Abstract
Objective
To compare the cost-effectiveness of genetic testing strategies in patients with epilepsy of unknown etiology.
Methods
This meta-analysis and cost-effectiveness study compared strategies involving 3 genetic tests: chromosomal microarray (CMA), epilepsy panel (EP) with deletion/duplication testing, and whole-exome sequencing (WES) in a cost-effectiveness model, using “no genetic testing” as a point of comparison.
Results
Twenty studies provided information on the diagnostic yield of CMA (8 studies), EP (9 studies), and WES (6 studies). The diagnostic yield was highest for WES: 0.45 (95% confidence interval [CI]: 0.33–0.57) (0.32 [95% CI: 0.22–0.44] adjusting for potential publication bias), followed by EP: 0.23 (95% CI: 0.18–0.29), and CMA: 0.08 (95% CI: 0.06–0.12). The most cost-effective test was WES with an incremental cost-effectiveness ratio (ICER) of $15,000/diagnosis. However, after adjusting for potential publication bias, the most cost-effective test was EP (ICER: $15,848/diagnosis) followed by WES (ICER: $34,500/diagnosis). Among combination strategies, the most cost-effective strategy was WES, then if nondiagnostic, EP, then if nondiagnostic, CMA (ICER: $15,336/diagnosis), although adjusting for potential publication bias, the most cost-effective strategy was EP ± CMA ± WES (ICER: $18,385/diagnosis). While the cost-effectiveness of individual tests and testing strategies overlapped, CMA was consistently less cost-effective than WES and EP.
Conclusion
WES and EP are the most cost-effective genetic tests for epilepsy. Our analyses support, for a broad population of patients with unexplained epilepsy, starting with these tests. Although less expensive, CMA has lower yield, and its use as the first-tier test is thus not supported from a cost-effectiveness perspective.
Epilepsy affects approximately 0.6% to 0.8% of the general population.1,2 Even after a thorough diagnostic evaluation, a large proportion of patients have epilepsy of unknown etiology.3 However, 4% to 78% of selected patients with initially unknown etiology have genetic variants of probable or definitive etiologic significance.4–23 A genetic diagnosis typically ends what has been called a “diagnostic odyssey,” limits the pursuit of additional diagnostic investigations, facilitates reproductive counseling, and can potentially guide a tailored treatment related to the specific genetic cause involved.24
Clinical features often drive the choice of a particular genetic test or testing strategy, but in many patients, their presentation is not suggestive of a specific gene, or set of genes. For these patients, a stepwise approach starting with chromosomal microarray (CMA), if negative epilepsy gene panel (EP), and if negative whole-exome sequencing (WES) is often recommended.25 This recommendation is based on (1) the diagnostic yield of genetic tests in epilepsy: 0.06–0.12 for CMA,4,5,9,11,13,14,16,18 0.18–0.29 for EP,5–7,12,15,17,20,21,23 and 0.33–0.57 for WES,5,8,10,16,19,22 and (2) their costs, with CMA being the most affordable and WES the most expensive. Of note, no studies have systematically compared the diagnostic yield and cost-effectiveness of genetic tests in patients with epilepsy.
We aimed to address this gap in knowledge by comparing the diagnostic yield and cost-effectiveness of individual tests and commonly used testing strategies in patients with epilepsy of unknown etiology.
Methods
Study design
This is a systematic review, meta-analysis, and cost-effectiveness study. We used decision analysis, which explicitly compares the costs and effectiveness of competing clinical strategies, and evaluates how the variation in input parameters—cost and effectiveness of each strategy—influences outcomes to inform clinical decision-making.
Population of interest
Our population of interest consisted of patients with epilepsy of unknown etiology in whom a genetic diagnosis is considered in the clinical setting. The current study considers the clinical scenario whereby a patient with epilepsy does not have clinical features suggestive of any specific genetic syndrome and has undergone an initially unrevealing etiologic evaluation. Genetic testing is performed as an additional step in the etiologic evaluation for epilepsy of unknown etiology.
Outcome
The outcome measure for this analysis was reaching a genetic diagnosis for epilepsy. A genetic test was considered diagnostic when a genetic variant was definitively pathogenic or likely pathogenic. Furthermore, a genetic variant was considered definitively pathogenic when an association with epilepsy was already described in the literature, and was considered likely pathogenic when the genetic variant was not found in controls, or was significantly more frequent in the epilepsy cohort, or disrupted a molecular pathway in a way that probably disrupted function and caused epilepsy. This is in keeping with current American College of Medical Genetics guidelines used in clinical laboratories.26
The decision model: Competing strategies
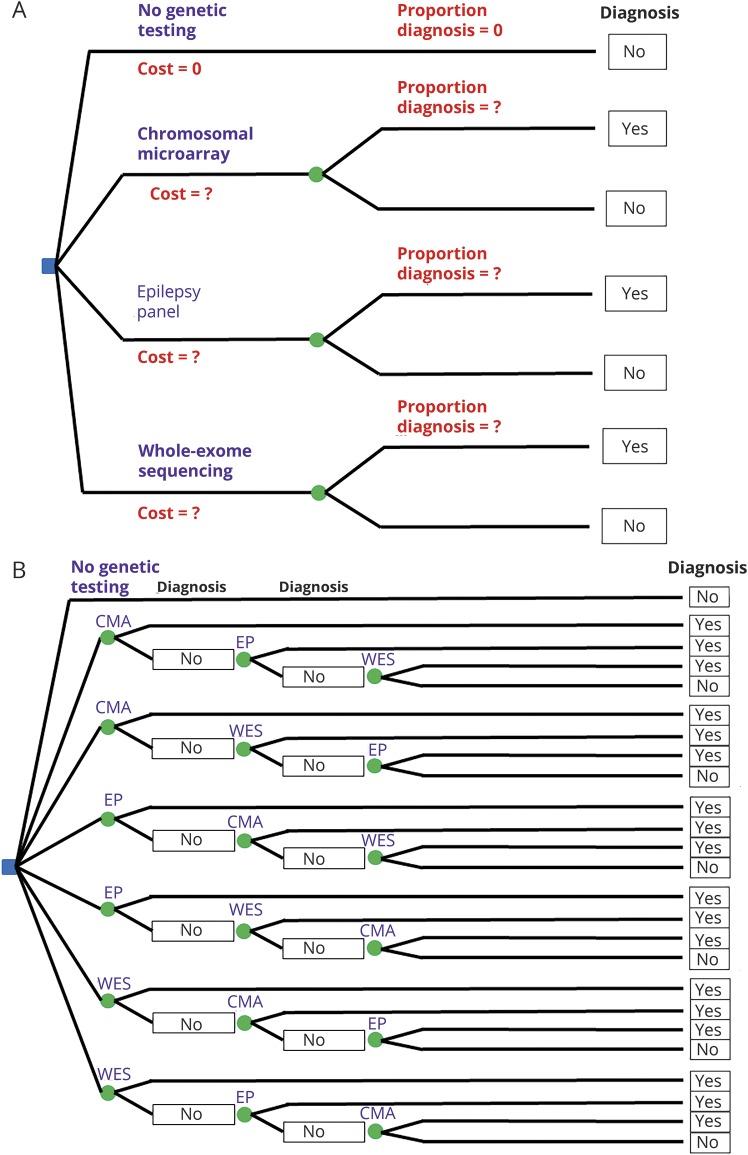
We compared 4 individual genetic testing strategies: CMA, EP, and WES vs “no genetic testing” (figure 1A), and the 6 diagnostic strategies that comprised all sequential combinations of these tests vs “no genetic testing” (figure 1B). In building our cost-effectiveness models, we used “no genetic testing” as a zero-cost point of comparison.
Figure 1. Decision trees for the comparison of individual tests (A) and of testing strategies (B).
(A) A patient with epilepsy of unknown etiology and no features suggestive of any particular genetic etiology can choose 4 individual tests: no genetic testing, CMA, EP, and WES. (B) A patient with epilepsy of unknown etiology and no features suggestive of any particular genetic etiology can choose 7 testing strategies: no genetic testing, CMA ± EP ± WES, CMA ± WES ± EP, EP ± CMA ± WES, EP ± WES ± CMA, WES ± CMA ± EP, and WES ± EP ± CMA. CMA = chromosomal microarray; EP = epilepsy panel; WES = whole-exome sequencing.
Input parameters for the model
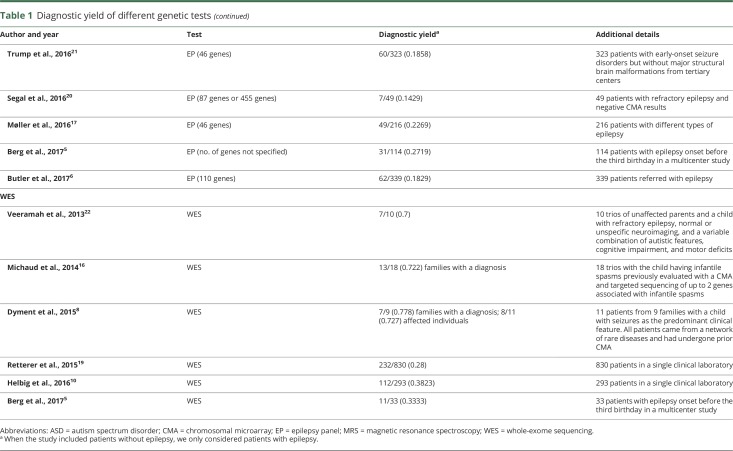
Effectiveness was estimated from the literature (table 1), with a systematic search of PubMed using the following terms: ([chromosomal microarray OR comparative genomic hybridization OR aCGH] OR [gene* panel OR epilepsy panel] OR [whole-exome sequencing, whole* sequencing]) AND (diagnos* OR utility OR yield) AND (epilep* OR seiz*). The search was restricted to full-length articles in the English language until November 2017 (data available from GitHub and Zenodo, figure e-1, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). We collected available genetic test list prices from major genetic laboratories in the United States. Because the analysis of parental samples is sometimes necessary to clarify the diagnostic significance of a variant, we considered the trio price when available.
Table 1.
Diagnostic yield of different genetic tests
Base case analysis
This analysis considers the most likely input parameters as if there were no uncertainty, and therefore, yields stable and fixed outcomes.27
Sensitivity analysis
To evaluate how robust the base case analysis is, sensitivity analyses were implemented to the extent to which the outcomes vary with variations of the input parameters.27 One-way sensitivity analysis varies one parameter at a time over a broad range but keeps other parameters constant.27 Second-order Monte Carlo simulations draw the values of the input parameters randomly from a distribution.27 Each parameter has a distribution that reflects the uncertainty for that parameter in the literature: values based on large studies or meta-analysis have distributions with little variance, but values based on small studies have wider distributions.27,28 Conventionally, to ensure the stability of the outputs, the model is run through 10,000 iterations, so that each distribution undergoes 10,000 random value draws.27 We confirmed that the model produced stable outputs with the conventional approach of 10,000 iterations.
Statistical analysis and statistical software
A meta-analysis of proportions was performed with R, version 3.4.1 (R Foundation for Statistical Computing: R-project.org/)29 and the meta30 package. In a meta-analysis, the sample size influences the weight of the study on the summary estimate. Because of expected heterogeneity among study populations and genetic tests, we considered a priori a random-effects model. This a priori choice was supported by the findings of between-study heterogeneity as measured by the I2 index.31 We evaluated for potential publication bias with funnel plots, and performed a sensitivity analysis correcting for publication bias using the trim and fill method by Duval and Tweedie.32 Input parameters were modeled with a β distribution for effectiveness and with a triangular distribution for cost. All cost-effectiveness analyses were performed using TreeAge Pro 2015 (TreeAge Software, Inc., Williamstown, MA). We quantified cost-effectiveness with the incremental cost-effectiveness ratio (ICER), which compares an option to the next most cost-effective alternative.27 Interactive versions of the meta-analysis and the cost-effectiveness models created with packages shiny,33 ggplot2,34 and plotly35 are available at app e-1 and bchepilepsygenetics.shinyapps.io/metaanalysisgenetictests/ for the interactive meta-analysis, app e-2 and bchepilepsygenetics.shinyapps.io/CEgeneticsindividualtests/ for the comparison of cost-effectiveness of individual tests, and app e-3 and bchepilepsygenetics.shinyapps.io/CEgeneticsstrategies/ for the comparison of cost-effectiveness of testing strategies. The comparison of strategies relies on the assumption of independent probabilities.
Data availability
The source code for the interactive models is available as an R script for Rstudio36 (app e-1–e-3). All supplementary files are available at GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and at Zenodo: zenodo.org/badge/latestdoi/138793909. All models, data, and results are available on request.
Results
Input parameters
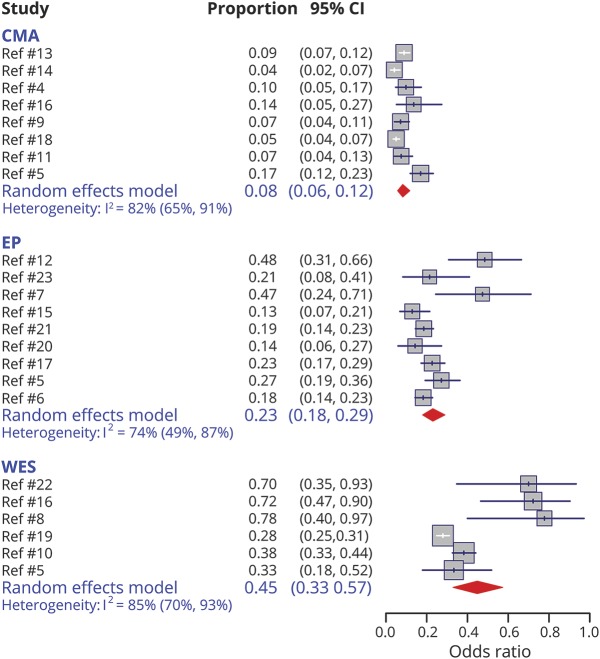
Twenty studies provided information on the diagnostic yield of CMA (8 studies), EP (9 studies), and WES (6 studies) (table 1). The meta-analysis showed that the diagnostic yield was highest for WES: 0.45 (95% confidence interval [CI]: 0.33–0.57), followed by EP: 0.23 (95% CI: 0.18–0.29), and CMA: 0.08 (95% CI: 0.06–0.12) (figure 2). Visual inspection of the funnel plots showed that, for WES, small studies were skewed toward higher diagnostic yield (data available from GitHub and Zenodo, figure e-2, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909).
Figure 2. Meta-analysis of the diagnostic yield of the different genetic tests.
CI = confidence interval; CMA = chromosomal microarray; EP = epilepsy panel; WES = whole-exome sequencing.
We therefore performed a sensitivity analysis using Duval and Tweedie's trim and fill method32 that showed an adjusted diagnostic yield for WES of 0.32 (95% CI: 0.22–0.44) (data available from GitHub and Zenodo, figure e-3, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909).
Therefore, in subsequent analyses, we present the values without and with correction for publication bias. Cost information relied on the list prices from major US genetic labs for CMA (6 laboratories), EP (4 laboratories), and WES (5 laboratories). The input parameters, as entered in the cost-effectiveness model, are presented in table 2.
Table 2.
Input parameters in the cost-effectiveness model
Comparison of individual tests
In the base case, the most cost-effective test was WES, with an ICER of $15,000/diagnosis. EP and CMA were not cost-effective alternatives to WES because a payer willing to pay $15,848/diagnosis for EP or $17,888/diagnosis for CMA will prefer to pay $15,000/diagnosis for WES as it yields more diagnoses per $ (figure 3A). One-way sensitivity analyses showed that CMA, with its current effectiveness, would only become the most cost-effective test if it were to cost $1,200 or less and, with its current cost, CMA would only become the most cost-effective test if it had a diagnostic yield of 0.1 or more (data available from GitHub and Zenodo, file e-1, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). Second-order Monte Carlo simulations showed that the cost-effectiveness of genetic tests markedly overlapped (figure 3B) and the acceptability curve showed that for a willingness to pay below approximately $13,500/diagnosis no genetic test was affordable, and WES was the most cost-effective test above a willingness to pay of approximately $13,500/diagnosis (figure 3C).
Figure 3. Comparison of individual tests: No genetic testing (orange diamond), CMA (red square), EP (blue triangle), and WES (green circle).
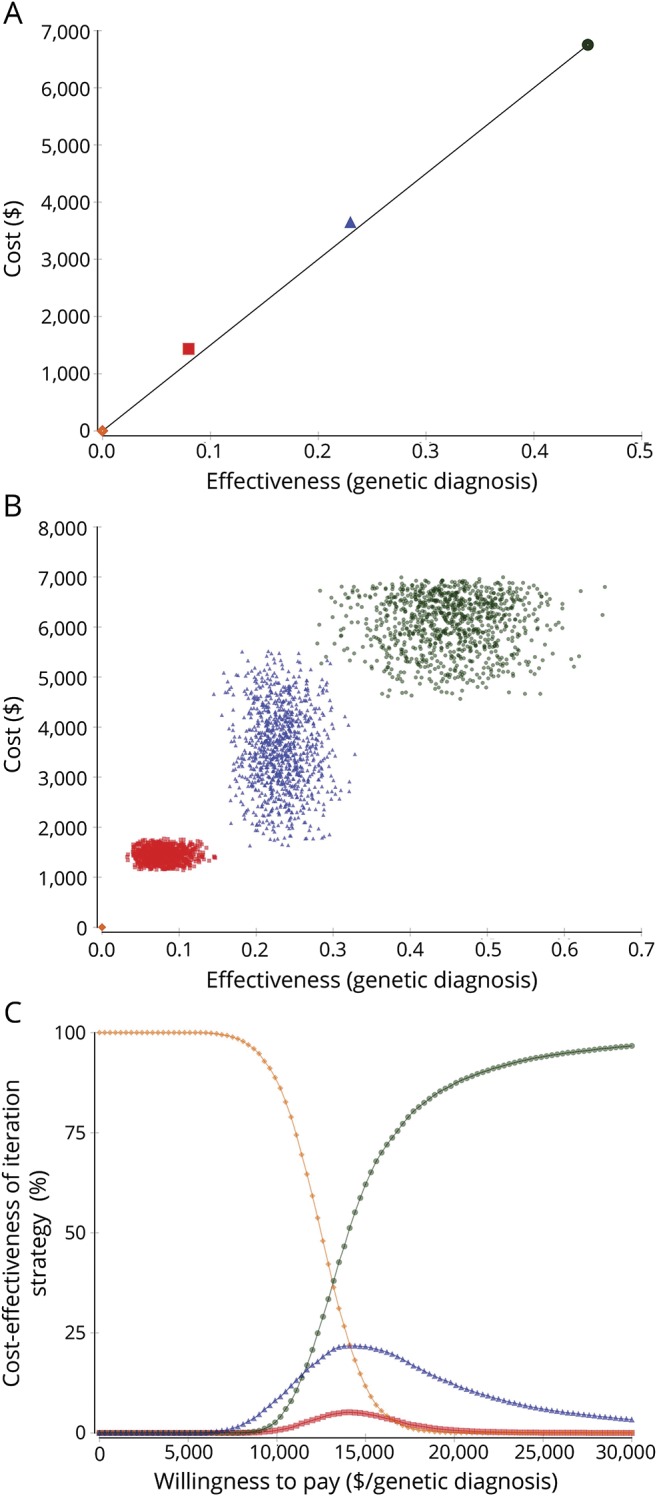
(A) Base case analysis. The x-axis measures effectiveness as diagnostic yield and the y-axis measures cost in US dollars. The most desirable genetic tests would have a high effectiveness (and would be close to 1 in the x-axis) and a low cost (and would be close to 0 in the y-axis). To compare cost-effectiveness, the competing options are compared based on the ICER. To calculate the ICER, the incremental (compared to the next most cost-effective strategy) cost of a strategy is divided by the incremental (compared to the next most cost-effective strategy) effectiveness.27,28 In cost-effectiveness, a lower ICER marks a more cost-effective option because that genetic test diagnoses more patients per dollar spent. The black line that links the most cost-effective strategies marks the efficiency frontier. Genetic tests above this line are less cost-effective than the alternatives in the line because they are less effective, more costly, more costly and less effective, or their increase in cost per unit of effectiveness (their ICER) is higher than the ICER of other strategies. Therefore, WES is the most cost-effective strategy. EP and CMA are slightly above the efficiency frontier. (B) Probabilistic sensitivity analysis (second-order Monte Carlo simulations). CMA, EP, and WES markedly overlap in cost-effectiveness. There is no possible efficiency frontier that leaves any strategy clearly above it. (C) Acceptability curve. The willingness to pay (in the x-axis) measures how many dollars a payer (a patient in this analysis) is willing to pay per a genetic diagnosis of epilepsy. The percentage of second-order Monte Carlo simulations where a particular option is the most cost-effective strategy is in the y-axis. For a willingness to pay of less than approximately $13,500/diagnosis, there is not enough money for any genetic test in most simulations. For a willingness to pay above approximately $13,500/diagnosis, WES is the most cost-effective strategy in most simulations. EP can be the most cost-effective alternative in some simulations between $10,000 and $20,000, while CMA is rarely the most cost-effective alternative. CMA = chromosomal microarray; EP = epilepsy panel; ICER = incremental cost-effectiveness ratio; WES = whole-exome sequencing.
With a sensitivity analysis adjusting for potential publication bias, in the base case, the most cost-effective option was EP with an ICER of $15,848/diagnosis followed by WES with an ICER of $34,500/diagnosis (figure 4A). One-way sensitivity analyses showed that CMA, with its current effectiveness, would only become the most cost-effective test if it were to cost $1,267 or less and, with its current cost, CMA would only become the most cost-effective test if it had a diagnostic yield of 0.1 or more (data available from GitHub and Zenodo, file e-1, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). Second-order Monte Carlo simulations showed that the cost-effectiveness of genetic tests markedly overlapped (figure 4B) and the acceptability curve showed that for a willingness to pay below approximately $15,200/diagnosis, no genetic test was affordable, EP was the most cost-effective option for a willingness to pay between approximately $15,200/diagnosis and $28,400/diagnosis, and WES was the most cost-effective test above a willingness to pay of approximately $28,400/diagnosis (figure 4C).
Figure 4. Comparison of individual tests: No genetic testing (orange diamond), CMA (red square), EP (blue triangle), and WES (green circle) adjusting for potential publication bias.
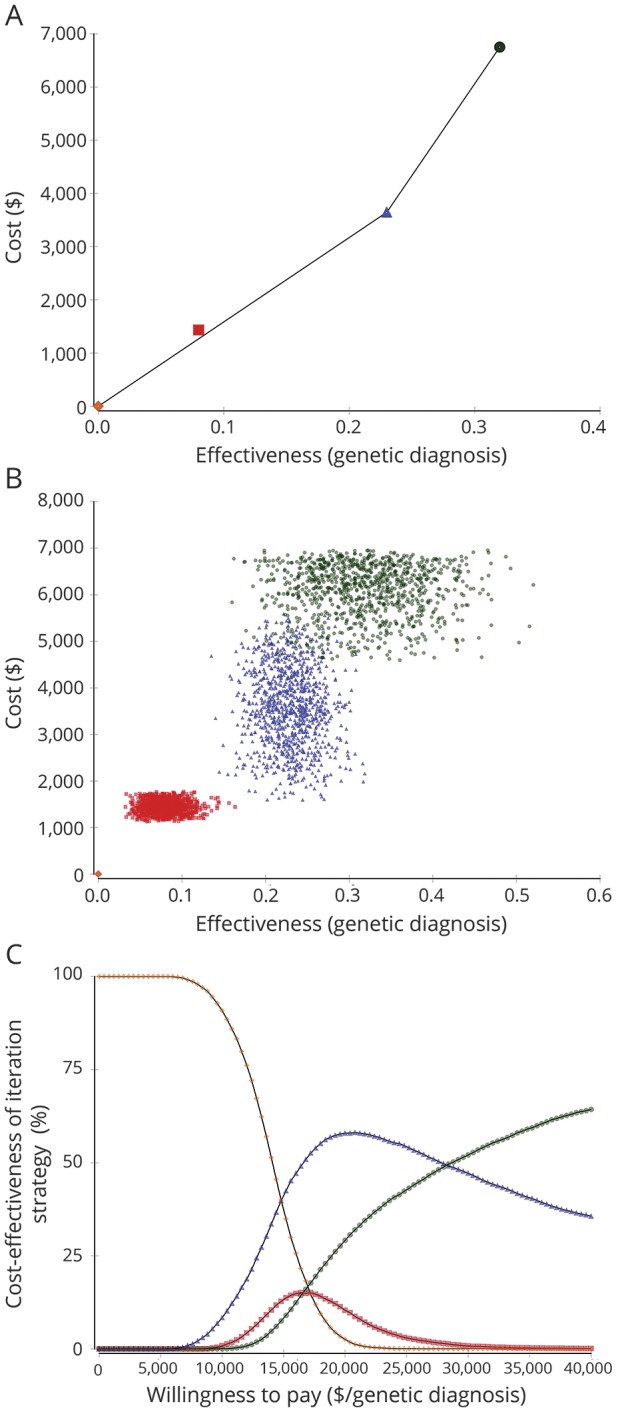
(A) Base case analysis. EP is the most cost-effective option followed by WES. CMA is slightly above the efficiency frontier. (B) Probabilistic sensitivity analysis (second-order Monte Carlo simulations). CMA, EP, and WES markedly overlap in cost-effectiveness. There is no possible efficiency frontier that leaves any strategy clearly above it. (C) Acceptability curve. For a willingness to pay of less than approximately $15,200/diagnosis, in most simulations, there is not enough money for any genetic test. For a willingness to pay between approximately $15,200/diagnosis and $28,400/diagnosis, in most simulations, EP is the most cost-effective strategy. For a willingness to pay above $28,400/diagnosis, WES is the most cost-effective option. CMA = chromosomal microarray; EP = epilepsy panel; WES = whole-exome sequencing.
Comparison of strategies
The comparison of strategies relies on the assumption of independent probabilities. In the base case, the most cost-effective strategy was WES ± EP ± CMA with an ICER of $15,336/diagnosis (data available from GitHub and Zenodo, figure e-4, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). Second-order Monte Carlo simulations showed that the cost-effectiveness of genetic tests markedly overlapped (figure e-4B), and the acceptability curve showed that for a willingness to pay below approximately $15,400/diagnosis, no genetic test was affordable, with WES ± EP ± CMA being the most cost-effective option above a willingness to pay of approximately $15,400/diagnosis (data available from GitHub and Zenodo, figure e-4C, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). With a sensitivity analysis adjusting for potential publication bias, in the base case, the most cost-effective option was EP ± CMA ± WES with an ICER of $18,385/diagnosis (data available from GitHub and Zenodo, figure e-5A). Second-order Monte Carlo simulations showed that the cost-effectiveness of genetic tests markedly overlapped (data available from GitHub and Zenodo, figure e-5B), and the acceptability curve showed that for a willingness to pay below approximately $19,500/diagnosis, no genetic test was affordable. EP ± WES ± CMA was the most cost-effective strategy for a willingness to pay between approximately $19,500/diagnosis and $21,000/diagnosis, and EP ± CMA ± WES for a willingness to pay above approximately $21,000/diagnosis (data available from GitHub and Zenodo, figure e-5C, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909).
Subpopulations
Our interactive web-based applications allow estimations of the most cost-effective test and the most cost-effective diagnostic strategy for each individual population (data available from GitHub and Zenodo, file e-2, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). We invite readers to evaluate in real time with our apps comparative cost-effectiveness of genetic tests with the inputs that better reflect their population of interest.
Discussion
The present study shows that the most cost-effective tests for obtaining a genetic diagnosis for patients with epilepsy are WES and EP. The recommendation to use the stepwise CMA ± EP ± WES testing strategy is not supported by current data on cost and effectiveness for these tests. An individualized evaluation of cost-effectiveness based on a priori diagnostic yields for each of the targeted populations and costs for each test is expected to optimize diagnostic yield and use of resources.
The choice of a particular genetic test is based, in part, on the patient phenotype. CMA is most frequently used in patients with epilepsy and developmental delay, autism, and/or dysmorphisms.37 This may in part reflect the early discovery of a role for copy number variations (CNVs) in epilepsy research9,13,18 and that CMA is generally recommended as the first-tier test for autism spectrum disorder, developmental delay, and intellectual disability.38,39 The rate of CNVs is likely higher in patients with epilepsy and intellectual disability than in patients with epilepsy without other neurologic conditions as shown by a series of 419 patients with genetic generalized epilepsy in which the rate of CNVs was higher in the 359 patients with intellectual disability than in the 60 patients without intellectual disability (10% vs 3%, p = 0.02).40 EP is typically the “second-step” genetic test when CMA is nonrevealing.25 WES is reserved for patients with epilepsy of unknown etiology in whom extensive clinical testing and extensive genetic testing have yielded nonrevealing results.37 Even the test with the highest diagnostic yield—WES—has limitations as it does not identify small deletions/duplications, CNVs, structural rearrangements, methylation abnormalities, or abnormalities in noncoding regions. Recent studies show that analysis of WES data can identify CNVs.41 While this practice has yet to be adopted widely in the clinical laboratory setting, it is likely that WES analysis will one day include detection of CNVs,41 which we anticipate will further improve the diagnostic yield and cost-effectiveness profile of WES since with limited additional cost, it will encompass the yield of CMA. Of note, there have been no cost-effectiveness studies comparing these tests with current yields and costs.
CMA may not be the most cost-effective test in a generic case of epilepsy of unknown etiology. However, in specific scenarios—epilepsy plus intellectual disability, epilepsy plus autism spectrum disorder, epilepsy with dysmorphic features—CMA may be the most cost-effective and clinically useful test. We provide interactive apps to evaluate in real time which tests and testing strategies are most cost-effective for specific patient populations, as exemplified in the data available from GitHub and Zenodo (file e-2, GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and Zenodo: zenodo.org/badge/latestdoi/138793909). Prior literature suggests that the decreasing costs of WES would make this approach more cost-effective than other genetic tests in the future.42 Our results show that in epilepsy the cost-effectiveness profile of either WES or EP is already better than that of CMA.
Decision analysis explicitly quantifies uncertainty in input parameters through sensitivity analyses. One source of uncertainty is the a priori probability of having a genetic etiology for epilepsy. The estimated proportion of individuals who carry a pathogenic variant that contributes substantially or causes epilepsy is approximately 17% of patients for epileptic encephalopathies, 5% of patients with genetic generalized epilepsies, and 2% for nonlesional focal epilepsies.43 In the literature reviewed for this analysis, the severity and prior evaluation were similar for patients studied with CMA and EP. In contrast, patients tested with WES often had been tested with CMA and even EP with nondiagnostic results. This can potentially underestimate or overestimate the diagnostic yield of WES: patients with negative CMA and EP might represent a population with lower incidence of a genetic etiology or they might represent a population in whom genetic testing is pursued further because of high clinical suspicion of a genetic etiology. The comparison of strategies relies on the assumption of independent probability, that is, the diagnostic yield of a test is independent of the diagnostic tests done before. The fact that the diagnostic yield may be influenced by prior genetic testing may limit the results of the comparison of strategies (figure 1B). However, until new studies evaluate the diagnostic yield of each test conditional on negative results of prior tests, we present the best available data for diagnostic yield at each step in the genetic evaluation. In part to incorporate additional information that becomes available, we have provided interactive applications so that the analysis can be updated with future, more granular information by the reader.
Genetic tests target heterogeneous genetic variants in different genes with commercially available EPs typically targeting from 70 to 465 genes.44 We used data from the literature, which comes from large reference centers and state-of-the-art laboratories. Excellence and expertise may optimize effectiveness and minimize costs in these centers, and these results may not be necessarily generalizable to other centers or laboratories. Another source of uncertainty is the rapidly evolving field of epilepsy genetics. The diagnostic yields and costs of different genetic tests are based on current data at the time of writing.
Cost-effectiveness studies are classified from the perspective or point of view that the analyses take. This analysis considered the patient perspective. Apart from cost-effectiveness, other factors may modify the preference for a genetic test. Results from CMA come back in 1–3 months, from EP in 2–4 months, and from WES in 3–6 months. Where prompt results may modify clinical management, faster turnaround may outweigh cost-effectiveness. Reaching a genetic diagnosis in epilepsy may modify treatment, although this occurs in a minority of cases. The most frequent benefits of a genetic diagnosis of epilepsy are difficult to quantify but include the answer to what is causing the disease, the ability to search for other symptoms associated with the gene variant, additional prognostic information, a sense of belonging to a specific support group for the families, informed reproductive choices, and possibly enrollment in clinical trials that are genotype specific.24
We focused on the most commonly used genetic tests for epilepsy. We did not evaluate the cost-effectiveness of other genetic tests such as targeted single-gene sequencing, single-gene duplication/deletion analysis, targeted mutation analysis, methylation studies, FISH (fluorescent in situ hybridization), or karyotype. We also did not evaluate whole-genome sequencing, which has shown a diagnostic yield of 32% in a series of 197 patients with epileptic encephalopathies45 and of 100% in a small series of 6 selected patients.46
Health care costs have been growing rapidly in recent decades and currently represent approximately one-third of the median household income for families in the United States.47 Health care costs and affordability are therefore considered an urgent problem in the United States that is leading more than a quarter of individuals to postpone care because of cost.47 Increases in health care costs in the United States are unsustainable,47,48 and one of the most realistic short-term strategies to address high prices include comparative cost-effectiveness analyses and competition based on transparent pricing and quality metrics.49,50 Decision analysis integrates results from the literature to provide insights that no individual series can yield but also identifies areas in which more data are needed. The diagnostic yield of CMA is well established. The heterogeneity in the diagnostic yield of EP likely reflects the different genes targeted by different EPs. In contrast, the heterogeneity in the diagnostic yield of WES likely reflects limited data and potential publication bias, hence future studies may prioritize this gap in knowledge. Heterogeneity in the diagnostic yield of each test also reflects different characteristics of the tested cohorts. Decision analysis (cost-effectiveness analysis) objectively evaluates the best available evidence to help guide clinical decision-making, where heterogeneity and uncertainty will always exist. The present study also provides a fully interactive analysis so that readers can tailor the analysis to the population of most interest to them and can update it as future evidence becomes available.
Parental testing for variants of unknown significance is performed free of charge by many laboratories,44 and when individual and trio testing had different prices, we considered the cost of trio testing. In this relatively simple model, we considered that the costs associated with DNA extraction, genetic counseling, and consultant clinical genetics were similar between CMA, EP, and WES. Future studies may take into account these more granular details to further refine the approach.
The validation of cost-effectiveness results usually entails corroboration with similar cost-effectiveness studies and third-order validation—comparison with actual clinical data. Neither cost-effectiveness studies nor clinical data are available for cost-effectiveness studies of genetic tests for epilepsy. The current report will fuel the development of the field of cost-effectiveness of genetic tests for epilepsy.
An individualized cost-effectiveness evaluation is expected to optimize diagnostic yield and use of resources. Although useful in many clinical scenarios, in our analysis, CMA does not prove to be the most cost-effective genetic test for epilepsy of unknown etiology when compared with WES or EP. The routine use of a stepwise CMA ± EP ± WES testing strategy is not supported by current data on cost-effectiveness for these tests. Rather, a strategy using initial evaluation with WES or EP, and then, if these are negative, CMA, is supported for patients with epilepsy of unknown etiology.
Acknowledgment
The authors appreciate the input from the Division of Epilepsy and Clinical Neurophysiology and the Epilepsy Genetics Program at Boston Children's Hospital for critical feedback and discussion.
Glossary
- CI
confidence interval
- CMA
chromosomal microarray
- CNV
copy number variation
- EP
epilepsy panel
- ICER
incremental cost-effectiveness ratio
- WES
whole-exome sequencing
Footnotes
Patient Page page e523
Author contributions
I.S.F. contributed to design and conceptualization of the study, data acquisition, data analysis, statistical analysis, data interpretation, and study drafting and revision for intellectual content. T.L. contributed to design and conceptualization of the study, data interpretation, and study drafting and revision for intellectual content. M.G.-L. contributed to design and conceptualization of the study, data acquisition, statistical analysis, data interpretation, and study drafting and revision for intellectual content. B.R.S. contributed to design and conceptualization of the study, data acquisition, data interpretation, and study drafting and revision for intellectual content. A.P. contributed to design and conceptualization of the study, data acquisition, data interpretation, and study drafting and revision for intellectual content.
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the content of this manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe: a systematic review. Eur J Neurol 2005;12:245–253. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 1996;71:576–586. [DOI] [PubMed] [Google Scholar]
- 3.Thomas RH, Berkovic SF. The hidden genetics of epilepsy: a clinically important new paradigm. Nat Rev Neurol 2014;10:283–292. [DOI] [PubMed] [Google Scholar]
- 4.Bartnik M, Szczepanik E, Derwinska K, et al. Application of array comparative genomic hybridization in 102 patients with epilepsy and additional neurodevelopmental disorders. Am J Med Genet B Neuropsychiatr Genet 2012;159B:760–771. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Coryell J, Saneto RP, et al. Early-life epilepsies and the emerging role of genetic testing. JAMA Pediatr 2017;171:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler KM, da Silva C, Alexander JJ, Hegde M, Escayg A. Diagnostic yield from 339 epilepsy patients screened on a clinical gene panel. Pediatr Neurol 2017;77:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Della Mina E, Ciccone R, Brustia F, et al. Improving molecular diagnosis in epilepsy by a dedicated high-throughput sequencing platform. Eur J Hum Genet 2015;23:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyment DA, Tetreault M, Beaulieu CL, et al. Whole-exome sequencing broadens the phenotypic spectrum of rare pediatric epilepsy: a retrospective study. Clin Genet 2015;88:34–40. [DOI] [PubMed] [Google Scholar]
- 9.Helbig I, Swinkels ME, Aten E, et al. Structural genomic variation in childhood epilepsies with complex phenotypes. Eur J Hum Genet 2014;22:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helbig KL, Farwell Hagman KD, Shinde DN, et al. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med 2016;18:898–905. [DOI] [PubMed] [Google Scholar]
- 11.Hrabik SA, Standridge SM, Greiner HM, et al. The clinical utility of a single-nucleotide polymorphism microarray in patients with epilepsy at a tertiary medical center. J Child Neurol 2015;30:1770–1777. [DOI] [PubMed] [Google Scholar]
- 12.Lemke JR, Riesch E, Scheurenbrand T, et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia 2012;53:1387–1398. [DOI] [PubMed] [Google Scholar]
- 13.Mefford HC, Muhle H, Ostertag P, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet 2010;6:e1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mefford HC, Yendle SC, Hsu C, et al. Rare copy number variants are an important cause of epileptic encephalopathies. Ann Neurol 2011;70:974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercimek-Mahmutoglu S, Patel J, Cordeiro D, et al. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia 2015;56:707–716. [DOI] [PubMed] [Google Scholar]
- 16.Michaud JL, Lachance M, Hamdan FF, et al. The genetic landscape of infantile spasms. Hum Mol Genet 2014;23:4846–4858. [DOI] [PubMed] [Google Scholar]
- 17.Møller RS, Larsen LH, Johannesen KM, et al. Gene panel testing in epileptic encephalopathies and familial epilepsies. Mol Syndromol 2016;7:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson H, Shen Y, Avallone J, et al. Copy number variation plays an important role in clinical epilepsy. Ann Neurol 2014;75:943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retterer K, Juusola J, Cho MT, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med 2016;18:696–704. [DOI] [PubMed] [Google Scholar]
- 20.Segal E, Pedro H, Valdez-Gonzalez K, et al. Diagnostic yield of epilepsy panels in children with medication-refractory epilepsy. Pediatr Neurol 2016;64:66–71. [DOI] [PubMed] [Google Scholar]
- 21.Trump N, McTague A, Brittain H, et al. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J Med Genet 2016;53:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeramah KR, Johnstone L, Karafet TM, et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia 2013;54:1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Gotway G, Pascual JM, Park JY. Diagnostic yield of clinical next-generation sequencing panels for epilepsy. JAMA Neurol 2014;71:650–651. [DOI] [PubMed] [Google Scholar]
- 24.Poduri A, Sheidley BR, Shostak S, Ottman R. Genetic testing in the epilepsies: developments and dilemmas. Nat Rev Neurol 2014;10:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ream MA, Patel AD. Obtaining genetic testing in pediatric epilepsy. Epilepsia 2015;56:1505–1514. [DOI] [PubMed] [Google Scholar]
- 26.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunink MMG, Weinstein MC, Wittenberg E, et al. Decision Making in Health and Medicine: Integrating Evidence and Values, 2nd ed. Cambridge, UK: Cambridge University Press; 2014. [Google Scholar]
- 28.Sánchez Fernández I, Gainza-Lein M, Loddenkemper T. Nonintravenous rescue medications for pediatric status epilepticus: a cost-effectiveness analysis. Epilepsia 2017;58:1349–1359. [DOI] [PubMed] [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing, version 3.4.1 [software]. Vienna: R Foundation for Statistical Computing; 2017. Available at: R-project.org/. [Google Scholar]
- 30.Meta: General Package for Meta-Analysis. R Package Version 4.3-2 [software]; 2015. Available at: CRAN.R-project.org/package=meta. [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 33.Shiny: Web Application Framework for R. R Package Version 0.14.2 [software]; 2016. Available at: CRAN.R-project.org/package=shiny. [Google Scholar]
- 34.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 35.Plotly: Create Interactive Web Graphics via 'plotly.js.' R package version 4.7.1 [software]; 2017. Available at: CRAN.R-project.org/package=plotly. [Google Scholar]
- 36.RStudio: Integrated Development for R, version 1.0.153 [software]. Boston: RStudio, Inc.; 2015. Available at: rstudio.com/. [Google Scholar]
- 37.Olson HE, Poduri A, Pearl PL. Genetic forms of epilepsies and other paroxysmal disorders. Semin Neurol 2014;34:266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010;86:749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaefer GB, Mendelsohn NJ; Professional Practice and Guidelines Committee. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med 2013;15:399–407. [DOI] [PubMed] [Google Scholar]
- 40.Mullen SA, Carvill GL, Bellows S, et al. Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology 2013;81:1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchida N, Nakashima M, Kato M, et al. Detection of copy number variations in epilepsy using exome data. Clin Genet 2018;93:577–587. [DOI] [PubMed] [Google Scholar]
- 42.Lu JT, Campeau PM, Lee BH. Genotype-phenotype correlation: promiscuity in the era of next-generation sequencing. New Engl J Med 2014;371:593–596. [DOI] [PubMed] [Google Scholar]
- 43.EpiPM Consortium. A roadmap for precision medicine in the epilepsies. Lancet Neurol 2015;14:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers C, Jansen LA, Dhamija R. Review of commercially available epilepsy genetic panels. J Genet Couns 2016;25:213–217. [DOI] [PubMed] [Google Scholar]
- 45.Hamdan FF, Myers CT, Cossette P, et al. High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am J Hum Genet 2017;101:664–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin HC, Kim GE, Pagnamenta AT, et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet 2014;23:3200–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emanuel EJ, Glickman A, Johnson D. Measuring the burden of health care costs on US families: the affordability index. JAMA 2017;318:1863–1864. [DOI] [PubMed] [Google Scholar]
- 48.Dieleman JL, Squires E, Bui AL, et al. Factors associated with increases in US health care spending. JAMA 2017;318:1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conway PH. Factors associated with increased US health care spending: implications for controlling health care costs. JAMA 2017;318:1657–1658. [DOI] [PubMed] [Google Scholar]
- 50.Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA 2016;316:858–871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The source code for the interactive models is available as an R script for Rstudio36 (app e-1–e-3). All supplementary files are available at GitHub: github.com/IvanSanchezFernandez/CostEffectivenessGeneticTests and at Zenodo: zenodo.org/badge/latestdoi/138793909. All models, data, and results are available on request.