Abstract
Mitochondria are the double membrane organelles providing most of the energy for cells. In addition, mitochondria also play essential roles in various cellular biological processes such as calcium signaling, apoptosis, ROS generation, cell growth, and cell cycle. Mitochondrial dysfunction is observed in various neurological disorders which harbor acute and chronic neural injury such as neurodegenerative diseases and ischemia, hypoxia-induced brain injury. In this review, we describe how mitochondrial dysfunction contributes to the pathogenesis of neurological disorders which manifest chronic or acute neural injury.
Keywords: mitochondrial dysfunction, neural injury, mitochondria, neurodegeneration, neurological disorders
Introduction
Mitochondria structurally consist of two membranes (the inner and outer membrane), an intermembrane space and an internal matrix which contains mtDNA. Mitochondria are known as the “power house,” which provide 95% energy for cells. Mitochondria are important for calcium signaling (Brini et al., 2014), cell apoptosis (Tait and Green, 2013), production and sequestration of ROS (Shadel and Horvath, 2015). Mitochondrial dysfunction causes impairment in these processes such as impaired energy supply, Ca2+ buffering, increased ROS production and enhanced apoptosis, which contribute to neurodegeneration. In addition, mitochondria modulate synaptic plasticity via regulating neurotransmitter production and inactivation, the formation and maintenance of synapses, neuronal development, neurogenesis, axonal transport, synaptic plasticity (Levy et al., 2003; Cheng et al., 2010, 2012; Lopez-Domenech et al., 2016; Todorova and Blokland, 2017), which are closely linked to neurological disorders.
Mitochondria are dynamic organelles that adapt to physiological needs in different tissues. Neurons are dependent on mitochondrial oxidative phosphorylation (OXPHO) to fulfill their energy demands. Neurons have a limited capacity to upregulate glycolysis. In comparison to neurons, astrocytes, and oligodendrocytes are highly glycolytic, and are resilient to mitochondrial dysfunction (Dimonte et al., 1992; Fernandez-Fernandez et al., 2012). The number and morphology of mitochondria vary among cell types. Even in the same type cells, mitochondria change their number and morphology in response to different environments. Mitochondria keep a balance in their number, structure and function (mitochondrial homeostasis) (Van Blerkom, 2009), which plays an important physiological role in maintaining cell homeostasis (Fischer et al., 2012; Marzetti et al., 2013; Muller et al., 2015). Impairment in mitochondrial homeostasis was observed in neurological disorders which manifest chronic or acute neural injury such as neurodegenerative diseases, cerebral ischemia, cerebral hypoxia and other brain injuries (Calkins et al., 2011; Witte et al., 2014; Liu et al., 2016; Suarez-Rivero et al., 2016; Ottolini et al., 2017; Cheng and Bai, 2018; Ludwig et al., 2018).
Mitochondrial Homeostasis
The morphology and function of mitochondrial networks are regulated by continuous fusion and fission cycles, which constitutes a quality control system to maintain mitochondrial function. When the mitochondria are damaged, the damaged mitochondria fuse with the surrounding healthy mitochondria, thus alleviating the slight damage. If the mitochondria are severely damaged, the damaged mitochondria will be transported to the lysosomes for degradation through a process called mitophagy. While new mitochondria continue to divide to maintain the number of qualified mitochondria. By these methods, the cells control dynamic balance of the mitochondrial network, thus maintaining cell homeostasis (Fischer et al., 2012; Marzetti et al., 2013; Muller et al., 2015).
Mitochondrial Fission and Fusion
Mitochondrial fission/fusion refers to the mutual fusion and recleavage of two lipid bilayers of mitochondria (Hoppins et al., 2007). Mitochondrial fission and fusion play critical roles in maintaining mitochondrial homeostasis when cells experience metabolic or environmental stresses. When the mitochondria are damaged, fusion rescues stress by allowing functional mitochondria to complement dysfunctional mitochondria by diffusion and sharing of components between organelles. Fission is needed to create new mitochondria. However, it also contributes to the mitochondrial quality control by enabling the removal of damaged mitochondria (Youle and van der Bliek, 2012; Meyer et al., 2017).
Fission is mediated by members of the cytoplasmic dynein family [Dynamin1 (Dnm1) in yeast, worms, and Dynamin-related protein1 (Drp1) in flies and mammals]. Drp1 is recruited from the cytoplasm to spiral around the mitochondria, contracting to sever internal and external membranes. Yeast shares the common function of Drp1 with mammals but requiring a unique accessory protein. Mitochondrial division protein 1 (Mdv1) recruits Dnm1 to the mitochondrial fission site in yeast. Whereas mitochondrial dynamics protein 49 (Mid49), Mid51, and mitochondrial fission factor (Mff) recruit Drp1 to sites of mitochondrial and endoplasmic reticulum contact in mammals (Friedman et al., 2011; Elgass et al., 2013). Fusion between mitochondrial outer membranes is mediated by mitofusin 1 (Mfn1) and Mfn2, members of the membrane anchoring motor protein family in mammals. Whereas fusion between mitochondrial inner membranes is mediated by a single dynamin family member, Opal (Dynamin-like 120 kDa protein, mitochondrial 1, in mammals). Mitochondrial fission and fusion mechanisms are regulated by protein levels and post-translational modifications (Hoppins et al., 2007).
Mitochondrial Transport
A variety of studies have shown that mitochondrial transport is impaired in patients with neurodegenerative diseases or brain injury. Mitochondrial transport in neuronal cells is closely related to neurological diseases (Ebrahimi-Fakhari et al., 2016). Mitochondria are a power factory for cells that supply a large amount of ATP to meet the physiological needs of neurons. However, due to the complex structure of neurons, it is difficult to compare the energy requirements with ordinary cells (Zhou et al., 2014). Compared to ordinary cells, neurons need to transport mitochondria to distal synapses and therefore have higher energy requirements. The process of mitochondrial transport from the cell body to the axon is anterograde transport, and the process of transporting from the axon back to the cell body is the retrograde transport. The anterograde transport of mitochondria in normal neurons maintains a dynamic balance with the retrograde transport. When the neurons are induced by external pressure or the integrity is impaired, the damaged mitochondria preferentially degenerate from the axon transported cysts (Cai et al., 2012). The balance with retrograde transportation is broken.
Mitophagy
Mitophagy refers to the process of cells selectively removing excess or damaged mitochondria through autophagy, which plays an important role in the mitochondrial quality control and cell survival (Pickles et al., 2018). There are many ways to regulate mitophagy. The PINK1/Parkin pathway has emerged as a critical pathway in the regulation of mitophagy as well as mitochondrial function (Clark et al., 2006; Narendra et al., 2008). Dysfunctional mitochondria fail to import and degrade PINK1, which stabilizes on the outer mitochondrial membrane (OMM). After PINK1 accumulation, PINK1 phosphorylates ubiquitin and Parkin to activate the E3 ligase activity of Parkin. Parkin ubiquitinates substrates on the outer mitochondria for two divergent processes: autophagosome recruitment and ubiquitin-proteasome degradation of ubiquitinated mitochondrial substrates. Then autophagy receptors such as NBR1, Nix, TAxBP1, NDP51, optineurin and FUNDC1 on the outer membrane binds LC3 to recruit the autophagosomes around the damaged mitochondria. The autophagosome is then delivered to the lysosome for degradation (Pickrell and Youle, 2015; Chu, 2018). If mitochondria are damaged irreversibly, they will initiate autophagic clearance. Impaired mitophagy is closely related to brain injury in ischemic stroke and neurodegenerative diseases.
Mitochondrial Dysfunction in Hypoxic-Ischemic Brain Injury
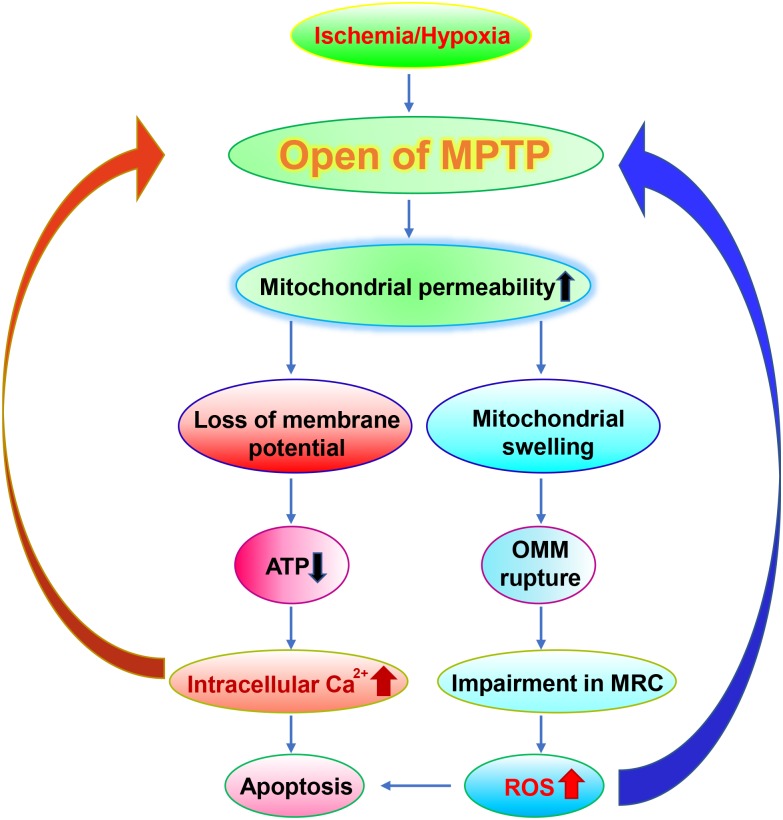
Mitochondria are composed of two membrane structures with high permeability of the outer membrane and relatively low permeability of the endometrium. The mitochondrial permeability transition pore (MPTP) is a non-specific voltage-dependent special protein complex which crosses the mitochondrial outer membrane and control the mitochondrial permeability (Halestrap, 2009). In the physiological state, MPTP is in the off state. Whereas MPTP is open during ischemia, which is triggered by both the Ca2+ overload in the mitochondrial matrix and the elevated oxidative stress (Malkevitch et al., 1997; Kushnareva and Sokolove, 2000; Kim et al., 2006). The opening of MPTP leads to the increase of mitochondrial permeability, which allows the solute such as water, large molecules and ions to enter freely into the mitochondrial matrix. The latter in turn causes mitochondrial swelling, the outer membrane to rupture, the impairment of the MRC, leading to an abundant release of reactive oxygen species (ROS) (Broekemeier et al., 1998; Krasnikov et al., 2005). Moreover, the increased mitochondrial permeability also causes loss of membrane potential, further leading to decreased cellular mitochondrial ATP levels and enhanced intracellular Ca2+ concentration (Zorov et al., 2000), and then activation of endogenous apoptotic pathways, consequently inducing ischemia-hypoxia-induced neuronal damage (Broughton et al., 2009) (Figure 1). Consistent with these observations, the immunosuppressant compound cyclosporine A, which is capable of inhibiting MPTP, exhibits a neuroprotective effect on acute brain injury caused by ischemia (Sullivan et al., 2000; Uchino, 2002) (Table 1).
FIGURE 1.
Roles of MPTP in ischemia. In the ischemia, MPTP is open, which leads to loss of the membrane potential and the rupture of OMM. The damaged mitochondria exhibit reduced ATP generation, impaired MRC and increased Ca2+ flux which results in elevated generation of ROS and apoptosis respectively. It is worth noting that elevated ROS levels and apoptosis in turn further exacerbate the damages on MPTP.
Table 1.
Compounds targeting mitochondria in the treatment of ischemia, PD, and AD.
| Compounds | Mechanisms of action | Disorders | Therapeutic effects | Reference |
|---|---|---|---|---|
| Cyclosporine A | Inhibiting MPTP | Ischemia-caused acute brain injury | Pre-clinical studies | Sullivan et al., 2000; Uchino, 2002 |
| Tirilazad mesylate | Lipid peroxidation inhibitor | Ischemia-caused brain injury | Effective in pre-clinical models; showing no effective in clinical studies | Park and Hall, 1994; Fleishaker et al., 1995 |
| Edaravone | Free radical scavenger | Ischemia-caused brain injury | Exhibited clinical improvements in acute ischemic stroke patients | Edaravone Acute Infarction Study Group, 2003 |
| Lubeluzole | Reduces NO levels and subsequent ONOO- production | Ischemia-caused brain injury | Effective in pre-clinical models; showing no effective in clinical studies | De Ryck et al., 1996; Diener et al., 1996 |
| DPQ | Inhibiting PARP-1 | Ischemia-caused brain injury | Pre-clinical studies; partial protection against the pathological process | Soane et al., 2007 |
| Mdivi-1 | Inhibitor of Drp-1 | Ischemia-caused brain injury | Pre-clinical studies; controversial effect | Baek et al., 2017 |
| 3-MA | Inhibiting autophagy/mitophagy | Ischemia-caused brain injury | Pre-clinical studies; controversial effect | Zhang X. et al., 2013; Zuo et al., 2014 |
| Rapamycin | Autophagy/mitophgy inducer through suppressing mTOR | Ischemia-caused brain injury | Pre-clinical studies | Li et al., 2014 |
| Selegiline | Inhibiting monoamine oxidase-B (MAO-B) and antioxidant | Parkinson’s disease | Beneficial effect and significantly delaying the time of using levodopa | Koller et al., 1993 |
| Rasagiline | MAO-B inhibitor | Parkinson’s disease | Change the Unified Parkinson Disease Rating Scale (UPDRS) modestly | Smith et al., 2015 |
| N-acetylcysteine (NAC) | Antioxidant | Parkinson’s disease | Improved UPDRS moderately | Monti et al., 2016 |
| Mitoquinone | Mitochondrial targeted antioxidant | Parkinson’s disease | Protected dopaminergic neurons in a 6-OHDA-induced model of PD; but no effective in clinical studies | Snow et al., 2010; Liu and Wang, 2014; Xi et al., 2018 |
| Ubiquinone | Antioxidant | Parkinson’s disease | Pre-clinical studies | Koller et al., 1993 |
| Creatine | Antioxidant | Parkinson’s disease | Pre-clinical studies | Kieburtz et al., 2015 |
| Curcumin | Antioxidant | Alzheimer’s disease | Pre-clinical studies | Mecocci and Polidori, 2012; Zhao and Zhao, 2013 |
| Mitoquinone | Mitochondria-targeted antioxidant | Alzheimer’s disease | Pre-clinical studies | Mecocci and Polidori, 2012; Zhao and Zhao, 2013 |
| CoQ10/Ubisol-Q10 | Antioxidants | Alzheimer’s disease | Pre-clinical studies | McManus et al., 2011; Muthukumaran et al., 2018 |
| Idebenone | An analog of CoQ10 | Alzheimer’s disease | Effective in clinical trails | Senin et al., 1992; Weyer et al., 1997 |
| Nicotinamide riboside (NR) | NAD+ precursors | Alzheimer’s disease | Pre-clinical studies | Wang et al., 2016; Hou et al., 2018 |
| Nicotinamide mononucleotide (NMN) | NAD+ precursors | Alzheimer’s disease | Pre-clinical studies | Wang et al., 2016; Hou et al., 2018 |
| Rapamycin | Autophagy/mitophgy inducer through suppressing mTOR | Alzheimer’s disease | Pre-clinical studies | Spilman et al., 2010; Caccamo et al., 2013 |
| Trehalose | mTOR-independent autophagy/mitophgy inducer | Alzheimer’s disease | Pre-clinical studies | Spilman et al., 2010; Caccamo et al., 2013 |
| 2, 4- Dinitrophenol (DNP) | Mitochondrial uncoupling agents | Alzheimer’s disease | Pre-clinical studies | Geisler et al., 2017 |
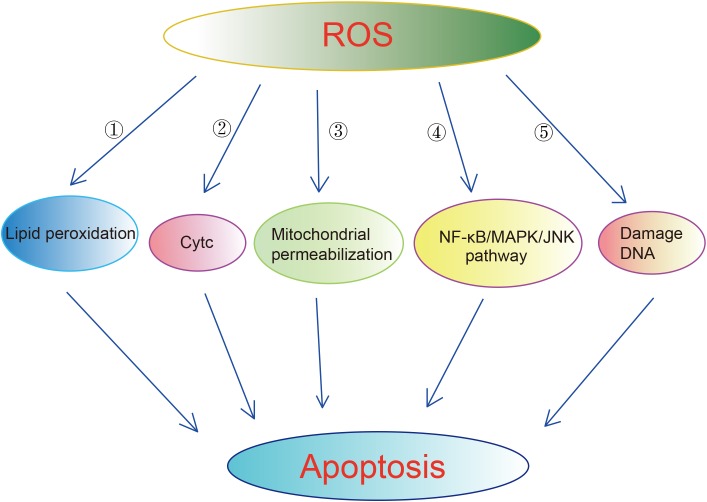
Mitochondria and its electron transport chain complexes are the main sources of ROS. Approximately 90% of cellular ROS production occurs in the mitochondria. Healthy mitochondria generate small levels of ROS. There is a set of scavenging system including enzymatic and non-enzymatic antioxidants to protect cells from the attack by ROS. The enzymatic antioxidants include superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). The non-enzymatic antioxidants include ascorbic acid (Vitamin C), α-tocopherol (Vitamin E), glutathione (GSH), carotenoids, flavonoids, and other antioxidants (Valko et al., 2007). Under normal circumstances, ROS exhibits little damage as it enters into a balance with antioxidant systems (Fridovich, 1995; Takizawa et al., 1998). However, excessive ROS damages proteins, mtDNA and lipids, resulting in apoptosis, neuroinflammation, and disruption of blood–brain barrier (BBB) in ischemic brains (Shirley et al., 2014). Excessive ROS induces apoptosis through lipid peroxidation. 4-Hydroxynoneal (4-HNE), one of byproducts of lipid peroxidation, has been shown to be upregulated in rat brains upon cerebral ischemia and to induce axonal damage and apoptosis of oligodendrocytes (McCracken et al., 2000; Matsuda et al., 2009). ROS can also lead to apoptosis through releasing of cytochrome C (CytC) (Kirkland et al., 2002; Sugawara et al., 2002), enhancing mitochondrial permeabilization (Kim et al., 2006), activation of NF-κB/MAPK/JNK pathway (Kim et al., 2010). Excessive ROS also can bring damages directly on DNA, leading to DNA base damage and single-strand breaks (SSBs) in rodent models of cerebral ischemia (Liu et al., 1996; Chen et al., 1997) (Figure 2). Excessive ROS results in the breakdown of BBB through direct activation of matrix metalloproteinase (MMPs) and regulating Ca2+ concentration (Mark and Davis, 2002; Yamagata et al., 2004; Cortez et al., 2007; Rosell et al., 2008). The generation of ROS is elevated in the ischemic brains. In ischemia, oxygen is deleted prior to glucose, which results in an accumulation of lactic acid. The latter promotes pro-oxidant and detrimental changes in neurons such as the inactivation of antioxidant defenses, release of oxidant ions and elevated glutamate toxicity (Manzanero et al., 2013). In addition, the enzyme xanthin oxidase and mitochondrial depolarization also contributes to the elevated ROS in the ischemic brains (Abramov et al., 2007). It is worth noting that blood reperfusion also leads to a substantial increase in mitochondrial ROS, which may be contributed by the complex I of MRC (Niatsetskaya et al., 2012). In this context, it is reported that ischemic stroke upregulates the antioxidant defense system mentioned above (Fridovich, 1995; Takizawa et al., 1998). In contrast, some studies detected reduced levels of antioxidants in stroke patients in the early hours of post-stroke (Kaur et al., 2011). Despite these controversial reports, it is believed that the antioxidant defense system fails to protect cells from the elevated ROS, eventually leading to cell death (Manzanero et al., 2013). Considering the roles of ROS in ischemia, anti-oxidants have been used to treat ischemia. One of the anti-oxidant strategies is using compounds capable of scavenging free radicals such as tirilazad mesylate, an inhibitor of lipid peroxidation. Tirilazad exhibited benefits in reducing infarct size in pre-clinical models (Xue et al., 1992; Park and Hall, 1994). However, tirilazad failed to improve mortality in clinical studies, which possibly due to differences metabolic rate between women and men (Fleishaker et al., 1995; Scott et al., 1996). Another free radical scavenger, edaravone, which has been widely used in clinics in Japan for the treatment of cerebral infarctions, exhibited clinical improvements in acute ischemic stroke patients (Edaravone Acute Infarction Study Group, 2003).
FIGURE 2.
Mechanisms underlying that ROS induced apoptosis. Excessive ROS can cause apoptosis via multiple ways:  enhancing lipid peroxidation.
enhancing lipid peroxidation.  Releasing of Cytc.
Releasing of Cytc.  Enhancing mitochondrial permeabilization.
Enhancing mitochondrial permeabilization.  Activation of NF-KB/MAPK/JNK pathway.
Activation of NF-KB/MAPK/JNK pathway.  Damaging DNA directly.
Damaging DNA directly.
In addition to ROS, mitochondrial dysfunction also leads to abnormal generation of nitrix oxide (NO), which in turn regulates mitochondrial function. One of mechanisms underlying that NO-regulating mitochondrial function is via S-nitrosylation of mitochondrial proteins. About 1% mitochondrial proteins are available for S-nitrosylation. NO inhibits Complex I (Clementi et al., 1998; Dahm et al., 2006) and Complex IV (Cleeter et al., 1994; Orsi et al., 2000) and other metabolic enzymes such as mitochondrial aldehyde dehydrogenase (ALDH2) (Moon et al., 2005) and aconitase (Tortora et al., 2007) via S-nitrosylation. S-nitrosylation is also involved in regulation of MPTP, which is closely linked to neural injury in stroke (Vieira and Kroemer, 2003). Moreover, S-nitrosylated SHP-2 and receptor-interacting protein 3 (RIP3) contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke (Shi et al., 2013; Miao et al., 2015). Consistently, mitochondria-targeted S-nitrosothiols (MitoSNOs), which generates NO and S-nitrosated thiol proteins within the mitochondria, has been observed protective in mouse model of cardiac ischemia-refusion injury (Prime et al., 2009). Lubeluzole, which reduces NO levels and subsequent ONOO- production, reduced infarct in a transient MCAO mouse model (Aronowski et al., 1996; De Ryck et al., 1996). However, lubeluzole showed no improvement in mortality in a large clinical study despite the fact that it shows benefits in two smaller clinical studies (Diener et al., 1996; Diener, 1998; Grotta, 1998) (Table 1).
A great amount of cellular NAD(H) is involved in mitochondria, the ratio of NAD/NADH plays an important role in glycolysis, TCA cycle and oxidative phosphorylation. In addition, it also mediates calcium homeostasis, genetic expression, protection against oxidative stress, and programmed cell death (Ziegler, 2000). Under pathological conditions, such as cerebral ischemia, oxidative stress, hypoglycemia, or excitotoxicity, poly (ADP-ribose) polymerase-1 (PARP-1) is the most efficient depleting enzyme for NAD (Narasimhan et al., 2003; Kosenko et al., 2004). In this case, PARP becomes extremely active because it can promote the repair of damaged DNA. Activated PARP-1 hydrolyzes NAD, which causes a large decrease in intracellular NAD, especially under conditions of metabolic stress. At the same time, the reduction of intracellular ATP also limits the production of NAD. When the concentration of NAD is less than 1 mM or 0.1 mM, ATP production is affected, resulting in a vicious circle that eventually leads to permanent metabolic failure and necrotic cell apoptosis. Specific PARP-1 inhibitors DPQ or cyclosporine A have partial protection against the pathological process (Soane et al., 2007) (Table 1).
Mitochondrial dynamics are closely linked to ischemic brain injury. Severe brain ischemia caused mitochondrial fragmentation and dendritic damage (Kislin et al., 2017). Proteins mediated mitochondrial fission and fusion such as Drp1, Opa1, and Mfn1/2 exhibited dynamic expression in MCAO mice (Kumari et al., 2012; Liu et al., 2012; Martorell-Riera et al., 2014; Owens et al., 2015). Drp1 and Mfn1/2 mediated mitochondrial fission is required for ischemic neuronal death (Barsoum et al., 2006; Peng et al., 2015). Consistent with these observations, reducing mitochondrial fission by using mdivi-1, an inhibitor for Drp-1, exhibited protective roles against OGD reperfusion injury and MCAO through suppressing the ROS production and decreasing the expression of cytochrome c (Zhang N. et al., 2013; Wang et al., 2014). However, another study observed mdivi-1 exacerbated brain injury in a mouse model of transient focal ischemia (Zuo et al., 2014).
Upregulation of autophagy/mitophagy has been observed in the brains of rats subjected to permanent middle cerebral artery occlusion (MCAO). The upregulated mitophagy seems neural protective since inhibition of autophagy using 3-methyladenine (3-MA) exacerbated brain injury (Zuo et al., 2014). However, another two studies observed converse results that inhibition of autophagy by using either 3-MA or knocking-down of ATG7 exhibited neuroprotective in ischemia-induced neural injury (Zhang X. et al., 2013; Zuo et al., 2014) Enhancing autophagy with rapamycin attenuated mitochondrial dysfunction and partly reversed the deleterious effects of 3-MA-treated neurons subjected to oxygen-glucose deprivation reperfusion (Li et al., 2014). However, it is worth noting that excessive induction of mitophagy leads to cell death in neonatal stroke (Shi et al., 2014). Thus, it seems that mitophagy is a double-edged sword that can be protective or destructive after experimental stroke.
Mitochondrial Dysfunction in Parkinson’s Disease
Parkinson’s disease (PD) is the second common neurodegenerative disease all over the world which demonstrates motor symptoms including myotonia, rest tremor, hypokinesia, and abnormal postures (Zeng et al., 2018) accompanied by non-motor symptoms such as anxiety, depression, constipation, frequent micturition, RBD and cognitive disorders (Marsili et al., 2018). Continuous loss of dopaminergic (DA) neurons and accumulation of α-synuclein (α-Syn) are regarded as the features of PD pathology (Bridi and Hirth, 2018; Song and Xie, 2018). Mitochondrial dysfunction plays essential roles in PD pathogenesis (Pozo Devoto and Falzone, 2017). One of evidence comes from the facts that treatment with toxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP), rotenone, trichloroethylene, yridaben, and other inhibitors of mitochondrial complex I reproduce the parkinsonian features including dopaminergic neurodegeneration in rodents and primates (Blesa et al., 2012). These toxins cause defects in the activity of the mitochondrial electron transport complex, mitochondrial movements and an increase in mitochondrial permeability transition and generation of ROS. Mutations in DJ-1, a mitochondrial peroxiredoxin-like peroxidase that plays a role in scavenging mitochondrial ROS, cause an autosomal recessive form of PD (Bonifati et al., 2003; Ammal Kaidery and Thomas, 2018), indicating a contribution of ROS in PD pathogenesis.
Accumulation of α-Syn in the brain is a noteworthy pathological feature in PD. Most of α-Syn localize at the cytoplasm, while a small fraction of α-Syn is present in mitochondria. α-Syn regulates the mitochondrial morphology through acting directly on the fusion-fission process by changing the membrane curvature (Kamp et al., 2010; Pozo Devoto and Falzone, 2017; Pozo Devoto et al., 2017). Distinct pathogenic variants that contain a single amino acid substitution in the N-terminal domain display different affinities for membranes, thus exhibiting distinct efficacy in inducing mitochondrial fragmentation: A53T mutations shows the most notable fragmentation effect, followed by mild fragmentation is observed by overexpression of WT α-Syn. Whereas almost no fragmentation is observed upon overexpression of the A30P variant (Nakamura et al., 2011; Butler et al., 2012; Gui et al., 2012; Pozo Devoto and Falzone, 2017; Pozo Devoto et al., 2017). However, some studies have reported that A30P α-Syn induces similar or even greater fragmentation defects to A53T (Kamp et al., 2010; Guardia-Laguarta et al., 2014). Striatal dopamine release deficits were restored after inhibition of Drp1 in animal models (Rappold et al., 2014), suggesting that the excessive fission contributes to the pathophysiology of PD. However, complete deletion of Drp1 in mice models leads to slower weight gain, parkinsonism, and loss of DA neuron in caudate–putamen (CPu) and nucleus accumbens (NAc) areas (Berthet et al., 2014), suggesting that the disruption of fission could also be a suspected factor in the pathogenesis of PD.
In addition to directly regulate the fusion-fission process of mitochondria, α-Syn also regulates mitochondrial transport. Overexpression of α-Syn leads to deficits of mitochondrial transport prior to axonal degeneration (O’Donnell et al., 2014; Pozo Devoto et al., 2017), which may be associated with impaired kinesin function and direct modulation of the fusion-fission dynamics (Pozo Devoto and Falzone, 2017).
Although there is still a lack of evidence as to whether α-Syn deregulates mitophagy in PD, studies have observed that α-Syn overexpression caused compromised autophagy activity, which was evidenced by increased levels of p62, decreased levels of LC3 and the number of LC3-II-positive vesicles (Winslow et al., 2010). α-Syn overexpression also leads to the mislocalization of autophagy related protein 9 (ATG9) via inhibiting Rab1 GTPase protein (Winslow et al., 2010). Moreover, mutant α-Syn, but not WT α-Syn, causes defects in the chaperon-mediated autophagy (CMA) pathway by binding to the LAMP2 transporter in the lysosomal membrane (Cuervo et al., 2004; Alvarez-Erviti et al., 2010). These evidences mentioned above hint that α-Syn accumulation or/and mutations participate in the pathogenesis of PD through regulating mitochondrial dynamics and mitophagy.
Other mutational genes coding Parkin, PINK1 (PTEN Induced Putative Kinase 1), LRRK2 (Leucine-rich repeat kinase 2), which are associated genetically with PD, have also been reported to alter mitochondrial dynamics. Mutations in Parkin and PINK1 are the common causes of autosomal recessive PD (Corti et al., 2011). These two genes operate together in a common genetic pathway. Recent studies show abnormality in either of them could cause mitochondrial dysfunction from unusual mitochondrial biogenesis, dynamics and mitophagy. For example, the reduced Complex I activity and impaired ATP production has been found in Parkin or/and PINK1 null PD patients or models, which may be resulted from impaired mitochondrial biogenesis (Muftuoglu et al., 2004; Morais et al., 2009). Supplementation of PINK1 restores PINK1-defect model fly’s ATP production and strength of flight muscles (Vos et al., 2012). Parkin exhibits protective function in maintaining mitochondrial genome integrity from mitochondrial DNA repair via an interaction with TFAM (Rothfuss et al., 2009). PARIS (Parkin Interacting Substrate, ZNF746), a strong pathogenic Parkin substrate, accumulates in familial PD with Parkin mutations, sporadic PD, Parkin knockout mice and MPTP- induced mice (Shin et al., 2011). PARIS upregulation is required for DA neuron degeneration caused by Parkin deficiency through repressing transcription of peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1α (PGC-1α) (Dawson and Dawson, 2014), a master regulator of mitochondrial biogenesis (Zhu et al., 2013). In this context, it is worth noting that PGC-1α levels are decreased in the SNc of PD patients (Shin et al., 2011). Parkin mediates mitophagy downstream of PINK1. PINK1 can act as a molecular sensor of damaged mitochondria. Parkin is an E3 ubiquitin ligase, which ubiquitylates numerous OMM proteins, which in turn recruits other proteins to mitochondria to initiate mitophagy. A recent study has observed that PINK1- and Parkin-mediated mitophagy restrains innate immunity through regulating STING levels (Sliter et al., 2018), highlighting mitochondrial dysfunction leads to neurodegeneration through neuroinflammation. In this context, it is worth noting that mitochondrial antigen presentation (MitAP), in which mitochondria-derived vesicles are targeted to endolysosomes for processing and presentation by MHC class molecules (MHC-1), is antagonized by Parkin and PINK1. MHC-1 are upregulated in DA neurons by inflammatory conditions. The absence of Parkin and PINK1 in familial PD promotes MitAP, results to the development of mitochondrial antigen-reactive CD8+ T cells, which might cross the BBB and bind to mitochondrial peptide: MHC-I complex, leading to the death of DA neurons in PD (Matheoud et al., 2016). PINK1/Parkin-mediated mitophagy can be attenuated by S-Nitrosylation of PINK1 (Oh et al., 2017), indicating a novel mechanism underlying that nitrosative stress damages mitochondria. Parkin and PINK1 also take part in the process of mitochondrial fusion. PINK1 phosphorylates Mfn, which tethers separated mitochondria together to induce fusion on the OMM, where Mfn is degraded by Parkin (Jin and Youle, 2012).
The LRRK2 (G2019S) mutation is another common cause of PD. LRRK2 regulates mitophagy by targeting RHOT1, an OMM protein, which anchors the microtubule motors to mitochondria. In both wild-type neurons and neurons differentiated from iPSC of human fibroblast, LRRK2 is recruited to RHOT-1 after mitochondrial depolarization or damage, and then removing RHOT-1 from OMM through promoting degradation by proteasomes. Through this way, LRRK2 leads to both anterograde and retrograde mitochondrial motility stop and subsequent mitophagy. While in mutant LRRK2 (such as LRRK2G2019S) cells, this phenomenon would be delayed, thus resulting in accumulation of depolarized or damaged mitochondria in cells, as well as higher oxidative stress (Hsieh et al., 2016). Consistently, knock-down RHOT1 in a drosophila model carrying human LRRK2G2019S restores its motor disabilities and reduced DA neuronal numbers (Wang, 2017), implying that partial inhibition of RHOT1 may remit PD symptoms.
Given the potential role of mitochondrial dysfunction in PD pathogenesis, some therapeutics targeting mitochondria have been investigated. Selegiline has shown beneficial effect and significantly delaying the time of using levodopa through inhibiting monoamine oxidase-B (MAO-B) and antioxidant (Koller et al., 1993). Rasagiline, a new MAO-B inhibitor, enables to change the Unified Parkinson Disease Rating Scale (UPDRS) modestly (Smith et al., 2015). N-acetylcysteine (NAC) has been reported to improved UPDRS moderately (Monti et al., 2016) through protecting cells from mitochondrial dysfunction and from enhanced oxidative stress (Chandramani Shivalingappa et al., 2012). While other agents such as mitoquinone (mitoQ) (Snow et al., 2010; Liu and Wang, 2014; Xi et al., 2018), ubiquinone (Koller et al., 1993), creatine (Kieburtz et al., 2015) are still under investigation or demonstrated as no improvement in UPDRS (Table 1).
Mitochondrial Dysfunction in Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disease, which exhibits dementia syndrome characterized by learning and memory loss. AD is characterized with two pathological markers: amyloid-β (Aβ) plaques formed by accumulation of Aβ and neurofibrillary tangles (NFTs) consisted of hyperphosphorylated tau (Sun et al., 2018). In addition to these two pathological markers, mitochondrial dysfunction has been taken as another feature in both the familial and sporadic AD (Swerdlow et al., 2014). AD patients brain exhibit changes in the mitochondrial mass, enzymes and mtDNA, which are accompanied with altered morphology of mitochondria such as disruption of mitochondrial cristae, intra-mitochondrial accumulation of osmiophilic materials and changes in mitochondrial sizes (Silva et al., 2012). Neurons in the brains of AD patients exhibit less glucose uptake as revealed by the PET scan, suggesting enzyme activities that involved in mitochondrial oxidative phosphorylation and the tricarboxylic acid cycle (TCA) are reduced (Kapogiannis and Mattson, 2011). Extensive oxidative stress has been observed in the early stage of AD brains (Nunomura et al., 2001; Zhao and Zhao, 2013). The levels of oxidative markers are directly correlated with the severity of cognitive impairment as well as the symptomatic progression from mild cognitive impairment to AD (Ansari and Scheff, 2010), further suggesting a close link of mitochondrial dysfunction to AD pathology.
The mitochondrial dysfunction even occurs prior to the accumulation of Aβ plaques in the brains of AD mouse models. Thus, Swerdlow et al. (2014), has proposed the “mitochondrial cascade hypothesis” that mitochondrial dysfunction is taken as a trigger of AD pathogenesis. Consistent with this hypothesis, mitochondrial dysfunction pushes APP processing toward Aβ production (Gabuzda et al., 1994; Gasparini et al., 1997; Webster et al., 1998), enhances tau phosphorylation (Blass et al., 1990; Szabados et al., 2004), makes cells more susceptible to either Aβ- or tau-induced toxicity (Zhao and Zhao, 2013), eventually leading to neurodegeneration. One of the mechanisms underlying the above effects of mitochondrial dysfunction is oxidative stress. The extensive oxidative damages in AD brains result in oxidative modification of proteins and lipids, eventually leading to neuronal dysfunction. Additionally, the key enzymes for the generation of Aβ including BACE1 (the β-secretase in the brain) and PS1 (the core component of γ-secretase) are upregulated by oxidative stress (Tamagno et al., 2002; Oda et al., 2010). Oxidative stress also involved in Aβ- or tau- induced toxicity. Consistently, antioxidants such as curcumin and mitochondrial-targeted antioxidant mitoQ can attenuate the pathology of AD in model mice (Mecocci and Polidori, 2012; Zhao and Zhao, 2013). Some antioxidants are even under clinical trials in the therapy of MCI and AD (Mecocci and Polidori, 2012).
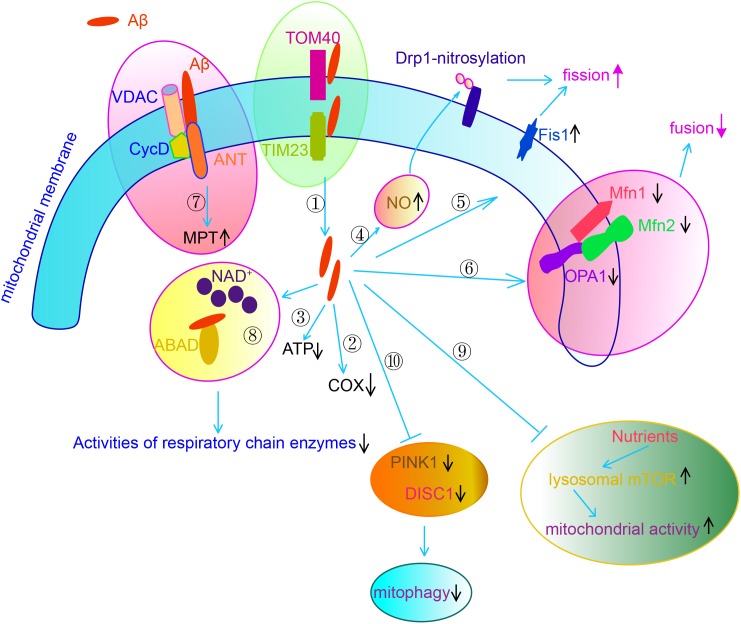
It is worth noting that accumulated Aβ and hyperphosphorylated tau also contribute to mitochondrial dysfunction in AD brains (Lu et al., 2018). Extracellular Aβ is internalized and imported to the mitochondria via interacting with TOM40 and TIM20, eventually localizing cross mitochondrial membrane (Hansson Petersen et al., 2008). Aβ interacts with adenine nucleotide translocase (ANT), cyclophilin D and voltage-dependent anion channel (VDAC), the key proteins in MPTP. Aβ also interacts with mitochondrial protein Aβ-binding ethanol dehydrogenase (ABAD), preventing the binding of NAD+ to ABAD, thereby changing MTP and decreasing the activities of RC enzymes (Lustbader et al., 2004). Aβ binds to ATP synthase subunit α and regulates the generation of ATP (Schmidt et al., 2008). Aβ accumulation also results in abnormalities of mitochondrial dynamics. APP decreases mitochondrial fusion/fission proteins such a as Drp1, OPA1, Mfn1 and Mfn2, while increases Fis1 significantly in AD neurons (Wang et al., 2008, 2009) through overproduction of Aβ (Wang et al., 2008). Another study observes that Aβ enhances mitochondrial fission via increasing generation of NO, which promotes S-nitrosylation of Drp1. Block nitrosylation of Drp1 through mutating cysteine abrogate Aβ-induced mitochondrial dysfunction and suppressed synaptic plasticity (Cho et al., 2009). Nutrients such as insulin or amino acids stimulates mitochondrial activity and regulates mitochondrial DNA synthesis in neurons by activating lysosomal mechanistic target of rapamycin complex 1 (mTORC1). However, Aβ oligomer suppresses such nutrient-induced mitochondrial activity through activating mTORC1 protein kinase activity at the plasma membrane but not at lysosomes in a way dependent on tau (Norambuena et al., 2018). Defective mitophagy also contributes to the accumulated damaged mitochondria in AD neurons. Both DISC1, a novel mitophagy receptor, and PINK1, which is a key regulator in mitophagy, exhibit reduced levels in the brains of transgenic mice. Overexpression of either DISC1 or PINK1 rescues mitochondrial dysfunction, cognitive, and synaptic deficits and Aβ accumulation in AD transgenic mice (Deng et al., 2016; Du et al., 2017; Wang et al., 2018) (Figure 3). Another recent study indicates that impaired syntaphilin-mediated retrograde axonal transport of mitochondria is involved in neurodegeneration of AD neurons in a way independent on Parkin-mediated mitophagy (Lin et al., 2017).
FIGURE 3.
Aβ causes mitochondrial dysfunction via multiple mechanisms.  Aβ is imported to the mitochondria through interacting with TOM40 and TIM20, eventually localizing cross mitochondrial membrane.
Aβ is imported to the mitochondria through interacting with TOM40 and TIM20, eventually localizing cross mitochondrial membrane.  Aβ decreases COX activity.
Aβ decreases COX activity.  Aβ decreases ATP generation via binding to ATP synthase subunit α.
Aβ decreases ATP generation via binding to ATP synthase subunit α.  Aβ increases NO generation, which results in S-nitrosylation of DLP.
Aβ increases NO generation, which results in S-nitrosylation of DLP.  Aβ increases the expression of Fis1. Through
Aβ increases the expression of Fis1. Through  and
and  , Aβ enhances mitochondrial fission.
, Aβ enhances mitochondrial fission.  Aβ decreases the expression of Mfn1/2 and OPA1, through which Aβ suppresses mitochondrial fusion.
Aβ decreases the expression of Mfn1/2 and OPA1, through which Aβ suppresses mitochondrial fusion.  Aβ regulates mitochondrial permeability transition (MPT) via forming an interacting complex with ANT, cyclophilin D (Cyc D) and VADC, the core components of MPTP.
Aβ regulates mitochondrial permeability transition (MPT) via forming an interacting complex with ANT, cyclophilin D (Cyc D) and VADC, the core components of MPTP.  Aβ interacts with ABAD, preventing NAD+ to ABAD, thereby changing MTP and decreasing the activities of RC.
Aβ interacts with ABAD, preventing NAD+ to ABAD, thereby changing MTP and decreasing the activities of RC.  Aβ suppresses nutrients induced mitochondrial activity through regulation mTOR activity.
Aβ suppresses nutrients induced mitochondrial activity through regulation mTOR activity.  Aβ impairs mitophagy through downregulation of DISC1 and PINK1.
Aβ impairs mitophagy through downregulation of DISC1 and PINK1.
Tau is a microtubule-binding protein mainly responsible for the organization of microtubules in neurons. In this regard, hyperphosphorylated tau causes disruption of preformed microtubules and mitochondrial perinuclear distribution (Zempel et al., 2010; Amadoro et al., 2014; Musiek and Holtzman, 2015). P301L transgenic mice which overexpress the mutant P301L human tau protein exhibited abnormal expression of metabolism-related proteins including mitochondrial respiratory chain complex components, antioxidant enzymes, and synaptic proteins that are associated with increased oxidative stress (David et al., 2005). Overexpression of mutant P301L human tau, rather than WT tau, in neuroblast cells induces complex I deficit, which is accompanied by decreased ATP levels and increased susceptibility to oxidative stress (Schulz et al., 2012). Overexpression of mutant P301L human tau also decreases fusion and fission rates concomitant with downregulation of OPA-1 and Drp1. A recent study indicates that overexpression of human P301L tau completely inhibit mitophagy by impairing Parkin recruitment to defective mitochondria by sequestering it in the cytosol (Cummins et al., 2018). These lines of evidence highlight a role of hyperphosphorylated tau in regulation of mitochondrial dynamics (Quintanilla et al., 2014). Hyperphosphorylated tau also interacts with VDAC1, through which hyperphosphorylated tau blocks the mitochondrial pores (Manczak and Reddy, 2012).
Therefore, a vicious cycle also exists between mitochondria dysfunction and Aβ- or tau-pathology. Accumulation of Aβ or hyperphosphorylated tau causes mitochondrial dysfunction, which in turn execrates Aβ accumulation and tau hyperphosphorylation. The latter cause further damages on mitochondria through multiple mechanisms (Lu et al., 2018). Thus, various strategies that enhancing mitochondrial homeostasis have been used to treat AD in both animal models and clinical trials. Antioxidants such as CoQ10 and MitoQ show beneficial efficacy in AD transgenic mice (McManus et al., 2011; Muthukumaran et al., 2018). Idebenone, an analog of CoQ10, showed benefit effect in AD patients (Senin et al., 1992; Weyer et al., 1997). Supplementation with NAD+ precursors like nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) ameliorates cognitive deficits, synaptic plasticity, phosphorylation of tau in AD model mice (Wang et al., 2016; Hou et al., 2018). Amelioration of mitochondrial dynamic deficits by the treatment of mitochondrial division inhibitor 1 (mdivi-1), a mitochondrial fission inhibitor, attenuates cognitive and synaptic deficits and Aβ accumulation in AD transgenic mice (Baek et al., 2017; Wang et al., 2017). Consistent with the contribution of compromised mitophagy in AD pathogenesis, compounds enhancing autophagy/mitophagy attenuate AD pathology in AD model mice. For example, both rapamycin, which induces autophagy/mitophagy through suppressing mTOR, and trehalose, which induces mTOR-independent autophagy/mitophagy, exhibit benefit in AD model mice (Spilman et al., 2010; Caccamo et al., 2013; Du et al., 2013; Portbury et al., 2017). 2, 4- Dinitrophenol (DNP), one of mitochondrial uncoupling agents, which can induce autophagy, exhibits protective functions in APP/PS1 transgenic mice (Geisler et al., 2017) (Table 1).
Outlook
Mitochondria play essential roles in various cellular functions. Mitochondrial dysfunction is closely linked to the pathogenic mechanisms of the acute or chronic neural injury in diseases. Dysregulation of mitochondrial biosynthesis, fission, fusion, and degradation are implicated as the potential mechanisms in these various neural injuries. Despite that differences in mechanisms underlying mitochondrial dysfunction in ischemia, PD and AD, enhancing mitochondrial homeostasis exhibits therapeutic effects in pre-clinical studies. Though few candidate drugs targeting mitochondria show efficacy in clinical trials, present knowledge of mitochondrial abnormalities in these diseases may still be helpful for us to make therapeutic schedules to slow down and even cure different kinds of neural injury by correcting the mitochondrial dysfunction mentioned above. Though challenges lie ahead, we are still full of confidence that we can overcome difficulties and eventually find the way out to cope with neural injury relating to dysfunctional mitochondria in the near future.
Author Contributions
X-YZ, M-HL, D-JY, and W-LL wrote the first draft and revised it. D-EX, P-PY, M-HL, and C-XY prepared the literature. W-LJ and HC drew the figures. JT, Y-YX, and SL helped to revise the manuscript. Q-HM designed and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81671111, 81870897, and 81601111), the Suzhou Clinical Research Center of Neurological Disease (Grant No Szzx201503), the Jiangsu Provincial Special Program of Medical Science (Grant No. BK20181436), and the Jiangsu Higher Education Institutions for funding the Priority Academic Program Development; Jiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases (Grant No. BM2013003).
References
- Abramov A. Y., Scorziello A., Duchen M. R. (2007). Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 27 1129–1138. 10.1523/JNEUROSCI.4468-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L., Rodriguez-Oroz M. C., Cooper J. M., Caballero C., Ferrer I., Obeso J. A., et al. (2010). Chaperone-mediated autophagy markers in Parkinson disease brains. Arch. Neurol. 67 1464–1472. 10.1001/archneurol.2010.198 [DOI] [PubMed] [Google Scholar]
- Amadoro G., Corsetti V., Florenzano F., Atlante A., Ciotti M. T., Mongiardi M. P., et al. (2014). AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol. Dis. 62 489–507. 10.1016/j.nbd.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Ammal Kaidery N., Thomas B. (2018). Current perspective of mitochondrial biology in Parkinson’s disease. Neurochem. Int. 117 91–113. 10.1016/j.neuint.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. A., Scheff S. W. (2010). Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 69 155–167. 10.1097/NEN.0b013e3181cb5af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J., Strong R., Grotta J. C. (1996). Treatment of experimental focal ischemia in rats with lubeluzole. Neuropharmacology 35 689–693. 10.1016/S0028-3908(96)00046-9 [DOI] [PubMed] [Google Scholar]
- Baek S. H., Park S. J., Jeong J. I., Kim S. H., Han J., Kyung J. W., et al. (2017). Inhibition of Drp1 ameliorates synaptic depression, a beta deposition, and cognitive impairment in an Alzheimer’s disease model. J. Neurosci. 37 5099–5110. 10.1523/Jneurosci.2385-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum M. J., Yuan H., Gerencser A. A., Liot G., Kushnareva Y. E., Graber S., et al. (2006). Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 25 3900–3911. 10.1038/sj.emboj.7601253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A., Margolis E. B., Zhang J., Hsieh I., Zhang J., Hnasko T. S., et al. (2014). Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J. Neurosci. 34 14304–14317. 10.1523/JNEUROSCI.0930-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass J. P., Baker A. C., Ko L., Black R. S. (1990). Induction of Alzheimer antigens by an uncoupler of oxidative phosphorylation. Arch. Neurol. 47 864–869. 10.1001/archneur.1990.00530080046009 [DOI] [PubMed] [Google Scholar]
- Blesa J., Phani S., Jackson-Lewis V., Przedborski S. (2012). Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012:845618. 10.1155/2012/845618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., et al. (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299 256–259. 10.1126/science.1077209 [DOI] [PubMed] [Google Scholar]
- Bridi J. C., Hirth F. (2018). Mechanisms of alpha-synuclein induced synaptopathy in Parkinson’s disease. Front. Neurosci. 12:80. 10.3389/fnins.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M., Cali T., Ottolini D., Carafoli E. (2014). Neuronal calcium signaling: function and dysfunction. Cell. Mol. Life Sci. 71 2787–2814. 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekemeier K. M., Klocek C. K., Pfeiffer D. R. (1998). Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry 37 13059–13065. 10.1021/bi980820c [DOI] [PubMed] [Google Scholar]
- Broughton B. R., Reutens D. C., Sobey C. G. (2009). Apoptotic mechanisms after cerebral ischemia. Stroke 40 e331–e339. 10.1161/STROKEAHA.108.531632 [DOI] [PubMed] [Google Scholar]
- Butler E. K., Voigt A., Lutz A. K., Toegel J. P., Gerhardt E., Karsten P., et al. (2012). The mitochondrial chaperone protein TRAP1 mitigates α-Synuclein toxicity. PLoS Genet. 8:e1002488. 10.1371/journal.pgen.1002488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A., Magri A., Medina D. X., Wisely E. V., Lopez-Aranda M. F., Silva A. J., et al. (2013). mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell 12 370–380. 10.1111/acel.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Zakaria H. M., Simone A., Sheng Z. H. (2012). Spatial parkin translocation and degradation of damaged mitochondria via mitophagy in live cortical neurons. Curr. Biol. 22 545–552. 10.1016/j.cub.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins M. J., Manczak M., Mao P., Shirendeb U., Reddy P. H. (2011). Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 20 4515–4529. 10.1093/hmg/ddr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramani Shivalingappa P., Jin H., Anantharam V., Kanthasamy A., Kanthasamy A. (2012). N-acetyl cysteine protects against methamphetamine-induced dopaminergic neurodegeneration via modulation of redox status and autophagy in dopaminergic cells. Parkinsons Dis. 2012:424285. 10.1155/2012/424285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jin K. L., Chen M. Z., Pei W., Kawaguchi K., Greenberg D. A., et al. (1997). Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J. Neurochem. 69 232–245. 10.1046/j.1471-4159.1997.69010232.x [DOI] [PubMed] [Google Scholar]
- Cheng A., Hou Y., Mattson M. P. (2010). Mitochondria and neuroplasticity. ASN Neuro 2:e00045. 10.1042/AN20100019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Wan R., Yang J. L., Kamimura N., Son T. G., Ouyang X., et al. (2012). Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 3:1250. 10.1038/ncomms2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Bai F. (2018). The association of tau with mitochondrial dysfunction in Alzheimer’s disease. Front. Neurosci. 12:163. 10.3389/fnins.2018.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D. H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., et al. (2009). S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324 102–105. 10.1126/science.1171091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. T. (2018). Multiple pathways for mitophagy: a neurodegenerative conundrum for Parkinson’s disease. Neurosci. Lett. 10.1016/j.neulet.2018.04.004 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., et al. (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441 1162–1166. 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- Cleeter M. W. J., Cooper J. M., Darleyusmar V. M., Moncada S., Schapira A. H. V. (1994). Reversible inhibition of cytochrome-C-oxidase, the terminal enzyme of the mitochondrial respiratory-chain, by nitric-oxide - implications for neurodegenerative diseases. FEBS Lett. 345 50–54. 10.1016/0014-5793(94)00424-2 [DOI] [PubMed] [Google Scholar]
- Clementi E., Brown G. C., Feelisch M., Moncada S. (1998). Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. U.S.A. 95 7631–7636. 10.1073/pnas.95.13.7631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D. M., Feldman M. D., Mummidi S., Valente A. J., Steffensen B., Vincenti M., et al. (2007). IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 293 H3356–H3365. 10.1152/ajpheart.00928.2007 [DOI] [PubMed] [Google Scholar]
- Corti O., Lesage S., Brice A. (2011). What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol. Rev. 91 1161–1218. 10.1152/physrev.00022.2010 [DOI] [PubMed] [Google Scholar]
- Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D. (2004). Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305 1292–1295. 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- Cummins N., Tweedie A., Zuryn S., Bertran-Gonzalez J., Gotz J. (2018). Disease-associated tau impairs mitophagy by inhibiting Parkin translocation to mitochondria. EMBO J. 10.15252/embj.201899360 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm C. C., Moore K., Murphy M. P. (2006). Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 281 10056–10065. 10.1074/jbc.M512203200 [DOI] [PubMed] [Google Scholar]
- David D. C., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., et al. (2005). Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 280 23802–23814. 10.1074/jbc.M500356200 [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Dawson V. L. (2014). Parkin plays a role in sporadic Parkinson’s disease. Neurodegener. Dis. 13 69–71. 10.1159/000354307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ryck M., Keersmaekers R., Duytschaever H., Claes C., Clincke G., Janssen M., et al. (1996). Lubeluzole protects sensorimotor function and reduces infarct size in a photochemical stroke model in rats. J. Pharmacol. Exp. Ther. 279 748–758. [PubMed] [Google Scholar]
- Deng Q. S., Dong X. Y., Wu H., Wang W., Wang Z. T., Zhu J. W., et al. (2016). Disrupted-in-schizophrenia-1 attenuates amyloid-beta generation and cognitive deficits in APP/PS1 transgenic mice by reduction of beta-site APP-cleaving enzyme 1 levels. Neuropsychopharmacology 41 440–453. 10.1038/npp.2015.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener H. C. (1998). Multinational randomised controlled trial of lubeluzole in acute ischaemic stroke. Cerebrovasc. Dis. 8 172–181. 10.1159/000015847 [DOI] [PubMed] [Google Scholar]
- Diener H. C., Hacke W., Hennerici M., Radberg J., Hantson L., DeKeyser J. (1996). Lubeluzole in acute ischemic stroke - A double-blind, placebo-controlled phase II trial. Stroke 27 76–81. 10.1161/01.Str.27.1.76 [DOI] [PubMed] [Google Scholar]
- Dimonte D. A., Wu E. Y., Delanney L. E., Irwin I., Langston J. W. (1992). Toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in primary cultures of mouse astrocytes. J. Pharmacol. Exp. Ther. 261 44–49. [PubMed] [Google Scholar]
- Du F., Yu Q., Yan S. J., Hu G., Lue L. F., Walker D. G., et al. (2017). PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain 140 3233–3251. 10.1093/brain/awx258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Liang Y., Xu F., Sun B., Wang Z. (2013). Trehalose rescues Alzheimer’s disease phenotypes in APP/PS1 transgenic mice. J. Pharm. Pharmacol. 65 1753–1756. 10.1111/jphp.12108 [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D., Saffari A., Wahlster L., Di Nardo A., Turner D., Lewis T. L., et al. (2016). Impaired mitochondrial dynamics and mitophagy in neuronal models of tuberous sclerosis complex. Cell Rep. 17 1053–1070. 10.1016/j.celrep.2016.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edaravone Acute Infarction Study Group (2003). Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 15 222–229. [DOI] [PubMed] [Google Scholar]
- Elgass K., Pakay J., Ryan M. T., Palmer C. S. (2013). Recent advances into the understanding of mitochondrial fission. Biochim. Biophys. Acta 1833 150–161. 10.1016/j.bbamcr.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez S., Almeida A., Bolanos J. P. (2012). Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 443 3–11. 10.1042/BJ20111943 [DOI] [PubMed] [Google Scholar]
- Fischer F., Hamann A., Osiewacz H. D. (2012). Mitochondrial quality control: an integrated network of pathways. Trends Biochem. Sci. 37 284–292. 10.1016/j.tibs.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Fleishaker J. C., Hulst-Pearson L. K., Peters G. R. (1995). Effect of gender and menopausal status on the pharmacokinetics of tirilazad mesylate in healthy subjects. Am. J. Ther. 2 553–560. 10.1097/00045391-199508000-00007 [DOI] [PubMed] [Google Scholar]
- Fridovich I. (1995). Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64 97–112. 10.1146/annurev.bi.64.070195.000525 [DOI] [PubMed] [Google Scholar]
- Friedman J. R., Lackner L. L., West M., DiBenedetto J. R., Nunnari J., Voeltz G. K. (2011). ER tubules mark sites of mitochondrial division. Science 334 358–362. 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda D., Busciglio J., Chen L. B., Matsudaira P., Yankner B. A. (1994). Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J. Biol. Chem. 269 13623–13628. [PubMed] [Google Scholar]
- Gasparini L., Racchi M., Benussi L., Curti D., Binetti G., Bianchetti A., et al. (1997). Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci. Lett. 231 113–117. 10.1016/S0304-3940(97)00536-3 [DOI] [PubMed] [Google Scholar]
- Geisler J. G., Marosi K., Halpern J., Mattson M. P. (2017). DNP, mitochondrial uncoupling, and neuroprotection: a little dab’ll do ya. Alzheimers Dement. 13 582–591. 10.1016/j.jalz.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotta J. (1998). Lubeluzole treatment of acute ischemic stroke - Response. Stroke 29 1067–1067. 10.1161/01.STR.29.5.1067 [DOI] [PubMed] [Google Scholar]
- Guardia-Laguarta C., Area-Gomez E., Rub C., Liu Y., Magrane J., Becker D., et al. (2014). alpha-Synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 34 249–259. 10.1523/JNEUROSCI.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y.-X., Wang X.-Y., Kang W.-Y., Zhang Y.-J., Zhang Y., Zhou Y., et al. (2012). Extracellular signal-regulated kinase is involved in alpha-synuclein-induced mitochondrial dynamic disorders by regulating dynamin-like protein 1. Neurobiol. Aging 33 2841–2854. 10.1016/j.neurobiolaging.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. (2009). What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 46 821–831. 10.1016/j.yjmcc.2009.02.021 [DOI] [PubMed] [Google Scholar]
- Hansson Petersen C. A., Alikhani N., Behbahani H., Wiehager B., Pavlov P. F., Alafuzoff I., et al. (2008). The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. U.S.A. 105 13145–13150. 10.1073/pnas.0806192105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S., Lackner L., Nunnari J. (2007). The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 76 751–780. 10.1146/annurev.biochem.76.071905.090048 [DOI] [PubMed] [Google Scholar]
- Hou Y., Lautrup S., Cordonnier S., Wang Y., Croteau D. L., Zavala E., et al. (2018). NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. U.S.A. 115 E1876–E1885. 10.1073/pnas.1718819115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. H., Shaltouki A., Gonzalez A. E., Bettencourt da Cruz A., Burbulla L. F., St Lawrence E., et al. (2016). Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell 19 709–724. 10.1016/j.stem.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. M., Youle R. J. (2012). PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 125(Pt 4), 795–799. 10.1242/jcs.093849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F., Exner N., Lutz A. K., Wender N., Hegermann J., Brunner B., et al. (2010). Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 29 3571–3589. 10.1038/emboj.2010.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D., Mattson M. P. (2011). Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 10 187–198. 10.1016/S1474-4422(10)70277-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J., Arora S., Singh B., Thakur L. C., Gambhir J., Prabhu K. (2011). Role of oxidative stress in pathophysiology of transient ischemic attack and stroke. Int. J. Biol. Med. Res. 2 611–615. [Google Scholar]
- Kieburtz K., Tilley B. C., Elm J. J., Babcock D., Hauser R., Ross G. W., et al. (2015). Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA 313 584–593. 10.1001/jama.2015.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. J., Raval A. P., Hirsch N., Perez-Pinzon M. A. (2010). Ischemic preconditioning mediates cyclooxygenase-2 expression via nuclear factor-kappa B activation in mixed cortical neuronal cultures. Transl. Stroke Res. 1 40–47. 10.1007/s12975-009-0006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Jin Y., Lemasters J. J. (2006). Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 290 H2024–H2034. 10.1152/ajpheart.00683.2005 [DOI] [PubMed] [Google Scholar]
- Kirkland R. A., Windelborn J. A., Kasprzak J. M., Franklin J. L. (2002). A Bax-induced pro-oxidant state is critical for cytochrome C release during programmed neuronal death. J. Neurosci. 22 6480–6490. 10.1523/JNEUROSCI.22-15-06480.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislin M., Sword J., Fomitcheva I. V., Croom D., Pryazhnikov E., Lihavainen E., et al. (2017). Reversible disruption of neuronal mitochondria by ischemic and traumatic injury revealed by quantitative two-photon imaging in the neocortex of anesthetized mice. J. Neurosci. 37 333–348. 10.1523/JNEUROSCI.1510-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller W., Olanow C. W., Rodnitzky R., Fink J. S., Growdon J. H., Paulson G., et al. (1993). Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N. Engl. J. Med. 328 176–183. 10.1056/NEJM199301213280305 [DOI] [PubMed] [Google Scholar]
- Kosenko E., Montoliu C., Giordano G., Kaminsky Y., Venediktova N., Buryanov Y., et al. (2004). Acute ammonia intoxication induces an NMDA receptor-mediated increase in poly(ADP-ribose) polymerase level and NAD metabolism in nuclei of rat brain cells. J. Neurochem. 89 1101–1110. 10.1111/j.1471-4159.2004.02426.x [DOI] [PubMed] [Google Scholar]
- Krasnikov B. F., Zorov D. B., Antonenko Y. N., Zaspa A. A., Kulikov I. V., Kristal B. S., et al. (2005). Comparative kinetic analysis reveals that inducer-specific ion release precedes the mitochondrial permeability transition. Biochim. Biophys. Acta 1708 375–392. 10.1016/j.bbabio.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Kumari S., Anderson L., Farmer S., Mehta S. L., Li P. A. (2012). Hyperglycemia alters mitochondrial fission and fusion proteins in mice subjected to cerebral ischemia and reperfusion. Transl. Stroke Res. 3 296–304. 10.1007/s12975-012-0158-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva Y. E., Sokolove P. M. (2000). Prooxidants open both the mitochondrial permeability transition pore and a low-conductance channel in the inner mitochondrial membrane. Arch. Biochem. Biophys. 376 377–388. 10.1006/abbi.2000.1730 [DOI] [PubMed] [Google Scholar]
- Levy M., Faas G. C., Saggau P., Craigen W. J., Sweatt J. D. (2003). Mitochondrial regulation of synaptic plasticity in the hippocampus. J. Biol. Chem. 278 17727–17734. 10.1074/jbc.M212878200 [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang T., Wang J. X., Zhang Z. J., Zhai Y., Yang G. Y., et al. (2014). Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem. Biophys. Res. Commun. 444 182–188. 10.1016/j.bbrc.2014.01.032 [DOI] [PubMed] [Google Scholar]
- Lin M. Y., Cheng X. T., Tammineni P., Xie Y. X., Zhou B., Cai Q., et al. (2017). Releasing syntaphilin removes stressed mitochondria from axons independent of mitophagy under pathophysiological conditions. Neuron 94 595–610.e6. 10.1016/j.neuron.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang L.-N. (2014). Mitochondrial enhancement for neurodegenerative movement disorders: a systematic review of trials involving creatine, coenzyme Q10, idebenone and mitoquinone. CNS Drugs 28 63–68. 10.1007/s40263-013-0124-4 [DOI] [PubMed] [Google Scholar]
- Liu K., Zhao Q., Liu P., Cao J., Gong J., Wang C., et al. (2016). ATG3-dependent autophagy mediates mitochondrial homeostasis in pluripotency acquirement and maintenance. Autophagy 12 2000–2008. 10.1080/15548627.2016.1212786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. K., Hsu C. Y., Dizdaroglu M., Floyd R. A., Kow Y. W., Karakaya A., et al. (1996). Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J. Neurosci. 16 6795–6806. 10.1523/JNEUROSCI.16-21-06795.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tian F., Kurata T., Morimoto N., Abe K. (2012). Dynamic changes of mitochondrial fusion and fission proteins after transient cerebral ischemia in mice. J. Neurosci. Res. 90 1183–1189. 10.1002/jnr.23016 [DOI] [PubMed] [Google Scholar]
- Lopez-Domenech G., Higgs N. F., Vaccaro V., Ros H., Arancibia-Carcamo I. L., MacAskill A. F., et al. (2016). Loss of dendritic complexity precedes neurodegeneration in a mouse model with disrupted mitochondrial distribution in mature dendrites. Cell Rep. 17 317–327. 10.1016/j.celrep.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. H., Zhao X. Y., Yao P. P., Xu D. E., Ma Q. H. (2018). The mitochondrion: a potential therapeutic target for Alzheimer’s disease. Neurosci. Bull. 34 1127–1130. 10.1007/s12264-018-0310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig P. E., Thankam F. G., Patil A. A., Chamczuk A. J., Agrawal D. K. (2018). Brain injury and neural stem cells. Neural Regen. Res. 13 7–18. 10.4103/1673-5374.224361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader J. W., Cirilli M., Lin C., Xu H. W., Takuma K., Wang N., et al. (2004). ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304 448–452. 10.1126/science.1091230 [DOI] [PubMed] [Google Scholar]
- Malkevitch N. V., Dedukhova V. I., Simonian R. A., Skulachev V. P., Starkov A. A. (1997). Thyroxine induces cyclosporin A-insensitive, Ca2+-dependent reversible permeability transition pore in rat liver mitochondria. FEBS Lett. 412 173–178. 10.1016/S0014-5793(97)00666-2 [DOI] [PubMed] [Google Scholar]
- Manczak M., Reddy P. H. (2012). Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease. Hum. Mol. Genet. 21 5131–5146. 10.1093/hmg/dds360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S., Santro T., Arumugam T. V. (2013). Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem. Int. 62 712–718. 10.1016/j.neuint.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Mark K. S., Davis T. P. (2002). Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 282 H1485–H1494. 10.1152/ajpheart.00645.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsili L., Rizzo G., Colosimo C. (2018). Diagnostic criteria for Parkinson’s disease: from James Parkinson to the concept of prodromal disease. Front. Neurol. 9:156 10.3389/fneur.2018.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell-Riera A., Segarra-Mondejar M., Munoz J. P., Ginet V., Olloquequi J., Perez-Clausell J., et al. (2014). Mfn2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J. 33 2388–2407. 10.15252/embj.201488327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E., Csiszar A., Dutta D., Balagopal G., Calvani R., Leeuwenburgh C. (2013). Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 305 H459–H476. 10.1152/ajpheart.00936.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheoud D., Sugiura A., Bellemare-Pelletier A., Laplante A., Rondeau C., Chemali M., et al. (2016). Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell 166 314–327. 10.1016/j.cell.2016.05.039 [DOI] [PubMed] [Google Scholar]
- Matsuda S., Umeda M., Uchida H., Kato H., Araki T. (2009). Alterations of oxidative stress markers and apoptosis markers in the striatum after transient focal cerebral ischemia in rats. J. Neural Trans. 116 395–404. 10.1007/s00702-009-0194-0 [DOI] [PubMed] [Google Scholar]
- McCracken E., Valeriani V., Simpson C., Jover T., McCulloch J., Dewar D. (2000). The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J. Cereb. Blood Flow Metab. 20 1529–1536. 10.1097/00004647-200011000-00002 [DOI] [PubMed] [Google Scholar]
- McManus M. J., Murphy M. P., Franklin J. L. (2011). The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 31 15703–15715. 10.1523/Jneurosci.0552-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P., Polidori M. C. (2012). Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta 1822 631–638. 10.1016/j.bbadis.2011.10.006 [DOI] [PubMed] [Google Scholar]
- Meyer J. N., Leuthner T. C., Luz A. L. (2017). Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 391 42–53. 10.1016/j.tox.2017.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W., Qu Z., Shi K., Zhang D., Zong Y., Zhang G., et al. (2015). RIP3 S-nitrosylation contributes to cerebral ischemic neuronal injury. Brain Res. 1627 165–176. 10.1016/j.brainres.2015.08.020 [DOI] [PubMed] [Google Scholar]
- Monti D. A., Zabrecky G., Kremens D., Liang T. W., Wintering N. A., Cai J., et al. (2016). N-acetyl cysteine may support dopamine neurons in Parkinson’s disease: preliminary clinical and cell line data. PLoS One 11:e0157602. 10.1371/journal.pone.0157602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K.-H., Kim B.-J., Song B. J. (2005). Inhibition of mitochondrial aldehyde dehydrogenase by nitric oxide-mediated S-nitrosylation. FEBS Lett. 579 6115–6120. 10.1016/j.febslet.2005.09.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais V. A., Verstreken P., Roethig A., Smet J., Snellinx A., Vanbrabant M., et al. (2009). Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol. Med. 1 99–111. 10.1002/emmm.200900006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuoglu M., Elibol B., Dalmizrak O., Ercan A., Kulaksiz G., Ogus H., et al. (2004). Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov. Disord. 19 544–548. 10.1002/mds.10695 [DOI] [PubMed] [Google Scholar]
- Muller M., Lu K., Reichert A. S. (2015). Mitophagy and mitochondrial dynamics in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1853(10 Pt B) 2766–2774. 10.1016/j.bbamcr.2015.02.024 [DOI] [PubMed] [Google Scholar]
- Musiek E. S., Holtzman D. M. (2015). Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat. Neurosci. 18 800–806. 10.1038/nn.4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaran K., Kanwar A., Vegh C., Marginean A., Elliott A., Guilbeault N., et al. (2018). Ubisol-Q10 (a nanomicellar water-soluble formulation of CoQ10) treatment inhibits Alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (TgAPEswe, PSEN1dE9) model of Alzheimer’s disease. J. Alzheimers Dis. 61 221–236. 10.3233/JAD-170275 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Nemani V. M., Azarbal F., Skibinski G., Levy J. M., Egami K., et al. (2011). Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 286 20710–20726. 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan P., Fujimura M., Noshita N., Chan P. H. (2003). Role of superoxide in poly(ADP-ribose) polymerase upregulation after transient cerebral ischemia. Mol. Brain Res. 113 28–36. 10.1016/s0169-328x(03)00062-7 [DOI] [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D. F., Youle R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183 795–803. 10.1083/jcb.200809125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niatsetskaya Z. V., Sosunov S. A., Matsiukevich D., Utkina-Sosunova I. V., Ratner V. I., Starkov A. A., et al. (2012). The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia-ischemia in neonatal mice. J. Neurosci. 32 3235–3244. 10.1523/JNEUROSCI.6303-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norambuena A., Wallrabe H., Cao R., Wang D. B., Silva A., Svindrych Z., et al. (2018). A novel lysosome-to-mitochondria signaling pathway disrupted by amyloid-beta oligomers. EMBO J. 37:e100241 10.15252/embj.2018100241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E. K., et al. (2001). Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 60 759–767. 10.1093/jnen/60.8.759 [DOI] [PubMed] [Google Scholar]
- Oda A., Tamaoka A., Araki W. (2010). Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J. Neurosci. Res. 88 1137–1145. 10.1002/jnr.22271 [DOI] [PubMed] [Google Scholar]
- O’Donnell K. C., Lulla A., Stahl M. C., Wheat N. D., Bronstein J. M., Sagasti A. (2014). Axon degeneration and PGC-1alpha-mediated protection in a zebrafish model of alpha-synuclein toxicity. Dis. Model. Mech. 7 571–582. 10.1242/dmm.013185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. K., Sultan A., Platzer J., Dolatabadi N., Soldner F., McClatchy D. B., et al. (2017). S-nitrosylation of PINK1 attenuates PINK1/parkin-dependent mitophagy in hiPSC-based Parkinson’s disease models. Cell Rep. 21 2171–2182. 10.1016/j.celrep.2017.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi A., Beltran B., Clementi E., Hallen K., Feelisch M., Moncada S. (2000). Continuous exposure to high concentrations of nitric oxide leads to persistent inhibition of oxygen Consumption by J774 cells as well as extraction of oxygen by the extracellular medium. Biochem. J. 346 407–412. 10.1042/bj3460407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini D., Cali T., Szabo I., Brini M. (2017). Alpha-synuclein at the intracellular and the extracellular side: functional and dysfunctional implications. Biol. Chem. 398 77–100. 10.1515/hsz-2016-0201 [DOI] [PubMed] [Google Scholar]
- Owens K., Park J. H., Gourley S., Jones H., Kristian T. (2015). Mitochondrial dynamics: cell-type and hippocampal region specific changes following global cerebral ischemia. J. Bioenerg. Biomembr. 47 13–31. 10.1007/s10863-014-9575-7 [DOI] [PubMed] [Google Scholar]
- Park C. K., Hall E. D. (1994). Dose-response analysis of the effect of 21-aminosteroid tirilazad mesylate (U-74006f) upon neurological outcome and ischemic brain-damage in permanent focal cerebral-ischemia. Brain Res. 645 157–163. 10.1016/0006-8993(94)91649-7 [DOI] [PubMed] [Google Scholar]
- Peng C., Rao W., Zhang L., Wang K., Hui H., Wang L., et al. (2015). Mitofusin 2 ameliorates hypoxia-induced apoptosis via mitochondrial function and signaling pathways. Int. J. Biochem. Cell Biol. 69 29–40. 10.1016/j.biocel.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Pickles S., Vigie P., Youle R. J. (2018). Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 28 R170–R185. 10.1016/j.cub.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell A. M., Youle R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85 257–273. 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portbury S. D., Hare D. J., Sgambelloni C., Perronnes K., Portbury A. J., Finkelstein D. I., et al. (2017). Trehalose improves cognition in the transgenic Tg2576 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 60 549–560. 10.3233/JAD-170322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo Devoto V., Dimopoulos N., Alloatti M., Pardi M. B., Saez T. M., Otero M. G., et al. (2017). α-Synuclein control of mitochondrial homeostasis in human-derived neurons is disrupted by mutations associated with Parkinson’s disease. Sci. Rep. 7:5042. 10.1038/s41598-017-05334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo Devoto V., Falzone T. (2017). Mitochondrial dynamics in Parkinson’s disease: a role for α-synuclein? Dis. Model. Mech. 10 1075–1087. 10.1242/dmm.026294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime T. A., Blaikie F. H., Evans C., Nadtochiy S. M., James A. M., Dahm C. C., et al. (2009). A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 106 10764–10769. 10.1073/pnas.0903250106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla R. A., von Bernhardi R., Godoy J. A., Inestrosa N. C., Johnson G. V. (2014). Phosphorylated tau potentiates Aβ-induced mitochondrial damage in mature neurons. Neurobiol. Dis. 71 260–269. 10.1016/j.nbd.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Rappold P. M., Cui M., Grima J. C., Fan R. Z., de Mesy-Bentley K. L., Chen L., et al. (2014). Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat. Commun. 5:5244. 10.1038/ncomms6244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A., Cuadrado E., Ortega-Aznar A., Hernandez-Guillamon M., Lo E. H., Montaner J. (2008). MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39 1121–1126. 10.1161/STROKEAHA.107.500868 [DOI] [PubMed] [Google Scholar]
- Rothfuss O., Fischer H., Hasegawa T., Maisel M., Leitner P., Miesel F., et al. (2009). Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum. Mol. Genet. 18 3832–3850. 10.1093/hmg/ddp327 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Lepsverdize E., Chi S. L., Das A. M., Pizzo S. V., Dityatev A., et al. (2008). Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol. Psychiatry 13 953–969. 10.1038/sj.mp.4002077 [DOI] [PubMed] [Google Scholar]
- Schulz K. L., Eckert A., Rhein V., Mai S., Haase W., Reichert A. S., et al. (2012). A new link to mitochondrial impairment in tauopathies. Mol. Neurobiol. 46 205–216. 10.1007/s12035-012-8308-3 [DOI] [PubMed] [Google Scholar]
- Scott P., Barsan W., Frederiksen S., Kronick S., Zink B. J., Domeier R. M., et al. (1996). A randomized trial of tirilazad mesylate in patients with acute stroke (RANTTAS). Stroke 27 1453–1458. 10.1161/01.STR.27.9.1453 [DOI] [PubMed] [Google Scholar]
- Senin U., Parnetti L., Barbagallosangiorgi G., Bartorelli L., Bocola V., Capurso A., et al. (1992). Idebenone in senile dementia of alzheimer type - a multicenter study. Arch. Gerontol. Geriatr. 15 249–260. 10.1016/0167-4943(92)90060-H [DOI] [PubMed] [Google Scholar]
- Shadel G. S., Horvath T. L. (2015). Mitochondrial ROS signaling in organismal homeostasis. Cell 163 560–569. 10.1016/j.cell.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R. Y., Zhu S. H., Li V., Gibson S. B., Xu X. S., Kong J. M. (2014). BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke. CNS Neurosci. Ther. 20 1045–1055. 10.1111/cns.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z. Q., Sunico C. R., McKercher S. R., Cui J., Feng G. S., Nakamura T., et al. (2013). S-nitrosylated SHP-2 contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke. Proc. Natl. Acad. Sci. U.S.A. 110 3137–3142. 10.1073/pnas.1215501110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. H., Ko H. S., Kang H., Lee Y., Lee Y. I., Pletinkova O., et al. (2011). PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell 144 689–702. 10.1016/j.cell.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley R., Ord E. N., Work L. M. (2014). Oxidative stress and the use of antioxidants in stroke. Antioxidants 3 472–501. 10.3390/antiox3030472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D. F., Selfridge J. E., Lu J., E L., Cardoso S. M., Swerdlow R. H. (2012). Mitochondrial abnormalities in Alzheimer’s disease: possible targets for therapeutic intervention. Adv. Pharmacol. 64 83–126. 10.1016/B978-0-12-394816-8.00003-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter D. A., Martinez J., Hao L., Chen X., Sun N., Fischer T. D., et al. (2018). Parkin and PINK1 mitigate STING-induced inflammation. Nature 561 258–262. 10.1038/s41586-018-0448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. M., Eyal E., Weintraub D. Adagio Investigators. (2015). Combined rasagiline and antidepressant use in Parkinson disease in the adagio study: effects on nonmotor symptoms and tolerability. JAMA Neurol. 72 88–95. 10.1001/jamaneurol.2014.2472 [DOI] [PubMed] [Google Scholar]
- Snow B. J., Rolfe F. L., Lockhart M. M., Frampton C. M., O’Sullivan J. D., Fung V., et al. (2010). A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 25 1670–1674. 10.1002/mds.23148 [DOI] [PubMed] [Google Scholar]
- Soane L., Kahraman S., Kristian T., Fiskum G. (2007). Mechanisms of impaired mitochondrial energy metabolism in acute and chronic neurodegenerative disorders. J. Neurosci. Res. 85 3407–3415. 10.1002/jnr.21498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N., Xie J. (2018). Iron, dopamine, and alpha-synuclein interactions in at-risk dopaminergic neurons in Parkinson’s disease. Neurosci. Bull. 34 382–384. 10.1007/s12264-018-0209-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P., Podlutskaya N., Hart M. J., Debnath J., Gorostiza O., Bredesen D., et al. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5:e9979. 10.1371/journal.pone.0009979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Rivero J. M., Villanueva-Paz M., de la Cruz-Ojeda P., de la Mata M., Cotan D., Oropesa-Avila M., et al. (2016). Mitochondrial dynamics in mitochondrial diseases. Diseases 5:1. 10.3390/diseases5010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T., Lewen A., Gasche Y., Yu F. S., Chan P. H. (2002). Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J. 16 1997–1999. 10.1096/fj.02-0251fje [DOI] [PubMed] [Google Scholar]
- Sullivan P. G., Thompson M., Scheff S. W. (2000). Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161 631–637. 10.1006/exnr.1999.7282 [DOI] [PubMed] [Google Scholar]
- Sun B. L., Li W. W., Zhu C., Jin W. S., Zeng F., Liu Y. H., et al. (2018). Clinical research on Alzheimer’s disease: progress and perspectives. Neurosci. Bull. 34 1111–1118. 10.1007/s12264-018-0249-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. H., Burns J. M., Khan S. M. (2014). The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim. Biophys. Acta 1842 1219–1231. 10.1016/j.bbadis.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados T., Dul C., Majtenyi K., Hargitai J., Penzes Z., Urbanics R. (2004). A chronic Alzheimer’s model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav. Brain Res. 154 31–40. 10.1016/j.bbr.2004.01.016 [DOI] [PubMed] [Google Scholar]
- Tait S. W., Green D. R. (2013). Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 5:a008706. 10.1101/cshperspect.a008706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa S., Hirabayashi H., Matsushima K., Tokuoka K., Shinohara Y. (1998). Induction of heme oxygenase protein protects neurons in cortex and striatum, but not in hippocampus, against transient forebrain ischemia. J. Cereb. Blood Flow Metab. 18 559–569. 10.1097/00004647-199805000-00011 [DOI] [PubMed] [Google Scholar]
- Tamagno E., Bardini P., Obbili A., Vitali A., Borghi R., Zaccheo D., et al. (2002). Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 10 279–288. 10.1006/nbdi.2002.0515 [DOI] [PubMed] [Google Scholar]
- Todorova V., Blokland A. (2017). Mitochondria and synaptic plasticity in the mature and aging nervous system. Curr. Neuropharmacol. 15 166–173. 10.2174/1570159X14666160414111821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora V., Quijano C., Freeman B., Radi R., Castro L. (2007). Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: mechanisms and relative contributions to aconitase inactivation. Free Radic. Biol. Med. 42 1075–1088. 10.1016/j.freeradbiomed.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Uchino H. (2002). Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol. Dis. 10 219–233. 10.1006/nbdi.2002.0514 [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39 44–84. 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. (2009). Mitochondria in early mammalian development. Semin. Cell Dev. Biol. 20 354–364. 10.1016/j.semcdb.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Vieira H., Kroemer G. (2003). Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life 55 613–616. 10.1080/15216540310001639652 [DOI] [PubMed] [Google Scholar]
- Vos M., Esposito G., Edirisinghe J. N., Vilain S., Haddad D. M., Slabbaert J. R., et al. (2012). Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 336 1306–1310. 10.1126/science.1218632 [DOI] [PubMed] [Google Scholar]
- Wang J., Wang P., Li S., Wang S., Li Y., Liang N., et al. (2014). Mdivi-1 prevents apoptosis induced by ischemia-reperfusion injury in primary hippocampal cells via inhibition of reactive oxygen species-activated mitochondrial pathway. J. Stroke Cerebrovasc. Dis. 23 1491–1499. 10.1016/j.jstrokecerebrovasdis.2013.12.021 [DOI] [PubMed] [Google Scholar]
- Wang W. Z., Yin J., Ma X. P., Zhao F. P., Siedlak S. L., Wang Z. L., et al. (2017). Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum. Mol. Genet. 26 4118–4131. 10.1093/hmg/ddx299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. (2017). Destructive cellular paths underlying familial and sporadic Parkinson disease converge on mitophagy. Autophagy 13 1998–1999. 10.1080/15548627.2017.1327511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu X., Yang Y., Takata T., Sakurai T. (2016). Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 1643 1–9. 10.1016/j.brainres.2016.04.060 [DOI] [PubMed] [Google Scholar]
- Wang X., Su B., Lee H. G., Li X., Perry G., Smith M. A., et al. (2009). Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 29 9090–9103. 10.1523/JNEUROSCI.1357-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su B., Siedlak S. L., Moreira P. I., Fujioka H., Wang Y., et al. (2008). Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. U.S.A. 105 19318–19323. 10.1073/pnas.0804871105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. T., Lu M. H., Zhang Y., Ji W. L., Lei L., Wang W., et al. (2018). Disrupted-in-schizophrenia-1 protects synaptic plasticity in a transgenic mouse model of Alzheimer’s disease as a mitophagy receptor. Aging Cell 10.1111/acel.12860 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M. T., Pearce B. R., Bowen D. M., Francis P. T. (1998). The effects of perturbed energy metabolism on the processing of amyloid precursor protein in PC12 cells. J. Neural Transm. 105 839–853. 10.1007/s007020050098 [DOI] [PubMed] [Google Scholar]
- Weyer G., BabejDolle R. M., Hadler D., Hofmann S., Herrmann W. M. (1997). A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology 36 73–82. 10.1159/000119366 [DOI] [PubMed] [Google Scholar]
- Winslow A. R., Chen C. W., Corrochano S., Acevedo-Arozena A., Gordon D. E., Peden A. A., et al. (2010). alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J. Cell Biol. 190 1023–1037. 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte M. E., Mahad D. J., Lassmann H., van Horssen J. (2014). Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 20 179–187. 10.1016/j.molmed.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Xi Y., Feng D., Tao K., Wang R., Shi Y., Qin H., et al. (2018). MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1alpha. Biochim. Biophys. Acta Mol. Basis Dis. 1864(9 Pt B) 2859–2870. 10.1016/j.bbadis.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Xue D., Slivka A., Buchan A. M. (1992). Tirilazad reduces cortical infarction after transient but not permanent focal cerebral-ischemia in rats. Stroke 23 894–899. 10.1161/01.Str.23.6.894 [DOI] [PubMed] [Google Scholar]
- Yamagata K., Tagami M., Takenaga F., Yamori Y., Itoh S. (2004). Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol. Dis. 17 491–499. 10.1016/j.nbd.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Zempel H., Thies E., Mandelkow E., Mandelkow E. M. (2010). Aβ oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30 11938–11950. 10.1523/JNEUROSCI.2357-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., van der Bliek A. M. (2012). Mitochondrial fission, fusion, and stress. Science 337 1062–1065. 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. S., Geng W. S., Jia J. J., Chen L., Zhang P. P. (2018). Cellular and molecular basis of neurodegeneration in Parkinson disease. Front. Aging Neurosci. 10:109 10.3389/fnagi.2018.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wang S., Li Y., Che L., Zhao Q. (2013). A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci. Lett. 535 104–109. 10.1016/j.neulet.2012.12.049 [DOI] [PubMed] [Google Scholar]
- Zhang X., Yan H., Yuan Y., Gao J., Shen Z., Cheng Y., et al. (2013). Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9 1321–1333. 10.4161/auto.25132 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao B. (2013). Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013:316523. 10.1155/2013/316523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Lin M. Y., Sun T., Knight A. L., Sheng Z. H. (2014). Characterization of mitochondrial transport in neurons. Methods Enzymol. 547 75–96. 10.1016/B978-0-12-801415-8.00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Wang K. Z., Chu C. T. (2013). After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 9 1663–1676. 10.4161/auto.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M. (2000). New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur. J. Biochem. 267 1550–1564. 10.1046/j.1432-1327.2000.01187.x [DOI] [PubMed] [Google Scholar]
- Zorov D. B., Filburn C. R., Klotz L. O., Zweier J. L., Sollott S. J. (2000). Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192 1001–1014. 10.1084/jem.192.7.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Zhang S., Xia C. Y., Guo X. F., He W. B., Chen N. H. (2014). Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: the role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology 86 103–115. 10.1016/j.neuropharm.2014.07.002 [DOI] [PubMed] [Google Scholar]