Abstract
Background
Chronic stress-induced inflammatory responses occur in part via danger associated molecular pattern (DAMP) molecules, such as HMGB1 but the receptor(s) underlying DAMP signaling have not been identified.
Method
DAMP signaling and microglia morphology were examined in enriched rat hippocampal microglia during the development and expression of chronic unpredictable stress (CUS)-induced behavioral deficits, including long-term, persistent changes after CUS.
Results
The results show that CUS promotes significant morphological changes and causes robust up-regulation of HMGB1 mRNA in enriched hippocampal microglia, an effect that persists for up to 6 weeks after CUS exposure. This coincides with robust and persistent up-regulation of receptor for advanced glycation end products (RAGE) mRNA, but not toll-like receptor 4 (TLR4) in hippocampal microglia. CUS also increased surface expression of RAGE protein on hippocampal microglia determined by flow cytometry and returned to basal levels 5 weeks after CUS. Importantly, exposure to short term stress was sufficient to increase RAGE surface expression as well as anhedonic behavior, reflecting a primed state that results from a persistent increase in RAGE mRNA expression. Further evidence for DAMP signaling in behavioral responses is provided by evidence that HMGB1 infusion into the hippocampus was sufficient to cause anhedonic behavior, and evidence that RAGE KO mice were resilient to stress-induced anhedonia.
Conclusions
Together, the results provide evidence of persistent microglial HMGB1-RAGE expression that increases vulnerability to depressive-like behaviors long after chronic stress exposure.
INTRODUCTION
Major Depressive Disorder (MDD) is a recurrent mental health illness that affects as many as 1 in 5 Americans (1). High levels of psychological or environmental stressors are associated with the initial development of MDD and also play a role in relapse (2–8). Subsequent episodes of MDD are often more severe and chronic, suggesting that the initial depressive episode causes priming or increased sensitivity to subsequent stressors or risk factors that cause reinstatement of depressive symptoms (9, 10). A potential underlying cause for this increased vulnerability is elevated inflammation (i.e., release of pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α) via activation of the immune system in response to physical and psychological stressors (11).
Microglia, the resident innate immune cells of the CNS, are robustly activated following stress exposure in brain regions implicated in depression and are thought to be the main contributors to central inflammation during stress. Our group and others have previously demonstrated that central inflammation is necessary for the development of depressive-like behaviors in rodents and may be mediated through activation of the cytoplasmic protein complex known as the NLRP3 inflammasome, which is required for the processing and release of IL-1β (12–17). Blockade of the ATP purinergic receptor P2X7 blocks activation of the inflammasome by stress and prevents the release of IL-1β, blocking stress-induced behavioral deficits (12). This work suggests that the inflammasome complex is an important mediator of depressive behavior, a possibility supported by studies demonstrating that mice with a null mutation of NLRP3 are protected from stress-induced behavioral deficits (12, 15–17)
Sterile activation of the inflammasome complex (i.e., in the absence of infectious agents) is induced by the release of endogenous factors collectively called danger associated molecular patterns (DAMPs). Physical, psychological and cellular stressors promote the release of DAMPs such as ATP, high mobility group box 1 protein (HMGB1), S100 proteins and heat shock proteins (18–20). Upon release, DAMPs can prime the assembly and activation of the inflammasome by binding to pattern recognition receptors (PRRs) such as toll-like receptors (TLRs) and the receptor for advanced glycation end products (RAGE) on the surface of immune competent cells, including microglia (18, 19, 21).
Recent evidence suggests that HMGB1 may be a critical mediator of stress-induced inflammasome activation of hippocampal microglia and may potentiate inflammatory responses to subsequent stimuli. Weber and colleagues (2015) reported that acute exposure to inescapable tail shock increases microglial HMGB1 release and sensitization to ex vivo LPS challenge 1 day after stress [19]. Pharmacological blockade of HMGB1 signaling using the selective antagonist BoxA, attenuated HMGB1-mediated potentiation of LPS responses in cultured primary microglia (22, 23). In addition, in vivo administration of glycyrrhizic acid (GZA), a non-selective antagonist of HMGB1, protects rodents against LPS-induced depressive-like behaviors (24) and cognitive deficits following fluid percussion injury (25). Together, these studies suggest that immune activation and microglial priming can promote HMGB1-mediated behavioral deficits. However, the receptor signaling mechanism by which HMGB1 potentiates subsequent inflammatory responses and contributes to the onset and relapse of depression remains to be elucidated.
One hypothesis is that severe acute or sustained chronic immune activation enhances the expression of DAMP receptors on the surface of microglia. However, it is unclear whether HMGB1 acts at via toll-like receptor 4 (TLR4) and/or RAGE, both of which are known to increase inflammatory signaling in various models of inflammation and neurodegeneration (18, 26–29). In this study, we demonstrate that chronic unpredictable stress (CUS) increases mRNA levels of RAGE, as well as HMGB1 in enriched hippocampal microglia and that these effects persist for up to 6 weeks after the end of CUS exposure. Importantly, flow cytometry shows that the up-regulation of surface RAGE levels on hippocampal microglia coincides with the onset and recurrence of depressive-like behaviors in post stress rats that are re-exposed to short-term unpredictable stress. Furthermore, preliminary evidence indicates that RAGE deletion mutant mice are resistant to CUS-induced behavioral deficits. Together, our findings indicate that stress-induced HMGB1-RAGE microglial signaling in the hippocampus plays a role in the development and persistent increase in vulnerability for recurrence of depressive behavior.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats and male RAGE constitutive knockout (KO) mice (30–32) (provided by Dr. Kevan Herold, Yale University) were group-housed and maintained in standard conditions with a 12h light/dark cycle. Animals had ad libitum access to food and water except during food or water deprivation stressors. Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committee.
Unpredictable stress exposure
Animals were subjected to short-term (3 to 7 days; US) or chronic (28 days; CUS) unpredictable stress exposure that consisted of a random combination of two stressors per day, including cold exposure (4°C for 1 h), cage rotation, overnight isolation, food or water deprivation, overnight lighting, light off during day (3h), odor, overnight stroboscope, crowding, cage tilting (45°) and immobilization (1h). Cold exposure and cage rotation were consistently used in combination on the last stress day. A subset of animals was subjected to 28 days of CUS followed by 28 days of stress recovery (Post Stress). Post stress animals were then randomly assigned to a non-stressed (Post Stress) or stress re-exposure group that received a 7 days re-exposure to unpredictable stress (Post Stress + US).
Stereotactic surgery and intracerebral ventricular (ICV) injections
Mice were anesthetized with a solution of ketamine/xylazine (100/10 mg/kg) and stereotaxically implanted with an intracerebral ventricular (ICV) guide cannula (26G, 2.4mm from the pedestal; Plastics One, VA) into the lateral ventricle using the following coordinates from bregma (33): −0.4mm anterior-posterior, −1.0mm medial-lateral, and −2.4mm dorsal-ventral. After 7 days of recovery, the free moving mice were infused with 5ul of saline or recombinant HMGB1 (dsHMGB1 certified LPS free (1ug/ul); HMGBiotech) using a 33G internal cannula (Plastics One, VA).
Behavioral Tests
Sucrose preference and consumption test
Animals were tested for sucrose preference as previously described (34). Briefly, animals were habituated to 1% sucrose for 48 hours, and the position of the bottle was counterbalanced across days. For sucrose preference test, the animals were water deprived for 6h and then presented with pre-weighed identical bottles of 1% sucrose and water on testing day. Sucrose and water consumption was quantified following one-hour bottle choice in rats and overnight bottle choice for mice. For sucrose consumption, the mice were given 1% sucrose (Day 1) and water (Day 2), and overnight consumption was quantified.
Novel object recognition test
Animals were tested for a single trial of novel object recognition as previously described (35). Briefly, animals were habituated to an open arena for 10 mins. The following day, the animals were placed back into the arena, and given 10 minutes to explore two identical objects placed near the northeast and northwest corners. 1h or 24h post familiar object exposure, the animals were placed back into the arena, which contained one of the familiar objects and a novel object placed near the northeast and northwest corners of the arena, and their positions were counterbalanced across animals and conditions. The animals were given 5 minutes to freely explore either object while being recorded. Exploration time was quantified for each object and used to determine preference.
Locomotor activity
Locomotor activity was quantified by placing animals in a clean, empty cage and ambulatory activity recorded for a 20-minute period. Distance traveled within that time was measured using the AnyMaze tracking system (36).
Microglia cell isolation
Hippocampal microglia were isolated as described previously (37). In brief, hippocampal tissue collected 4 h after the last stress exposure or after the 28–42 days post stress time period were homogenized in HBSS, pH 7.4, by passing through a 70 μm nylon cell strainer. Homogenates were centrifuged at 600 × g for 6 min. Supernatants were removed and cell pellets were resuspended in 70% isotonic Percoll (GE Healthcare) at room temperature. A discontinuous Percoll density gradient was layered as follows: 70%, 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged for 20 min at 2000 × g and microglia were collected from the interphase between the 70% and 50% Percoll layers (38, 39). Each hippocampi extraction yielded ~3 × 104 viable cells >90% microglia.
Flow cytometry
Cells were washed and then incubated with the conjugated antibodies CD45-FITC (BD Biosciences), CD11b-APC (BD Biosciences), TLR4-PerCP (Novus Biologicals) , and RAGE-PE (Bioss Antibodies) for 1 h. Cells were washed and then re-suspended in FACS buffer (2% FBS in 1X PBS with 1 mg/ml sodium azide) for analysis. Nonspecific binding was assessed by using nonspecific, isotype-matched antibodies. Antigen expression was determined using the BD FACSAria cell sorter. Twenty thousand events were recorded for each sample and isotype-matched conjugate. Data were analyzed using FlowJo software (Tree Star) and gating for each antibody was determined based on nonspecific binding of appropriate negative isotype stained controls.
Immunofluorescent labeling of microglia
Microglia were immunostained for Iba-1 detection as previously described (40). Briefly, animals were deeply anesthetized and transcardially perfused with sterile PBS (pH 7.4) and 10% formalin. Brains were removed, post-fixed in formalin for 2 days and incubated in 30% sucrose for cryoprotection. Brains were sectioned (25μm) and brain regions were located anatomically in accordance with the stereotaxic rat brain atlas (Paxinos and Watson, 2004). Hippocampal brain sections were incubated overnight at 4°C with IBA1 antibody (1:1000, Wako), washed several times and incubated overnight at 4°C in secondary antibody Alexa Fluor 546-conjugated anti-rabbit IgG (1:10000; Invitrogen). Sections were then mounted and kept at 4°C until processed.
Image analysis
Images were captured using a confocal microscope (Zeiss Axiovert LSM510, Carl Zeiss). 20X images were used to assess microglial density by counting Iba-1+ cells within the dentate gyrus (DG), CA1 and CA3 of the hippocampus. The computer-based cell tracing software Neurolucida 360 (MBF Bioscience, VT) was used for 3D reconstruction of Iba-1+ cells within the CA3 pyramidal layer of the hippocampus. NeuroExplorer software (MBF Bioscience, VT) was used to analyze microglial soma size and branch length and volume for ≥ 15 cells per animal. Sholl analysis was used to determine branch tree morphology by placing 3D concentric circles in 5 μ m increments starting at 5μ m from the soma.
Quantitative Real-Time PCR
RNA was purified from freshly isolated enriched hippocampal microglia using the RNAqueous kit (Ambion). RNA concentration was determined by Nanodrop spectrophotometry. cDNA was synthesized using 250ng of total RNA was used to synthesize cDNA using dNTP primers and reverse transcriptase (Genisphere, USA). Gene-specific primers for TLR2, TLR4, RAGE and HMGB1 were designed using Primer 3 software, and qPCR was run on the Applied Biosystems 7900HT using SYBR Green (Qiagen). CT values of genes of interest were normalized to that of housekeeping gene HMBS. Results were expressed as fold difference.
| GENE | FORWARD | REVERSE |
|---|---|---|
| TLR4 | CCA GAA TGA GGA CTG GGT GAG AAA | CCA CCA CAA TAA CTT TCC GGC TCT TG |
| RAGE | TGA CCC TGA CCT GTG CCA TC | CCT CAT CCT CAT GCC CTA CCT C |
| HMGB1 | CAC TGC TGC GGA TGA CAA GC | CCT CCT CGT CGT CTT CCT CTT |
| HMBS | GGA GGT CCG AGC CAA GGA CCA | CTG GCA CGC TAC AGC CTC CTT CC |
Statistical analysis
Data were analyzed using GraphPad Prism7 software. Data points greater than two standard deviations from the mean were considered outliers and excluded from all analyses. For two group comparisons, statistical differences were determined by two-tailed Student t-test. Group effects were determined by one-way ANOVA followed by Bonferonni post hoc test for multiple comparison. For 2X2 comparison statistical differences were determined by two-way ANOVA followed by Bonferroni or Tukey post hoc test for multiple comparison. Statistical significance was considered if p≤ 0.05 for all test.
RESULTS
CUS increases microglial reactivity and RAGE expression in hippocampal microglia
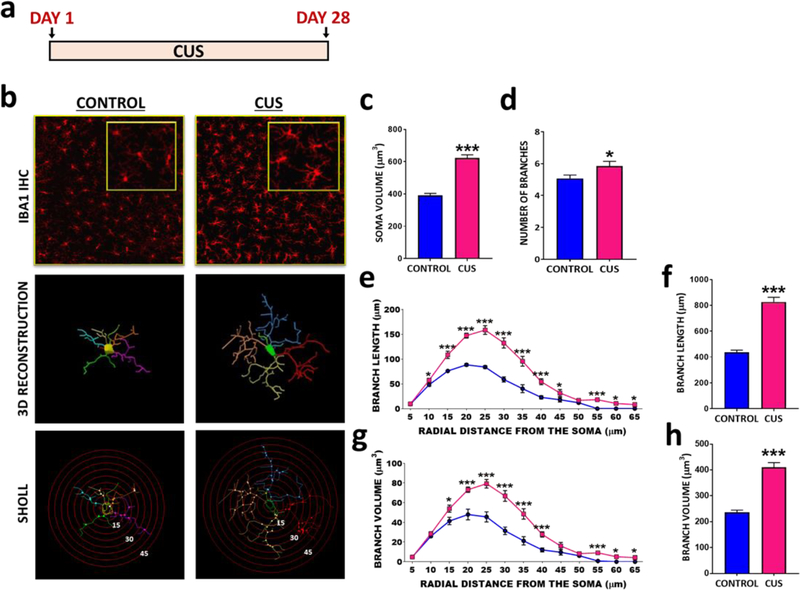
Under basal conditions, microglia are found in a ramified state during which they dynamically survey the microenvironment and take part in homeostatic processes such as synaptic plasticity and pruning (41–48). Stress can increase microglial density and reactivity, leading to hyper-ramification and increased soma size (49, 50). Using the CUS paradigm (Fig 1A) known to promote anhedonic behavior, a core symptom of depression (51), microglia density and morphology was examined with 3D reconstructed confocal images obtained within the CA3 pyramidal cell layer of hippocampus. CUS exposure increased soma size (Fig 1B-C) and caused hyper-ramification of microglia, characterized by significantly increased branch number, length and volume (Fig 1D-H), demonstrating robust microglial reactivity in the hippocampus. We did not observe any changes in microglial density following CUS (Supplemental Fig 1,2).
Figure 1. CUS alters microglial morphology in dorsal hippocampus of rats.
(A) CUS paradigm. (B) Immunohistochemical detection of microglia marker IBA1 within the CA3 pyramidal cell layer, followed by 3D reconstruction and Sholl analysis in Control and CUS rats. Average (C) soma size (t(466)=9.90, p<0.0001), (D) branch number (t(97)=2.18, p=0.0314), (E) total branch volume (t(97)=9.49, p<0.0001) and (G) total branch length (t(97)=10.89, p<0.0001). Sholl analysis for (F) branch length and (H) branch volume in control and CUS animals as a function of distance from soma. The results are expressed as the mean ± SEM., N=4 animals per group, > 60 cells per group. *p<0.05 and ***p<0.001. Student t-test performed for average soma size, branch number and total branch length and volume. Student t-test performed for each distal point of Sholl analysis and significance was determined based on adjusted p values.
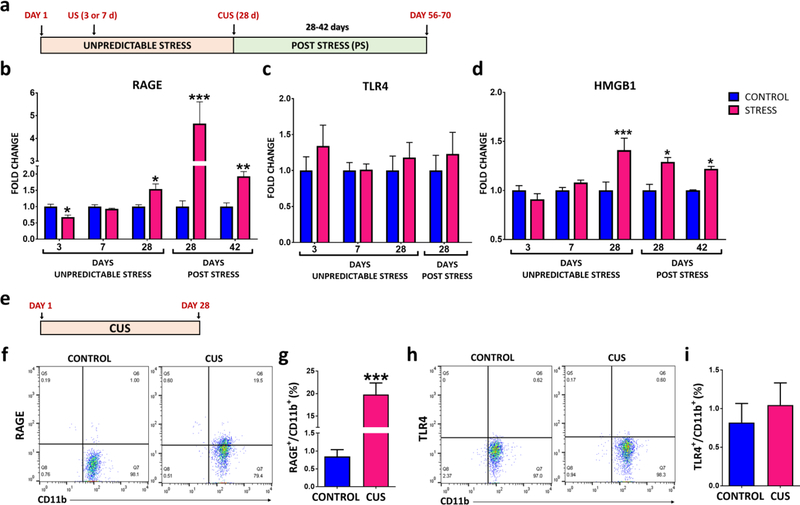
In follow-up experiments CUS-induced transcriptional alterations that influence microglia activation were investigated (Fig 2A). Several factors that may contribute to priming of the NLRP3 inflammasome pathway were examined, including the PRRs RAGE and TLR4, as well as a DAMP ligand for these receptors, HMGB1 (18, 19). We found that CUS increased the expression of RAGE (Fig 2B), but not TLR4 (Fig 2C) in enriched hippocampal microglia samples. We also examined levels of these factors 4 and 6 weeks after the last CUS, when there is no longer evidence of an elevated corticosterone response (Supplemental Fig 3A). Rats displayed normal weight gain at these time points, although weights were still decreased relative to controls (Supplemental Fig 3B). Interestingly, the stress-induced up-regulation of microglial RAGE was still present 4 weeks after the end of CUS exposure (~5 fold) and persisted for up to 6 weeks (Fig 2B). We also found that CUS increased the expression of HMGB1 mRNA in enriched hippocampal microglia and that this effect was also persistent for up to 6 weeks after the cessation of CUS exposure (Fig 2D).
Figure 2. CUS increases the expression and causes long lasting up-regulation of RAGE in hippocampal microglia.
(A) Experimental paradigm of unpredictable stress (7 or 28 days) exposure and post stress period in rats. Enriched microglia were prepared from hippocampus, mRNA was extracted and levels of each target were determined by PCR analysis. (B) RAGE (Stress Day 28 t(8)=2.639, p=0.0297; Post stress Day 28 t(9)=3.416, p=0.0076; Post stress Day 42 t(8)=4.078, p=0.0046), (C) TLR4 (Stress Day 28 t(10)=0.6207, p=0.5486; Post Stress Day 28 t(8)=0.6281, p=0.5474), and (D) HMGB1 (Stress Day 28 t(8)=2.452, p=0.0397; Post Stress Day 28 t(9)=3.855, p=0.0038; Post stress Day 42 t(7)=6.102, p=0.0004) mRNA expression during stress and post stress. All genes were normalized to housekeeping gene HMBS. (E) Experimental paradigm for flow cytometry analysis. (F-G) RAGE (t(9)=5.505, p=0.0004) and (H-I) TLR4 (t(9)=0.5066, p=0.628) surface expression was quantified by flow cytometry on enriched hippocampal microglia samples collected 4hrs following the last stressor on day 28. Flow cytometry diagrams for (G) RAGE and (I) TLR4. Results are represented as the percentage of RAGE+/CD11b+ or TLR4+/CD11b+ cells out of the total number of cells. The results are expressed as the mean ± SEM., N=4–8 per group. *P<0.05, **P<0.01 and ***P<0.001. Student t-test performed at each time point for mRNA analysis. Student t-test performed for flow cytometry.
In the absence of stress, HMGB1 is primarily localized in the nucleus where it acts as a transcription regulator. Upon stress exposure, immune competent cells actively translocate and release HMGB1 out of the cell where it acts as a DAMP with inflammatory actions via binding to TLR4 and RAGE (52, 53). To determine if HMGB1 localization is altered following CUS exposure, we examined the levels of HMGB1 in sub-cellular fractions of hippocampus. We found a significant reduction in nuclear HMGB1 on the last day of CUS exposure that persisted for up to 4 weeks after stress exposure (Supplemental Fig 4B-C).
Next, we assessed if the persistent increase in RAGE mRNA in enriched hippocampal microglia influenced protein expression. For these studies we assessed the number of hippocampal microglia expressing surface RAGE or TLR4 by flow cytometry. These studies showed that CUS significantly increased the proportion of microglia, determined by sorting based on the ration of CD11b+/CD45lo, with surface expression of RAGE (Fig 2F-I). These findings are consistent with the observed up-regulation of RAGE mRNA levels. In contrast, there was no significant effect on the number of TLR4+ microglia (Fig 2H-I).
CUS causes long-term, persistent stress-induced behavioral deficits
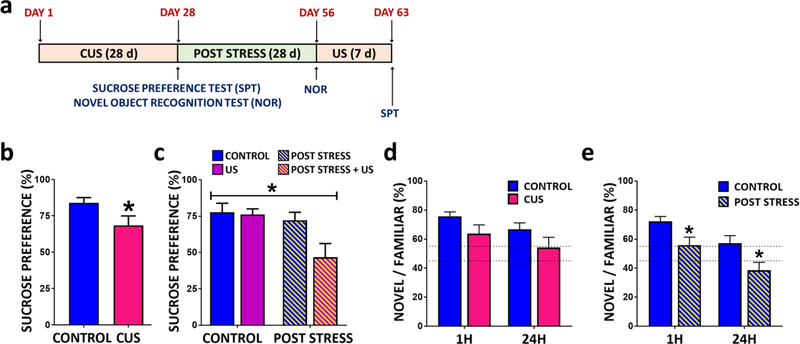
As previously reported (12) we found that CUS exposure significantly decreases sucrose preference, a measure of anhedonia (Fig 3A-B). This effect was no longer observed when animals were tested 4 weeks after CUS exposure (Fig 3C, naïve vs. CUS/post stress). We next challenged CUS/post stress rats with an acute restraint stress to determine if stress sensitivity was elevated. CUS/post stress rats displayed a non-significant elevation of serum corticosterone levels in response to restraint stress (Supplemental Fig 5A). We also found that this short-term restraint stress was insufficient to reveal increased behavioral sensitivity in the sucrose preference test (Supplemental Fig 5B). Next we tested a 7 d US paradigm, which also had no effect on sucrose preference in naïve rats (i.e., control), consistent with previous reports that anhedonic behavior develops after several weeks of CUS exposure (54, 55). However, animals previously exposed to CUS 4 weeks earlier (post stress) and then exposed to 7 d US showed a significant reduction in sucrose preference, indicating increased sensitivity and reinstatement of anhedonia (Fig 3C). These findings demonstrate that previous stress exposure increases vulnerability to stress-induced behavioral deficits, even to short-term stress that is insufficient to cause depressive-like behavior.
Figure 3. CUS exposure leads to long lasting vulnerability to anhedonia and cognitive deficits in rats.
(A) Paradigm for CUS plus post stress re-exposure to unpredictable stress. (B) Rats were tested for sucrose preference 4 h following CUS (t(22)=2.08, p=0.0494), or (C) following 4 weeks post stress with or without re-exposure to unpredictable stressors for 7 days (group effect F(3,21)=7.441; p=0.0014). Preference for novel object exploration was tested 1h or 24h after familiar object exposure. Rats were tested for novel object recognition (NOR) (D) 4 hrs (NOR (1h) t(12)=1.551, p=0.1520; NOR (24h) t(22)=1.523, p=0.1419)) or (E) 28 days (NOR (1h) t(12)=2.304, p=0.0399; NOR (24h) t(24)=2.334, p=0.0283) following exposure to the last CUS stressor. The results are expressed as the mean ± SEM. N=10–16 animals per group. *P<0.05. Student t-test was performed for sucrose preference in CUS animals. Two-way ANOVA was performed for post stress sucrose preference test followed by Bonferroni post hoc test. Student t-test performed at each time point for novel object recognition test.
Previous studies have reported that increased RAGE signaling results in cognitive deficits in a number of psychiatric/neurological disorder and rodent models (25, 56–60). Here we found that CUS animals showed a trend for a reduction (P≤ 0.1) in novel object exploration compared to age-matched controls at both 1 and 24 h after familiar object exposure (Fig 3D) as previously reported (61–63). Interestingly, this deficit significantly worsened 4 weeks post stress exposure, suggesting that chronic stress results in long-lasting cognitive deficits (Fig 3D-E).
CUS causes long-term, persistent stress-induced microglia reactivity and increased RAGE
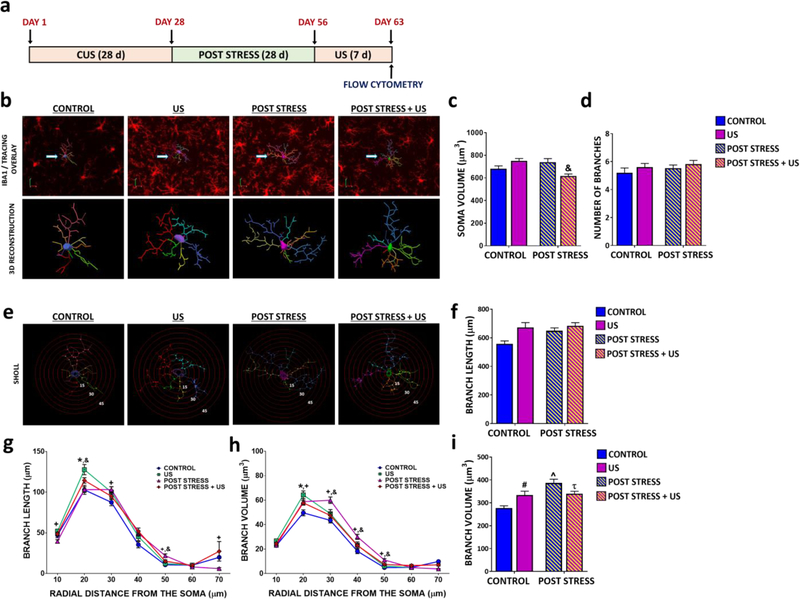
To determine if prolonged vulnerability to depression-like behaviors coincides with long lasting microglial reactivity in post stress animals, we assessed microglial morphology (Fig 4A-I). Remarkably, changes in soma size, branch length and branch volume still persisted at 5 weeks after stress cessation, indicating that microglia from post stress animals remain hyper-ramified long after CUS exposure (Fig 4B-I). These morphological changes were reduced compared to the effects observed immediately after CUS exposure (Fig 1) but were comparable to those observed in naïve animals exposed to 7 d US (Fig 4B-I). Surprisingly, 7 d US re-exposure decreased soma size and branch volume in previously stressed animals (Fig 4C and Fig 4I), indicating that stress causes differential morphological changes depending on prior experiences.
Figure 4. CUS induces long lasting morphological effects on hippocampal microglia in rats.
(A) Paradigm for CUS plus post stress re-exposure to unpredictable stress. (B) Immunohistochemical detection of microglia within the CA3 pyramidal cell layer for the microglial marker IBA1, followed by 3D reconstruction in age-matched controls and following US, Post stress and Post stress + US. (C) Average soma size (interaction of stress and prior exposure, F(3,916)=16.06; p<0.0001) and (D) branch number (no effect, F(3,199)=0.0.04141; p<0.8390). (E) Sholl analysis was performed on 3D reconstructed microglia within the CA3 pyramidal cell layer following short term US exposure in naïve and post stress animals. Branch length following (F) 3D analysis (main effect of stress (F(3,199)=4.254; p=0.0404) and prior stress exposure (F(3,199)=9.146; p=0.0028), no interaction) and (G) Sholl analysis (interaction of distance and stress, F(36,2574)=2.943; p<0.0001). Branch volume following (H) 3D analysis (interaction of stress and prior exposure, F(3,199)=12.63; p=0.0005) and (I) Sholl analysis (F(36,2574)=2.597; p<0.0001). The results are expressed as the mean ± SEM., N=4 animals per group, > 60 cells per group. # P≤0.1 US compared to controls. ^P<0.05 Post stress animals compared to controls. τ P<0.05 Post stress + US compared to Post Stress. & P<0.05 Post stress + US compared to Post Stress. Two-way ANOVA was performed followed by Bonferroni post hoc test for average soma size, branch number and total branch length and volume. Two-way ANOVA was performed followed by Bonferroni post hoc test for each distal point of Sholl analysis.
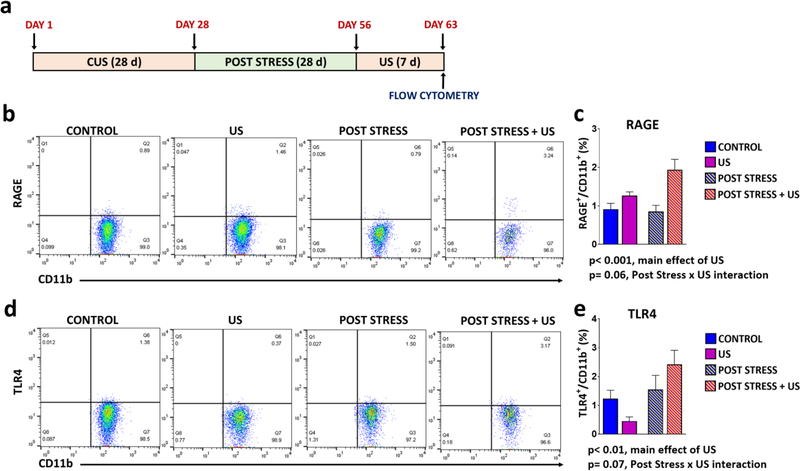
We next determined if CUS exposure resulted in persistent sensitivity of microglial RAGE expression in response to stress re-exposure. Using flow cytometry, we found that the number of hippocampal microglia (CD11b+/CD45lo cells) expressing RAGE was normalized in rats exposed to CUS 5 weeks earlier (Fig 5B-C). Exposure to 7 d US resulted in a significant main effect on surface RAGE expression (p < 0.001), and there was a strong trend for a US × post stress interaction (p = 0.06), with a robust elevation of RAGE surface expression in the post stress plus US group (Fig 5B-C). There was also a main effect of post stress on surface expression of TLR4 (p < 0.01) and a trend for a US × post stress interaction (p = 0.07) (Fig 5D-E). These results demonstrate that chronic stress primes microglia, in part via increased RAGE mRNA expression to display increased levels of extracellular RAGE upon subsequent short-term stress exposure. We also observed evidence post stress plus US causes priming of IL-1β and NLRP3 protein, but not mRNA levels in hippocampus (Supplemental Fig 6).
Figure 5. Previous CUS exposure increases vulnerability to short-term US-induced up-regulation RAGE and TLR4 in stress recovery rats.
(A) Paradigm for CUS plus post stress re-exposure to unpredictable stress. (B-E) RAGE and TLR4 were quantified by flow cytometry of enriched hippocampal microglia samples collected 4 h following the last US stressor. Flow cytometry diagrams for (C) RAGE (main effect of US, F(1,33)=14.17; p=0.0007; strong trend towards interaction of stress and prior exposure, F(1,33)=3.649; p=0.0648) or (E) TLR4 ((main effect of prior exposure, F(1,35)=6.764; p=0.0135; strong trend for interaction of US and prior exposure, F(1,35)=3.48; p=0.0705). Results are represented as the percentage of TLR4+/CD11b+ or RAGE+/CD11b+ cells out of the total number of cells. The results are expressed as the mean ± SEM. N=8–14 per group. Statistics were calculated by two-way ANOVA followed by Bonferroni post hoc test.
RAGE null mutant mice are resilient to CUS exposure
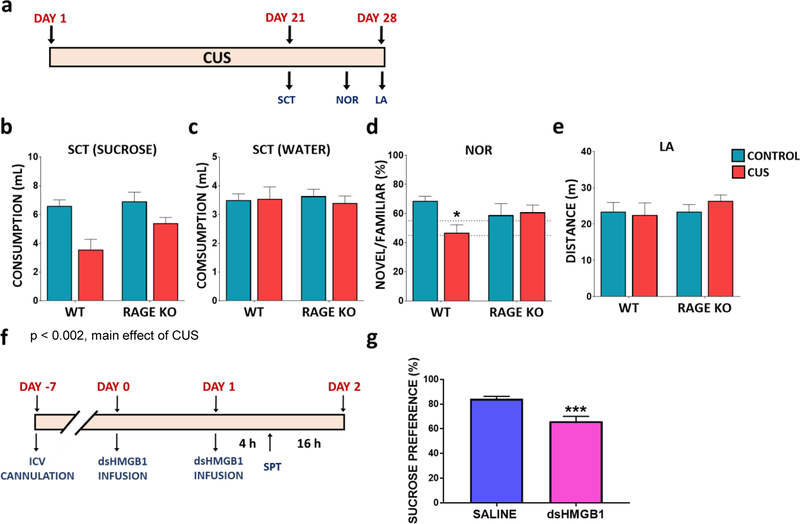
Due to the parallel changes in RAGE expression and the onset and reinstatement of stress-induced behavioral deficits, we next examined the effects of CUS on RAGE knockout (KO) mice (Fig 6A). Under basal conditions, RAGE KO mice showed no differences in sucrose consumption or novel object recognition compared to their wild-type littermates (Fig 6B-E). Analysis of CUS shows a significant main effect on sucrose, but not water consumption (Fig 6 B-C). In addition, RAGE KO mice displayed an attenuated, but non-significant response to CUS-induced deficits in sucrose consumption (Fig 6B-C), as well as object recognition (Fig 6D), compared to wild-type littermates. Taken together, these findings suggest that RAGE signaling plays a role in the development of stress-induced behavioral deficits.
Figure 6. RAGE deletion attenuates CUS induced behavioral deficits in mice.
(A) Experimental paradigm. Wild type (WT) and RAGE deletion mutant mice were tested for (B) sucrose consumption (main effect of stress F(1,33)=11.39, p=0.0019; no interaction of stress and genotype) and (C) water consumption (no effect F(1,33)=0.0806, p=0.7783 ). Mice were tested for (D) novel object recognition 24h after familiar object exposure (interaction of stress and genotype F(1,15)=4.853, p=0.0437). (E) Total cage locomotor activity within a 20 min period (no effect F(1,30)=0.1782, p=0.6759). The results are expressed as the mean ± SEM. N=6–10 animals per group. (F) Experimental paradigm for disulfide HMGB1 (dsHMGB1) infusion in naïve C57BL6 mice. (G) Mice were tested for sucrose preference 4 hrs post dsHMGB1 ICV infusion (t(24)=4.399, p=0.0002). The results are expressed as the mean ± SEM. N=12–14 animals per group. *p<0.05 and ***p<0.001. Two-way ANOVA was performed followed by Bonferroni post hoc test for behavioral analysis in RAGE KO mice. Student t-test was performed for sucrose preference test following dsHMGB1 infusion.
We next assessed if increased HMGB1 was sufficient to promote anhedonic behavior by infusion of disulfide HMGB1 (dsHMGB1, 5ug, i.c.v.). DsHMGB1 has previously been shown to robustly activate inflammatory signaling in the hippocampus (64, 65) and to induce depressive-like behaviors 20 hrs after infusion (16). We found that dsHMGB1 infusion robustly decreased sucrose preference compared to mice infused with saline (Fig 6G) in as little as 4h post infusion, in support of the hypothesis that enhanced HMGB1 DAMP signaling can quickly promote the development of depressive-like behaviors.
DISCUSSION
While physiological responses to acute stress allows an organism to adapt to changes in the environment, severe and/or chronic stress exposure can lead to maladaptive alterations that may contribute to the pathophysiology of mood disorders. This can include persistent molecular and cellular alterations that contribute to long-lasting priming and/or sensitivity to subsequent stressors and recurrence of depressive behaviors.
In the current study we examine microglial reactivity and characterize the endogenous factors that mediate stress-induced priming and activation of the inflammasome pathway. The results demonstrate that CUS exposure causes robust microglial hyper-ramification, which surprisingly persists for up to 5 weeks after CUS exposure. The results also show that short-term US (7 d) increases microglia reactivity although the effects were reduced compared to CUS. In contrast to these strong effects on microglia morphology, there were no significant effects on microglial density following either CUS or 7 d US exposure (Supplemental Fig 2). The literature on stress and microglia density is mixed with reports of increased, no effects, or decreased density (49, 66, 67). The reasons for these differences could be due to multiple variables, including the stress paradigm (timing and types of stressors) and methods for labeling microglia. Nevertheless, the current findings demonstrate robust and persistent effects of CUS on microglia morphology.
Previous reports have shown increased expression and activity of TLR4 following exposure to severe short-term stressors such as inescapable tail shock stress, suggesting that TLR4 up-regulation contributes to stress-induced priming (68–70). In the current study, we found that CUS, considered one of the most valid rodent models of depression (51), increased mRNA levels of RAGE, but not TLR4 in enriched hippocampal microglia, and the increase in RAGE mRNA was persistent for up to 6 weeks after CUS. Flow cytometry analysis also demonstrates increased number of hippocampal microglia expressing RAGE, but not TLR4 after CUS exposure. Taken together, these findings are consistent with the hypothesis that stress-induction of RAGE mRNA promotes the onset of depressive-like behaviors by increasing microglial receptor availability upon subsequent stress exposure, thereby priming DAMP signaling (Fig 7). However, TLR4 may be dynamically regulated by CUS and altered expression of this PRR may have been missed due to the time point examined. Therefore, the degree to which microglial RAGE, TLR4 or other PRRs contribute to the development of behavioral deficits following chronic stress remains unclear, and the results of the current study do not rule out a role for TLR4. We are currently conducting studies using conditional knockouts to selectively ablate microglial RAGE and TLR4 to further elucidate the role of these PRRs in chronic stress and post stress conditions.
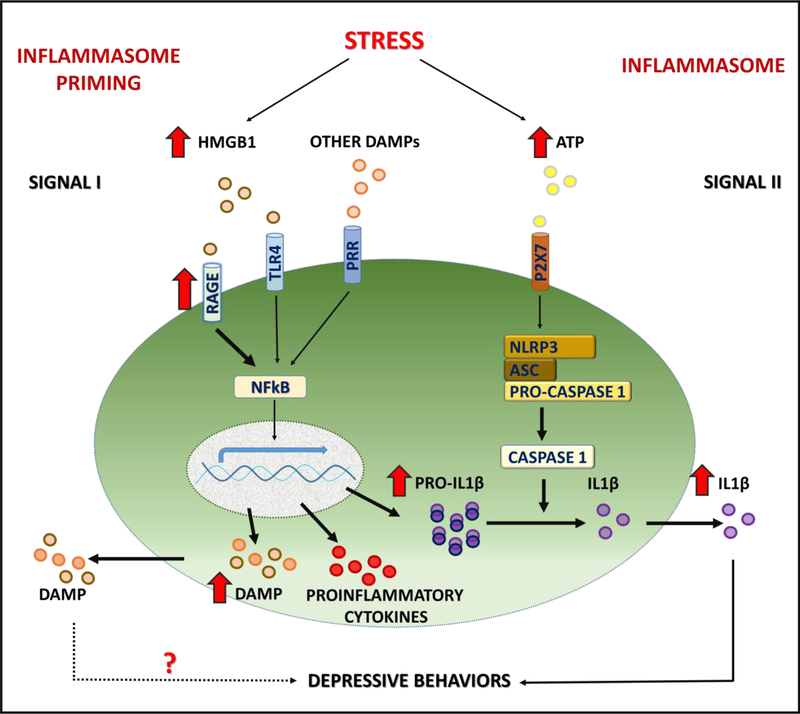
Figure 7. Schematic of stress-induced inflammasome priming.
Exposure to stress activates DAMP-PRR (e.g., HMGB1-RAGE) and downstream NFκΒ signaling to increase transcription of the proinflammatory cytokine IL1β, as well as further increase the expression of HMGB1. Microglial response to stress also requires activation of P2X7 receptors, for example via release of ATP, and activation of the NLRP3 inflammasome complex, which then stimulates the processing of pro-caspase 1 to caspase 1, which in turn cleaves pro-IL1β to IL1β for release. Exposure to CUS results in long-lasting, persistent elevation of HMGB1 and RAGE mRNA expression, and increases sensitivity to subsequent short-term stress exposure, which increases surface/extracellular levels of microglial RAGE. Elevated HMGB1-RAGE underlies increased vulnerability to depressive behaviors such as anhedonia that is blocked in RAGE deletion mutant mice.
Similarly, the results demonstrate that CUS exposure increases the expression of HMGB1 mRNA in hippocampal microglia, an effect that also persists for up to 6 weeks after CUS. In addition, CUS exposure resulted in altered levels of HMGB1 protein levels in nuclear fractions of hippocampus, an effect that also persisted for up to 5 weeks in post stress animals. These findings are consistent with the possibility that CUS exposure causes translocation and release of HMGB1 into the extracellular space where it activates DAMP-PRR signaling. It is important to note that stress-induced alterations in DAMP signaling may differ from model to model. In our stress study, we observed significant up regulation of HMGB1 in hippocampal microglia after 4 weeks of CUS exposure (Fig 2D). In contrast, Weber et al report robust HMGB1 up-regulation after an acute inescapable foot-shock stress session (23). These findings indicate that severe or chronic stress exposure can induce robust HMGB1 signaling in hippocampal microglia.
Previous studies report that stress-induced priming was unmasked by administration of exogenous ligands such as E.Coli or LPS, which exert inflammatory actions via binding to TLR4 (23, 68, 69, 71–74). In contrast, here we use short-term US (7 d), a sterile inflammatory stimulus that is more clinically relevant as precipitation of depression or relapse are often associated with short-term and/or traumatic stress (3). The results demonstrate that CUS/post stress animals exposed to 7 d US displayed anhedonic behavior in the sucrose preference test, while naïve animals exposed to short-term US had no change in anhedonic behavior. Coinciding with increased vulnerability to depressive behavior in CUS/post stress animals, we also found that 7 d US exposure robustly increased the number of RAGE expressing microglia. The rapid induction of surface RAGE by 7 d US could result from the persistent elevation of RAGE mRNA expression. RAGE up-regulation and signaling has been implicated in the development and progression of several inflammatory and neurodegenerative diseases (75–85), many of which show high levels of comorbidity with depression. However, this is the first report of long-term, persistent elevated hippocampal RAGE expression in the brain following chronic psychological stress exposure.
The role of RAGE in the behavioral response to CUS was also examined, and the results provide preliminary evidence that RAGE KO mice have a blunted response to stress-induced deficits in sucrose consumption and cognitive testing, supporting the hypothesis that RAGE signaling plays a critical role in the development of depressive-like behaviors. However, it is important to note that these are constitutive mutant mice and that RAGE deletion would occur in peripheral immune cells as well as microglia. Our future studies of microglial specific deletion of RAGE will address this question. The results also demonstrate that administration of dsHMGB1 into the CNS rapidly decreased sucrose consumption. These findings are consistent with a previous report of intracerebroventricular dsHMGB1 infusion (16), and demonstrate that sub-chronic increases in HMGB1-PRR signalingis sufficient to cause depressive-like behavior. Together, the results support the hypothesis that chronic stress leads to persistent enhancement of HMGB1-RAGE expression and signaling that causes a long-lasting increase in vulnerability to stress-induced reinstatement of depressive-like behaviors (Fig 7).
In conclusion, the results demonstrate an important role of HMGB1-RAGE signaling in prolonged microglial activation following chronic stress, and stress-induced recurrence of depressive-like behaviors. Further understanding of the microglial molecular signaling events involved in the persistently high levels of HMGB1-RAGE mRNA expression, such as epigenetic alterations of the transcriptional promoter elements, will be necessary to fully understand the molecular mechanisms underlying inflammation mediated onset and recurrence of depressive-like behaviors. These studies could also lead to identification of novel HMGB1-RAGE related drug targets and development of new antidepressant medications that could potentially reduce or block vulnerability due to prior stress exposure.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by funds from the State of Connecticut and a Yale University endowment (RSD).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Kessler RC, Bromet EJ (2013): The epidemiology of depression across cultures. Annu Rev Public Health 34:119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moylan S, Maes M, Wray NR, Berk M (2013): The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606. [DOI] [PubMed] [Google Scholar]
- 3.Smoller JW (2016): The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology 41:297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendler KS, Halberstadt LJ (2013): The road not taken: life experiences in monozygotic twin pairs discordant for major depression. Mol Psychiatry 18:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilman SE, Trinh NH, Smoller JW, Fava M, Murphy JM, Breslau J (2013): Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychol Med 43:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendler KS, Karkowski LM, Prescott CA (1999): Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin KA, Conron KJ, Koenen KC, Gilman SE (2010): Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med 40:1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paykel ES (2001): Stress and affective disorders in humans. Semin Clin Neuropsychiatry 6:4–11. [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Nunes PV, Oliveira KC, Young LT, Lafer B (2016): Neuropathological relationship between major depression and dementia: A hypothetical model and review. Progress in neuropsychopharmacology & biological psychiatry [DOI] [PubMed]
- 10.Sibille E, French B (2013): Biological substrates underpinning diagnosis of major depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 16:1893–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AH, Raison CL (2016): The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. (2016): Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol Psychiatry 80:12–22. [DOI] [PubMed] [Google Scholar]
- 13.Koo JW, Duman RS (2008): IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 105:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo JW, Duman RS (2009): Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett 456:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcocer-Gomez E, Ulecia-Moron C, Marin-Aguilar F, Rybkina T, Casas-Barquero N, Ruiz-Cabello J, et al. (2016): Stress-Induced Depressive Behaviors Require a Functional NLRP3 Inflammasome. Mol Neurobiol 53:4874–4882. [DOI] [PubMed] [Google Scholar]
- 16.Su WJ, Zhang Y, Chen Y, Gong H, Lian YJ, Peng W, et al. (2017): NLRP3 gene knockout blocks NF-kappaB and MAPK signaling pathway in CUMS-induced depression mouse model. Behav Brain Res 322:1–8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, et al. (2015): NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen GY, Nunez G (2010): Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez C, Huebener P, Schwabe RF (2016): Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene [DOI] [PMC free article] [PubMed]
- 20.Fleshner M, Frank M, Maier SF (2016): Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology [DOI] [PMC free article] [PubMed]
- 21.Guo H, Callaway JB, Ting JP (2015): Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine 21:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ID, Lee JK (2013): HMGB1-Binding Heptamer Confers Anti-Inflammatory Effects in Primary Microglia Culture. Exp Neurobiol 22:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF (2015): Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci 35:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu TY, Liu L, Zhang W, Zhang Y, Liu YZ, Shen XL, et al. (2015): High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J Psychiatr Res 64:99–106. [DOI] [PubMed] [Google Scholar]
- 25.Okuma Y, Liu K, Wake H, Liu R, Nishimura Y, Hui Z, et al. (2014): Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1-RAGE interaction. Neuropharmacology 85:18–26. [DOI] [PubMed] [Google Scholar]
- 26.Cai Z, Liu N, Wang C, Qin B, Zhou Y, Xiao M, et al. (2016): Role of RAGE in Alzheimer’s Disease. Cell Mol Neurobiol 36:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heneka MT, Kummer MP, Latz E (2014): Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477. [DOI] [PubMed] [Google Scholar]
- 28.Juranek J, Ray R, Banach M, Rai V (2015): Receptor for advanced glycation end-products in neurodegenerative diseases. Rev Neurosci 26:691–698. [DOI] [PubMed] [Google Scholar]
- 29.Richards RI, Robertson SA, O’Keefe LV, Fornarino D, Scott A, Lardelli M, et al. (2016): The Enemy within: Innate Surveillance-Mediated Cell Death, the Common Mechanism of Neurodegenerative Disease. Front Neurosci 10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, et al. (2008): RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol 181:4272–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, et al. (2004): Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 113:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, et al. (2003): RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 162:1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin KBJ (2013): Paxinos and Franklin’s the mouse brain in stereotaxic coordinates 4th ed Amsterdam: Boston: : Elsevier/Academic Press. [Google Scholar]
- 34.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. (2015): Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol Psychiatry [DOI] [PubMed]
- 35.Bevins RA, Besheer J (2006): Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1:1306–1311. [DOI] [PubMed] [Google Scholar]
- 36.Iwata M, Ota KT, Duman RS (2013): The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain, behavior, and immunity 31:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. (2011): beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF (2006): Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. Journal of neuroscience methods 151:121–130. [DOI] [PubMed] [Google Scholar]
- 39.Nair A, Hunzeker J, Bonneau RH (2007): Modulation of microglia and CD8(+) T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain, behavior, and immunity 21:791–806. [DOI] [PubMed] [Google Scholar]
- 40.Hinwood M, Morandini J, Day TA, Walker FR (2012): Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 22:1442–1454. [DOI] [PubMed] [Google Scholar]
- 41.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003): Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 100:13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, et al. (2016): TAM receptors regulate multiple features of microglial physiology. Nature 532:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monje ML, Toda H, Palmer TD (2003): Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765. [DOI] [PubMed] [Google Scholar]
- 44.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. (2011): Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. [DOI] [PubMed] [Google Scholar]
- 45.Paolicelli RC, Gross CT (2011): Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol 7:77–83. [DOI] [PubMed] [Google Scholar]
- 46.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. (2012): Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schafer DP, Lehrman EK, Stevens B (2013): The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia 61:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, et al. (2013): Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16:543–551. [DOI] [PubMed] [Google Scholar]
- 49.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. (2014): Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19:699–709. [DOI] [PubMed] [Google Scholar]
- 50.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. (2010): Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, behavior, and immunity 24:1058–1068. [DOI] [PubMed] [Google Scholar]
- 51.Menard C, Hodes GE, Russo SJ (2016): Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 321:138–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, et al. (2014): HMGB1 in health and disease. Molecular aspects of medicine 40:1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsung A, Tohme S, Billiar TR (2014): High-mobility group box-1 in sterile inflammation. Journal of internal medicine 276:425–443. [DOI] [PubMed] [Google Scholar]
- 54.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, et al. (2013): Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng L, Zheng X (2014): Integration of animal behaviors under stresses with different time courses. Neural Regen Res 9:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen C, Li XH, Tu Y, Sun HT, Liang HQ, Cheng SX, et al. (2014): Abeta-AGE aggravates cognitive deficit in rats via RAGE pathway. Neuroscience 257:1–10. [DOI] [PubMed] [Google Scholar]
- 57.Dal-Pizzol F, Pasquali M, Quevedo J, Gelain DP, Moreira JC (2012): Is there a role for high mobility group box 1 and the receptor for advanced glycation end products in the genesis of long-term cognitive impairment in sepsis survivors? Molecular medicine 18:1357–1358; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu YW, Zhu X, Yang QQ, Lu Q, Wang JY, Li HP, et al. (2013): Suppression of methylglyoxal hyperactivity by mangiferin can prevent diabetes-associated cognitive decline in rats. Psychopharmacology 228:585–594. [DOI] [PubMed] [Google Scholar]
- 59.Lv C, Wang L, Liu X, Yan S, Yan SS, Wang Y, et al. (2015): Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 89:175–184. [DOI] [PubMed] [Google Scholar]
- 60.Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, et al. (2008): Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci 28:3521–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gumuslu E, Mutlu O, Sunnetci D, Ulak G, Celikyurt IK, Cine N, et al. (2014): The Antidepressant Agomelatine Improves Memory Deterioration and Upregulates CREB and BDNF Gene Expression Levels in Unpredictable Chronic Mild Stress (UCMS)-Exposed Mice. Drug Target Insights 8:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu D, Zhang Q, Gu J, Wang X, Xie K, Xian X, et al. (2014): Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry 49:21–29. [DOI] [PubMed] [Google Scholar]
- 63.Wu R, Shui L, Wang S, Song Z, Tai F (2016): Bilobalide alleviates depression-like behavior and cognitive deficit induced by chronic unpredictable mild stress in mice. Behav Pharmacol 27:596–605. [DOI] [PubMed] [Google Scholar]
- 64.Frank MG, Weber MD, Fonken LK, Hershman SA, Watkins LR, Maier SF (2016): The redox state of the alarmin HMGB1 is a pivotal factor in neuroinflammatory and microglial priming: A role for the NLRP3 inflammasome. Brain Behav Immun 55:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y, Yang YY, et al. (2017): Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid-HMGB1. Brain, behavior, and immunity 59:322–332. [DOI] [PubMed] [Google Scholar]
- 66.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD (2016): Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl) 233:1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker FR, Nilsson M, Jones K (2013): Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets 14:1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng Y, Pardo M, Armini Rde S, Martinez A, Mouhsine H, Zagury JF, et al. (2016): Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain, behavior, and immunity 53:207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF (2007): Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, behavior, and immunity 21:47–59. [DOI] [PubMed] [Google Scholar]
- 70.Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF (2002): Prior stressor exposure sensitizes LPS-induced cytokine production. Brain, behavior, and immunity 16:461–476. [DOI] [PubMed] [Google Scholar]
- 71.Espinosa-Oliva AM, de Pablos RM, Villaran RF, Arguelles S, Venero JL, Machado A, et al. (2011): Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiol Aging 32:85–102. [DOI] [PubMed] [Google Scholar]
- 72.Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, et al. (2006): Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26:3813–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber MD, Frank MG, Sobesky JL, Watkins LR, Maier SF (2013): Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain, behavior, and immunity 32:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP (2012): Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber DJ, Allette YM, Wilkes DS, White FA (2015): The HMGB1-RAGE Inflammatory Pathway: Implications for Brain Injury-Induced Pulmonary Dysfunction. Antioxidants & redox signaling 23:1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vetreno RP, Crews FT (2012): Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience 226:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, et al. (2008): The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 28:12023–12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barbezier N, Tessier FJ, Chango A (2014): [Receptor of advanced glycation endproducts RAGE/AGER: an integrative view for clinical applications]. Annales de biologie clinique 72:669–680. [DOI] [PubMed] [Google Scholar]
- 79.Bartoli F, Carra G, Crocamo C, Carretta D, La Tegola D, Tabacchi T, et al. (2016): Association between depression and neuropathy in people with type 2 diabetes: a meta-analysis. International journal of geriatric psychiatry [DOI] [PubMed]
- 80.Cohen MM Jr., (2013): Perspectives on RAGE signaling and its role in cardiovascular disease. American journal of medical genetics Part A 161A:2750–2755. [DOI] [PubMed] [Google Scholar]
- 81.Kouidrat Y, Amad A, Arai M, Miyashita M, Lalau JD, Loas G, et al. (2015): Advanced glycation end products and schizophrenia: A systematic review. J Psychiatr Res 66-67:112–117. [DOI] [PubMed] [Google Scholar]
- 82.Ray R, Juranek J, Rai V (2015): RAGE axis in neuroinflammation, neurodegeneration and its emerging role in the pathogenesis of amyotrophic lateral sclerosis. Neuroscience and biobehavioral reviews [DOI] [PubMed]
- 83.Salahuddin P, Rabbani G, Khan RH (2014): The role of advanced glycation end products in various types of neurodegenerative disease: a therapeutic approach. Cellular & molecular biology letters 19:407–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Y, Ye RD (2015): Microglial Abeta receptors in Alzheimer’s disease. Cell Mol Neurobiol 35:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao DC, Lu HW, Huang ZH (2015): Association between the receptor for advanced glycation end products gene polymorphisms and cancer risk: a systematic review and meta-analysis. Journal of BUON : official journal of the Balkan Union of Oncology 20:614–624. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.