary syndrome. Dermatology. 2006;213(4):284–292. doi: 10.1159/000096191. [DOI] [PubMed] [Google Scholar]
- 57.Nakagawa M, Schmitz R, Xiao W, et al. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med. 2014;211(13):2497–2505. doi: 10.1084/jem.20140987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kataoka K, Iwanaga M, Yasunaga JI, et al. Prognostic relevance of integrated genetic profiling in adult T-cell leukemia/lymphoma. Blood. 2018;131(2):215–225. doi: 10.1182/blood-2017-01-761874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakamoto Y, Ishida T, Masaki A, et al. CCR4 mutations associated with superior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Blood. 2018;132(7):758–761. doi: 10.1182/blood-2018-02-835991. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47(12):1426–1434. doi: 10.1038/ng.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use of transcriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL) Clin Canc Res. 2015;21(12):2820–2829. doi: 10.1158/1078-0432.CCR-14-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litvinov IV, Tetzlaff MT, Thibault P, et al. Gene expression analysis in cutaneous T-cell lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. OncoImmunology. 2017;6(5):e1306618. doi: 10.1080/2162402X.2017.1306618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lefrancois P, Xie P, Wang L, et al. Gene expression profiling and immune cell-type deconvolution highlight robust disease progression and survival markers in multiple cohorts of CTCL patients. OncoImmunology. 2018;7(8):e1467856. doi: 10.1080/2162402X.2018.1467856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitadate A, Ikeda S, Abe F, et al. Histone deacetylase inhibitors downregulate CCR4 expression and decrease mogamulizumab efficacy in CCR4-positive mature T-cell lymphomas. Haematologica. 2018;103(1):126–135. doi: 10.3324/haematol.2017.177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zinzani PL, Horwitz SM, Kim YH, et al. Efficacy of mogamulizumab by prior systemic therapy in patients with previously treated cutaneous T-cell lymphoma: post hoc analysis from the phase 3 Mavoric study. Blood. 2018;132(Suppl 1):1619. [Google Scholar]
- 66.Baatar D, Olkhanud P, Newton D, Sumitomo K, Biragyn A. CCR4-expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J Immunol. 2007;179(3):1996–2004. doi: 10.4049/jimmunol.179.3.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perera LP, Zhang M, Nakagawa M, et al. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am J Hematol. 2017;92(9):892–901. doi: 10.1002/ajh.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Canc Res. 2010;16(5):1520–1531. doi: 10.1158/1078-0432.CCR-09-2697. [DOI] [PubMed] [Google Scholar]
- 69.Yano H, Ishida T, Inagaki A, et al. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: a novel immunotherapy for aggressive/refractory mycosis fun-goides and Sezary syndrome. Clin Canc Res. 2007;13(21):6494–6500. doi: 10.1158/1078-0432.CCR-07-1324. [DOI] [PubMed] [Google Scholar]
- 70.Ito A, Ishida T, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rγ null mouse model. Cancer Immunol Immunother. 2009;58(8):1195–1206. doi: 10.1007/s00262-008-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niwa R, Shoji-Hosaka E, Sakurada M, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64(6):2127–2133. doi: 10.1158/0008-5472.can-03-2068. [DOI] [PubMed] [Google Scholar]
- 72.Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 73.Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278(5):3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 74.Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. MAbs. 2009;1(3):230–236. doi: 10.4161/mabs.1.3.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- 76.Yu X, Marshall MJE, Cragg MS, Crispin M. Improving antibody-based cancer therapeutics through glycan engineering. BioDrugs. 2017;31(3):151–166. doi: 10.1007/s40259-017-0223-8. [DOI] [PubMed] [Google Scholar]
- 77.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 78.Ishida T, Utsunomiya A, Jo T, et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: updated follow-up analysis of phase I and II studies. Cancer Sci. 2017;108(10):2022–2029. doi: 10.1111/cas.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phillips A, Fields P, Hermine O, et al. A prospective, multicenter, randomized study of Anti-CCR4 monoclonal antibody mogamulizumab versus investigator’s choice in the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma: overall response rate, progression-free survival, and overall survival. Blood. 2016;128(22):4159. [Google Scholar]
- 80.Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for cutaneous lymphomas, the United States cutaneous lymphoma Consortium, and the cutaneous lymphoma Task Force of the European Organisation for research and treatment of cancer. J Clin Oncol. 2011;29(18):2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevens SR, Ke MS, Parry EJ, Mark J, Cooper KD. Quantifying skin disease burden in mycosis fungoides-type cutaneous T-cell lymphomas: the severity-weighted assessment tool (SWAT) Arch Dermatol. 2002;138(1):42–48. doi: 10.1001/archderm.138.1.42. [DOI] [PubMed] [Google Scholar]
- 82.Hudgens S, Porcu P, Quaglino P, et al. Evaluation of symptom and side effect bother in cutaneous T-cell lymphoma patients treated with mogamulizumab or vorinostat. Blood. 2018;132(Suppl 1):3592. [Google Scholar]
- 83.Ishida T, Ito A, Sato F, et al. Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia/lymphoma. Cancer Sci. 2013;104(5):647–650. doi: 10.1111/cas.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosoi H, Mushino T, Nishikawa A, et al. Severe graft-versus-host disease after allogeneic hematopoietic stem cell transplantation with residual mogamulizumab concentration. Int J Hematol. 2018;107(6):717–719. doi: 10.1007/s12185-018-2456-9. [DOI] [PubMed] [Google Scholar]
- 85.Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation Anti-CCR4 antibody mogamulizumab against adult T-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34(28):3426–3433. doi: 10.1200/JCO.2016.67.8250. [DOI] [PubMed] [Google Scholar]
- 86.Ishitsuka K, Yurimoto S, Kawamura K, et al. Safety and efficacy of mogamulizumab in patients with adult T-cell leukemia–lymphoma in Japan: interim results of postmarketing all-case surveillance. Int J Hematol. 2017;106(4):522–532. doi: 10.1007/s12185-017-2270-9. [DOI] [PubMed] [Google Scholar]
- 87.Ni X, Jorgensen JL, Goswami M, et al. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Canc Res. 2015;21(2):274–285. doi: 10.1158/1078-0432.CCR-14-0830. [DOI] [PubMed] [Google Scholar]
- 88.Ishida T, Jo T, Takemoto S, et al. Follow-up of a randomised phase II study of chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: impact on allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2019;184(3):479–483. doi: 10.1111/bjh.15123. [DOI] [PubMed] [Google Scholar]
- 89.Dai J, Almazan TH, Hong EK, et al. Potential association of anti-CCR4 antibody mogamulizumab and graft-vs-host disease in patients with mycosis fungoides and Sézary syndrome. JAMA Dermatol. 2018;154(6):728–730. doi: 10.1001/jamadermatol.2018.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishida T, Jo T, Takemoto S, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol. 2015;169(5):672–682. doi: 10.1111/bjh.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]