Abstract
Background:
Point-of-care ultrasound (POCUS) can potentially help distinguish cellulitis from abscess, which can appear very similar on physical examination but necessitate different treatment approaches.
Objective:
To compare POCUS guidance vs. clinical assessment alone on the management of pediatric skin and soft tissue infections (SSTI) in the emergency department (ED) setting.
Methods:
Children ages 6 months to 18 years presenting to participating EDs with SSTIs ≥ 1 cm were eligible. All treatment decisions, including use of POCUS, were at the discretion of the treating clinicians. Patients were divided into those managed with POCUS guidance (POCUS group) and those managed using clinical assessment alone (non-POCUS group). Primary outcome was clinical treatment failure at 7–10 days (unscheduled ED return visit or admission, procedural intervention, change in antibiotics therapy). Secondary outcomes were ED length of stay, discharge rate, use of alternative imaging, and need for procedural sedation. POCUS utility and impact on management decisions were also assessed by treating clinicians.
Results:
In total, 321 subjects (327 lesions) were analyzed, of which 299 (93%) had completed follow-up. There was no significant difference between the POCUS and non-POCUS groups in any of the primary or secondary outcomes. Management plan was changed in the POCUS group in 22.9% of cases(13.8% from medical to surgical, 9.1% from surgical to medical). Clinicians reported increased benefit of POCUS in cases of higher clinical uncertainty.
Conclusions:
Use of POCUS was not associated with decreased ED treatment failure rate or process outcomes in pediatric SSTI patients. However, POCUS changed the management plan in approximately one in four cases.
Keywords: ultrasonography, pediatrics, emergency, skin and soft tissue infections
INTRODUCTION
Over the past two decades, ambulatory and emergency department (ED) visits for skin and soft tissue infections (SSTIs) have doubled in number, with a current estimate of more than 14 million encounters annually in the United States. This rise in SSTIs is largely attributable to the increase in incidence of cellulitis and abscess, as well as the rising prevalence of community-associated methicillin resistant Staphylococcus aureus (1–4). Among patients younger than 18 years, SSTI-related outpatient visits, incision and drainage rate, and hospitalization rate have nearly doubled from 1997 to 2005, more than any other age group (2,5).
Skin abscess is typically managed with incision and drainage (surgical management), whereas antibiotics alone (medical management) is generally sufficient for cellulitis. Nevertheless, abscess and cellulitis can have very similar appearance on physical examination, creating a clinical dilemma given the differing treatment modalities. Prior investigations have found that clinical assessment alone has a high rate of inaccuracy with respect to distinguishing abscess from cellulitis, or whether an incision and drainage is indicated (6,7).
Recently, point-of-care ultrasound (POCUS) has emerged as a promising tool to aid in distinguishing abscess from cellulitis in the ED setting. Several studies attest to the superior sensitivity and specificity of POCUS compared with physical assessment alone, and the positive effect of POCUS in improving bedside treatment decisions for pediatric SSTI (8–11). Limitations of these studies include small sample sizes, single-site studies, and lack of a control group. Most importantly, there are no published studies directly comparing the outcomes of pediatric SSTI patients treated with POCUS guidance vs. those treated based solely on physical examination findings.
The current study examines the outcome of POCUS vs. clinical assessment alone on the management of pediatric SSTIs in the ED setting. We examined the effect of POCUS utilization on treatment failure rate as well as process outcomes including ED length of stay and resource utilization.
MATERIALS AND METHODS
Study Design and Setting
This was a prospective, multicenter, cohort observational study of children presenting to the ED with SSTIs between March 1, 2014 and July 31, 2016. Study sites included seven institutions across North America (six in the United States, one in Canada) with a combined annual volume of more than 350,000 pediatric visits. All participating institutions had separate pediatric EDs where children were seen by board certified/eligible emergency physicians or pediatric emergency physicians. All were urban, academic, tertiary medical centers with designated POCUS directors and quality assurance processes in place. Credentialed POCUS users at each site were required to watch a video prior to enrollment of subjects to ensure that SSTI POCUS study images were obtained and recorded using a standardized approach. The study was registered on ClinicalTrials.gov website (identification number NCT02099227).
Subject Recruitment and Study Procedure
Eligible subjects were children 6 months to 18 years of age presenting to the participating EDs with history and examination findings consistent with SSTI as determined by the treating clinician. For study inclusion, the lesion diameter on physical examination had to be at least 1 cm. Exclusion criteria included suspected soft tissue infections involving, or near, mucosal membranes (e.g., perirectal, peritonsillar, vulvovaginal areas), facial lesions, paronychia or felons, and subjects deemed unsuitable by treating clinicians.
Subjects were recruited using a convenience sampling approach when a member of the study team was available. Potential subjects were identified from the electronic patient tracking board at each participating ED. Those meeting inclusion criteria were then approached for enrollment. Written informed consents were obtained from parents/guardians; assents were obtained when appropriate. All ED treatments then proceeded according to the standard of care at each institution, and all management decisions, including the use of POCUS, were at the discretion of the treatment team. The study was approved by Institutional Review Boards at all participating study sites.
Data Collection
Immediately after the initial clinical evaluation, ED treating clinicians completed a standardized data collection form for all study subjects. Data collected during the visit included subject demographics, history and physical examination findings, degree of clinical suspicion for presence of an abscess (rated on a 1–100 visual analog scale), and the initial management plan (e.g., antibiotics administration, incision and drainage, ordering of further studies, consultation of other specialists). After the visit, a member of the study team also extracted from the medical record any use of procedural sedation, ED length of stay, disposition, results of any intervention, and test/imaging results if obtained for the index ED visits, and repeat visits to the ED.
Two ad hoc study groups were defined in the cohort: the POCUS group, which had POCUS performed as part of their ED management, and the clinical assessment (non-POCUS) group, which had care based solely on clinical grounds. In the POCUS group, ultrasound was performed by the treating clinicians and its findings used to guide treatment decisions. Not all providers were POCUS credentialed. Thus, the decision to use POCUS for treatment guidance was based on provider training/credentialing, as well as provider clinical judgement. All POCUS images were saved electronically in still or clip format, and included at least two views of the suspected lesion, as well as Doppler interrogation of the site concerned. POCUS findings, perceived utility of POCUS (rated on a 1–100 visual analog scale), and whether there was a change in management plan after POCUS was performed (e.g., changed incision location/size, added packing, medical to surgical management, surgical to medical management, consultation of specialist, other) were recorded on the data sheet. Deidentified POCUS images from the study were reviewed blindly by all site ultrasound directors at the completion of data collection for quality assurance purposes.
Follow-up occurred at 7–10 days after ED discharge via telephone interview. Three separate attempts were made to contact the parent/guardian/subject at the telephone number provided. Electronic medical records at each hospital were also reviewed by study team members in cases of hospitalized patients or patients lost to follow-up. The goal was to identify incidences of unscheduled ED or outpatient visits, unscheduled admissions, recurrent abscesses, repeat or new incisions, or initiation/change in antibiotics. Each of these cases was reviewed carefully to make sure that it qualified as “treatment failure” of the initial visit.
Sample Size Estimation and Statistical Analysis
Based on prior literature, we estimated an ED SSTI treatment failure rate of 15% (12). Assuming that POCUS would reduce treatment failure rate to 5%, we estimated that a sample size of 280 (140 in each group) would give us a two-tailed α of 0.05, and a power of 0.8 for the primary outcome.
All locally recorded data were uploaded anonymously onto a REDCap (Research Electronic Data Capture) database hosted at the University of Illinois Center for Clinical and Translational Science. Study data were summarized using descriptive statistics. Chi-squared test, Wilcoxon rank–sum test, or independent sample t-test was used for comparison between the POCUS and non-POCUS groups when appropriate. The primary outcome of interest was clinical treatment failure rate, defined as presence of one or more of the following at 7–10 days: unscheduled ED return visit, need for subsequent procedural intervention or admission, or change in antibiotics therapy. Chi-squared tests were used in the comparative analysis. Data were stratified by site in addition to the overall results to detect meaningful differences between groups while taking into account practice variation across institutions. Secondary outcomes included comparisons of ED length of stay, discharge rate, use of alternative imaging, and need for procedural sedation between POCUS and non-POCUS groups. These were assessed using chi-squared test or Wilcoxon rank–sum test. Additionally, utility of POCUS was compared among groups with different levels of clinical suspicion for the presence of an abscess using analysis of variance. Type I error rate was set at 0.05 for all comparisons. Statistical analysis was performed with SAS Studio v3.5 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 328 subjects (334 lesions) were consented and enrolled. Seven subjects were excluded due to age, lesion size, or POCUS performance by noncredentialed staff. A total of 321 subjects were analyzed, of which 299(93.0%; 305 lesions) had complete follow-up. There were 209 subjects (69.9% of total included) with 214 lesions in the POCUS group and 90 subjects with 91 lesions in the non-POCUS group (Figure 1). Patient demographics are summarized in Table 1. Locations and clinical examination findings for lesions are listed in Table 2. Treatment outcomes are compared in Table 3. Overall there was no significant difference between the POCUS and non-POCUS groups in terms of demographic characteristics, history, physical examination findings, test results, discharge rate, or ED length of stay. Clinician-estimated likelihood of drainable fluid collection after physical examination, overall frequency of surgical procedures, and frequency of incision and drainage performed were significantly greater in the POCUS group. There was also no significant difference between subjects with complete follow-up and those who were lost to follow-up in any of the above measurements. Table 4 lists the clinician type by hospital. The majority of clinician respondents (305 subjects, 95% of total) were attending or fellow physicians.
Figure 1.
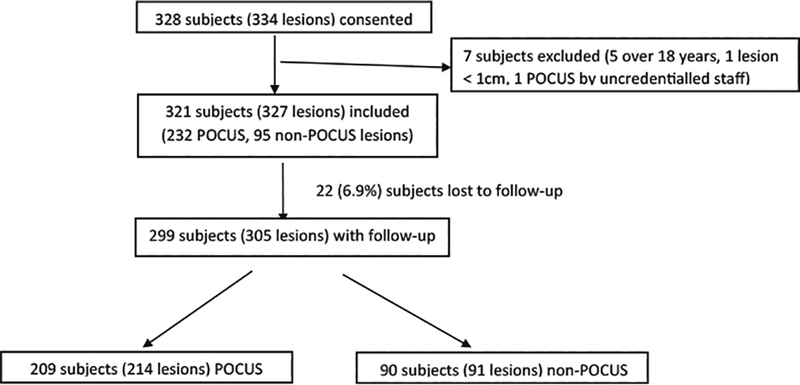
Inclusion of study subject for analysis. POCUS = point-of-care ultrasound.
Table 1.
Summary of Patient Demographic Characteristics
| Variable | POCUS (n = 232) | Non-POCUS (n = 95) | p-Value |
|---|---|---|---|
| Age (years)* | 7.8 (6.3)* | 7.5 (5.9)* | 0.766 |
| Sex, male, n (%) | 107(46.1) | 45 (47.4) | 0.837 |
| Duration of symptoms (days)† | 3 (2–6)† | 3 (2–5)† | 0.246 |
| Currently on antibiotics, n (%) | 65 (28.0) | 29 (30.5) | 0.641 |
| History of prior abscess, n (%) | 67 (28.8) | 29 (30.5) | 0.750 |
| Presence of comorbidity, n (%) | |||
| Diabetes | 4(1.7) | 2(2.1) | 0.850 |
| Obesity | 9 (3.9) | 3 (3.2) | 0.709 |
| Asthma | 11 (4.7) | 10 (10.5) | 0.0649 |
| Eczema | 14 (6.0) | 5 (5.3) | 0.729 |
| Homelessness | 0 (0) | 0 (0) | - |
| Sickle Cell disease | 0 (0) | 0 (0) | - |
| Immunosuppression | 6 (2.6) | 0 (0) | 0.107 |
| Others | 22 (9.5) | 7 (7.4) | 0.481 |
| Likelihood of drainable fluid (1–100)* | 62.5 (33.6)* | 50.7 (40.8)* | 0.0138 |
POCUS = point-of-care ultrasound.
Mean (SD).
Median (interquartile range).
Table 2.
Location and Physical Examination Findings of theIncluded SSTI Lesions
| POCUS (n = 232) | Non-POCUS (n = 95) | p-Value | |
|---|---|---|---|
| Location of lesions, n (%) | |||
| Head/neck (excluding face) | 9 (3.9) | 6 (6.3) | 0.339 |
| Chest/abdomen/back | 38 (16.4) | 19(20.0) | 0.923 |
| Genital/perineum | 44 (19.0) | 6 (6.3) | 0.0031 |
| Upper extremity | 39 (16.8) | 21 (22.1) | 0.261 |
| Lower extremity | 102 (44.0) | 43 (45.2) | 0.999 |
| Characteristics of lesions | Mean (SD) | Mean (SD) | |
| Erythema length (cm) | 5.3 (4.4) | 5.5 (3.8) | 0.613 |
| Erythema width (cm) | 4.5 (3.8) | 5.1 (4.0) | 0.238 |
| Induration length (cm) | 3.7 (3.1) | 3.1 (2.9) | 0.129 |
| Induration width (cm) | 3.2 (2.5) | 3 (3.2) | 0.650 |
| Fluctuance length (cm) | 1.5 (2.1) | 1.1 (1.6) | 0.119 |
| Fluctuance width (cm) | 1.3 (1.7) | 1.2 (1.5) | 0.632 |
| n (%) | n (%) | ||
| Lymphangitis | 2 (0.9) | 3 (3.2) | 0.125 |
SSTI = skin and soft tissue infection; POCUS = point-of-care ultrasound.
Table 3.
Comparison of Treatment Outcomes of Subjects in POCUS and Non-POCUS Groups
| Variable | POCUS (n = 232) | Non-POCUS (n = 95) | p-Value |
|---|---|---|---|
| Surgical procedure, n (%) | 141 (60.8) | 38 (40.0) | <0.001 |
| Incision & drainage | 122 (52.6) | 33 (34.7) | 0.0033 |
| Needle aspiration | 7 (3.0) | 1 (1.1) | 0.446 |
| Manual expression | 15 (6.5) | 5 (5.3) | 0.680 |
| Blood culture positive, n (%) | 1 (0.43) | 0 (0) | 1.000 |
| Wound culture positive, n (%) | 105(45.2) | 28 (29.5) | 0.517 |
| Discharge, n (%) | 166 (71.6) | 66 (69.5) | 0.876 |
| Length of stay (minutes)* | 139.5(99.5–245)* | 162.0 (92–264)* | 0.935 |
POCUS = point-of-care ultrasound.
Median (interquartile range).
Table 4.
Treating Clinician Level by Hospital (Based onSurvey Responses)
| Attending | Fellow | Resident | Midlevel | Total | |
|---|---|---|---|---|---|
| Hospital | |||||
| A | 68 | 2 | 3 | 0 | 73 |
| B | 32 | 3 | 0 | 0 | 35 |
| C | 35 | 2 | 1 | 0 | 38 |
| D | 36 | 9 | 1 | 0 | 46 |
| E | 39 | 3 | 0 | 0 | 42 |
| F | 34 | 9 | 1 | 2 | 46 |
| G | 25 | 8 | 7 | 1 | 41 |
POCUS was performed on a total of 232 (71%) lesions. The median perceived utility of POCUS by clinicians was 95 out of 100. Figure 2 plots POCUS utility as rated on a continuous 1–100 scale by clinicians against clinical likelihood (also on 1–100 scale, divided into quintiles) of presence of drainable fluid collection. Perceived utility of POCUS was highest in cases with the most clinical uncertainty (41–60% clinical suspicion for drainable fluid collection). Many providers commented on the utility of POCUS in confirming their clinical suspicion/diagnoses, therefore, a separate category was created post hoc to tally this response (Table 5). The management plan was changed as a result of POCUS performance in 22.9% of cases, including 13.8% from medical to surgical, and 9.1% from surgical to medical.
Figure 2.
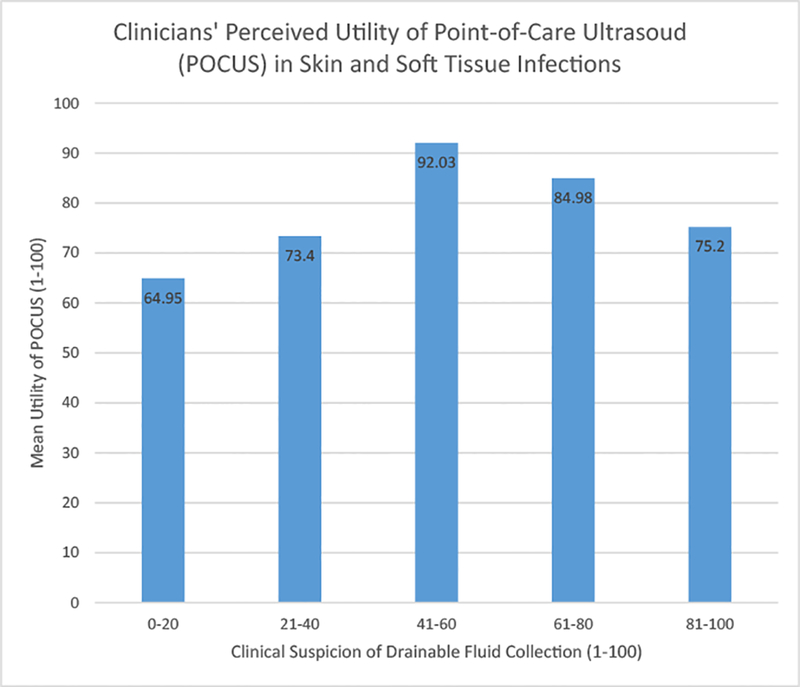
Plot of POCUS utility against clinical likelihood of drainable fluid collection.
Table 5.
Clinicians’ Perceived POCUS Utility and Impact onLesion Management
| Utility of POCUS | ||
|---|---|---|
| Median (interquartile range) helpfulness of POCUS (1–100) | 95 (66–100) | |
| Ultrasound impact (percentage, total n = 232) | n | % |
| Changed incision location/size | 46 | 19.8 |
| Added packing | 5 | 2.2 |
| Medical to surgical | 32 | 13.8 |
| Surgical to medical | 21 | 9.1 |
| Confirmation of clinical plan | 67 | 28.9 |
| Consultation of specialist(s) | 7 | 3.0 |
| Other | 27 | 11.6 |
POCUS = point-of-care ultrasound.
On analysis of those subjects with complete follow-up, rate of treatment failure was not significantly different between the POCUS and non-POCUS groups, in aggregate or in any of the individual outcome criterion. Furthermore, there was no significant difference between the two groups in any of the process outcomes, including discharge rate, ED length of stay, use of sedation, or order of alternative imaging by provider (Table 6). Analysis of the data using patients (instead of lesions) as the unit of measurement yielded the same conclusions. Stratified analysis by hospital or level of experience (attendings vs. fellows/residents) also did not yield any significant difference between the two groups (data not shown).
Table 6.
Comparison of Primary and Secondary Outcomes in POCUS and Non-POCUS Groups (n = Number of Lesions)
| POCUS (n = 214) | Non-POCUS (n = 91) | p-Value | |
|---|---|---|---|
| Primary outcome Resolution of symptoms, n (%) | 179(83.6) | 74(81.3) | 0.621 |
| Treatment failure, n (%) | 30 (14.0) | 13(14.3) | 0.855 |
| Hospital admission | 6 (2.8) | 3 (3.3) | 0.816 |
| Unscheduled return to ED | 6 (2.8) | 6 (6.6) | 0.119 |
| New incision/reinstrumentation | 15(7.0) | 8 (8.8) | 0.590 |
| Antibiotics started/changed | 15(7.0) | 5 (5.5) | 0.625 |
| Recurrent abscess | 3(1.4) | 2 (2.2) | 0.617 |
| Secondary outcomes Discharge, n (%) | 148(69.2) | 63 (69.2) | 0.946 |
| ED LOS (time to disposition), minutes* | 138.0 | 163.5 | 0.935 |
| Sedation, n (%) | 41 (19.2) | 11 (12.1) | 0.133 |
| Alternative imaging, n (%) | 24 (11.2) | 16(17.5) | 0.132 |
POCUS = point-of-care ultrasound; ED = emergency department; LOS = length of stay.
Median values.
Table 7 compares the sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of clinical assessment alone vs. POCUS guidance in management of pediatric SSTI. These test characteristics were calculated for all cases with complete follow-up, as well as stratified into high certainty and equivocal cases. The overall sensitivity and specificity of POCUS guidance was 90.3% and 80%, respectively, compared with an overall sensitivity and specificity of 76% and 94% with clinical assessment alone. Overall POCUS guidance positive and negative likelihood ratios were 4.5 and 0.12, respectively, while the overall clinical assessment positive and negative likelihood ratios were 12.4 and 0.25. Clinical assessment tended to perform better in high certainty cases, whereas POCUS guidance tended to perform better in equivocal cases.
Table 7.
Test Characteristics of Clinical Assessment Alone vs. POCUS Guidance in Pediatric SSTI Management
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | +LR (95% CI) | −LR (95% CI) | |
|---|---|---|---|---|
| All (n = 305) | ||||
| Clinical assessment only | 76.2 (60.2–87.4) | 93.9 (82.1–98.4) | 12.4 (4.1–37.7) | 0.25(0.15–0.44) |
| POCUS guidance | 90.3 (83.4–94.7) | 80.0 (70.0–87.4) | 4.5 (2.98–6.85) | 0.12 (0.07–0.21) |
| High certainty cases* (n = 183) | ||||
| Clinical assessment only | 79.3 (59.7–91.3) | 94.9(81.4–99.1) | 15.5 (4.0–60.4) | 0.22 (0.11–0.44) |
| POCUS guidance | 89.9 (79.6–95.5) | 73.9 (58.6–85.2) | 3.4 (2.10–5.64) | 0.14(0.07–0.28) |
| Equivocal casest† (n = 122) | ||||
| Clinical assessment only | 69.2 (38.9–89.6) | 90.0 (54.1–99.5) | 6.9 (1.0–46.0) | 0.34(0.15–0.79) |
| POCUS guidance | 90.9 (79.2–96.6) | 86.3 (72.0–94.3) | 6.7 (3.2–14.1) | 0.11 (0.05–0.24) |
POCUS = point-of-care ultrasound; SSTI = skin and soft tissue infection; CI = confidence interval; +LR = positive likelihood ratio; −LR = negative likelihood ratio.
Clinical suspicion of drainable fluid collection 0–20 or 81–100 on visual analog scale.
Clinical suspicion of drainable fluid collection 21–80 on visual analog scale.
DISCUSSION
In this prospective, multicenter, cohort study, we found that the use of POCUS did not reduce the overall treatment failure rate in pediatric SSTIs when compared with clinical assessment alone. In addition, management of SSTIs with POCUS did not reduce hospital admission rate after ED discharge, recurrent abscesses, unscheduled returns to ED, re-instrumentation or new incision and drainage, or initiation/change in antibiotics. There was also no significant difference between the POCUS and non-POCUS groups in terms of ED length of stay, discharge rate, need for procedural sedation, or ordering of additional imaging. POCUS guidance and clinical assessment alone had compatible outcomes in pediatric ED SSTI patients.
Compared with previous studies of POCUS in pediatric SSTI management, our study had a larger sample size, recruited a more diverse patient population from multiple EDs, and had a high follow-up rate (8–11). Hence, we believe our study may have improved external validity. This was also the first study to include a comparison (non-POCUS) group in evaluating the effect of POCUS on pediatric SSTI management. Prior SSTI studies focused mainly on elaborating the test characteristic of POCUS compared with physical examination alone (8–15). Our study further examined the impact of POCUS in terms of tangible patient and ED process outcomes.
We found that the use of POCUS led to management change in approximately a quarter of the SSTI cases. We also observed that clinicians tend to find POCUS more useful in cases of higher clinical uncertainty, when it was unclear whether a drainable fluid collection was present. Our results were consistent with those reported by other pediatric SSTI studies, reaffirming the value of POCUS in the clinical decision-making process (8–11). However, the percentage of management change after POCUS was much lower than that reported by Tayal et al. (56%) in adult ED patients with SSTI (12). This may be due to body habitus difference between adults and children, as larger body sizes in adults render physical assessment less accurate in determining the presence of drainable abscesses.
We were surprised that a substantial proportion of management plan change did not translate into improved patient outcomes in our study. In examining treatment failures in pediatric SSTIs, Mistry et al. reported that 10 of the 11 failures were in cases of abscess, and six of them had incision and drainage performed on the initial ED encounter (16). A possible explanation may be that when compared with cellulitis, abscess has a higher inherent treatment failure rate despite proper management on initial encounter. Given that the POCUS group in our study had a significantly higher clinical suspicion of drainable fluid collection on examination as well as higher incision and drainage rate than the non-POCUS group (therefore, likely a higher incidence of abscess), confounding by indication may have occurred because of the observational nature of the study, leading to a higher baseline failure rate in the POCUS group (17,18).
In addition to treatment plan change in 23% of cases, 22% of the clinicians in the POCUS group utilized ultra-sound to better visualize the anatomy and guide their procedures (location and size of incision, insertion of packing), and 29% used POCUS to confirm their clinical treatment plan. The benefit of POCUS was further highlighted by multiple provider comments (in the POCUS group) on how it facilitated parental/patient “buy-in” of the potentially painful and invasive incision and drainage procedure. Given its impact on many aspects of care, we believe that POCUS adds value to the ED management of pediatric SSTI patients. This is also supported by findings and evidence from other published studies on the topic (8–11). Future investigations may focus on how to translate provider utility into improved patient outcomes, such as performing selective POCUS on patients with equivocal physical examination findings, identifying POCUS features potentially predictive of treatment failure, and exploring the potential cost-saving effect of POCUS.
Previous studies on POCUS in SSTI have reported the general sensitivity and specificity of POCUS to be around 90–95% and 80–90%, respectively, and those of clinical assessment to be around 75–85% and 60–80% (8–11). Our calculated test characteristics of clinical assessment and POCUS were similar to these reported values, with the exception of much higher specificity in the clinical assessment group. This might be due to selection bias of subjects with higher clinical certainty into the non-POCUS group. In our patient population we found that POCUS has higher sensitivity than clinical assessment alone, hence it was useful in ruling out the presence of drainable fluid collection, whereas clinical assessment alone has very high specificity, hence it was sufficient to rule in drainable fluid collection. The test characteristics of POCUS and clinical assessment alone improved and deteriorated, respectively, in equivocal cases, concurring with higher perceived utilities in these cases by the clinicians.
Limitations
There are several limitations to this study. First, we used a convenience sampling approach, potentially leading to selection bias. For example, clinicians in the non-POCUS group might have been more experienced with a lower baseline treatment failure rate, or might have utilized other modalities (e.g., imaging by radiology) to compensate for their clinical uncertainty. It is also possible that study investigators enrolled more clinically equivocal cases in the POCUS group, whereas obvious cases of cellulitis or abscess were managed without POCUS guidance. These cases might represent lesions at an early stage and thus be more prone to “treatment failure” as they progress. Although a randomized controlled trial might have been a better study design, most of the investigators in our study felt that POCUS management of SSTI had become the standard of care at their institutions for many of their clinicians, making a randomized controlled trial infeasible and unethical. Second, all of the study sites already had established POCUS teaching programs in place. Thus, our results may not be generalizable to all ED settings. Third, the validity and reliability of our visual analog rating scales (likelihood of fluid collection, utility of POCUS) have not been proven in previous studies, and some of their values might have been affected by provider bias. Fourth, we did not reach the predetermined sample size in our non-POCUS group, potentially limiting the power of our findings. Although our recruitment exceeded the total calculated sample size, the percentage of POCUS patients was higher than expected. At the prevailing distribution, it would have taken at least an additional year to recruit enough non-POCUS patients to meet the calculated sample size in that group. Furthermore, we recalculated the power of our study using our enrolled subject numbers, and found that our study power was, in fact, slightly improved compared with our original calculation. Therefore, we decided to terminate our study prematurely without reaching the predetermined sample size in the non-POCUS group.
CONCLUSIONS
Point-of-care ultrasound use did not result in a lower treatment failure rate compared with clinical assessment alone in pediatric ED patients presenting with SSTIs in our study. However, providers who used POCUS reported it to be frequently beneficial in terms of formulation and change in patient management plans, particularly in cases of high clinical uncertainty. Future studies focusing on limiting POCUS to those with equivocal physical examination findings and using randomization with larger sample sizes may be needed to assess the true value of POCUS for this indication.
ARTICLE SUMMARY.
1. Why is this topic important?
Point-of-care ultrasound (POCUS) is increasingly being used to help with management of pediatric skin and soft tissue infections (SSTIs) in the emergency department (ED) setting. Yet, to date, no published study has directly compared the outcomes of pediatric SSTI cases treated with POCUS guidance vs. those treated based solely on physical examination findings alone.
2. What does this study attempt to show?
The current study compares the treatment failure rate, ED length of stay, and resource utilization of POCUS vs. clinical assessment alone on the management of pediatric SSTIs in the ED setting. POCUS utility and its impact on management decisions are also assessed in those who use the technology.
3. What are the key findings?
There is no significant difference between the POCUS and non-POCUS groups in terms of hospital admission rate after ED discharge, recurrent abscesses, unscheduled returns to ED, re-instrumentation or new incision and drainage, initiation/change in antibiotics, ED length of stay, discharge rate, need for procedural sedation, or ordering of additional imaging. The management plan was changed in the POCUS group in 22.9% of cases. The median utility of POCUS was 95/100 in this group. Clinicians reported increased benefit of POCUS in cases of higher clinical uncertainty.
4. How is patient care impacted?
Given its impact on many aspects of care, we believe that POCUS adds value to the ED management of pediatric SSTI patients. Future investigations may focus on how to translate provider utility into improved patient outcomes.
Acknowledgment—
The initial phase of this study was supported by the American College of Emergency Physicians Section of Pediatric Emergency Medicine Research Seed Grant.
REFERENCES
- 1.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008;168:1585–91. [DOI] [PubMed] [Google Scholar]
- 2.Pallin DJ, Bisno DJ, Pelletier AJ, et al. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008;51:291–8. [DOI] [PubMed] [Google Scholar]
- 3.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistantS. aureus infections among patients in the emergency department. N Engl J Med 2006;355:666–74. [DOI] [PubMed] [Google Scholar]
- 4.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis 2009;49: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez MA, Cruz AT, Kowalkowski MA, Raphael JL. Trends in resource utilization for hospitalized children with skin and soft tissue infection. Pediatrics 2013;131:e718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin JR, Bilker W, Lautenbach E, Alpern ER. Reliability of clinical examinations for pediatric skin and soft-tissue infections. Pediatrics 2010;126:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovanni JE, Dowd MD, Kennedy C, Michael JG. Interexaminer agreement in physical examination for children with suspected soft tissue abscesses. Pediatr Emerg Care 2011;27:475–8. [DOI] [PubMed] [Google Scholar]
- 8.Sivitz AB, Lam SH, Ramirez-Schrempp D, Valente JH, Nagdev AD. Effect of bedside ultrasound on management of pediatric soft-tissue infection. J Emerg Med 2010;39:637–43. [DOI] [PubMed] [Google Scholar]
- 9.Iverson K, Haritos D, Thomas R, Kannikeswaran N. The effect of bedside ultrasound on diagnosis and management of soft tissue infections in a pediatric ED. Am J Emerg Med 2012;30:1347–51. [DOI] [PubMed] [Google Scholar]
- 10.Marin JR, Dean AJ, Bilker WB, Panebianco NL, Brown NJ, Alpern ER. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med 2013;20:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams CM, Neuman MI, Levy JA. Point-of-care ultrasonography for the diagnosis of pediatric soft tissue infection. J Pediatr 2016; 169:122–1271. [DOI] [PubMed] [Google Scholar]
- 12.Tayal VS, Hasan N, Norton HJ. The effect of soft tissue ultrasound on the management of cellulitis in the emergency department. Acad Emerg Med 2006;13:384–8. [DOI] [PubMed] [Google Scholar]
- 13.Squire BT, Fox JC, Anderson C. ABSCESS: applied bedside sonography for convenient evaluation of superficial soft tissue infections. Acad Emerg Med 2005;12:601–6. [DOI] [PubMed] [Google Scholar]
- 14.Berger T, Garrido F, Green J, Lema PC, Gupta J. Bedside ultra-sound performed by novices for the detection of abscess in ED patients with soft tissue infections. Am J Emerg Med 2012;30: 1569–73. [DOI] [PubMed] [Google Scholar]
- 15.Gaspari R, Dayno M, Briones J, Blehar D. Comparison of computerized tomography and ultrasound for diagnosing soft tissue abscesses. Crit Ultrasound J 2012;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mistry RD, Scott HF, Zaoutis TE, Alpern ER. Emergency department treatment failure for skin infections in the era of community acquired methicillin-resistant Staphylococcus aureus. Pediatr Emerg Care 2011;27:21–6. [DOI] [PubMed] [Google Scholar]
- 17.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol 1999;149:981–3. [DOI] [PubMed] [Google Scholar]
- 18.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016;316:1818–9. [DOI] [PubMed] [Google Scholar]


