Abstract
Objective
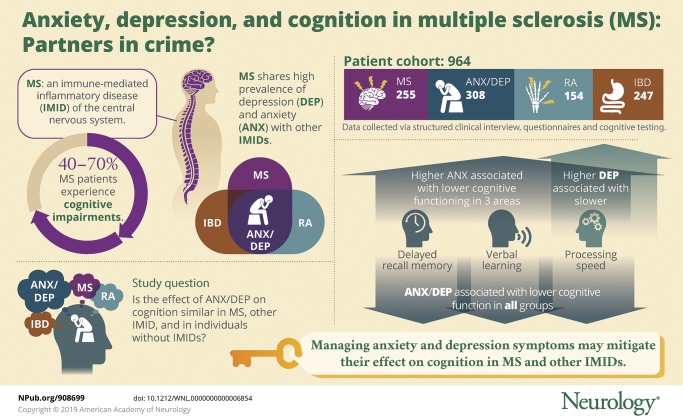
To determine whether anxiety and depression are associated with cognition in multiple sclerosis (MS), and whether these associations are similar in other immune-mediated inflammatory diseases (IMID; including inflammatory bowel disease [IBD] and rheumatoid arthritis [RA]) and in anxious/depressed individuals (ANX/DEP) without an IMID.
Methods
Participants (MS: n = 255; IBD: n = 247; RA: n = 154; ANX/DEP: n = 308) completed a structured psychiatric interview, the Hospital Anxiety and Depression Scale, and cognitive testing, including the Symbol Digit Modalities Test, the California Verbal Learning Test, and Letter Number Sequencing test. Test scores were converted to age-, sex-, and education-adjusted z scores. We evaluated associations of anxiety and depression with the cognitive z scores using multivariate linear models, adjusting for disease cohort.
Results
All cohorts exhibited higher rates of impairment (i.e., z less than or equal to −1.5) in the domains of processing speed, verbal learning, and delayed recall memory relative to general population norms. Higher levels of anxiety symptoms were associated with slower processing speed, lower verbal learning, and lower working memory performance (all p < 0.001); higher levels of depression symptoms were associated with slower processing speed. These associations did not differ across cohorts.
Conclusion
Anxiety and depression are associated with lower cognitive function in MS, with a similar pattern observed in persons with other IMID, including IBD and RA, and persons without an IMID. Managing symptoms of anxiety and of depression in MS, as well as other IMIDs, is important to mitigate their effect on cognition.
Multiple sclerosis (MS) is an immune-mediated inflammatory disease (IMID) of the CNS that shares features of inflammation and immune system dysregulation with other chronic diseases, such as inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), that affect different organ systems. Like MS, IBD and RA also share an increased prevalence of depression and anxiety as compared to the general population.1–3
Cognitive impairment is common in MS, affecting an estimated 40%–70% of individuals4; deficits in information processing speed are most often identified.5 Several studies suggest that comorbidities,6 including depression and anxiety, influence physical impairment in MS.7 Prior studies also suggest that depression influences cognition in MS, although findings have been inconsistent.8,9 While recent studies have suggested that anxiety is associated with impaired information processing speed in MS,10 few have jointly assessed the effects of depression and anxiety on cognition in MS.5 Moreover, it is uncertain whether the nature and magnitude of the effects of depression and anxiety on cognition in MS are the same as observed in other IMID without direct CNS involvement,11–13 or the same as for persons with depression and anxiety without an IMID.14 Such knowledge is of interest given the potential role of inflammation in psychiatric disorders including depression and anxiety.15
We aimed to examine the association of anxiety and depression on cognitive function in MS, and to determine whether the effects of anxiety and depression on cognition in MS were similar to those observed in IBD and RA, and in individuals with anxiety and depressive disorders (ANX/DEP) without an IMID. We hypothesized that those with MS, IBD, or RA who reported symptoms of anxiety and depression would exhibit greater cognitive impairments than those without such symptoms. We also hypothesized that such impairments would be more common in an IMID cohort with comorbid anxiety and depression than in a non-IMID cohort living with anxiety and depression.
Methods
Participants
As described elsewhere, 4 cohorts of participants from Manitoba, Canada, were enrolled in a longitudinal study of the effects of comorbid anxiety and depression in IMID.16 Individuals were eligible to participate if, in their lifetime, they had had any of the following: (1) definite MS,17 (2) definite IBD—including Crohn disease or ulcerative colitis,18 (3) definite RA,19 or (4) major depressive disorder (MDD) or any diagnosed anxiety disorder or both (ANX/DEP group).20 Diagnoses of MS, IBD, and RA were confirmed by medical records review. Current or lifetime diagnoses of MDD or an anxiety disorder were confirmed by the Structured Clinical Interview for DSM-IV (SCID-IV),20 since DSM-IV was in widespread use at the time of study inception. Posttraumatic stress disorder and obsessive-compulsive disorder were included as anxiety disorders as per the DSM-IV classification. Participants were required to be aged ≥18 years and able to provide informed consent. Participants were recruited through multiple methods: general community announcements (i.e., posters), approaching patients during or before their scheduled medical clinic visits, and contacting patients via established registries/clinic mailing lists.16
Standard protocol approvals, registrations, and patient consents
The study was approved by the University of Manitoba Health Research Ethics Board. All participants provided written informed consent.
Measures
Questionnaires captured sociodemographic characteristics including sex, date of birth, race, and total years of formal education. Participants completed questionnaires and cognitive testing on the day of enrollment. Structured psychiatric interviews were completed the same day for 37.4% of participants, within 1 week of enrollment for 97.8%, and within 2 weeks for the remainder.
We assigned diagnoses of MDD or an anxiety disorder using the SCID-IV20 in all participants. Although individuals without IMID were included in the ANX/DEP group if they had MDD or any diagnosed anxiety disorder in their lifetime, for these analyses, we considered only current diagnoses of MDD and any anxiety disorder. The lone exception to this was the exclusion of specific phobias that have not been found to influence cognition as much as other psychiatric disorders.21 Per the DSM-IV, current MDD and obsessive-compulsive disorder were defined as occurring over the last 2 weeks; posttraumatic stress disorder and panic disorder were classified as current if occurring in the last month; generalized anxiety disorder and social phobia were classified as current if symptoms persisted over the last 6 months.
Severity of current symptoms of depression and anxiety was determined via the Hospital Anxiety and Depression Scale (HADS),22 a 14-item questionnaire that assesses symptoms of depression (HADS-D) and anxiety (HADS-A) in the last week. Total scores range from 0 to 21 on each scale. The HADS has been validated for use in MS, IBD, RA, and the general population.23–26
Three neuropsychological tests were selected to broadly assess core domains of cognitive functioning: the California Verbal Learning Test, Second Edition (CVLT-II),27 Letter Number Sequencing (LNS) from the Wechsler Memory Scale, Third Edition,28 and the Symbol Digit Modalities Test (SDMT).29 The CVLT-II assesses the capacity to learn and remember verbal information, the LNS assesses working memory capacity, and the SDMT assesses processing speed. These measures were chosen to assess cognitive domains commonly reported as affected in those with MS, IBD, RA,4,30–32 and anxiety and depression disorders.21 The Wechsler Test of Adult Reading (WTAR)33 was included to estimate premorbid (i.e., relatively resistant to brain disease) cognitive functioning, based on single word reading.
Cognitive test scores were divided into 4 cognitive domains of interest. Raw scores in processing speed (SDMT), verbal learning (CVLT-II trials 1–5), and delayed recall memory (CVLT-II long-delay free-recall trial) were converted to z scores using regression-based norms adjusted for age, sex, and years of education.34 As regression-based norms were not available, raw scores for working memory (LNS) were converted to z scores based on normative data controlling for age.28 Participants were classified as unimpaired or impaired (i.e., z of −1.5 or lower)35 on each domain. Raw scores for estimated premorbid IQ (WTAR) were converted to standard scores adjusting for age and years of education.33
Analysis
To examine the effect of clinically meaningful symptoms of anxiety or depression on cognitive functioning, participants within each disease cohort were dichotomized based on their self-reported symptoms of anxiety (HADS-A score) and of depression (HADS-D score) (i.e., ≥11 vs <11). We chose this threshold based on several considerations. First, the initial publication of the HADS proposed that scores of 8–10 indicated possible anxiety or depression, while scores ≥11 indicated probable anxiety or depression.22 Second, although an initial validation study suggested that a cutpoint of 8 was optimal for the HADS in MS,36 more recent studies have suggested that scores ≥9–11 would be optimal, particularly for the HADS-A.26,37,38 Third, the specificity of the HADS-A and HADS-D are high (>90%) in IBD and RA at this threshold.23,25 Since the continuous HADS-A and HADS-D scores were highly correlated (r = 0.67), as expected,24 dichotomizing these results also reduced the correlation (r = 0.31), allowing us to examine both variables in the same models.
Given that the normative databases available to obtain z scores for the cognitive domains adjusted for different demographic variables, we developed 2 sets of multivariate models. First, multivariate analyses of variance (MANOVAs) were conducted using disease cohort (all 4 cohorts), HADS-A dichotomized, and HADS-D dichotomized as the independent variables. Three cognitive domains were included as the dependent variables in this model: processing speed (SDMT), verbal learning (CVLT-II Trials 1–5), and verbal delayed recall memory (CVLT-II Delayed Recall). Combining correlated cognitive domains that used the same normative data reduced the number of comparisons and increased power. Since these z scores were adjusted for age, sex, and years of education, these variables were not included in the model. Second, analyses of covariance (ANCOVAs) were conducted with working memory (LNS) as the dependent variable. Disease cohort, sex, and years of education were included as independent variables because this z score was only adjusted for age. In both models, HADS-A dichotomized and HADS-D dichotomized were also included as independent variables. For each multivariate model, we also included interaction terms between disease cohort and HADS-A dichotomized, and disease cohort and HADS-D dichotomized, to examine whether the effects of anxiety and depression on cognition differed across the disease cohorts. Statistically significant associations identified in the MANOVA/ANCOVA were followed by pairwise comparisons using the Tukey-Kramer adjustment. Partial eta-squared (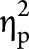 ), which measures the proportion of variance explained after accounting for other independent variables, was reported as a measure of effect size, interpreted as small (0.01), medium (0.09), or large (0.25).39
), which measures the proportion of variance explained after accounting for other independent variables, was reported as a measure of effect size, interpreted as small (0.01), medium (0.09), or large (0.25).39
Next, we conducted complementary analyses. First, because our initial threshold for dichotomizing the HADS optimized specificity over sensitivity, we repeated the analyses using more liberal thresholds of 9 for anxiety and 8 for depression.23,26 Second, we repeated the analyses using SCID-based diagnoses of any anxiety disorder and of MDD rather than HADS-A and HADS-D scores. Third, recognizing that panic disorder is characterized by episodic symptoms (panic attacks) whereas the other anxiety disorders are not, we repeated these latter analyses after excluding panic disorder from the SCID-based definition of any anxiety disorder. Finally, we examined the joint effects of symptoms of anxiety or depression by substituting a categorical variable representing 4 groups: HADS-A < 11 and HADS-D < 11, HADS-A < 11 and HADS-D ≥ 11, HADS-A ≥ 11 and HADS-D < 11, and both HADS-A and HADS-D ≥ 11.
Assumptions of multivariate normality were tested using P-P and Q-Q plots. Homogeneity of variance and covariance was assessed using discriminant function analysis. We report the Pillai trace as it is relatively robust to departures from these assumptions. Equality of error variances across dependent variables was assessed using the Levene test for ANCOVAs. Statistical analyses were conducted using SPSS Statistics version 23 (IBM SPSS Statistics for Windows, Armonk, NY).
Data availability
Ethical approval precludes the data being used for another purpose or being provided to researchers who have not signed the appropriate confidentiality agreement, per the Bannatyne Health Research Ethics Board, University of Manitoba.
Results
Participants
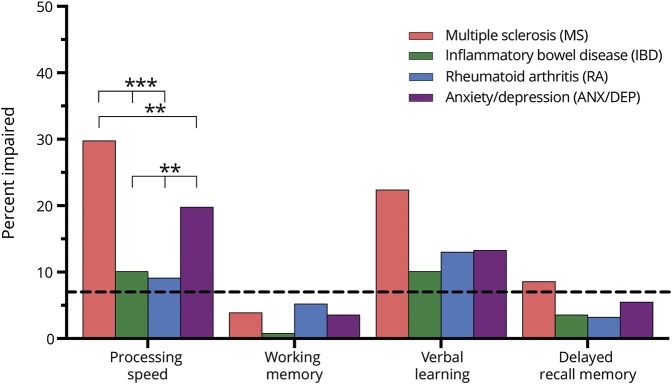
We enrolled 964 participants (table 1), who were predominantly female. Participants were generally highly educated, and the average age ranged from 43 to 59 years across cohorts. Although this was overall a midlife sample, younger and older individuals were represented in all cohorts. Participants were predominantly Caucasian. At the time of testing, 72.2% of the MS cohort was relapsing-remitting, 18.8% were secondary progressive, and 9.0% were primary progressive.
Table 1.
Participant characteristics
Anxiety and depression
As expected, the ANX/DEP cohort had the highest proportion of participants with current anxiety disorders as identified with the SCID (51.6%), followed by the IBD cohort (17.4%; table 1). Similarly, this cohort had the most participants reporting clinically meaningful symptoms of anxiety (HADS-A score ≥ 11; 61.4%) followed by the IBD cohort (16.7%). The ANX/DEP cohort also had the most participants meeting criteria for a current diagnosis of MDD (27.6%) using the SCID, and the highest percentage of individuals reporting clinically meaningful symptoms of depression (HADS-D score ≥ 11; 26.9%). The RA cohort had the next highest frequency of current MDD (11.0%) and of clinically meaningful depressive symptoms (9.8%). Notably, however, rates of clinically meaningful symptoms of anxiety and depression and current diagnoses of anxiety disorder and MDD did not differ statistically across the 3 IMID cohorts. Clinically meaningful symptoms of anxiety were more common than were depressive symptoms in all cohorts.
Across cohorts, the number of participants with clinically meaningful symptoms of depression but not anxiety was small (MS: 14 [5.5%], IBD: 6 [2.4%], RA: 7 [4.5%], ANX/DEP: 17 [5.5%]). Using thresholds of 8 for the HADS-D and of 9 for the HADS-A, rather than 11, the number of participants with clinically meaningful symptoms of depression without anxiety was modestly larger (MS: 34 [13.3%], IBD: 36 [14.6%], RA: 22 [14.3%], ANX/DEP: 92 [29.9%]). Symptom severity varied among individuals with current SCID-based diagnoses of an anxiety disorder or MDD. Among those with any anxiety disorder (n = 307), the mean (SD) HADS-A score was 11.8 (3.9), but 25% had scores <9, and 25% had scores of ≥15. Among those with current MDD (n = 150), the mean (SD) HADS-D score was 10.8 (4.0), but 25% had scores <8, and 25% had scores of ≥14. Among the 607 participants without an anxiety disorder or MDD, the mean (SD) HADS-A score was 5.8 (3.8) and 25% had scores of ≥8, and the mean (SD) HADS-D score was 4.15 (3.4). Thus, reasonable concordance existed between the symptom-based measures of anxiety and depression and the interview-based diagnostic measures, but there were some differences.
Cognitive function
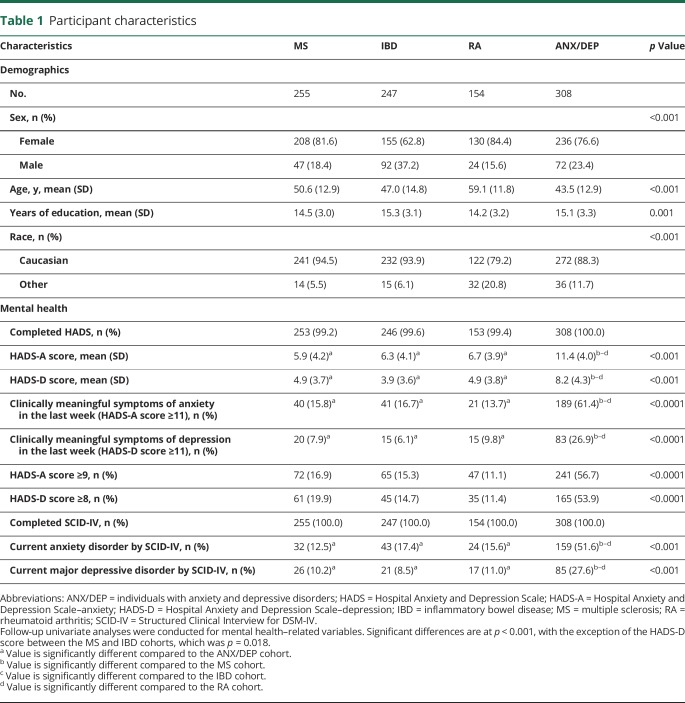
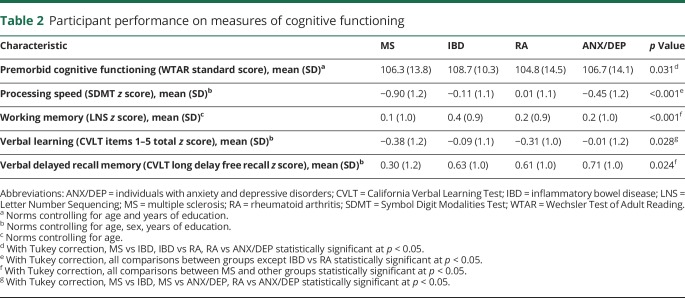
Participants differed little in their estimated premorbid IQ (WTAR), although the IBD cohort had slightly higher WTAR scores than the RA cohort (p = 0.026). Across the IMID cohorts, processing speed, verbal learning, and delayed recall memory were more commonly impaired (−1.5 SD below the mean or lower) and few participants (≤5.2%) had impaired working memory (figure 1). As expected, more persons in the MS cohort were impaired with respect to processing speed compared to the other 3 cohorts (table 2, p ≤ 0.001). The MS cohort also had the highest proportion of participants with impaired processing speed (30%), verbal learning (22%), and delayed recall memory (8%) (figure 1). Among participants in the MS cohort without clinically meaningful symptoms of either anxiety or depression (HADS-A and HADS-D < 11), impaired processing speed (26.6%), verbal learning (20.6%), and delayed recall memory (8.5%) were relatively common, although impaired working memory was not (3.0%).
Figure 1. Proportion of participants cognitively impaired by domain.
Impairment was defined as a score less than or equal to 1.5 SD below the mean (z score ≤−1.5); the proportion of individuals that could be expected to fall 1.5 SD below the mean is represented by the dotted line; *significant at α = 0.05, **significant at α = 0.01, ***significant at α = 0.001.
Table 2.
Participant performance on measures of cognitive functioning
When examining cognitive performance z scores, participants with MS performed worse than all other cohorts with respect to processing speed, falling approximately 1 SD below the other 2 IMID cohorts and the ANX/DEP cohort (table 2). Participants with MS also performed worse than all other cohorts with respect to delayed recall memory. The MS cohort performed below the IBD and ANX/DEP cohort on working memory, and below the IBD and ANX/DEP cohorts on verbal learning. The other 3 cohorts differed from one another only with respect to processing speed; the ANX/DEP performed worse than the IBD and RA cohorts (table 2).
Associations of anxiety and depression with cognition
In the MANOVA, the presence of clinically meaningful anxiety symptoms (HADS-A scores ≥11) was associated with cognitive test performance when adjusting for disease cohort (Pillai trace = 0.024, F3,951 = 7.67, p < 0.0001; 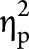 = 0.01). The subsequent analysis of variance (ANOVA) indicated that the presence of clinically meaningful anxiety symptoms was associated with decreased processing speed specifically (F1,959 = 20.73, p < 0.0001;
= 0.01). The subsequent analysis of variance (ANOVA) indicated that the presence of clinically meaningful anxiety symptoms was associated with decreased processing speed specifically (F1,959 = 20.73, p < 0.0001; 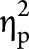 = 0.02), but not with verbal learning or delayed recall memory. In contrast, clinically meaningful symptoms of depression were not associated with cognitive functioning (p = 0.52). The MS cohort had lower processing speed performance than the other 3 cohorts (all p < 0.0001, Tukey corrected), lower verbal learning than the ANX/DEP cohort (p = 0.0001), and lower delayed recall memory than the IBD (p = 0.0031), RA (p = 0.026), and ANX/DEP cohorts (p < 0.0001).
= 0.02), but not with verbal learning or delayed recall memory. In contrast, clinically meaningful symptoms of depression were not associated with cognitive functioning (p = 0.52). The MS cohort had lower processing speed performance than the other 3 cohorts (all p < 0.0001, Tukey corrected), lower verbal learning than the ANX/DEP cohort (p = 0.0001), and lower delayed recall memory than the IBD (p = 0.0031), RA (p = 0.026), and ANX/DEP cohorts (p < 0.0001).
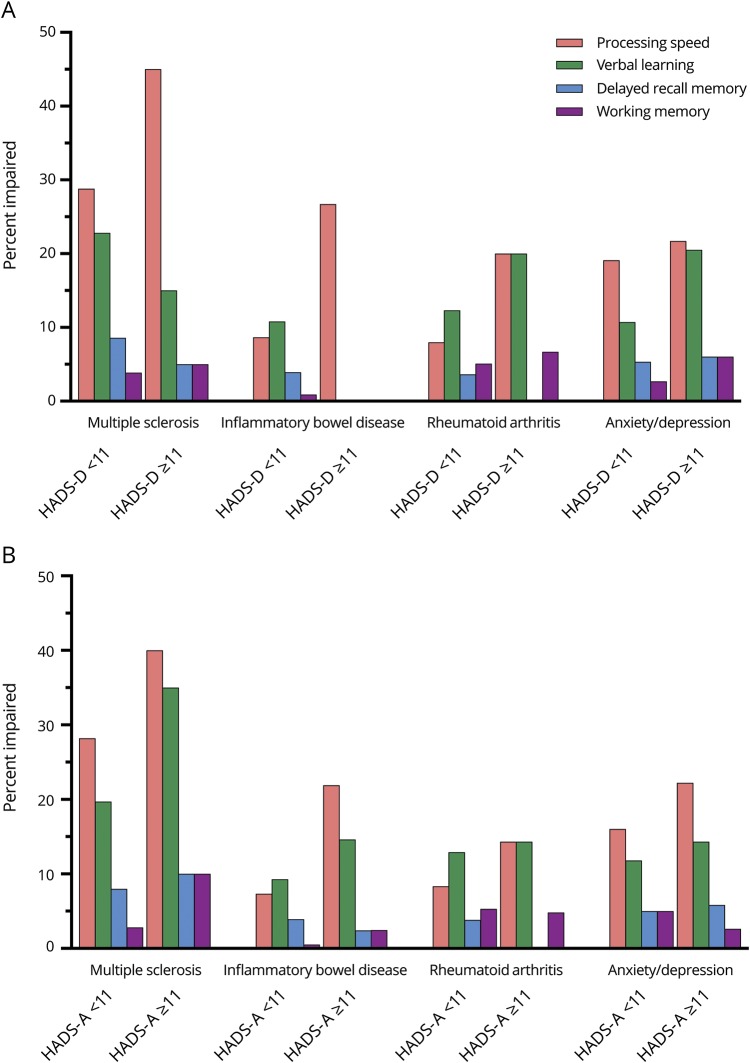
In the ANCOVA with working memory as the dependent variable, clinically meaningful symptoms of anxiety were associated with reduced working memory (F1,946 = 13.49, p < 0.001; 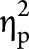 = 0.01) although symptoms of depression were not (p = 0.80). Working memory performance was lower in the MS and RA cohorts than in the IBD or ANX/DEP cohorts. We did not observe interactions between anxiety/depression symptoms and cognitive performance by disease cohort (figure 2).
= 0.01) although symptoms of depression were not (p = 0.80). Working memory performance was lower in the MS and RA cohorts than in the IBD or ANX/DEP cohorts. We did not observe interactions between anxiety/depression symptoms and cognitive performance by disease cohort (figure 2).
Figure 2. Proportion of participants cognitively impaired by domain, stratified by disease group and Hospital Anxiety and Depression Scale (HADS) scores.
(A) Depression. (B) Anxiety. Impairment was defined as a score less than or equal to 1.5 SD below the mean (z score ≤−1.5).
Complementary analyses
When we repeated the MANCOVA analyses above, using a threshold of 9 to dichotomize the HADS-A and a threshold of 8 to dichotomize the HADS-D, symptoms of anxiety were again associated with cognitive function (Pillai trace = 0.023, F3,951 = 7.48, p < 0.0001). Specifically, anxiety symptoms were associated with reduced processing speed (F1,953 = 18.7, p < 0.0001; 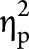 = 0.02) and reduced verbal learning (F1,953 = 7.14, p = 0.0077; np2 = 0.01). In this analysis, symptoms of depression were associated with cognitive function as well (Pillai trace = 0.0092, F3,951 = 2.94, p = 0.033), specifically with reduced processing speed (F1,953 = 8.58, p < 0.035;
= 0.02) and reduced verbal learning (F1,953 = 7.14, p = 0.0077; np2 = 0.01). In this analysis, symptoms of depression were associated with cognitive function as well (Pillai trace = 0.0092, F3,951 = 2.94, p = 0.033), specifically with reduced processing speed (F1,953 = 8.58, p < 0.035; 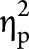 = 0.009). Depression symptoms were not associated with working memory in the ANOVA even when anxiety symptoms were not included in the model. However, symptoms of anxiety remained associated with working memory (F1,953 = 12.3, p = 0.0003;
= 0.009). Depression symptoms were not associated with working memory in the ANOVA even when anxiety symptoms were not included in the model. However, symptoms of anxiety remained associated with working memory (F1,953 = 12.3, p = 0.0003; 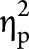 = 0.013).
= 0.013).
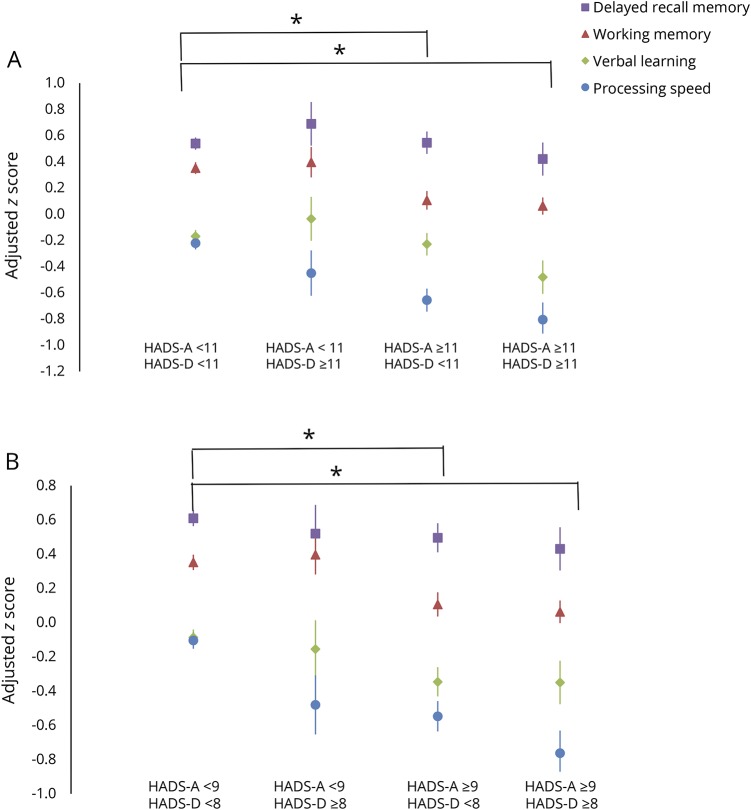
When we repeated the analyses above after substituting SCID-based diagnoses of an anxiety disorder or MDD for the HADS-A and HADS-D scores, diagnoses of an anxiety disorder were associated with cognitive test performance (Pillai trace = 0.012, F3,953 = 3.94, p = 0.0082) but MDD diagnoses were not (p = 0.058). Specifically, an anxiety disorder diagnosis was associated with reduced processing speed (F1,953 = 11.3, p = 0.0008; 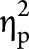 = 0.012). Removing panic disorder from the anxiety disorder category did not change our findings (data not shown). The joint effects of depression and anxiety symptoms were not statistically significantly greater than the effects of depression or anxiety alone on cognition, whether we used the HADS threshold of 11 (figure 3A) or the lower thresholds (figure 3B), indicating no synergistic effects.
= 0.012). Removing panic disorder from the anxiety disorder category did not change our findings (data not shown). The joint effects of depression and anxiety symptoms were not statistically significantly greater than the effects of depression or anxiety alone on cognition, whether we used the HADS threshold of 11 (figure 3A) or the lower thresholds (figure 3B), indicating no synergistic effects.
Figure 3. Adjusted mean (standard error) z scores for cognitive domains according to presence of clinically meaningful symptoms of depression and anxiety.
(A) Depression. (B) Anxiety. HADS = Hospital Anxiety and Depression Scale (A = anxiety, D = depression). *Statistically significant with Tukey correction for processing speed and working memory.
Discussion
We examined the association of current symptoms of anxiety and depression with cognitive function in MS as compared to 2 other IMID cohorts (IBD and RA) and in a non-IMID group with anxiety or depression (ANX/DEP). Based on the normal distribution, 7% of the population would be projected to meet criteria for impairment but these proportions were higher in the MS and the other study cohorts, particularly for the domains of processing speed and verbal learning. In the MS cohort, the observed pattern of cognitive impairments was consistent with previous demonstrations of impaired processing speed, verbal learning, and delayed recall memory in persons with MS.5 We also observed differences in the cognitive test z scores, whereby the MS cohort performed worse (0.6–0.8 SDs) than the other IMID cohorts and the ANX/DEP cohort. Thus, the MS cohort did appear to experience lower cognitive functioning compared to the other disease cohorts, though not necessarily at a level that met a formal threshold for impairment. Previous studies have also reported deficits in processing speed in IBD,30 and deficits in verbal memory in IBD and RA, in keeping with our findings.12,31 Working memory was the only cognitive domain examined in the current study in which elevated rates of impairment were not seen. However, as previous studies have identified working memory as an affected cognitive domain in MS, IBD, and RA,40–42 it is possible that the test of working memory that we employed lacks sensitivity for identifying impairment in these populations. Further studies with a broader range of working memory measures are clearly required.
Consistent with previous literature, we found that the prevalence of anxiety and depression in MS exceeded that expected for the general population,3 and was similar to the other IMID cohorts. Across all disease cohorts, current symptoms of anxiety were associated with reduced cognitive functioning. Specifically, greater self-reported symptoms of anxiety were associated with slower processing speed, lower working memory performance, and reduced verbal learning, but not with delayed recall memory. Symptoms of depression were associated with cognition in complementary analyses, specifically with reduced processing speed. However, this was only seen when using a more liberal threshold for identifying individuals as having clinically meaningful symptoms, which doubled the number of those individuals with depression without anxiety symptoms and thereby enhanced our power to detect an effect. Similar findings to our main analyses were also reported in a recent study examining anxiety, depression, and stress in 322 persons with MS, in that only anxiety remained independently associated with cognitive function.43 Neuroticism, which is associated with chronically elevated negative affect, has also been found to be associated with worse cognitive performance in MS.44
Notably, the influence of anxiety on cognitive functioning did not differ across cohorts regardless of the cutpoints used to identify meaningful symptoms, or the use of SCID-based disorder criteria. This indicates that clinically meaningful symptoms of anxiety had effects on cognitive functioning that may contribute directly to the cognitive impairments identified for those with MS, as well as for those with other IMID including IBD and RA. Associations of anxiety symptoms with poorer cognitive functioning have also been reported previously in studies of otherwise healthy adults living with a psychiatric condition,21 and these were evident in our ANX/DEP cohort as well.
Most studies examining the effect of psychiatric symptoms on cognitive functioning in MS have focused on depression. Mild symptoms of depression have typically not been associated with problematic cognitive functioning,9,45 although more severe depression has been associated with impaired working memory and information processing speed.46 Most studies of MS have found depression to be associated with deficits on tests that place demands on information processing speed as well as more complex aspects of attentional control and working memory.47,48 In the general population, depression has been found to commonly affect working memory, a domain in which few participants were impaired in the current study, and to have broad effects on executive functioning, which was not specifically assessed herein.21,45 However, the challenges in disentangling the potential role of comorbid anxiety symptoms on cognitive functioning in those with MDD are recognized.45 Moreover, clinically meaningful effects of anxiety symptoms (i.e., state anxiety) on processing efficiency and the control of attention by executive functions have been hypothesized as occurring in healthy populations.46
In our study, clinically meaningful symptoms of anxiety were much more common than were symptoms of depression (30.2% vs 13.8% of the total sample), and relatively few participants had meaningful depressive symptoms in the absence of meaningful anxiety symptoms. These characteristics of our study sample may have influenced the limited associations that were detected with depression. However, given that anxiety and depression may have similar influences on cognitive functioning despite the differences in their presenting symptoms, and given that they are commonly comorbid, future studies will also need to examine both types of symptoms concurrently. It is also important to note that while the effect sizes were small in our cohorts, the observation that anxiety and depression symptoms remained associated with cognitive functioning after accounting for other established large-magnitude contributors, such as age and years of education, demonstrates that these symptoms are indeed important factors affecting cognitive function in MS, as well as in other populations with IMID. As depression and anxiety symptoms did not fully explain cognitive impairment in IBD and RA, further study of the reasons for this is warranted in these cohorts. Potential contributors include systemic or intestinal inflammation,19 adverse treatment effects, and other comorbidities.
In all disease cohorts studied, a diagnosis of current anxiety disorder based on the SCID was associated with a decrease in cognitive performance but a diagnosis of MDD was not, based on our a priori analytic approach. This absence of an association with a diagnosis of MDD, despite the presence of an association with symptoms of depression, could reflect several factors. First, diagnoses of a disorder require a specific constellation of symptoms over a specific period of time, associated with some impairment of function. However, among those meeting diagnostic criteria, symptom severity can vary, as was seen in the variability in HADS scores that we observed among those with MDD and those with an anxiety disorder. Prior studies of individuals with MDD have reported that severity of symptoms is associated with the degree of cognitive impairment.49 Thus, even those individuals who do not meet formal diagnostic criteria for a disorder may still experience elevated symptoms of anxiety or depression that interfere with cognitive functions.
Our study has important limitations. Although the characteristics of our IMID study participants are largely consistent with the epidemiology of these conditions, we noted a slightly greater female predominance than expected in all cohorts.16 This may reflect an increased willingness of women to participate in research studies than men,47 thereby reducing the generalizability of our findings. Women are at increased risk of depression and anxiety in the general population as well as in those with MS, IBD, and RA.48 While sex-specific risks of cognitive impairment and sex-specific associations with symptoms of depression and anxiety may differ by disease, we did not pursue sex-specific analyses to explore effect modification by sex given the relatively small numbers of men in our study cohorts. Future studies specifically designed to address this issue may be needed. To reduce participant burden, cognitive assessments were limited to commercially available tests that could be readily administered in a clinic-type setting. Relevant cognitive domains, such as executive functions, were not assessed and more demanding tests of working memory and complex attention were not included. Future studies using more complex cognitive tasks may detect differences between persons with MS and other IMID such as IBD and RA, and may also reveal differences in the effects of comorbid anxiety and depression on cognitive functions in persons with these conditions. Other factors that may influence cognition, such as use of psychotropic and immunomodulatory therapies, as well as measures of disease activity and specific disease phenotypes (which differ across cohorts), were also not included. The inclusion of healthy controls in future studies would also support analyses to determine whether there are synergistic effects of psychiatric disorders and IMID on cognition. Finally, our analyses employed only the initial assessment of a planned longitudinal study and these additional data should eventually allow for better causal inferences regarding the role of anxiety/depressive symptoms on cognitive performance in these populations.
Strengths of our study include the use of 2 IMID and one non-IMID cohort for comparison with our MS cohort. Our analyses were also based on both self-reported symptom severity of anxiety and depression as well as on diagnostic classification for current anxiety and depression disorder as established via the SCID-IV.20 Although the range of cognitive domains assessed was limited, we examined multiple domains that are commonly affected in MS and other IMID and the proportions of cognitive impairments were considered in relation to base rates. Importantly, our analyses also allowed us to account for the presence of comorbid anxiety and depression, both at the level of symptom severity and disorder classification, when examining their effect on cognitive functioning.
We found that MS is associated with impairments in new learning, delayed recall memory, and processing speed and that impairment of information processing speed is more common in MS than other IMID populations. Anxiety disorders, and symptoms of anxiety and depression, were associated with reduced cognitive functioning in MS and these same associations were seen to a similar degree in those with IBD or RA, and in a cohort with psychiatric disorders without an IMID. While our ability to detect the effect of depression may have been limited, we found that greater self-reported symptoms of depression were associated with slower processing speed. Our findings suggest that in individuals with MS and other IMID in which anxiety and depression are common, and in individuals with a history of anxiety disorder or major depression, the severity of current anxiety and depression symptoms is more strongly associated with cognitive function than is a current formal diagnosis by DSM-IV criteria. Since symptoms of anxiety and depression represent potentially modifiable factors that influence the cognitive functioning of persons with MS and other populations, our findings highlight the importance of recognizing and managing these symptoms to mitigate their effect on the functioning of persons with these chronic conditions.
Glossary
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- ANX/DEP
individuals with anxiety and depressive disorders
- CVLT-II
California Verbal Learning Test, Second Edition
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HADS
Hospital Anxiety and Depression Scale
- HADS-A
Hospital Anxiety and Depression Scale–anxiety
- HADS-D
Hospital Anxiety and Depression Scale–depression
- IBD
inflammatory bowel disease
- IMID
immune-mediated inflammatory disease
- LNS
Letter Number Sequencing
- MANOVA
multivariate analysis of variance
- MDD
major depressive disorder
- MS
multiple sclerosis
- RA
rheumatoid arthritis
- SCID-IV
Structured Clinical Interview for DSM-IV
- SDMT
Symbol Digit Modalities Test
- WTAR
Wechsler Test of Adult Reading
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Editorial page 211
CME Course: NPub.org/cmelist
Contributor Information
Collaborators: CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease, Alan Katz, Lisa M Lix, Scott B Patten, Alexander Singer, Renee El-Gabalawy, and Ryan Zarychanski
Study funding
This study was funded by the Canadian Institutes of Health Research (THC-135234), Crohn's and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis (to R.A.M.). Dr. Bernstein is supported in part by the Bingham Chair in Gastroenterology. Dr. Sareen is supported by CIHR #333252. Dr. Stewart is supported through a Tier 1 Canada Research Chair in Addictions and Mental Health. The sponsors had no role in the design and conduct of the study, collection and interpretation of the data, or in the decision to submit the manuscript for publication.
Disclosure
C. Whitehouse is supported by a Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. J. Fisk receives research grant support from the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, the Nova Scotia Health Authority Research Fund, and the Dalhousie Medical Research Fund. C. Bernstein has consulted to AbbVie Canada, Ferring Canada, Janssen Canada, Pfizer Canada, Shire Canada, Takeda Canada, Napo Pharmaceuticals, 4D-Pharma, and Mylan Pharmaceuticals. He has received unrestricted educational grants from AbbVie Canada, Janssen Canada, Shire Canada, Pfizer Canada, and Takeda Canada. He has been on speaker's bureaus of AbbVie Canada, Ferring Canada, and Shire Canada. L. Berrigan receives research funding from the Canada Foundation for Innovation (CFI) and SSHRC. J. Bolton receives research funding from CIHR, Brain and Behavior Research Foundation, and the MS Society of Canada. L. Graff receives research funding from CIHR, the MS Society of Canada, and the Health Sciences Centre Foundation. C. Hitchon has research funds for unrelated studies from UCB Canada and Pfizer. J. Marriott has conducted trials for Biogen Idec and Roche and receives research funding from the MS Society of Canada and the MS Scientific Foundation and Research Manitoba. C. Peschken receives research funds from CIHR and the Lupus Research Alliance; has consulted to Astra Zeneca, Baxalta, and GlaxoSmithKline; and has conducted clinical trials for Celgene. J. Sareen receives research funding from CIHR and holds stocks in Johnson and Johnson. J. Walker receives research funding from CIHR. S. Stewart receives research funding from Canadian Institutes of Health Research, Social Sciences and Humanities Research Council, Manitoba Gambling Research Program, Nova Scotia Health Research Foundation, National Center for Responsible Gaming, and Nova Scotia Health Authority Research Fund. R.A. Marrie receives research funding from CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn's and Colitis Canada, National Multiple Sclerosis Society, and CMSC. She serves on the Editorial Board of Neurology®. Go to Neurology.org/N for full disclosures.
References
- 1.Marrie RA, Hitchon CA, Walld R, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res 2018;70:970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis 2016;22:752–762. [DOI] [PubMed] [Google Scholar]
- 3.Marrie R, Reider N, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler J 2015;21:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocca MA, Amato MP, De Stefano N, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 2015;14:302–317. [DOI] [PubMed] [Google Scholar]
- 5.Ruano L, Portaccio E, Goretti B, et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler 2017;23:1258–1267. [DOI] [PubMed] [Google Scholar]
- 6.Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol 2017;13:375–382. [DOI] [PubMed] [Google Scholar]
- 7.McKay KA, Tremlett H, Fisk JD, et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology 2018;90:e1316–e1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moller A, Wiedemann G, Rohde U, Backmund H, Sonntag A. Correlates of cognitive impairment and depressive mood disorder in multiple sclerosis. Acta Psychiatr Scand 1994;89:117–121. [DOI] [PubMed] [Google Scholar]
- 9.Good K, Clark CM, Oger J, Paty D, Klonoff H. Cognitive impairment and depression in mild multiple sclerosis. J Nerv Ment Dis 1992;180:730–732. [DOI] [PubMed] [Google Scholar]
- 10.Goretti B, Viterbo RG, Portaccio E, et al. Anxiety state affects information processing speed in patients with multiple sclerosis. Neurol Sci 2014;35:559–563. [DOI] [PubMed] [Google Scholar]
- 11.Berrill JW, Gallacher J, Hood K, et al. An observational study of cognitive function in patients with irritable bowel syndrome and inflammatory bowel disease. Neurogastroenterol Motil 2013;25:918-e704. [DOI] [PubMed] [Google Scholar]
- 12.Shin SY, Katz P, Wallhagen M, Julian L. Cognitive impairment in persons with rheumatoid arthritis. Arthritis Care Res 2012;64:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simos P, Ktistaki G, Dimitraki G, et al. Cognitive deficits early in the course of rheumatoid arthritis. J Clin Exp Neuropsychol 2016;38:820–829. [DOI] [PubMed] [Google Scholar]
- 14.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry 2008;69:1122–1130. [DOI] [PubMed] [Google Scholar]
- 15.Lotrich FE, El-Gabalawy H, Guenther LC, Ware CF. The role of inflammation in the pathophysiology of depression: different treatments and their effects. J Rheumatol 2011;88:48–54. [DOI] [PubMed] [Google Scholar]
- 16.Marrie RA, Graff LA, Walker JR, et al. A prospective study of the effects of psychiatric comorbidity in immune-mediated inflammatory disease: rationale, protocol and participation. JMIR Res Protoc 2018;7:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(suppl A):5–36. [DOI] [PubMed] [Google Scholar]
- 19.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–1588. [DOI] [PubMed] [Google Scholar]
- 20.First M, Gibbon M, Spitzer R, Williams J. User's Guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders: Research Version (SCID-I for DSM-IV-TR, November 2002 Revision). New York: New York Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 21.Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 2012;11:141–168. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein C, Zhang L, Lix L, et al. The validity and reliability of screening measures for depression and anxiety disorders in inflammatory bowel disease. Inflamm Bowel Dis Epub 2018 Apr 13. [DOI] [PMC free article] [PubMed]
- 24.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosomatic Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 25.Hitchon C, Zhang L, Graff L, et al. Validity of screening tools for depression and anxiety in rheumatoid arthritis. In: Canadian Rheumatology Association Meeting. Vancouver: British Columbia; 2018. [Google Scholar]
- 26.Marrie RA, Zhang L, Lix LM, et al. The validity and reliability of screening measures for depression and anxiety disorders in multiple sclerosis. Mult Scler Relat Disord 2017;20:9–15. [DOI] [PubMed] [Google Scholar]
- 27.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Second Edition, Adult Version Manual. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- 28.The Psychological Corporation. Wechsler Memory Scale–III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 29.Smith A. Symbol Digit Modalities Test, 9th ed. Torrance, CA: Western Psychological Services; 2002. [Google Scholar]
- 30.Golan D, Gross B, Miller A, et al. Cognitive function of patients with Crohn’s disease is associated with intestinal disease activity. Inflamm Bowel Dis 2016;22:364–371. [DOI] [PubMed] [Google Scholar]
- 31.Attree EA, Dancey CP, Keeling D, Wilson C. Cognitive function in people with chronic illness: inflammatory bowel disease and irritable bowel syndrome. Appl Neuropsychol 2003;10:96–104. [DOI] [PubMed] [Google Scholar]
- 32.Appenzeller S, Bertolo MB, Costallat LT. Cognitive impairment in rheumatoid arthritis. Methods Find Exp Clin Pharmacol 2004;26:339–343. [DOI] [PubMed] [Google Scholar]
- 33.The Psychological Corporation. The Wechsler Test of Adult Reading (WTAR). San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 34.Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RHB. The utility of regression-based norms in interpreting The Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). J Int Neuropsychological Soc 2010;16:6–16. [DOI] [PubMed] [Google Scholar]
- 35.Benedict RHB, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 2002;16:381–397. [DOI] [PubMed] [Google Scholar]
- 36.Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler 2009;15:1518–1524. [DOI] [PubMed] [Google Scholar]
- 37.Patten SB, Burton JM, Fiest KM, et al. Validity of four screening scales for major depression in MS. Mult Scler J 2015;21:1064–1071. [DOI] [PubMed] [Google Scholar]
- 38.Watson TM, Ford E, Worthington E, Lincoln NB. Validation of mood measures for people with multiple sclerosis. Int J MS Care 2014;16:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen J. A power primer. Psychol Bull 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 40.Berrigan LI, Lefevre JA, Rees LM, Berard J, Freedman MS, Walker LA. Cognition in early relapsing-remitting multiple sclerosis: consequences may be relative to working memory. J Int Neuropsychol Soc 2013;19:938–949. [DOI] [PubMed] [Google Scholar]
- 41.van Erp S, Ercan E, Breedveld P, et al. Cerebral magnetic resonance imaging in quiescent Crohn’s disease patients with fatigue. World J Gastroenterol 2017;23:1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum 2002;47:639–644. [DOI] [PubMed] [Google Scholar]
- 43.Ribbons K, Lea R, Schofield PW, Lechner-Scott J. Anxiety levels are independently associated with cognitive performance in an Australian multiple sclerosis patient cohort. J Neuropsychiatry Clin Neurosci 2017;29:128–134. [DOI] [PubMed] [Google Scholar]
- 44.Leavitt VM, Buyukturkoglu K, Inglese M, Sumowski JF. Protective personality traits: high openness and low neuroticism linked to better memory in multiple sclerosis. Mult Scler J 2017;23:1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 2013;139:81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion 2007;7:336–353. [DOI] [PubMed] [Google Scholar]
- 47.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol 2007;17:643–653. [DOI] [PubMed] [Google Scholar]
- 48.Marrie RA, Walld R, Bolton JM, et al. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosomatic Res 2017;101:17–23. [DOI] [PubMed] [Google Scholar]
- 49.Hartlage S, Alloy LB, Vazquez C, Dykman B. Automatic and effortful processing in depression. Psychol Bull 1993;113:247–278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Ethical approval precludes the data being used for another purpose or being provided to researchers who have not signed the appropriate confidentiality agreement, per the Bannatyne Health Research Ethics Board, University of Manitoba.