Abstract
AG-30/5C is an angiogenic host defense peptide (HDP) that activates human mast cells (MC) via an unknown mechanism. Using shRNA-silenced human MC line (LAD2) and stably transfected RBL-2H3 cells, we demonstrate that AG-30/5C induces MC degranulation via Mas-related G protein coupled receptor (GPCR) X2 (MRGPRX2). Most GPCRs signal via parallel and independent pathways mediated by G proteins and β-arrestins. AG-30/5C and compound 48/80 induced similar maximal MC degranulation via MRGPRX2, which was abolished by pertussis toxin (PTx). However, compound 48/80 induced a robust β-arrestin activation as determined by transcriptional activation following arrestin translocation (Tango) but AG-30/5C did not. Overnight culture of MCs with compound 48/80 resulted in reduced cell surface MRGPRX2 expression and this was associated with a significant decrease in subsequent MC degranulation in response to compound 48/80 or AG-30/5C. However, AG-30/5C pretreatment had no effect on cell surface MRGPRX2 expression or degranulation in response to compound 48/80 or AG-30/5C. Icatibant, a bradykinin B2 receptor antagonist, promotes MC degranulation via MRPGRX2 and causes pseudo-allergic drug reaction. Icatibant caused MC degranulation via a PTx-sensitive G-protein but did not activate β-arrestin. A screen of the NIH Clinical Collection library (NCC-1) led to the identification of resveratrol as an inhibitor of MRGPRX2. Resveratrol inhibited compound 48/80-induced Tango and MC degranulation in response to compound 48/80, AG-30/5C and Icatibant. This study demonstrates the novel finding that AG-30/5C and Icatibant serve as G-protein biased agonists for MRGPRX2, but compound 48/80 signals via both G protein and β-arrestin with distinct differences in receptor regulation.
INTRODUCTION
Antibiotics have been used for the treatment of microbial infections since the early 1900s but emergence of multidrug resistant strains of microbes poses a tremendous public health concern globally (1). Thus, there is an urgent need to develop novel therapy for the treatment of infections caused by antibiotic resistant organisms. Antimicrobial peptides (AMPs), also known as host defense peptides (HDPs), represent an evolutionarily ancient mechanism of innate immunity found in both animal and plant kingdoms (2–4). These amphipathic peptides provide protection against a variety of organisms including antibiotic-resistant bacteria, fungi, and parasites via two pathways; one involving the direct killing of microbes and the other via the activation of immune cells (3, 5). Mast cells (MC) are granule-containing immune cells that are widely distributed in tissues such as the skin and mucosal tissues that interact with the environment. Although MC are best known for their roles in allergic and hypersensitivity diseases, they act as sentinel cells that sense microbial pathogens to initiate protective innate and adaptive immune responses via the recruitment of circulating leukocytes and lymphocytes (6–12). The well characterized HDPs, the cathelicidin LL-37, human β-defensins, retrocyclins, and protegrins activate human MC via a G-protein coupled receptor (GPCR) known as MRGPRX2 (13–15). Thus, HDPs that harness MCs’ immunomodulatory property in additional to their antimicrobial activity may serve as novel targets for the treatment of infections caused by antibiotic-resistant organisms.
A small angiogenic amphipathic peptide (AG-30) was identified from a screen of a human library of angiogenic factors (16). It has direct antibacterial activity, induces growth of endothelial cells and augments angiogenesis (16). Because AG-30 is easily degraded by proteolysis, a modified version of the peptide was generated by replacing several of its neutral amino acids with cationic amino acids, resulting in a new peptide known as AG-30/5C (17). Compared to the original AG-30 peptide, AG-30/5C displays greater antimicrobial activity and shows enhanced ability to induce endothelial cell migration, angiogenesis, and wound healing (17). Interestingly, topical application of AG-30/5C on a mouse diabetic wound healing model infected with methicillin-resistant Staphylococcus aureus (MRSA) results in clearance of the microbe and promotes accelerated wound healing (17). This in vivo effect of AG-30/5C likely reflects its ability to kill microbes directly, to harness MCs’ immunomodulatory property, to promote angiogenesis and to induce keratinocyte migration and proliferation (17–19). Although AG-30/5C induces mediator release in human MCs via signaling pathways involving G-protein and phospholipase C, the possibility that it does so via a cell surface GPCR has not been determined (19).
GPCRs are also known as seven-transmembrane receptors (7TMRs) because their structures are characterized by the presence of seven α-helices traversing the plasma membrane. In addition to G-proteins, most agonists for 7TMRs activate an additional signaling pathway that involves the recruitment of adapter proteins known as β-arrestins. This pathway was initially characterized for its role in GPCR desensitization (uncoupling of the G-protein from the cognate receptor), endocytosis, and internalization (20). However, it also serves an important role in G-protein-independent downstream signaling for cell migration, growth, and differentiation (21, 22). Agonists of 7TMRs that preferentially activate G-proteins and β-arrestins are known as G-protein-biased and β-arrestin-biased, respectively. However, agonists that activate both pathways are known as balanced agonists.
MRGPRX family are primate-specific GPCRs and contain four members (23–25). MRGPRX2 is expressed predominantly on human MC whereas MRGPRX3 and MRGPRX4 are present on human keratinocytes (18, 26, 27). A diverse group of cationic ligands that include compound 48/80, HDPs and neuropeptides activate human MCs via MRGPRX2 and likely contributes to host defense and chronic inflammatory diseases (26, 28–30). A number of US Food and Drug Administration (FDA)-approved peptidergic drugs also induce MC degranulation via MRGPRX2, resulting in injection-site pseudo-allergic drug reactions (31). Lansu et al., (32) recently used a novel high throughput β-arrestin activation assay, known as transcriptional activation following arrestin translocation (Tango), to screen ~6000 small molecules for MRGPRX2 activation. A surprising observation from these studies was that while compound 48/80 activates β-arrestin-dependent gene expression, many of the FDA-approved drugs that induce pseudo-allergic drug reactions do not (31, 32). These findings raise the interesting possibility that if AG-30/5C activates human MC via MRGPRX2, it may act either as a balanced or biased agonist for the receptor. The stilbenoid resveratrol activates sirtuin 1 (sirt1) to inhibit IgE-mediated MC degranulation (33). A recent screen of the NIH Clinical Collection library (NCC-1) led to the identification of resveratrol as an inhibitor of MRGPRX2-mediated Tango (34). However, the possibility that resveratrol inhibits AG-30/5C-induced MC degranulation has not been determined.
The purpose of the present study was to determine if AG-30/5C activates human MC via MRGPRX2 and to test if it utilizes G-protein, β-arrestin, or both signaling pathways. We utilized the well-established MRGPRX2 agonist, compound 48/80, for comparison. Icatibant, a bradykinin B2 receptor antagonist used for the treatment of hereditary angioedema, promotes MC degranulation via MRPGRX2 and causes injection site pseudo-allergic drug reaction in nearly every patient (31, 35). However, because Icatibant does not activate β-arrestin-mediated gene expression, it was used as an additional control (32). The data presented herein demonstrate that both AG-30/5C and Icatibant serve as G-protein-biased agonists for MRGPRX2 with clinical implications for MC-mediated host defense and pseudo-allergy
MATERIALS AND METHODS
Materials:
All cell culture and Lipofectamine 2000 transfection reagents were purchased from Invitrogen Life Technologies (Carlsbad, CA); recombinant human stem cell factor cytokine (hSCF) was from Peprotech (Rocky Hill, NJ); p-nitrophenyl-N-acetyl-β-D-glucosamine (PNAG) was from Sigma-Aldrich (St. Louis, MO); PE conjugated anti-MRGPRX2 antibody was from Biolegend (San Diego, CA); polyclonal MRGPRX2 antibody was purchased from Novus Biologicals (Littleton, CO); PE conjugated anti-FLAG antibody, HRP labeled goat anti-rabbit IgG and β-actin antibodies were obtained from Cell Signaling Technology (Danvers, MA); SuperSignal® West PicoMaximum Sensitivity Substrate was from Thermo Scientific (Rockford, IL); Compound 48/80, and Icatibant were from AnaSpec (Fremont, CA); Nateglinide was from Tocris Biosciences and AG-30/5C was from Peptide International Inc. (Louisville, KY), respectively. MRGPRX2-Tango (Addgene #66440) and MRGPRX4-Tango (Addgene#66442) plasmids were gifts from Bryan Roth (32).
Cell culture:
The human MC line, LAD2, was maintained in complete StemPro-34 medium supplemented with L-glutamine (2 mM), penicillin (100 IU/mL), streptomycin (100 μg/mL), and 100 ng/mL recombinant human stem cell factor (rhSCF). Hemidepletion was performed weekly with media containing rhSCF (100 ng/mL) (36). Rat basophilic leukemia (RBL-2H3) cells were maintained as monolayer cultures in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, L-glutamine (2 mM), penicillin (100 IU/mL) and streptomycin (100 μg/mL). RBL-2H3 cells stably expressing MRGPRX2 were maintained similarly in presence of 1mg/mL G418 (37). HTLA (HEK-293T cells stably expressing a β-arrestin2-TEV fusion gene) cells were cultured in DMEM medium supplemented with 10% FBS, L-glutamine (2 mM), penicillin (100 IU/mL) streptomycin (100 μg/mL), hygromycin (200 μg/mL), puromycin (5 μg/mL), and G418 (500 μg/mL) (32, 34).
Lentivirus-mediated knockdown of MRGPRX2 in LAD2 cells:
MRGPRX2-targeted Mission shRNA lentiviral plasmids were purchased from Sigma. A non-target vector was used as a control. Lentivirus generation was performed according to the manufacturer’s manual. Cell transduction was conducted by mixing 1.5 mL of viral supernatant with 3.5 ml of LAD2 (5 × 106 ) cells. Eight hours post-infection, medium was changed to virus-free complete medium, and antibiotic (puromycin, 4 μg/mL, Sigma) was added 16 h later (14, 38). Cells were analyzed for MRGPRX2 knockdown by Western blotting and subsequently used for degranulation experiments.
Generation of HTLA cells stably expressing MRGPRX2 and MRGPRX4:
HTLA cells (0.8 × 106/well) were plated in a six well plate in antibiotic-free medium (DMEM, 10% FBS, and L-glutamine). The following day, the cells were transfected with MRGPRX2-Tango or MRGPRX4-Tango plasmids using the Lipofectamine 2000 DNA transfection reagent (32). Briefly, DNA (2 μg) was mixed with Lipofectamine 2000 (12 μL) in Opti-MEM and incubated with cells for 6 h at 37°C. Transfection reagent was removed and cells were incubated overnight in antibiotic free medium. Receptor expression was determined by flow cytometry using PE-conjugated anti-FLAG antibody. Transfected HTLA cells were serially diluted such that a single cell was present in 200 μL of medium and were cultured in a 96-well plate. Cells from the each well were analyzed by flow cytometry and one population derived from a single transfected cell was used for the present study.
Transcriptional activation following arrestin translocation (Tango) assay:
HTLA cells stably expressing either MRGPRX2 or MRGPRX4 were plated (50,000 cells/well) on a poly-L-lysine-coated 96 well white, clear bottomed cell culture plate in triplicates in 160 μL of antibiotic free medium. After 6 h at 37°C, the medium was aspirated and cells were incubated with MRGPRX2 or MRGPRX4 agonists in 160 μL of antibiotic-free medium for additional 16 h at 37°C. The ligands were aspirated, Bright-Glo solution (100 μL) was added to each well, and relative luminescence unit was measured in a Thermo Labsystems Luminoskan Ascent 392 Microplate Luminometer (32).
Calcium mobilization:
Most of the Ca2+ mobilization studies in HTLA cells were conducted using a high throughput FLIPR-Tetra (Molecular Devices). Cells were plated (104 cells/well) on a collagen coated black wall, clear bottom plate (Corning 3764) and incubated in a 37ºC incubator for 24 h. Cells were loaded with Calcium 6 dye (Molecular Devices) for 2 h at 37ºC. Assay plate was allowed to equilibrate to room temperature for 1 h prior to assay. Compound 48/80 (1.0 and 30 μg/mL) AG-30/5C (1.0 and 10 μM) and Icatibant (30 μg/mL) were added to their respective wells and imaged every 1s for 5 min (31). Ca2+ mobilization studies in RBL-MRGPRX2 cells were conducted using a Hitachi F-2700 Fluorescence Spectrophotometer. Cells were cultured in the absence or presence of PTx (100 ng/mL, 16h) and loaded with 1 mM Indo-1 acetoxymethyl ester for 30 min at room temperature. Cells were then washed and suspended in HEPES-buffered saline containing 0.1% BSA and then stimulated with compound 48/80 (1.0 μg/mL), AG-30/5C (1.0 μM) and Icatibant (20 μg/mL). Calcium mobilization was determined in excitation wavelength of 355 nm and an emission wavelength of 410 nm (12).
Degranulation:
LAD2 cells were washed in HEPES buffer containing 0.1% BSA and seeded at 104 cells/well in a 96 well plate in 45 μL of HEPES buffer containing 0.1% BSA. RBL-2H3 and RBL-MRGPRX2 cells (5 × 104/well) were seeded in a 96 well plate and cultured overnight in presence and absence of pertussis toxin (PTx). Cells were washed twice in HEPES buffer containing 0.1% BSA, suspended in 45 μL of buffer. For resveratrol pretreatment, RBL-MRGPRX2 cells were suspended in 45 μL of buffer containing resveratrol (100 μM) and incubated for 5 min. Untreated and treated LAD2/RBL-MRGPRX2 cells were stimulated with compound 48/80, AG-30/5C, Icatibant at 37°C for 30 min. For total β-hexosaminidase release, unstimulated cells were lysed in 50 μL of 0.1% Triton X-100. Aliquots (20 μL) of supernatant or cell lysates were incubated with 20 μL of 1 mM p-nitrophenyl-N-acetyl-β-D-glucosamine for 1 h at 37°C. The reaction was stopped by adding 250 μL of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer and absorbance was measured at 405 nm (39).
Flow cytometry:
RBL-MRGPRX2 cells (control, compound 48/80 or AG-30/5C-treated) were washed and suspended in FACS buffer (PBS containing 2% fetal calf serum [FCS] and 0.02% sodium azide). Cells (0.5 × 106) were incubated with the PE-conjugated anti-MRGPRX2 antibody for 1 h at 4ºC in dark. Transfected HTLA cells (HTLA-MRGPRX2, HTLA-MRGPRX4) were incubated with PE-conjugated anti-FLAG antibody similarly. Cells were washed in FACS buffer, fixed in 1.5% paraformaldehyde and acquired using a BD LSR II flow cytometer (San Jose, CA) (28, 40). The resulting data were analyzed using WinList software, version 8.
Statistical analysis:
Data shown are mean ± SEM value derived from at least three independent experiments. Statistical significance was determined by non-parametric t-test and one or two-way ANOVA. Error-bars represent mean ± SD from three independent experiments. Significant differences were set at *p≤ 0.05, **p≤ 0.01, ***p≤ 0.001, and analyzed by GraphPad Prism version 5.01.
RESULTS
AG-30/5C induces MC degranulation via MRGPRX2
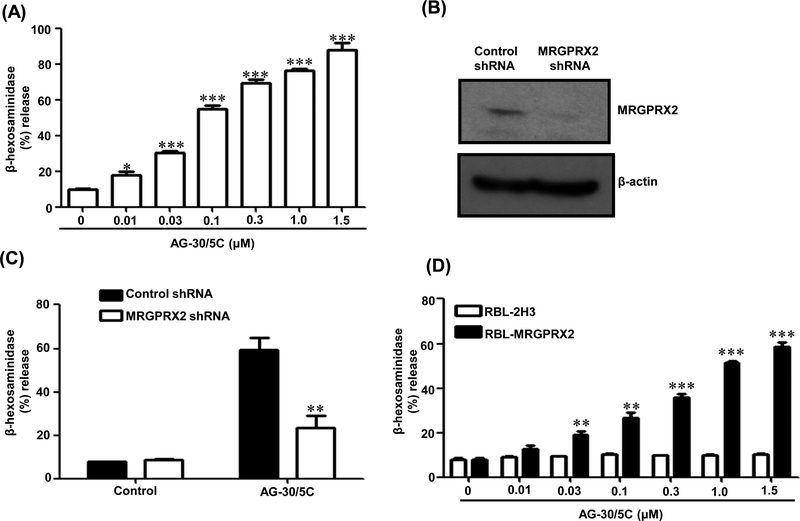
We utilized a human MC line (LAD2) that endogenously expresses MRGPRX2 and found that AG-30/5C induced significant degranulation at a concentration of 0.01 μM (p≤ 0.05), with an EC50 value of <0.1 μM and reaching a maximal response at 1.0 −1.5 μM (Figure 1A). To determine if MRGPRX2 contributes to AG-30/5C-induced degranulation, we used lentiviral shRNA to silence the expression of MRGPRX2. Using western blotting, we confirmed >90% MRGPRX2 knockdown (Figure 1B) and this resulted in significantly (p≤ 0.01) reduced AG-30/5C-induced degranulation when compared to non-targeted shRNA pretreatment (Figure 1C). To further confirm the role of MRGPRX2 on MC degranulation in response to AG-30/5C, we utilized RBL-2H3 cells stably expressing MRGPRX2 (RBL-MRGPRX2) (28). As shown in Figure 1D, AG-30/5C induced dose dependent (0.1 – 1.5 μM) degranulation in RBL-MRGPRX2 cells, but native RBL-2H3 cells did not respond to AG-30/5C. These findings demonstrate that AG-30/5C activates human MC via MRGPRX2.
Figure. 1: AG-30/5C induces MC degranulation via MRGPRX2:
(A) LAD2 cells were stimulated with different concentrations of AG-30/5C (0.01 – 1.5 μM) for 30 min. Percent degranulation (β-hexosaminidase release) was determined. (B) LAD2 cells were stably transduced with scrambled control shRNA or lentivirus shRNA targeting human MRGPRX2. Western blotting was performed to determine MRGPRX2 expression. β-actin was used as a loading control. (C) Control and MRGPRX2 knockdown LAD2 cells were stimulated with AG-30/5C (1 μM) and degranulation was determine. (D) RBL-2H3 and RBL-2H3 cell stably expressing MRGPRX2 (RBL-MRGPRX2) were stimulated with different concentrations of AG-30/5C (0.01 – 1.5 μM) and percent degranulation was determined. All data are expressed as a mean ± SEM of three experiments. Statistical significance was determined by non-parametric t-Test and two way ANOVA (P value<.05). Western blotting data presented are representative of three similar experiments. *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
Pertussis toxin inhibits AG-30/5C-induced MC degranulation but not Ca2+ mobilization.
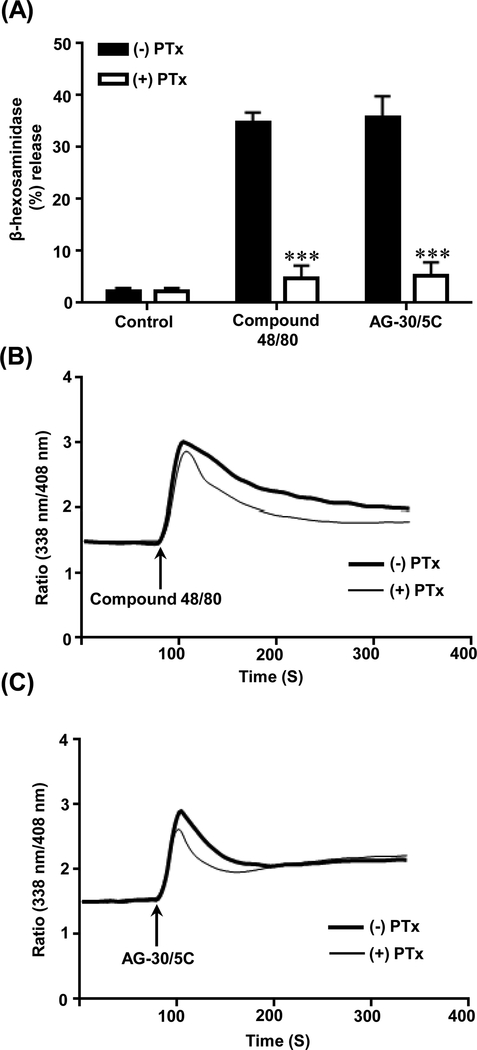
We previously showed that pertussis toxin (PTx) inhibits MC degranulation in response to host defense peptide, human β-defensin 3 without affecting Ca2+ mobilization (19). We therefore tested the effect of PTx on degranulation and Ca2+ mobilization to the well-established MRGPRX2 agonists compound 48/80 and AG-30/5C. Pretreatment of RBL-MRGPRX2 cells with PTx (100 ng/mL, 16h) abolished degranulation in response to compound 48/80 (1 μg/mL) and AG-30/5C (1 μM) (p ≤ 0.001) (Figure 2A). However, PTx had little to no effect on calcium mobilization to both agonists (Figure 2B-C). These findings suggest that MRGPRX2-mediated MC degranulation via diverse ligands (compound 48/80 and AG-30/5C) require the interaction of a Gαi-independent Ca2+ influx.
Figure. 2: Pertussis toxin inhibits AG-30/5C and compound 48/80-induced MC degranulation but not calcium mobilization.
(A) RBL-MRGPRX2 cells cultured in absence (-PTx) or presence of pertussis toxin (+PTx, 100 ng/ml, 16h). Cells were stimulated with compound 48/80 (1μg/mL) or AG-30/5C (1 μM) and percent degranulation was determined. (B-C) RBL-MRGPRX2 cells were cultured in the absence or presence of PTx, loaded with Indo-1 (1 μM, 30 min) and stimulated with either compound 48/80 (1 μg/mL) or AG-30/5C (1 μM) as indicated by arrows and time course of calcium mobilization was determined. Representative traces of 3 similar experiments are shown. All data points for degranulation are expressed as mean ± SEM of three experiments. Statistical significance was determined by non-parametric t-Test and two way ANOVA (P value<.05). *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
AG-30/5C acts as a G protein-biased agonist for MRGPRX2 and MRGPRX4
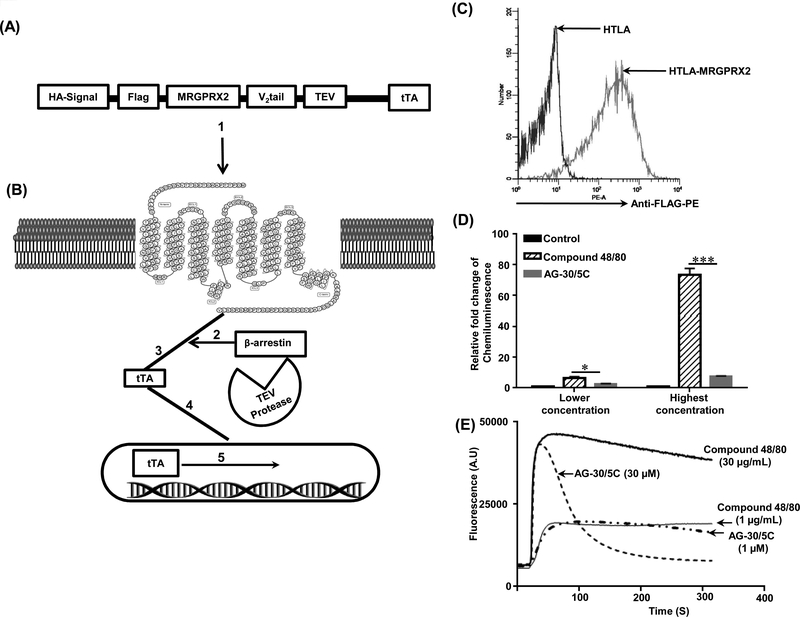
To determine if AG-30/5C activates β-arrestin-dependent signaling in addition to G protein, we utilized a newly developed assay that allows for the measurement of both pathways in the same cell line. The β-arrestin activation assay involves transcriptional gene activation; the design of this assay is shown in Figure 3A-B. HTLA cells stably expressing FLAG-tagged MRGPRX2 were incubated with PE-conjugated anti-FLAG antibody to demonstrate cell surface receptor expression by flow cytometry (Figure 3C). For β-arrestin-mediated gene expression study, cells were exposed to buffer (control) or comparable concentrations of compound 48/80 (1 μg/mL), and AG-30/5C (1 μM), which induce robust mast cell degranulation (27) (Figure 1 and 2). At these concentrations, compound 48/80 showed significantly (p≤ 0.05) higher β-arrestin-mediated gene expression when compared to AG-30/5C (Figure 3D) but induced comparable level of Ca2+ mobilization (Figure 3E). At higher concentrations, compound 48/80 (30 μg/mL) induced ~75 fold increase in β-arrestin-mediated gene expression when compared to buffer control. However, AG-30/5C (10 μM) induced a weak response, which was <10% of that observed with compound 48/80 (Figure 3D). Despite this difference in β-arrestin-mediated gene expression, both compound 48/80 and AG-30/5C induced similar peak in Ca2+ mobilization (Figure 3E). However, compound 48/80 induced a more sustained Ca2+ response than AG-30/5C did. These findings demonstrate that unlike compound 48/80, AG-30/5C serves as a G protein biased agonist for MRGPRX2.
Figure. 3: Compound 48/80 is a balanced agonist whereas AG-30/5C is a β-arrestin-biased agonist for MRGPRX2.
(A) Modular design of Tango constructs. (B) Upon activation (1), β-arrestin is recruited to the C terminus of the receptor (2). This is followed by cleavage of the GPCR fusion protein at the TEV protease–cleavage site (3). Cleavage results in the release of the tTA transcription factor (4), which after transport to the nucleus activates transcription of the luciferase reporter gene (5). (C) Untransfected (HTLA) and stably transfected HTLA-MRGPRX2-Tango (HTLA-MRGPRX2) cells were incubated with anti-FLAG-PE antibody and cell surface receptor expression was determined by flow cytometry. A representative overlay histogram of MRGPRX2 expression is shown. (D) HTLA-MRGPRX2 cells were exposed to compound 48/80 (1.0 or 30 μg/mL) and AG-30/5C (1.0 and 10 μM) respectively and β-arrestin-mediated gene expression was determined. (E) Representative Ca2+ measurement traces for compound 48/80 (1 μg/mL and 30 μg/mL) and AG-30/5C (1 μM and 10 μM) are shown. All data points for the β-arrestin activation assay are expressed as a mean ± SEM of three experiments. Statistical significance was determined by non-parametric t-Test and two way ANOVA (P value<.05). *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
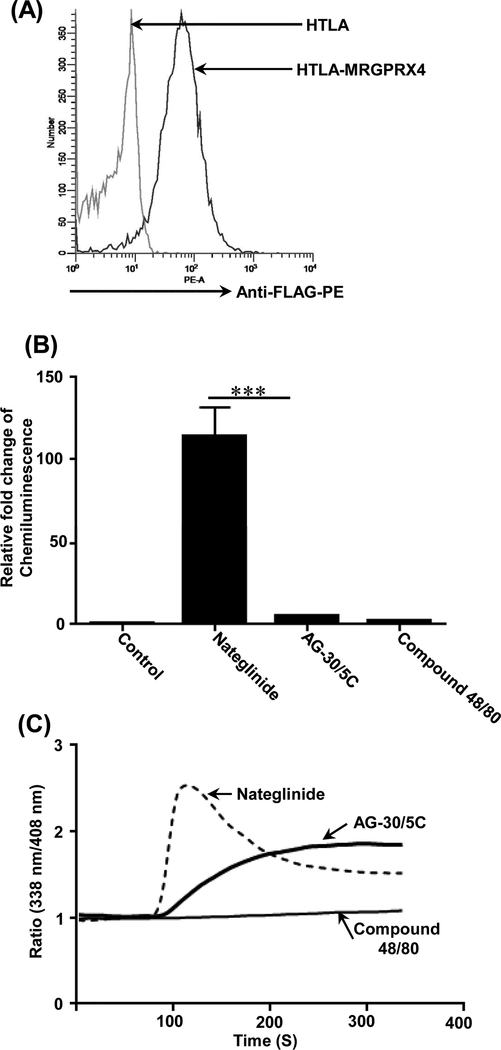
AG-30/5C activates human keratinocytes via MRGPRX3 and MRGPRX4 (18). Because validated plasmid is available only for MRGPRX4, we sought to determine if it also serves as a G protein biased agonist for this receptor. We generated HTLA cells stably expressing FLAG-tagged MRGPRX4-Tango and confirmed cell surface receptor expression by flow cytometry (Figure 4A). Kroeze, et al., (34) recently showed that the KATP-channel blocker nateglinide serves as a balanced agonist for MRGPRX4; activating both G protein and β-arrestin-mediated signaling pathways. Therefore, to validate the transfection system, we first confirmed that nateglinide induces β-arrestin-mediated gene expression (Figure 4B) and causes an increase in Ca2+ mobilization (Figure 4C). Compound 48/80, a balanced agonist for MRGPRX2 (Figure 3), did not activate MRGPRX4 either for β-arrestin (Tango) or G -protein (Ca2+ mobilization) (Figure 4, B and C). By contrast, AG-30/5C caused Ca2+ mobilization in HTLA cells expressing MRGPRX4 but did not activate Tango (Figure 4B-C). These findings suggest that AG-30/5C performs its biological function by acting as a G protein-biased agonist for MRGPRX2 in MCs and MRGPRX4 in keratinocytes.
Figure. 4: Nateglinide is a balanced agonist whereas AG-30/5C is a G protein -biased agonist for MRGPRX4.
(A) Untransfected (HTLA) and stably transfected HTLA-MRGPRX4-Tango (HTLA-MRGPRX4) cells were incubated with anti-FLAG-PE antibody and receptor expression was determined by flow cytometry. A representative overlay histogram of MRGPRX4 expression is shown. (B) Effects of nateglinide (30 μM), AG-30/5C (10 μM), compound 48/80 (30 μg/mL) on β-arrestin-mediated gene expression was determined as described in Figure 3. (C) Representative Ca2+ measurements traces in response to high concentrations of nateglinide (30 μM), AG-30/5C (10 μM) and compound 48/80 (30 μg/mL) are shown. All data points for the Tango assay are expressed as a mean ± SEM of three experiments. Statistical significance was determined by non-parametric t-Test and two way ANOVA (P value<.05). *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
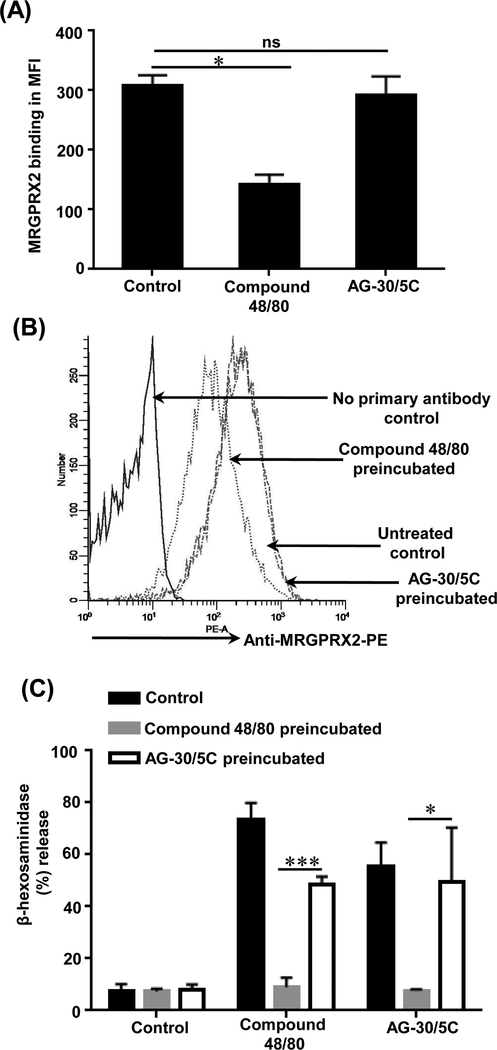
AG-30/5C does not cause MRGPRX2 downregulation but compound 48/80 does.
For most GPCRs, β-arrestin recruitment is associated with receptor internalization (41). Given that compound 48/80 caused robust β-arrestin activation and that AG-30/5C did not, we hypothesized that these compounds would have different effect on receptor internalization. To test this possibility, RBL-MRGPRX2 cells were incubated with compound 48/80 or AG-30/5C (16 h) and cell surface receptor expression was determined by flow cytometry. Compound 48/80 pretreatment caused significant (p≤0.05) reduction in cell surface MRGPRX2 expression when compared to untreated control (Figure 5A). By contrast, AG-30/5C pretreatment had no significant effect on cell surface MRGPRX2 expression. A representative overlay histogram is shown in Figure 5B. We then sought to determine if this difference in cell surface receptor expression correlates with a similar difference in MC degranulation. Consistent with β-arrestin activation (Figures 3D), and receptor internalization (Figure 5A), overnight preincubation of LAD2 cells with compound 48/80 (1.0 μg/mL) resulted in complete inhibition of degranulation in response to the same ligand or AG-30/5C (Figure 5C). However, cells preincubated with AG-30/5C (1 μM) did not lead to substantial inhibition of degranulation to subsequent stimulation by compound 48/80 or AG-30/5C (Figure 5C).
Figure. 5: Compound 48/80 causes MRGPRX2 internalization and reduced MC degranulation whereas AG-30/5C does not.
(A) RBL-MRGPRX2 cells (0.5 × 106) were cultured in the absence (unstimulated) or presence of compound 48/80 (1 μg/mL) or AG-30/5C (1 μM) for 16 h and cells surface expression of MRGPRX2 was determined by flow cytometry using anti-MRGPRX2-PE antibody. The data is presented as mean fluorescent intensity (MFI) of three experiments (B) A representative histogram of cell surface MRGPRX2 receptor expression is shown. (C) LAD2 cells were cultured in the absence (Control) or presence of in compound 48/80 (1 μg/mL) or AG-30/5C (1 μM) for 16h, washed and plated in a 96 well plate (10,000 cells/well). Cells were stimulated with compound 48/80 (1.0 μg/mL) and AG-30/5C (1 μM) respectively for 30 min and percent degranulation was determined by β-hexosaminidase release assay. All the points are expressed as a mean ± SEM of three experiments. Statistical significance was determined by non-parametric t-Test and two way ANOVA. *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
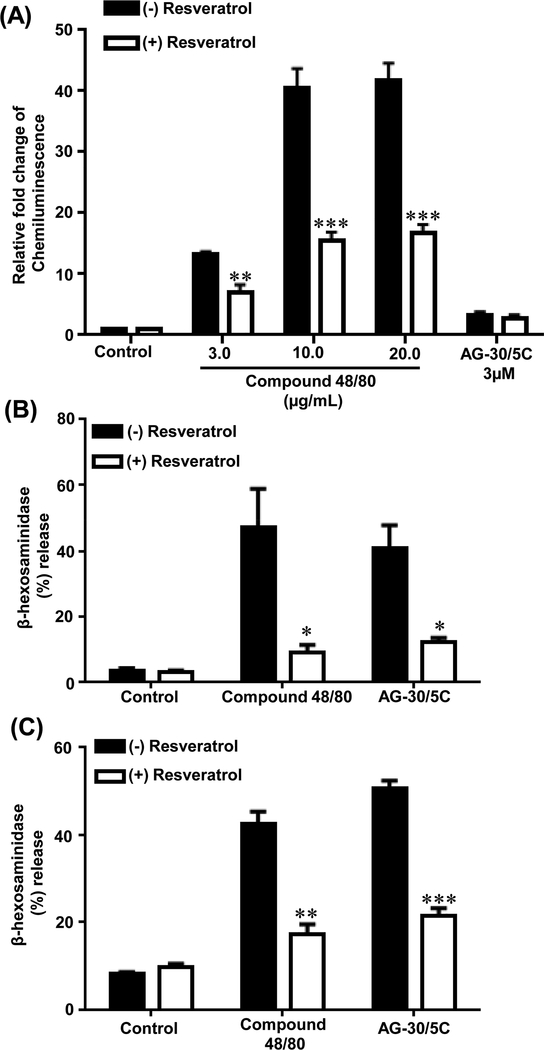
Resveratrol inhibits both G protein and β-arrestin-mediated MRGPRX2 signaling
A screen of the NIH Clinical Collection library (NCC-1) led to the identification of resveratrol as an inhibitor of constitutive MRGPRX2-mediated Tango (34). However, we did not observe a significant constitutive MRGPRX2 activation in stably transfected HTLA cells. We therefore sought to determine if it inhibits agonist-induced response. Pretreatment of HTLA cells stably expressing MRGPRX2 resulted in significant (p≤ 0.01 and p≤ 0.001) inhibition of compound 48/80-induced β-arrestin gene expression (Figure 6A). As AG-30/5C had a minimal effect on β-arrestin signaling resveratrol pretreatment has no effect on Tango for this agonist (Figure 6A). To determine if resveratrol inhibits agonist-induced MRGPRX2-mediated G-protein-mediated response, we utilized RBL-MRGPRX2 and LAD2 cells and determined the effects of resveratrol on degranulation in response to compound 48/80 and AG-30/5C. Preincubation of both cell types with resveratrol (100 μM) significantly (p ≤0.05, 0.01 and 0.001) reduced degranulation in response to both compound 48/80 and AG-30/5C (Figure 6B-C).
Figure. 6: Resveratrol inhibits β-arrestin-mediated gene expression and MC degranulation.
(A) HTLA cells stably expressing MRGPRX2-Tango were incubated with resveratrol (100 μM, 5 min) followed by overnight stimulation with compound 48/80 (3.0, 10 and 20 μg/mL) or AG-30/5C (10 μM) respectively and relative luminescence was determined. (B) RBL-MRGPRX2 cells and (C) LAD2 cells either left untreated (Control) or incubated with resveratrol (100 μM, 5 min) and stimulated with compound 48/80 (1μg/mL) or AG-30/5C (1 μM) for 30 min and percent degranulation was determined. Statistical significance was determined by non-parametric t-Test and two way ANOVA. *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
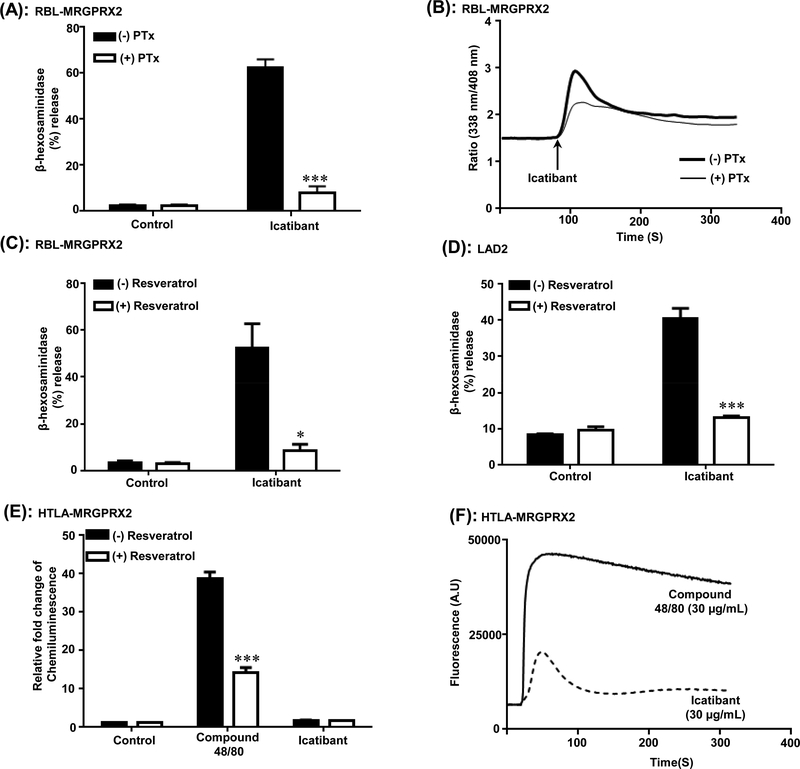
Icatibant is a G protein biased agonist for MRGPRX2; inhibition of degranulation by resveratrol
Icatibant, a bradykinin B2 receptor antagonist used for the treatment of hereditary angioedema, promotes MC degranulation via MRPGRX2 and causes injection site pseudo-allergic drug reaction in nearly every patient (31, 35). To determine the role of Gαi family of G proteins on MC activation by Icatibant, RBL-MRGPRX2 cells were incubated with PTx and the effect of Icatibant on degranulation and Ca2+ mobilization was determined. As shown in Figure 7A and B, PTx completely blocked Icatibant-induced degranulation but not Ca2+ mobilization. This finding is similar to what was observed with compound 48/80 and AG-30/5C (Figure 2). Using RBL-MRGPRX2 cells and LAD2 cells we found that resveratrol caused significant (p≤ 0.05 and p≤ 0.001) inhibition of Icatibant-induced degranulation (Figure 7C-D). We sought to determine if Icatibant also act as a biased ligand for MRGPRX2. Tango assay showed Icatibant induced minimal or no β-arrestin signaling compared to compound 48/80 (Figure 7E) however it induced Ca2+ mobilization although the peak was much shorter than compound 48/80 (Figure 7F).
Figure. 7: Icatibant is a G protein-biased agonist for MRGPRX2 and inhibition of degranulation by resveratrol:
(A and B) RBL-MRGPRX2 cells were cultured in the absence or presence of PTx (100 ng/mL, 16 h) and percent degranulation and Ca2+ mobilization in response to Icatibant (20 μg/mL) was determined as described for compound 48/80 and AG-30/5C (Figure 2). (C) RBL-MRGPRX2 and (D) LAD2 cells were incubated with resveratrol (100 μM, 5 min), stimulated with Icatibant (20 μg/mL) for 30 min and percent degranulation was determined. (E) HTLA cells expressing MRGPRX2 (HTLA-MRGPRX2) were exposed to medium (Control) or resveratrol (100 μM of 5 min) followed by overnight stimulation with compound 48/80 (10 μg/mL) or Icatibant (30 μg/mL) and chemiluminescence was measured (F) Representative Ca2+ traces for compound 48/80 (30 μg/mL) and Icatibant (30 μg/mL) in HTLA-MRGPRX2 cells are shown. Statistical significance was determined by non-parametric t-Test and two way ANOVA. *** indicates P value<.001,** indicates P value <.01 and * indicates P value <.05.
DISCUSSION
AG-30/5C is a synthetic angiogenic HDP that promotes new blood vessel formation, induces human keratinocyte proliferation and effectively clears microbial infection and induces wound healing in mice (17). The ability of AG-30/5C to harness MCs’ immunomodulatory property likely contributes to its host defense but the mechanism via which it activates MC remains unknown (19). The data present herein clearly demonstrate that AG-30/5C induces MC degranulation via MRGPRX2. In addition, we demonstrate that AG-30/5C serves as a G-protein biased agonist for MRGPRX2 (MC) and MRGPRX4 (keratinocyte) with important consequences for host defense and wound healing. Furthermore, Icatibant also acts as a G-protein-biased agonist for MRGPRX2 and the inhibition of MC degranulation by resveratrol has important clinical implication for modulating pseudo-allergic drug reactions.
Kanazawa et al., (19) recently showed that AG-30/5C induces MC degranulation and cytokine generation via signaling pathways that require the activation of a PTx-sensitive G-protein. They proposed that AG-30/5C activates MCs through a non-selective membrane receptor or via direct activation of G proteins. The data presented herein with shRNA-silenced LAD2 cells and transfected RBL-2H3 cells clearly demonstrate that AG-30/5C activates MC via MRGPRX2. MCs play an important role in innate immunity by causing increased vascular permeability and by initiating the recruitment of neutrophils to the sites of infection (6–8, 10, 42–44). In addition, MCs orchestrate the development of adaptive immunity and play an important role in wound healing (11, 12, 45–47). Thus, at sites of microbial infection, mediators released from MCs promote migration of dendritic cells, which are subsequently increased in draining lymph nodes (48–50). Furthermore, MC-derived histamine directly modulates dendritic cell activation to enhance antigen presentation to T cells (51). Interestingly, compound 48/80 and the host defense peptide, LL-37, which activate human MCs via MRGPRX2 are safe and effective vaccine adjuvant in mice (27, 47, 52, 53). Thus, AG-30/5C likely contributes to host defense by harnessing MC’s innate and adaptive immune functions via the activation of MRGPRX2.
It is well documented that G-protein-independent, β-arrestin-mediated signaling for many GPCRs involves phosphorylation of serine/threonine residues at the C-terminus by G-protein coupled receptor kinases (GRKs) (20, 41, 54). Functionally, this is associated with receptor desensitization and β-arrestin-mediated internalization. Interestingly, recent studies with MRGPRX2 using a HDP as an agonist (LL-37) demonstrated that the receptor is resistant to phosphorylation, desensitization and internalization (14). These findings are consistent with the data presented in this study showing that AG-30/5C functions as a G-protein biased agonist for MRGPRX2 without activating β-arrestin-mediated gene expression. Furthermore, preincubation of cells with AG-30/5C did not induce receptor internalization nor was there a decrease in MC degranulation in response to compound 48/80 or AG-30/5C. An important clinical implication of these findings is that lack of functional desensitization of MRGPRX2 by AG-30/5C likely enhances its potential therapeutic efficacy as an immunomodulator in host defense.
In addition to MC activation via MRGPRX2, AG-30/5C induces keratinocyte migration and proliferation via MRPGRX3 and MRGPRX4 (18). However, it is unclear if AG-30/5C also acts as a G-protein-biased ligand for MRPGRX3 and MRGPRX4. Our studies with a validated MRGPRX4-tango system demonstrated that a known MRGPRX4 agonist, nateglinide induced >100 fold increase in β-arrestin-mediated gene expression. However, AG-30/5C at concentrations that induce robust keratinocyte migration and proliferation (18) induced little to no β-arrestin response. These findings suggest that the immunomodulatory and wound healing properties of AG-30/5C reflects its ability to serve as a G-protein-biased agonist for MRGPRX2 in MCs and MRGPRX3/MRGPRX4 in keratinocytes.
In addition to host defense, MRGPRX2 contributes pseudo-allergy in response to a variety of FDA approved cationic drugs. It has been previously reported that Icatibant does not activate β-arrestin-mediated gene expression in HTLA cells expressing MRGPRX2-Tango (32). Our study confirmed these findings and demonstrated that similar to AG-30/5C, it also serves as a G-protein-biased agonist for MRGPRX2. Thus, unlike the situation of AG-30/5C, lack of desensitization of Icatibant-induced degranulation will likely exacerbate pseudo-allergic reaction. However, the finding in the present study showed that resveratrol inhibits Icatibant-induced MC degranulation suggests that it by may be used to modulate pathologic effects of MRGPRX2 activation.
The molecular mechanism by which compound 48/80, AG-30/5C and Icatibant activate MRGPRX2 via shared (G-protein) and distinct (β-arrestin) signaling pathways is not clear. Based on studies with other GPCRs, it is likely that biased ligands induce a unique receptor conformation, activating a particular signaling pathway. Thus, for arginine-vasopressin-type-2 receptor transmembrane helix 6 (TM6) and the third intracellular loop are associated with selective G-protein signaling, whereas TM7 is required for selective β-arrestin recruitment (55). For β2-adrenergic receptor, an unbiased ligand’s binding to the receptor primarily shifts the equilibrium toward the G-protein-specific active state of helix 6, while β-arrestin-biased ligands predominantly regulate the conformational states of helix 7 (56). It is therefore possible that compound 48/80, AG-30/5C and Icatibant induce different conformations of MRGPRX2 to activate distinct signaling pathways. Resveratrol was identified as an antagonist of constitutive activation of MRGPRX2-mediated Tango in HTLA cells (34). However, we did not observe a significant constitutive MRGPRX2 activation but we found that it effectively blocked both G protein (degranulation) and β-arrestin (Tango) activation. These findings suggest that resveratrol inhibits MRGPRX2 function by preventing ligand-receptor interaction. This possibility will be the subject of future investigation.
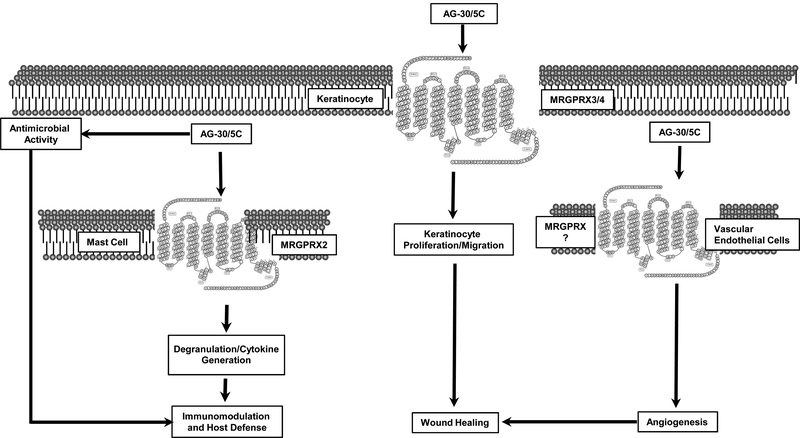
In conclusion, we have shown that a peptide identified from a human library of angiogenic factors (AG-30) and structurally modified to enhance both direct antimicrobial and wound healing properties (AG-30/5C) serves as a novel G-protein-biased ligand for MRGPRX2 (MC) and MRGPRX4 (keratinocytes). Interestingly, topical application of AG-30/5C on in a diabetic wound healing model with methicillin-resistant Staphylococcus aureus (MRSA) infection results in clearance of the microbe and promotes accelerated wound healing (17). This effect of AG-30/5C likely reflects its direct antimicrobial activity, its ability to induce angiogenesis and to promote keratinocyte migration and proliferation via MRGPRX3 and MRGPRX4, in addition to harnessing MCs’ immunomodulatory property via MRGPRX2 (Figure. 8). In addition to MC, MRGPRX2 is expressed in the dorsal root ganglia (57). Thus, it is possible that topical application of AG-30/5C at infected-wounds may sensitize the subject to the perception of pain. However, we believe that the potential benefit of AG-30/5C on healing antibiotic-resistant infected wound outweighs this potential risk.
Figure. 8: Model for the possible mode of action of AG-30/5C on host defense and wound healing.
We propose that in addition to its direct antimicrobial activity, AG-30/5C contributed to host defense via MC degranulation through MRGPRX2. AG-30/5C contributes to wound healing via its action on vascular endothelial cells keratinocyte proliferation and migration through MRGPRX3 and MRGPRX4.
ACKNOWLEDGEMENTS
We thank Drs. Arnold Kirshenbaum and Dean Metcalfe (NIAID/NIH) for providing LAD2 mast cells and Dr. Gilad Barnea, Brown University for providing HTLA cells. We also thank High Throughput Screening Core, Perelman School of Medicine, University of Pennsylvania for performing the calcium mobilization assay and FACS core facilities of the School of Dental Medicine, University of Pennsylvania for acquisition and analysis.
REFERENCES
- 1.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, and Cars O 2013. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 13: 1057–1098. [DOI] [PubMed] [Google Scholar]
- 2.Diamond G, Beckloff N, Weinberg A, and Kisich KO 2009. The roles of antimicrobial peptides in innate host defense. Current pharmaceutical design 15: 2377–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock RE, and Diamond G 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8: 402–410. [DOI] [PubMed] [Google Scholar]
- 4.Mahlapuu M, Hakansson J, Ringstad L, and Bjorn C 2016. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front Cell Infect Microbiol 6: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shai Y 2002. From innate immunity to de-novo designed antimicrobial peptides. Current pharmaceutical design 8: 715–725. [DOI] [PubMed] [Google Scholar]
- 6.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, and Adachi R 2007. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol.Chem 282: 20809–20815. [DOI] [PubMed] [Google Scholar]
- 7.Echtenacher B, Mannel DN, and Hultner L 1996. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381: 75–77. [DOI] [PubMed] [Google Scholar]
- 8.Malaviya R, and Abraham SN 2000. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J Leukoc Biol 67: 841–846. [DOI] [PubMed] [Google Scholar]
- 9.Piliponsky AM, and Romani L 2018. The contribution of mast cells to bacterial and fungal infection immunity. Immunol Rev 282: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JS, and Jawdat DM 2004. Mast cells in innate immunity. J Allergy Clin Immunol 114: 21–27. [DOI] [PubMed] [Google Scholar]
- 11.Abraham SN, and St John AL 2010. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 10: 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson-Weaver B, Choi HW, Abraham SN, and Staats HF 2018. Mast cell activators as novel immune regulators. Curr Opin Pharmacol 41: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian H, Gupta K, and Ali H 2016. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol 138: 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian H, Gupta K, Guo Q, Price R, and Ali H 2011. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J. Biol.Chem 286: 44739–44749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta K, Kotian A, Subramanian H, Daniell H, and Ali H 2015. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 6: 28573–28587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa T, Nakagami H, Maeda A, Morishita R, Miyazaki N, Ogawa T, Tabata Y, Kikuchi Y, Hayashi H, Tatsu Y, Yumoto N, Tamai K, Tomono K, and Kaneda Y 2009. Development of a novel antimicrobial peptide, AG-30, with angiogenic properties. J Cell Mol Med 13: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagami H, Nishikawa T, Tamura N, Maeda A, Hibino H, Mochizuki M, Shimosato T, Moriya T, Morishita R, Tamai K, Tomono K, and Kaneda Y 2012. Modification of a novel angiogenic peptide, AG30, for the development of novel therapeutic agents. J Cell Mol Med 16: 1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiatsurayanon C, Niyonsaba F, Chieosilapatham P, Okumura K, Ikeda S, and Ogawa H 2016. Angiogenic peptide (AG)-30/5C activates human keratinocytes to produce cytokines/chemokines and to migrate and proliferate via MrgX receptors. J Dermatol Sci 83: 190–199. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa K, Okumura K, Ogawa H, and Niyonsaba F 2016. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-kappaB pathways. Immunol Res 64: 594–603. [DOI] [PubMed] [Google Scholar]
- 20.Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ, Lamerdin JE, Triest S, Shukla AK, Berger B, Little J. t., Antar A, Blanc A, Qu CX, Chen X, Kawakami K, Inoue A, Aoki J, Steyaert J, Sun JP, Bouvier M, Skiniotis G, and Lefkowitz RJ 2017. Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci U S A 114: 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenoy SK, and Lefkowitz RJ 2011. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Violin JD, Crombie AL, Soergel DG, and Lark MW 2014. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci 35: 308–316. [DOI] [PubMed] [Google Scholar]
- 23.Burstein ES, Ott TR, Feddock M, Ma JN, Fuhs S, Wong S, Schiffer HH, Brann MR, and Nash NR 2006. Characterization of the Mas-related gene family: structural and functional conservation of human and rhesus MrgX receptors. Br J Pharmacol 147: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X, Han S, Zylka MJ, Simon MI, and Anderson DJ 2001. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632. [DOI] [PubMed] [Google Scholar]
- 25.Zylka MJ, Dong X, Southwell AL, and Anderson DJ 2003. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A 100: 10043–10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, Ra C, and Okayama Y 2014. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol 134: 622–633 e629. [DOI] [PubMed] [Google Scholar]
- 27.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, and Naito T 2006. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 28.Manorak W, Idahosa C, Gupta K, Roy S, Panettieri R Jr., and Ali H 2018. Upregulation of Mas-related G Protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respiratory research 19: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babina M, Guhl S, Artuc M, and Zuberbier T 2018. Allergic FcepsilonRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy 73: 256–260. [DOI] [PubMed] [Google Scholar]
- 30.Gupta K, Idahosa C, Roy S, Lee D, Subramanian H, Dhingra A, Boesze-Battaglia K, Korostoff J, and Ali H 2017. Differential Regulation of Mas-Related G Protein-Coupled Receptor X2-Mediated Mast Cell Degranulation by Antimicrobial Host Defense Peptides and Porphyromonas gingivalis Lipopolysaccharide. Infect Immun 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, and Dong X 2015. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, Che T, Nagase H, Carroll FI, Jin J, Shoichet BK, and Roth BL 2017. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 13: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Lee YJ, Jin F, Park YN, Deng Y, Kang Y, Yang JH, Chang JH, Kim DY, Kim JA, Chang YC, Ko HJ, Kim CH, Murakami M, and Chang HW 2017. Sirt1 negatively regulates FcepsilonRI-mediated mast cell activation through AMPK- and PTP1B-dependent processes. Sci Rep 7: 6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroeze WK, Sassano MF, Huang XP, Lansu K, McCorvy JD, Giguere PM, Sciaky N, and Roth BL 2015. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol 22: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumry WR, Li HH, Levy RJ, Potter PC, Farkas H, Moldovan D, Riedl M, Li H, Craig T, Bloom BJ, and Reshef A 2011. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol 107: 529–537. [DOI] [PubMed] [Google Scholar]
- 36.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, and Metcalfe DD 2003. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk Res 27: 677–682. [DOI] [PubMed] [Google Scholar]
- 37.Gupta K, Subramanian H, and Ali H 2016. Modulation of host defense peptide-mediated human mast cell activation by LPS. Innate Immun 22: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, and Ali H 2013. beta-Defensins activate human mast cells via Mas-related gene X2. J Immunol 191: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali H, Richardson RM, Tomhave ED, DuBose RA, Haribabu B, and Snyderman R 1994. Regulation of stably transfected platelet activating factor receptor in RBL-2H3 cells. Role of multiple G proteins and receptor phosphorylation. J. Biol.Chem 269: 24557–24563. [PubMed] [Google Scholar]
- 40.Alkanfari I, Gupta K, Jahan T, and Ali H 2018. Naturally Occurring Missense MRGPRX2 Variants Display Loss of Function Phenotype for Mast Cell Degranulation in Response to Substance P, Hemokinin-1, Human beta-Defensin-3, and Icatibant. J Immunol 201: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vibhuti A, Gupta K, Subramanian H, Guo Q, and Ali H 2011. Distinct and shared roles of β-arrestin-1 and β-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One 6: e19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gekara NO, and Weiss S 2008. Mast cells initiate early anti-Listeria host defences. Cell Microbiol 10: 225–236. [DOI] [PubMed] [Google Scholar]
- 43.Malaviya R, Navara C, and Uckun FM 2001. Role of Janus kinase 3 in mast cell-mediated innate immunity against gram-negative bacteria. Immunity 15: 313–321. [DOI] [PubMed] [Google Scholar]
- 44.Malaviya R, and Georges A 2002. Regulation of mast cell-mediated innate immunity during early response to bacterial infection. Clin Rev Allergy Immunol 22: 189–204. [DOI] [PubMed] [Google Scholar]
- 45.Weller K, Foitzik K, Paus R, Syska W, and Maurer M 2006. Mast cells are required for normal healing of skin wounds in mice. FASEB journal 20: 2366–2368. [DOI] [PubMed] [Google Scholar]
- 46.Douaiher J, Succar J, Lancerotto L, Gurish MF, Orgill DP, Hamilton MJ, Krilis SA, and Stevens RL 2014. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol 122: 211–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, and Abraham SN 2008. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med 14: 536–541. [DOI] [PubMed] [Google Scholar]
- 48.Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, Staats HF, and Abraham SN 2009. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe 6: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galli SJ, Nakae S, and Tsai M 2005. Mast cells in the development of adaptive immune responses. Nat Immunol 6: 135–142. [DOI] [PubMed] [Google Scholar]
- 50.Jawdat DM, Rowden G, and Marshall JS 2006. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol 177: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 51.Amaral MM, Davio C, Ceballos A, Salamone G, Canones C, Geffner J, and Vermeulen M 2007. Histamine improves antigen uptake and cross-presentation by dendritic cells. J Immunol 179: 3425–3433. [DOI] [PubMed] [Google Scholar]
- 52.McGowen AL, Hale LP, Shelburne CP, Abraham SN, and Staats HF 2009. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine 27: 3544–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SH, Yang IY, Kim J, Lee KY, and Jang YS 2015. Antimicrobial peptide LL-37 promotes antigen-specific immune responses in mice by enhancing Th17-skewed mucosal and systemic immunities. Eur J Immunol 45: 1402–1413. [DOI] [PubMed] [Google Scholar]
- 54.Guo Q, Subramanian H, Gupta K, and Ali H 2011. Regulation of C3a receptor signaling in human mast cells by G protein coupled receptor kinases. PLoS One 6: e22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahmeh R, Damian M, Cottet M, Orcel H, Mendre C, Durroux T, Sharma KS, Durand G, Pucci B, Trinquet E, Zwier JM, Deupi X, Bron P, Baneres JL, Mouillac B, and Granier S 2012. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc Natl Acad Sci U S A 109: 6733–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu JJ, Horst R, Katritch V, Stevens RC, and Wuthrich K 2012. Biased signaling pathways in beta2-adrenergic receptor characterized by 19F-NMR. Science 335: 1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robas N, Mead E, and Fidock M 2003. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J. Biol.Chem 278: 44400–44404. [DOI] [PubMed] [Google Scholar]