Abstract
Larvae of the black soldier fly, Hermetia illucens (L.), have been widely studied for their capacity of generating value-added products through waste bioconversion. Several efforts have been made towards optimization of rearing methods of the larval stages. Despite this, less information regarding the feeding behavior of adults and its effects on life parameters is available. We studied the impacts of subjecting adults to four diets: no diet (control); drinking water (water); a mix of sugar, bacteriological peptone, and milk powder (milk); and agar with sugar (agar). In total five replicates with 50 females and 50 males per treatment were performed. Oviposition and mortality were recorded daily. Additionally, egg hatchability and number of eggs per mass were measured. Results from this study indicate that the amount of eggs was 3 times higher for diets containing a protein source. Furthermore, milk treatment increased the oviposition period by 10 d compared with that of the standard water treatment. No significant difference was found for male longevity; however, females fed with milk diet lived 5 d longer than the control group. Egg hatchability ranged 70–85%, but was not significantly different across treatments. In terms of egg production for both industrial and research purposes, we suggest using a protein-rich diet to maximize oviposition and longevity parameters as adults do benefit from feeding.
Keywords: black soldier fly, feeding behavior, mating, reproduction
Broad-based scientific consensus has been reached about the necessity of finding sustainable sources of protein in order to meet the requirements of our growing population (van Huis 2013). The increase in demanding consumers will raise food production and thus exacerbate human-triggered depletion of natural resources. In this challenging context, also feed production would need to increase about 70% by 2050, without fully relying on neither fish meal nor soybean as main components (FAO 2006). All this with livestock accounting for 70% of agricultural land usage and aquaculture hitting production records in 2016 with 5.8% annual growth since 2001 (FAO 2018).
With the aim of finding alternatives, entomophagy and insects as a substitute for animal feeding have gained attention in recent years (Veldkamp et al. 2012, Sánchez-Muros et al. 2014, Tran et al. 2015). Especially for their nutritional value, low environmental impact, lower greenhouse gases (GHGs) emissions than livestock or low-cost applicability, insects are seen as a promising option for providing a sustainable protein source (van Huis 2013).
The black soldier fly, Hermetia illucens (Linnaeus, 1758), is a well-known candidate with applications in several sectors (Wang and Shelomi 2017). For instance, larvae of H. illucens have been successfully introduced as a partial component of several livestock diets such as swine (Newton et al. 1977), chicken (Onsongo et al. 2018), or rabbit (Dalle Zotte et al. 2018). Also, several studies have suggested the potential application of larvae of H. illucens as a feed source in the aquaculture sector (Bondari and Sheppard 1981, Cummins et al. 2017, Stadtlander et al. 2017). Further applications are manure and waste bioconversion (Diener et al. 2011) as well as antibacterial properties (Lalander et al. 2015, Vogel et al. 2018). Recently, even the cosmetic sector might become a potential target as H. ilucens fatty acid profile resembles those of coconut oil or palm kernel oil, which could be used to produce surfactants (Verheyen et al. 2018).
Despite this, adult stages from H. illucens (L.) still need to be researched in order to optimize egg production (Čičková et al. 2015). The shiny black wasp-like stratiomyid has a rather complicated mating behavior and oviposition pattern. First, studies regarding this topic focused mainly on the amount of eggs per female, ranging 500–1,000 eggs (Furman et al. 1959), 119–502 eggs (Gonzalez et al. 1963), and 205–820 eggs (Stephens 1975). In addition, Booth and Sheppard (1984) did observations about oviposition sites and estimated mean number of eggs per mass (998 eggs) and individual egg weight of 0.0276 mg. However, the differences in terms of egg production among studies suggest that external factors such as food availability might influence the reproduction capacity of adults of H. illucens.
Following this, Sheppard et al. (2002) recorded mating and oviposition to occur 2 and 4 d after emergence, respectively. Since providing water for adults was found to significantly increase longevity about 1–2 d, Tomberlin et al. (2002) established misting water as a standard method for rearing adults in a colony. Additionally, Tomberlin and Sheppard (2002) described light intensity (sunlight), temperature, and humidity as factors positively correlating with oviposition, with mating occurring rather early in the day. Lately, studies about life history traits of adult flies have focused on indoor-rearing methods by testing different light sources (Zhang et al. 2010, Heussler et al. 2018) or adding sugar as food source (Nakamura et al. 2016, Oonincx et al. 2016). Nonetheless, there is a lack of information about the effects of diets for adults on life parameters of H. illucens in a colony.
There are several reasons for suggesting that adults of H. illucens feed in wild conditions. Based on ecological observations, Stratiomyidae are known to feed on honeydew (Beuk 1990), nectar (James 1981), or pollen (Oldroyd 1973) and have been described as pollinators (Srikanth et al. 2013, Roy et al. 2014). In terms of insect anatomy, Rozkošný (1982) described them as nectar feeders having fully developed muscoid (sponge-like)-type mouthparts, which was confirmed by scanning electron microscope (SEM) images (Oliveira et al. 2016). Also, colonization of beehives might be explained due to their reservoir being exploited by H. illucens as energy source for both adults and larvae (Riley and Howard 1889, Copello 1926). Nevertheless, evidence about the ability of adults to benefit from nutritious diets in terms of egg production is not found in the literature.
The main purpose of this study was to determine the influence of different diets on longevity and egg production parameters of colony-reared adults of H. illucens, as well as to clarify discrepancies across studies regarding amount of eggs per female by applying more controlled diet treatments. Therefore, our research aims to extend adult lifespan, increase total oviposition, and enhance the hatching performance of laid eggs by providing adults with a food source. By this means, we expect to find a feasible diet for optimizing rearing methods by unraveling the nutritional requirements of H. illucens which more closely resemble the conditions of their natural habitat.
Materials and Methods
Colony Rearing
Eggs were obtained from a year round colony in the greenhouses of the Department of Entomology at National Chung Hsing University in Taichung, Taiwan. Adults were reared in 1 × 1 × 1 m screen cages at 28°C, 70 ± 5% RH, and sunlight. Water was misted twice daily and plants for resting and lekking were provided (Tomberlin and Sheppard 2001).
Egg collection was performed using small 3-cm2 corrugated cardboards as oviposition sites located on the top of mesh-covered soufflé plastic cups (250 ml, 9 cm upper diameter). Organic waste composed mainly of pineapple scraps and kitchen leftovers were mixed to ensure homogeneity and provided in the cups as attractant. Cardboards containing approximately two egg masses of newly laid eggs (<6 h) were kept in grip seal plastic bags (85 × 60 mm) and incubated at 28°C, 80% RH. Upon hatching, larvae were transferred to plastic boxes (43 × 35 × 11 cm) and reared with artificial diet composed of three parts wheat bran per one part of chicken feed (>20% protein) adding 250-ml water per 100 g dry weight (Samayoa et al. 2016). The containers were covered with mesh nets to avoid larvae escaping and water and diet were added until first prepupae appeared. Finally, prepupae were separated by sieving and transferred to plastic trays (23 × 22 × 9 cm) containing potting soil (Known-You Seed Co., Ltd., Kaohsiung, Taiwan) as pupation substrate according to Holmes et al. (2013). The adults used in the experiments had a mean (±SE) egg-to-adult development time of 33.6 ± 0.7 d at 28°C, 80% RH and 12:12 (L:D) h photoperiod.
Experimental Design
In total five replicates containing 100 newly emerged (<12 h) adults (50 females and 50 males) per treatment were marked according to their sex using 2-mm2 tags glued to the thorax with nail polish to facilitate recording daily mortality and reproductive females (Samayoa et al. 2016). Adults were then released in a wire-framed polyester mosquito net tent per treatment (Baohe E-Commerce Co., Ltd., New Taipei City, Taiwan). Each tent measured 120 × 87 × 75 cm and contained one plant, one oviposition site as described above, and the corresponding diet. Temperature and relative humidity were monitored with HOBO data logger (Onset Computer, Co., Bourne, MA). The temperature measured 28.1 ± 0.8°C (mean ± SE) and relative humidity was 70.8 ± 8%. All experiments were performed in the same greenhouse used for colony rearing during April–August 2018 with natural daylight and 10:14 (L:D) h photoperiod.
Diet Composition of the Treatments
Testing natural food sources such as flowering plants or honey was avoided due to the high variability in composition and the low applicability of such diets in rearing facilities. Instead, four diets were chosen prioritizing practical feasibility and low cost of artificial diets used for other dipterans. The treatments were named according to the main component of the diet: water, agar, milk, and control.
The diets were described as follows: the first treatment was water, consisting of a plastic tray (29 × 23 × 3 cm) containing 200-ml water and hard tissue paper allowing adults to drink without drowning. Misting water was avoided due to the difficulty of equal spreading and to ensure ad libitum access to water. The second treatment was agar, a gelatinous diet made of 37.5-g agar (Fei Kung Agar-Agar Co., Ltd., Tainan, Taiwan), water, and sugar with a concentration of 43.75 g/liter based on the recipe from the Mediterranean fruit fly California Preventive Release Program (CDFA 2014). Same trays as first treatment containing 300-g diet were provided with mesh net over the surface to avoid adults sticking to it.
The third treatment was milk: composed of 3:1:1 by weight combination of sugar (Yi-Feng Food Co., Ltd., New Taipei, Taiwan), milk powder (Fonterra Brands, Pte., Ltd., Taiwan Branch), and bacteriological peptone (USB Corporation, Cleveland, OH), respectively. The diet was adapted from Pastor et al. (2011) and Samayoa et al. (2016). The peptone component was included based on Turner and Hair (1967). After mixing the components, 50 g of the diet was spread over a tray, moisturized by spraying water, and covered with mesh net as landing surface. The fourth treatment was the control, provided with an empty plastic tray with same dimensions as the other treatments.
Influence of Different Diets on Adult Longevity
To measure longevity, the treatments were checked twice daily (morning and afternoon). Dead individuals were removed, and individual tag number and sex of individuals were recorded. Adults which died during the first day of emergence were replaced with ones of the same age as some insects were damaged while attaching the tags to them.
Influence of Different Diets on Oviposition
For the purpose of measuring egg production, cardboards from each treatment were collected daily at sunset to avoid interrupting oviposition. Eggs were separated using brushes and weighed to asses daily oviposition. With the aim of measuring mean number of eggs per mass in a single oviposition event, an additional cardboard was presented to each treatment. By doing so, 10 different females per treatment were allowed to individually oviposit by using an empty soufflé plastic cup (250 ml, 9 cm upper diameter) to cover them in order to avoid others from laying eggs in the same cardboard. Females were covered when landing on the oviposition site and extending the ovipositor. Then these were observed until they stopped laying eggs and the ovipositor was pulled back, as sometimes oviposition was shortly interrupted and females searched around the cardboard, which resulted in more than one egg clutch per laying event. This step usually took about 30 min. Finally, tag number was recorded to ensure sampling different females and all eggs were weighed and counted.
Influence of Different Diets on Egg Hatchability
Egg hatchability was calculated as the number of successfully hatched eggs (fertilized) divided by the estimated number of eggs, with single egg weight of 0.02557 mg as conversion factor, similar to Booth and Sheppard (1984). Individual egg weight was calculated by counting eggs of newly (<8 h) laid egg masses (n = 20) ranging 1–30 mg under binocular microscope in a 1:1 mix of glycerin and water to facilitate the counting (Samayoa et al. 2016).
Fertilized eggs were evaluated by counting hatched larvae under stereomicroscope. Egg clusters of about 10 mg were selected from each cardboard for sampling (n = 84). The samples were isolated inside Petri dishes using Parafilm M (Bemis Company, Inc., Oshkosh, WI) and then kept at 28°C and 80% RH inside grip seal plastic bags (120 × 85 mm) to avoid hatched larvae from escaping (Nakamura et al. 2016). Once hatching occurred, the samples remained for two more days in same conditions to ensure all eggs hatched.
Statistical Analysis
Data were analyzed using SAS Software v. 9.4 (SAS Institute 2001). Differences between treatments were tested by one-way ANOVA using Tukey–Kramer HSD as post hoc test for comparison with 5% significance level.
Results
Influence of Different Diets on Adult Longevity
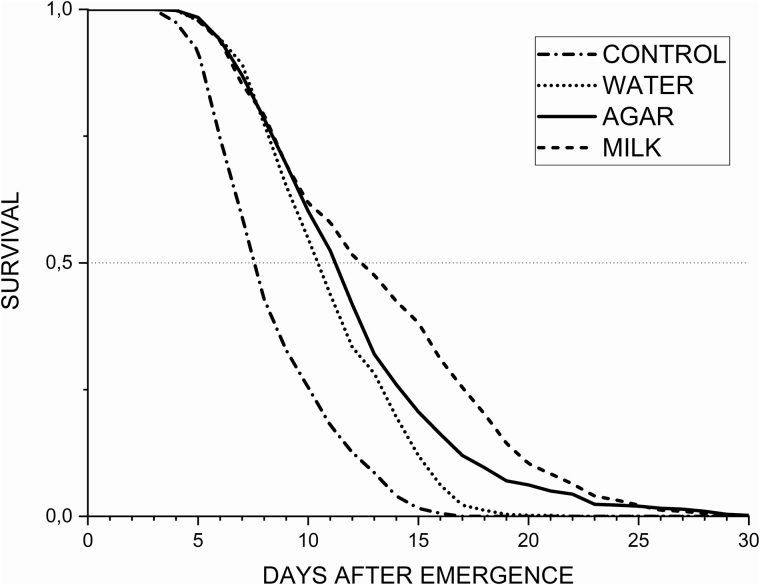
Mortality was observed starting at day 5 after emergence for all treatments and longevity reached a maximum of 30 d under milk treatment. The control treatment was the first to reach 50% of mortality at day 8, followed by water (day 10), agar (day 11), and milk (day 12) as seen on the survival curves (Fig. 1). Only 10% of adults remained alive by days 12 and 15, for the control and water treatments, respectively. In contrast, 10% of adults were still alive by day 18 under agar treatment and day 20 for milk diet.
Fig. 1.
Survival curves for adults of Hermetia illucens (L.) across treatments (5 replicates).
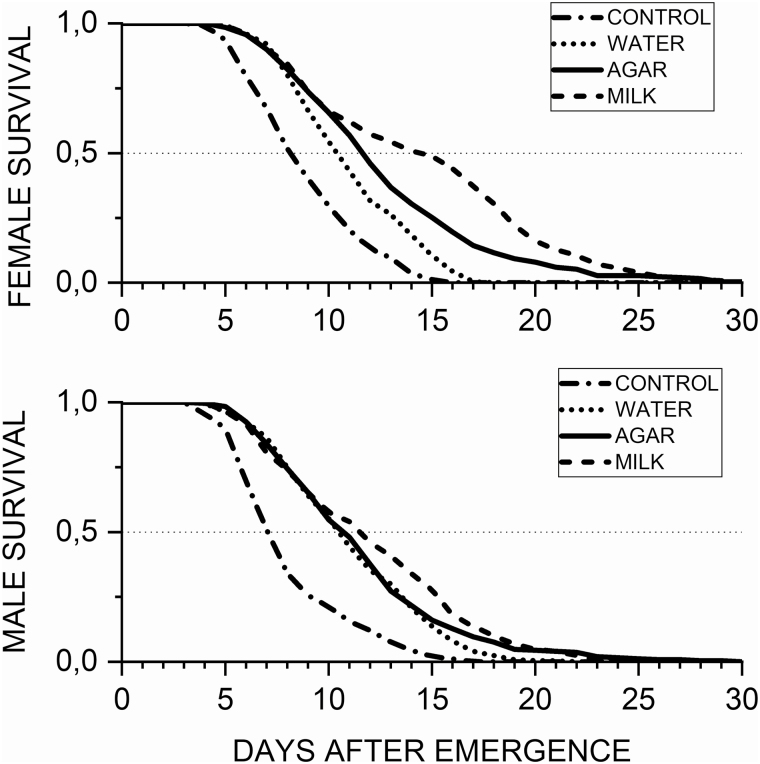
The mean longevity for males was greater for milk treatment, followed by agar and water (Fig. 2). Control treatment showed the shortest male longevity (Table 1). However, the difference between treatments for male longevity was not significant (F = 1.81; df = 3; P = 0.1865). In contrast to this, female longevity did significantly differ (F = 3.59; df = 3; P = 0.0371). On average females lived longer with milk as diet, followed by agar, water, and control treatment (Fig. 2).
Fig. 2.
Survival curves across sexes of adults of Hermetia illucens (L.) across treatments (5 replicates).
Table 1.
Effects of different treatments on life history parameters of adults of Hermetia illucens (L.)
| Control | Water | Agar | Milk | |
|---|---|---|---|---|
| Egg mass (mg) | 532.06 ± 23.45a | 555.74 ± 24.75a | 930.78 ± 62.50b | 1551.94 ± 106.44c |
| Preoviposition period (d) | 4.40 ± 0.40a | 4.20 ± 0.20a | 4.00 ± 0.00a | 4.00 ± 0.00a |
| Oviposition period (d) | 6.40 ± 0.98a | 7.00 ± 1.26a | 10.80 ± 1.20a | 17.20 ± 1.98b |
| Male longevity (d) | 8.70 ± 0.79a | 11.49 ± 1.12a | 11.93 ± 1.68a | 12.08 ± 0.95a |
| Female longevity (d) | 9.49 ± 0.68a | 11.43 ± 0.74ab | 13.09 ± 1.74ab | 14.58 ± 1.13b |
| Single egg mass (mg) | 10.53 ± 1.46a | 11.11 ± 0.78a | 12.23 ± 1.44a | 11.37 ± 1.64a |
| Hatchability1 % | 75.74 ± 9.31a | 71.56 ± 9.67a | 75.36 ± 6.06a | 81.81 ± 4.79a |
Mean ± SE.
1Individual egg weight = 0.02557 mg.
Means followed by different letters within the same rows are significantly different between treatments at the level of P < 0.05, Tukey–Kramer HSD post hoc test, SAS 9.4 (SAS Institute Inc., Cary, NC).
Influence of Different Diets on Oviposition
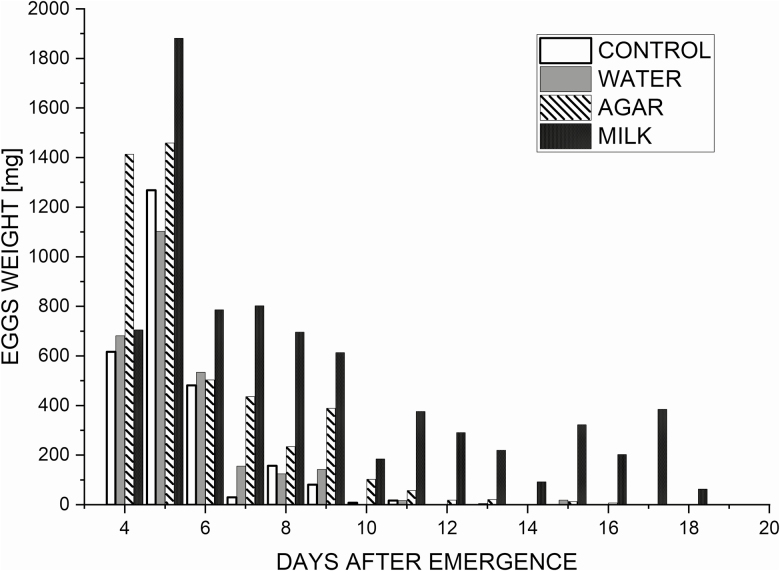
None of the treatments produced eggs before day 4 after emergence and no mating could be observed before the second day. The maximum daily egg production for all treatments was observed at day 5 after emergence (Fig. 3). Females in the milk treatment laid eggs during a period of 17 d, significantly longer than the agar treatment (11 d). Milk treatment also increased by 10 d the period for laying eggs compared with the water and control treatment (Table 1), with an overall significant effect of the treatments on the oviposition period (F = 13.24; df = 3; P < 0.001).
Fig. 3.
Daily oviposition for adults of Hermetia illucens (L.) across four treatments (5 replicates).
In terms of total oviposition, adults in the control (20,808 eggs) and water (21,734 eggs) treatments showed the lowest mean egg production. The agar diet was the second best egg producer with 36,401 eggs and adults provided milk diet showed the best performance with 60,694 eggs. The influence of diet on overall oviposition weight showed a significant difference among treatments (F = 55.26; df = 3; P < 0.001).
The biggest egg mass recorded by a single female laid at once weighed 27.1 mg (1,060 eggs) and was found in the control group. In addition, the control treatment showed the smallest value for mean number of eggs per mass with 412 eggs (Table 1), followed by water (434 eggs), milk (445 eggs), and agar (478 eggs). Interestingly, the smallest egg mass was found in the agar treatment with 1.8 mg (70 eggs). The mean number of eggs per mass did not significantly differ across diets (F = 0.27; df = 3; P = 0.8494).
Influence of Different Diets on Egg Hatchability
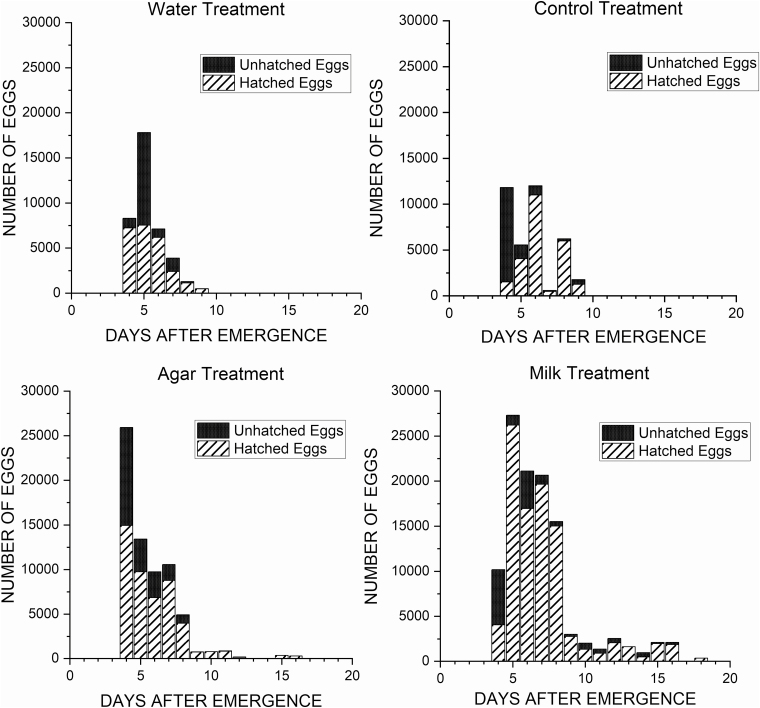
The lowest value for egg hatchability was measured on day 4 (Fig. 4); for control (13.01%), milk (39.90%), and agar (57.61%); and day 5 for water (42.32%). The mean (±SE) individual egg weight measured 0.02557 ± 0.00001 mg. The overall mean egg hatchability was lower for water, followed by agar, control, and milk treatment (Table 1), but the difference across treatments was not significant (F = 1.36; df = 3; P = 0.2605). Taking into account the mean hatchability and the total oviposition, the lowest amount of fertilized eggs was laid when provided water (15,552 eggs) followed by control (15,760 eggs), agar (27,432 eggs), and milk (49,654 eggs).
Fig. 4.
Daily mean egg hatchability of Hermetia illucens (L.) for n = 84 cardboards.
Discussion
In this study, we focused on the influence of different food sources on life parameters of H. illucens adults reared under artificial conditions. In terms of longevity, our results show that adults without any food source or provided only with water survived a mean time of 8 to 11 d. Furthermore, feeding adults with milk or agar diet increased longevity by 1–2 d for females, reaching a maximal mean lifetime of 14 d for females in the milk treatment. For instance, Tomberlin et al. (2009) mentioned mean longevity of 14 d for females and 17 d for males at 27°C provided with water. Additionally, Nakamura et al. (2016) reported 21 d for adults provided with water and introduced a sugar solution as diet increasing longevity up to 73 d for males and 47 d for females. Nevertheless, in the previously mentioned studies, adults were maintained in individual cups. Hence, we believe that the longer longevity periods compared with our study might be explained by the lack of mating and its associated side effects on lifespan (Chapman et al. 1995, Barnes et al. 2008). However, potential life-shortening due to other activities such as flying, foraging, or lekking also need to be taken into account for future studies measuring adult longevity.
Concerning the influence of diet sources on survival among sexes, we observed that longevity of males was not significantly affected by diets. However, longevity was increased when mating was not present as shown for individually reared adults provided with sugar (Nakamura et al. 2016). This might suggest the shortening of male longevity as a trade-off for increasing mating events, effect which has been shown for other dipteran species (Clutton-Brock and Langley 1997, Partridge and Prowse 1997, Papadopoulos et al. 2010).
Interestingly, females in our study lived 1–2 d longer than males, especially for milk and agar treatment, contrary to previous studies where males survived longer than females (Tomberlin et al. 2009, Samayoa et al. 2016, Heussler et al. 2018). Taking into account that milk and agar diet resulted in the highest oviposition values, we suggest that females in wild populations might profit from food sources and thus present longer longevity periods than males. A similar pattern is observed in the neriid fly, Telostylinus angusticollis (Enderlein 1922) (Diptera: Neriidae), where longevity of individuals with better resources increases in females, but decreases in males (Hooper et al. 2018). Hence, more studies about the effects of mating on the lifespan of H. illucens when provided with nutritious diets are needed.
Regarding the time of oviposition events, our study found that the preoviposition period had very small variation with no oviposition observed before day 4 after emergence. Hence, we suggest that the preoviposition period is rather short and fixed, with mating occurring soon after emergence and relying on fat reserves stored during the larval feeding stages (Nagar 1968). In contrast, total oviposition period differs greatly, ranging from 6 to 17 d, a pattern which is also observed in other studies (Zhang et al. 2010, Nakamura et al. 2016, Oonincx et al. 2016, Samayoa et al. 2016, Heussler et al. 2018).
The oviposition period might depend on several factors, such as food availability, temperature, or sunlight among others. Hence, feeding and improving conditions of adult stages might mainly influence the oviposition period, whereas factors affecting larval stages could rather affect the preoviposition period and first egg-laying events. Following this hypothesis, egg production could be maximized by improving diets for both larval stages: increasing initial oviposition and hatchability success between 4 and 7 d after emergence and adult stages: triggering longer lifespans and constant production of eggs during the second and third week after emergence.
According to the available literature, mean egg masses per female range 120–1000 eggs (Furman et al. 1959, Gonzalez et al. 1963, Stephens 1975, Tomberlin et al. 2002). In this term, our results show intermediate values of 412–478 eggs per egg mass and no significant difference across treatments. This suggests that providing adults with a food source did not have any relevant effect on the size or weight of egg masses, discarding the hypothesis of eggs being heavier or numerous in relation to the kind of diet provided to adults. Therefore, factors related to larval stages might determine the maximal size of egg masses which females are able to oviposit in a single event.
Taking into account the influence of diets on adults of H. illucens, we consider that adults in wild conditions do feed from available sources, for instance, pollen grains which consist of 22.7% protein and 30.8% carbohydrates percent mass (Komosinska-Vassev et al. 2015). Although adult flies might have difficulties absorbing solid food particles with low water content, they might use salivary secretions to facilitate the absorption (Oliveira et al. 2016). Hence, we suggest pollen grains as a possible food source in wild populations of H. illucens, similar to other Stratiomyidae (Oldroyd 1973). Furthermore, the influence of amino acid profile of the diets should be considered for future studies, as deficiency in essential amino acids decreased female fecundity in Ceratitis capitata (Wiedemann 1824) (Diptera: Tephritidae) (Chang 2004).
Other natural energy sources which might be consumed by adults of H. illucens are nectar (James 1981) or honeydew (Beuk 1990). Nonetheless, their chemical composition might not meet the nutritional requirements for inducing a high increase in oviposition as they are mainly composed of carbohydrates (7–70%) with rather low protein content (Nicolson et al. 2007). Despite this, adult flies in wild populations might balance their foraging behavior in relation to their necessities and thus benefit from different kinds of food sources and nutritional compositions as shown for Drosophila melanogaster (Meigen 1830) (Diptera: Drosophilidae) (Ribeiro and Dickson 2010).
According to the nutritional values of the different diets (Table 2), we suggest that the lower oviposition in the agar treatment compared with the milk diet might be due to both lower protein and fat content. However, the development of different microbial communities might affect the nutritional composition of the diets. Furthermore, the peak observed on day 4 in the agar treatment might be explained by carbohydrates being more easy to absorb due to the high water content. In contrast to this, because of the hygroscopic character of the peptone component, the milk diet progressively gained in moisture, slowly making it more accessible for the muscoid mouthparts of H. illucens and thus facilitating food incorporation. Nevertheless, factors related to the physiological mechanisms underlying nutrient assimilation need further research.
Table 2.
Nutritional composition of diets from the milk and agar treatment
| Agar | Milk | |
|---|---|---|
| Protein | 2.50% | 9.00% |
| Carbohydrates | 96.43% | 82.50% |
| Fats | <0.01% | 4.30% |
| Minerals | 0.05% | 1.60% |
All values given in mass percent (m/m) %.
When comparing other methods where water was provided to adults (Sheppard et al. 2002, Tomberlin and Sheppard 2002, Nakamura et al. 2016) with our data, it has been shown that egg production is not significantly improved compared with the control and that longevity is extended by 2 d at least, which means more nonreproductive individuals are maintained. Apart from this, females which are not provided any diet survive a mean of 9 d, which is long enough to allow individuals to oviposit. Hence, misting water should be reconsidered at least under tolerated temperatures as avoiding it might increase functionality and reduce rearing costs.
Summarizing the values obtained for daily egg production and hatchability rates, it is possible to extrapolate egg yield for a colony under different diets. Our study was performed over a period of 5 mo. In this time, adults in the control treatment laid 99.3% of the eggs between days 4 and 10 after emergence, from which only 66,178 eggs were fertilized. In the same period, 73.1% of the eggs were laid in the milk treatment, with 174,724 eggs being fertilized. In contrast, the remaining 26.9% of the eggs were laid between days 11 and 20, accounting for 61,072 fertilized eggs.
Hence, two different methods regarding egg production and workflow are proposed. On the one side, short cycles of production for unfed colonies, where adults are removed after the 10 d period to reduce cost and increase viability. On the other side, longer life cycles of about 20 d for adults provided with milk diet, thus maximizing the amount of eggs and favoring higher yields as the second half-period of the milk treatment produced almost the same amount of fertilized eggs as the first half-period of the control colony. In this sense, rearing conditions can be chosen according to the demands and easily be adapted for different purposes.
Our research showed that adding a nutritious food source enhanced egg production up to 3 times, extended the oviposition period by 10 d, and did not compromise the hatchability rate of the eggs. The milk diet had a cost of 1.20 USD (50 g) with one portion lasting enough to feed several generations. However, microbial degradation was an important disadvantage of this diet. Hence, we suggest diets containing protein-rich components as the optimal for egg production as also reported by Turner and Hair (1967). In the past, this trait was omitted when assessing feasibility of rearing conditions and several attempts have been regarded as inefficient due to low oviposition rates. Therefore, feeding adults might be especially important for experimental designs dealing with unsuitable conditions such as indoor-rearing or low temperatures. However, the factors enabling maximal oviposition parameters such as minimal nutritional requirements of diets, population density, or individual reproductive potential of females still need further research. This study contributes to reconsider some aspects regarding actual rearing methods of H. illucens based on biological traits of the organism and its habitat natural conditions in order to maximize life history parameters of adults through optimization of their diets.
Acknowledgments
We would like to express our sincere thanks to everybody involved in this project, especially to Dr. Georg Raiser for his comments and feedback on the manuscript as well as the anonymous reviewers for contributing to improve the quality of this paper. Furthermore, C.B. would like to thank the Deutscher Akademischer Austauschdienst (DAAD) for partially founding him through the PROMOS Program and the International Office of National Chung Hsing University for facilitating his stay in Taiwan.
References Cited
- Barnes A. I., Wigby S., Boone J. M., Partridge L., and Chapman T.. . 2008. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc. Biol. Sci. 275: 1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuk P. L. T. 1990. Honeydew as a food source for insects and in particular for soldierflies (Diptera: Stratiomyidae). Phegea 18: 137–140. [Google Scholar]
- Bondari K., and Sheppard D. C.. . 1981. Soldier fly larvae as feed in commercial fish production. Aquaculture 24: 103–109. [Google Scholar]
- Booth D. C., and Sheppard C.. . 1984. Oviposition of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): eggs, masses, timing, and site characteristics. Environ. Entomol. 13: 421–423. [Google Scholar]
- CDFA 2014. Mediterranean fruit fly preventive release program; preparation of fruit fly diet Available from https://www.cdfa.ca.gov/plant/PDEP/prpinfo/ Accessed 21 January 2019.
- Chang C. L. 2004. Effect of amino acids on larvae and adults of Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 97: 529–535. [Google Scholar]
- Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., and Partridge L.. . 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 373: 241–244. [DOI] [PubMed] [Google Scholar]
- Čičková H., Newton G. L., Lacy R. C., and Kozánek M.. . 2015. The use of fly larvae for organic waste treatment. Waste Manag. 35: 68–80. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T., and Langley P.. . 1997. Persistent courtship reduces male and female longevity in captive tsetse flies Glossina morsitans morsitans Westwood (Diptera: Glossinidae). Behav. Ecol. 8: 392–395. [Google Scholar]
- Copello A. 1926. Biologia de Hermetia illucens. Latr. Rev. Sco. Entomol. Argent. 1: 23–27. [Google Scholar]
- Cummins V., Rawles S. D., Thompson K., Velasquez A., Kobayashi Y., Hager J., and Webster C.. . 2017. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 473: 337–344. [Google Scholar]
- Dalle Zotte A., Cullere M., Martins C., Alves S. P., Freire J. P. B., Falcão-E-Cunha L., and Bessa R. J. B.. . 2018. Incorporation of black soldier fly (Hermetia illucens L.) larvae fat or extruded linseed in diets of growing rabbits and their effects on meat quality traits including detailed fatty acid composition. Meat Sci. 146: 50–58. [DOI] [PubMed] [Google Scholar]
- Diener S., Studt Solano N. M., Roa Gutiérrez F., Zurbrügg C., and Tockner K.. . 2011. Biological treatment of municipal organic waste using black soldier fly larvae. Waste Biomass Valori. 2: 357–363. [Google Scholar]
- FAO 2006. Food aid for food security? FAO agriculture series, vol. 37. FAO, Rome, Italy. [Google Scholar]
- FAO 2018. The state of world fisheries and aquaculture 2018. FAO, Rome, Italy. [Google Scholar]
- Furman D. P., Young R. D., and Catts P. E.. . 1959. Hermetia illucens (Linnaeus) as a factor in the natural control of Musca domestica linnaeus. J. Econ. Entomol. 52: 917–921. [Google Scholar]
- Gonzalez J. V., Young W. R., and Genel. 1963. Reduccion de la poblacion de mosca domestica en gallinaza por la mosca soldado en el tropico. Agricultura Técnica en México 2: 53–57. [Google Scholar]
- Heussler C. D., Walter A., Oberkofler H., Insam H., Arthofer W., Schlick-Steiner B. C., and Steiner F. M.. . 2018. Influence of three artificial light sources on oviposition and half-life of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS One. 13: e0197896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes L. A., Vanlaerhoven S. L., and Tomberlin J. K.. . 2013. Substrate effects on pupation and adult emergence of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 42: 370–374. [DOI] [PubMed] [Google Scholar]
- Hooper A. K., Lehtonen J., Schwanz L. E., and Bonduriansky R.. . 2018. Sexual competition and the evolution of condition-dependent ageing. Evol. Lett. 2: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huis A., Van Itterbeeck J., Klunder H., Mertens E., Halloran A., Muir G., and Vantomme P.. . 2013. Edible insects: future prospects for food and feed security. FAO forestry paper, vol. 171. FAO, Rome, Italy. [Google Scholar]
- James M. T.(ed.). 1981. Stratiomyidae, pp. 497–511. InMcAlpine J. f., Peterson B. V., Shewell G. E., Teskey H. J., Vockeroth J. R., and D. M. Wood (cds.), Manual of Nearctic Diptera, chapter 36, Research Branch of Agriculture, Canada, Ottawa. [Google Scholar]
- Komosinska-Vassev K., Olczyk P., Kaźmierczak J., Mencner L., and Olczyk K.. . 2015. Bee pollen: chemical composition and therapeutic application. Evid. Based. Complement. Alternat. Med. 2015: 297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalander C. H., Fidjeland J., Diener S., Eriksson S., and Vinnerås B.. . 2015. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustainable Dev. 35: 261–271. [Google Scholar]
- Nagar S. K. 1968. On the significance of the duration of preoviposition and oviposition peroids in ixodid ticks. Acarologia. 10: 621–629. [PubMed] [Google Scholar]
- Nakamura S., Ichiki R. T., Shimoda M., and Morioka S.. . 2016. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: low-cost and year-round rearing. Appl. Entomol. Zool. 51: 161–166. [Google Scholar]
- Newton G. L., Booram C. V., Barker R. W., and Hale O. M.. . 1977. Dried Hermetia illucens larvae meal as a supplement for swine. J. Anim. Sci. 44: 395–400. [Google Scholar]
- Nicolson S. W., Nepi M., and Pacini E.. . 2007. Nectaries and nectar. Springer, Netherlands. [Google Scholar]
- Oldroyd H. 1973. Diptera brachycera. section (a) tabanoidea and asiloidea. Handbk. Ident. Br. Insects IX 4: 10–14. [Google Scholar]
- Oliveira F. R., Doelle K., and Smith R. P.. . 2016. External morphology of Hermetia illucens Stratiomyidae: Diptera (L.1758) based on electron microscopy. Annu. Res. Rev. Biol. 9: 1–10. [Google Scholar]
- Onsongo V. O., Osuga I. M., Gachuiri C. K., Wachira A. M., Miano D. M., Tanga C. M., Ekesi S., Nakimbugwe D., and Fiaboe K. K. M.. . 2018. Insects for income generation through animal feed: effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J. Econ. Entomol. 111: 1966–1973. [DOI] [PubMed] [Google Scholar]
- Oonincx D. G., Volk N., Diehl J. J., van Loon J. J., and Belušič G.. . 2016. Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. J. Insect Physiol. 95: 133–139. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N. T., Liedo P., Müller H. G., Wang J. L., Molleman F., and Carey J. R.. . 2010. Cost of reproduction in male medflies: the primacy of sexual courting in extreme longevity reduction. J. Insect Physiol. 56: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., and Prowse N.. . 1997. The effects of reproduction on longevity and fertility in male Drosophila melanogaster. J. Insect Physiol. 43: 501–512. [DOI] [PubMed] [Google Scholar]
- Pastor B., Čičková H., Kozánek M., Martí A., Takáč P., and Rojo S.. . 2011. Effect of the size of the pupae, adult diet, oviposition substrate and adult population density on egg production in Musca domestica (Diptera: Muscidae). Eur. J. Entomol. 108: 587–596. [Google Scholar]
- Ribeiro C., and Dickson B. J.. . 2010. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 20: 1000–1005. [DOI] [PubMed] [Google Scholar]
- Riley C. V., and Howard L. O.. . 1889. Hermetia mucens infesting bee-hives. Insects Life 1: 353–354. Secretary of Agriculture, Washington, D.C. [Google Scholar]
- Roy S., Kumar Gayen A., Mitra B., and Duttagupta A.. . 2014. Diversity, foraging activities of the insect visitors of Mustard (Brassica juncea Linnaeus) and their role in pollination in West Bengal. J. Zool. Stud. 1: 7–12. [Google Scholar]
- Rozkošný R. 1982. A biosystematic study of the European Stratiomyidae (Diptera). vol. 1 - Series Entomologica 21, p. 401. Dr. W Junk, The Hague, Boston, London. [Google Scholar]
- Samayoa A. C., Chen W. T., and Hwang S. Y.. . 2016. Survival and development of Hermetia illucens (Diptera: Stratiomyidae): a biodegradation agent of organic waste. J. Econ. Entomol. 109: 2580–2585. [DOI] [PubMed] [Google Scholar]
- Sánchez-Muros M.-J., Barroso F. G., and Manzano-Agugliaro F.. . 2014. Insect meal as renewable source of food for animal feeding: a review. J. Cleaner Prod. 65: 16–27. [Google Scholar]
- SAS Institute 2001. SAS/STAT(R) 9.3 User’s Guide, 1st electronic book. SAS Institute, Cary, NC. [Google Scholar]
- Sheppard D. C., Tomberlin J. K., Joyce J. A., Kiser B. C., and Sumner S. M.. . 2002. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 39: 695–698. [DOI] [PubMed] [Google Scholar]
- Srikanth C. D., Kuberappa G. C., and Shwetha B. V.. . 2013. Role of attractants on insect pollinators diversity with special reference to pollination in increasing the productivity of bottle gourd, Lagenaria siceraria L. Mysore J. Agric. Sci. 47: 16–21. [Google Scholar]
- Stadtlander T., Stamer A., Buser A., Wohlfahrt J., Leiber F., and Sandrock C.. . 2017. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 3: 165–175. [Google Scholar]
- Stephens C. S. 1975. Hermetia illucens (Diptera: Stratiomyidae) as a banana pest in Panama. Tropic. Agric. 52: 173–178. [Google Scholar]
- Tomberlin J. K., and Sheppard D. C.. . 2001. Lekking behavior of the black soldier fly (Diptera: Stratiomyidae). Florida Entomol. 84: 729–730. [Google Scholar]
- Tomberlin J. K., and Sheppard D. C.. . 2002. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 37: 345–352. [Google Scholar]
- Tomberlin J. K., Sheppard D. C., and Joyce J. A.. . 2002. Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Ann. Entomol. Soc. Am. 95: 379–386. [Google Scholar]
- Tomberlin J. K., Adler P. H., and Myers H. M.. . 2009. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 38: 930–934. [DOI] [PubMed] [Google Scholar]
- Tran G., Heuzé V., and Makkar H. P. S.. . 2015. Insects in fish diets. Anim. Front. 5: 37–44. [Google Scholar]
- Turner E. C., and Hair J. A.. . 1967. Effect of diet on longevity and fecundity of laboratory-reared face flies. J. Econ. Entomol. 60: 857–860. [Google Scholar]
- Veldkamp T., van Duinkerken G., Huis A., Lakemond C. M. M., Ottevanger E., Bosch G., and van Boekel T.. . 2012. Insects as a sustainable feed ingredient in pig and poultry diets: a feasibility study. Report 638. Wageningen UR Livestock Research, Netherlands. [Google Scholar]
- Verheyen G. R., Ooms T., Vogels L., Vreysen S., Bovy A., Van Miert S., and Meersman F.. . 2018. Insects as an alternative source for the production of fats for cosmetics. J. Cosmet. Sci. 69: 187–202. [PubMed] [Google Scholar]
- Vogel H., Müller A., Heckel D. G., Gutzeit H., and Vilcinskas A.. . 2018. Nutritional immunology: diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 78: 141–148. [DOI] [PubMed] [Google Scholar]
- Wang Y.-S., and Shelomi M.. . 2017. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 6: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Huang L., He J., Tomberlin J. K., Li J., Lei C., Sun M., Liu Z., and Yu Z.. . 2010. An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. J. Insect Sci. 10: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]