Abstract
Purpose
To determine the relationship between demographic and sonographic characteristics of thyroid nodules and malignancy in a pediatric population.
Materials and Methods
All thyroid nodules in patients younger than 19 years that underwent ultrasound (US)-guided fine-needle aspiration biopsy between January 2004 and July 2017 were retrospectively identified. Age, sex, and background appearance of the thyroid gland were recorded for each patient, and sonographic characteristics and pathologic diagnosis were recorded for each nodule. Demographic and sonographic characteristics were assessed to determine which were associated with malignancy. Categorical and continuous variables and interobserver variability were assessed.
Results
A total of 404 nodules in 314 patients (82.8% female) (age range, 2–18 years; mean age, 14.9 years) were analyzed. A total of 77 nodules (19.1%) were malignant, the majority of which were papillary thyroid carcinoma (n = 68 [88.3%]). The likelihood of malignancy did not differ between boys and girls (27.8% vs 22.7%, P = .64), nor did it differ between prepubertal and pubertal patients (18.8% vs 19.1%, P > .99). The cancer rate in patients with a solitary nodule was higher than that in patients with multiple nodules (29.4% vs 14.2%, P = .003). Sonographic characteristics associated with malignant nodules included larger size, solid parenchyma, taller-than-wide shape, presence of speckled calcifications, lack of a smooth margin, and presence of abnormal lymph nodes. Interobserver variability for assessment of sonographic characteristics ranged from moderate to very strong.
Conclusion
In children with thyroid nodules, solitary nodules, larger nodule size, solid parenchyma, taller-than-wide shape, speckled calcifications, irregular margins, and abnormal lymph nodes raise concern for malignancy.
© RSNA, 2018
Introduction
Thyroid nodules are less common in the pediatric population than in the adult population, occurring in 1.0%–1.5% of children but up to 68% of adults at high-resolution ultrasonography (US) (1–5). The reported rate of malignancy of 22%–26% in pediatric thyroid nodules is considerably higher than the reported rate of 5%–15% in adults (6–10), and several studies suggest that the overall incidence of thyroid cancer in the pediatric population may be increasing (11,12). In addition, children with thyroid cancer are more likely to have lymph node and pulmonary metastases at diagnosis than are adults (1).
Children who have been exposed to radiation therapy (13–15) or chemotherapy (15) have an increased risk of developing thyroid nodules and thyroid cancer, as do patients with certain tumor predisposition syndromes, such as phosphatase and tensin hamartoma tumor and familial adenomatous polyposis syndromes (1). Although the American Thyroid Association does not currently recommend screening US of the thyroid in children in high-risk categories, it does recommend annual physical examination of the gland in these children (1). When a thyroid nodule is identified in a pediatric patient at physical examination or imaging, it is important to recognize which nodules should undergo US-guided fine-needle aspiration (FNA) biopsy to avoid overtreatment of a benign lesion or undertreatment of a malignant one.
The US features of malignant thyroid nodules in the adult population have been well characterized and include microcalcifications, solid composition, taller-than-wide shape, hypoechoic or markedly hypoechoic echotexture, and irregular margins (8,16–23). While current American Thyroid Association guidelines recommend applying these criteria in the evaluation of pediatric thyroid nodules (1), few large studies have investigated the sonographic features of malignant pediatric thyroid nodules (9,11,12,24).
Our goal was to characterize the sonographic features of thyroid nodules in a large pediatric cohort and to determine which demographic and sonographic characteristics are associated with malignancy.
Materials and Methods
Permission from our institutional review boards was obtained for this study. Informed consent was waived due to the retrospective nature of this study. Patient confidentiality was maintained, in accordance with Health Insurance Portability and Accountability Act guidelines.
We reviewed databases of pediatric thyroid biopsies and surgeries and identified all patients younger than 19 years who were referred for US and possible biopsy to our multidisciplinary pediatric thyroid nodule clinic between January 2004 and July 2017 and who underwent US-guided FNA of a thyroid nodule. Ninety-six patients in the current study were part of a prior study (9). The prior study described a diagnostic approach to and management of thyroid nodules, whereas in this study we assessed the sonographic characteristics associated with thyroid cancer in a much larger cohort of children. All patients underwent thyroid sonography performed by a radiologist (C.B.B., P.M.D., H.E.P., E.A., M.C.F.) with special expertise in pediatric thyroid sonography (4–36 years of experience) using a 10–15-MHz or 12–18-MHz linear transducer (Logiq E9, GE Healthcare, Milwaukee, Wis; Acuson Sequoia, Siemens, Mountain View, Calif). Only nodules with definitive results and sonographic images available for review were included.
For each patient, we recorded age, sex, cytology or pathology results, background echotexture of the thyroid gland, sonographic characteristics of each nodule at the time of biopsy, and whether the nodule was solitary or one of multiple nodules. Onset of puberty was defined as 11 years of age in girls and as 13 years of age in boys. Demographic and pathology results were obtained via electronic medical record review. Sonographic findings were obtained via retrospective review of US images. Six radiologists (D.M.R., C.B.B, P.M.D., H.E.P., E.A., M.C.F.) with 2–36 years of US subspecialty experience analyzed the US images. For each nodule, US images were reviewed by two radiologists so that each nodule was double read. If there was a discrepancy in the interpretation of a nodule characteristic, the readers sat together to adjudicate the characteristic and to determine the final interpretation that would be used for analysis.
Background echotexture of the gland was recorded as normal or abnormal (Fig 1). A nodule was classified as solitary if there were no other nodules larger than 5 mm in the gland and as nonsolitary if the gland contained at least one other nodule larger than 5 mm in either lobe. Patients with no discrete nodule because the process filled the entire lobe were considered to have a solitary nodule. The following characteristics were recorded for each nodule: size, location, composition, predominant echogenicity, margins, presence and type of calcifications, presence and pattern of color flow in the nodule, and presence or absence of abnormal lymph nodes. Size was recorded in the anteroposterior, transverse, and sagittal dimensions. Taller-than-wide shape was defined as an anteroposterior measurement larger than the transverse measurement. Location was subjectively divided into upper, middle, and lower lobes bilaterally and in the isthmus. Parenchymal composition was recorded as completely solid, less than 25% cystic, 25%–49% cystic, 50%–75% cystic, or more than 75% cystic. Predominant echogenicity of nodules that were less than 50% cystic was determined by comparing the solid component of the nodule to the surrounding gland. The nodule was considered hyperechoic when it was more echogenic than thyroid tissue, isoechoic when its echogenicity was similar to that of thyroid tissue, hypoechoic when it was less echogenic than thyroid tissue, and markedly hypoechoic when it was less echogenic than the adjacent strap muscles (Fig 2). Margin appearance was classified as smooth, irregular, poorly defined, or no discrete mass (Fig 3). Presence or absence of calcifications was recorded for each nodule, and calcifications were classified as rim, speckled, coarse, or a combination thereof (Fig 4). Vascularity on color Doppler US images, when obtained, was subjectively recorded as predominant rim flow, hypervascular (central equal to or greater than rim), or minimal flow. Abnormal lymph nodes, defined as nodes with calcifications, abnormally round shape, no visible echogenic hilum, or abnormal echotexture, were recorded, if present.
Figure 1a:
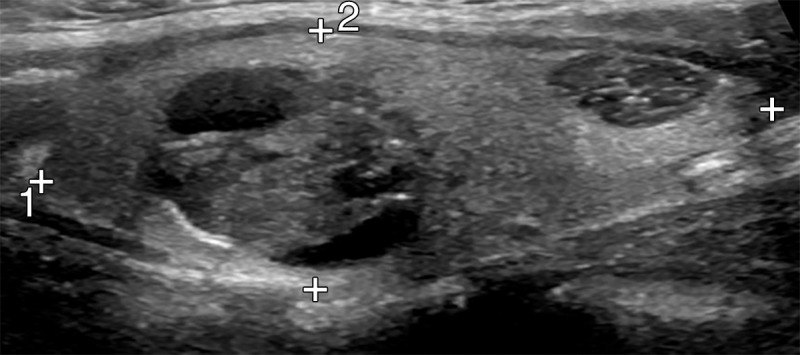
US images show background echotexture of the gland. (a) Sagittal gray-scale image of the left lobe of the thyroid in an 18-year-old girl with normal background echotexture and two benign thyroid nodules. Calipers outline the thyroid gland. (b) Sagittal gray-scale image of the left lobe of the thyroid in an 18-year-old girl with abnormal background echotexture (arrowheads) consistent with Hashimoto thyroiditis.
Figure 2a:
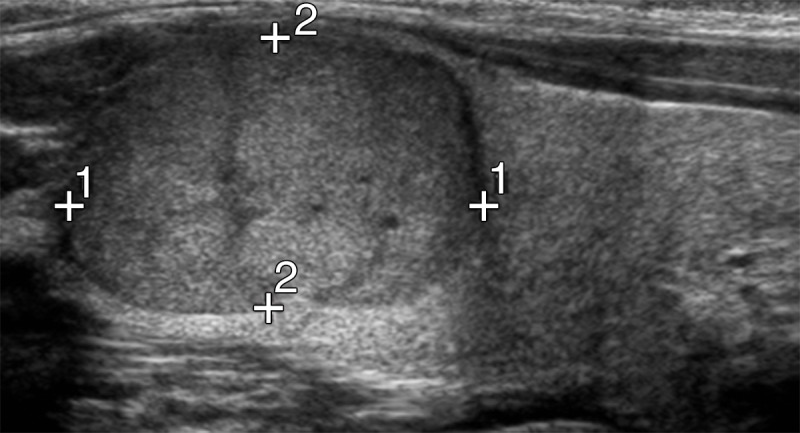
US images show follicular thyroid cancer in a 12-year-old boy. (a) Sagittal gray-scale US image of the left lobe of the thyroid shows a solid hypoechoic mass with smooth margins and no calcifications in the upper pole (calipers), consistent with biopsy-proven follicular cancer. (b) Corresponding color Doppler US image shows central flow equal to rim flow.
Figure 3:
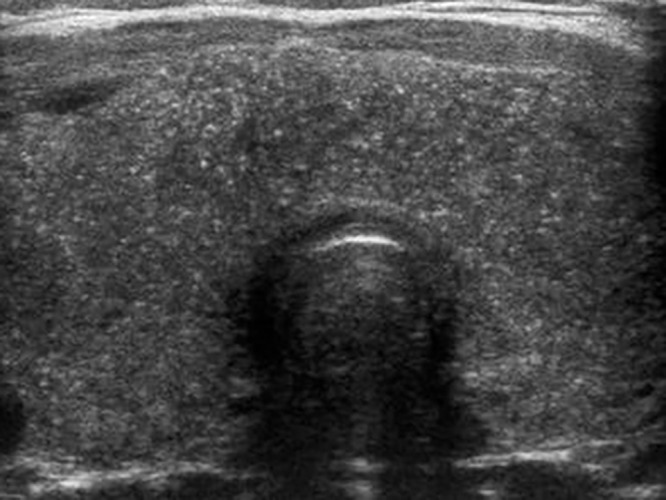
Diffuse sclerosing papillary cancer in a 14-year-old girl. Transverse gray-scale US image of the thyroid shows an enlarged gland with extensive speckled calcifications without a discrete mass, consistent with biopsy-proven diffuse sclerosing papillary cancer.
Figure 4a:
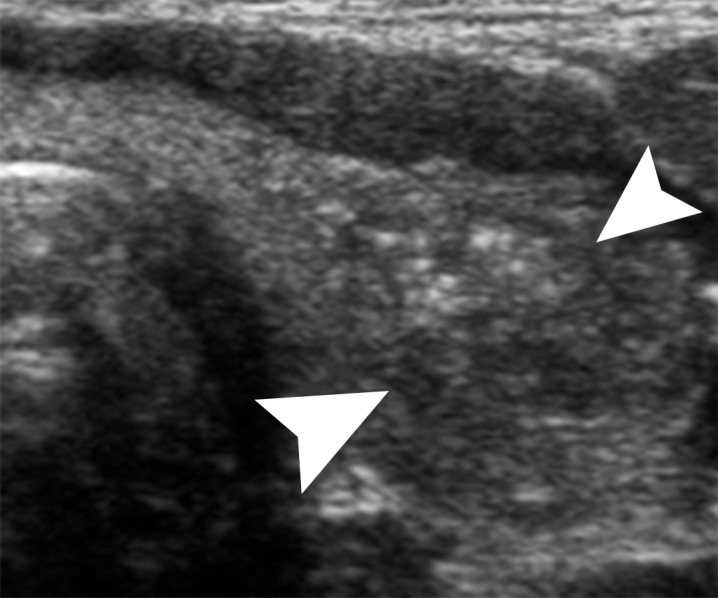
Papillary thyroid cancer in a 16-year-old girl. (a) Transverse gray-scale US image of the left lobe of the thyroid shows a poorly defined hypoechoic solid mass (arrowheads) with associated speckled calcifications consistent with biopsy-proven papillary cancer. (b) Corresponding color Doppler US image shows minimal flow within the lesion.
Figure 1b:
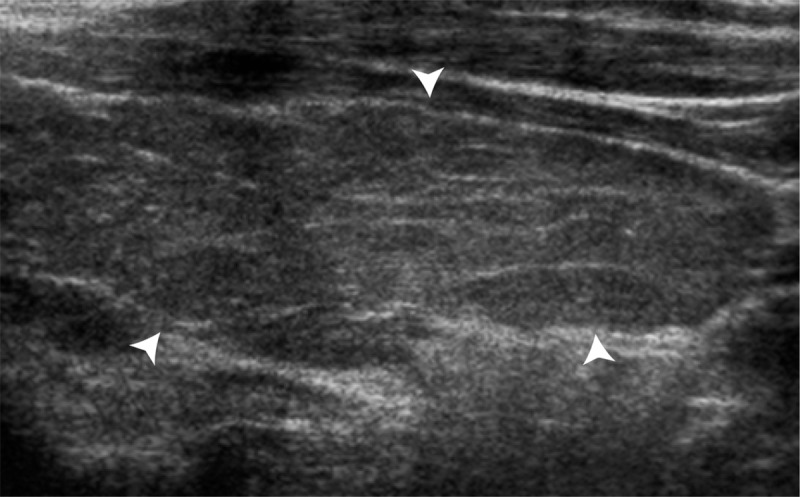
US images show background echotexture of the gland. (a) Sagittal gray-scale image of the left lobe of the thyroid in an 18-year-old girl with normal background echotexture and two benign thyroid nodules. Calipers outline the thyroid gland. (b) Sagittal gray-scale image of the left lobe of the thyroid in an 18-year-old girl with abnormal background echotexture (arrowheads) consistent with Hashimoto thyroiditis.
Figure 2b:

US images show follicular thyroid cancer in a 12-year-old boy. (a) Sagittal gray-scale US image of the left lobe of the thyroid shows a solid hypoechoic mass with smooth margins and no calcifications in the upper pole (calipers), consistent with biopsy-proven follicular cancer. (b) Corresponding color Doppler US image shows central flow equal to rim flow.
Figure 4b:
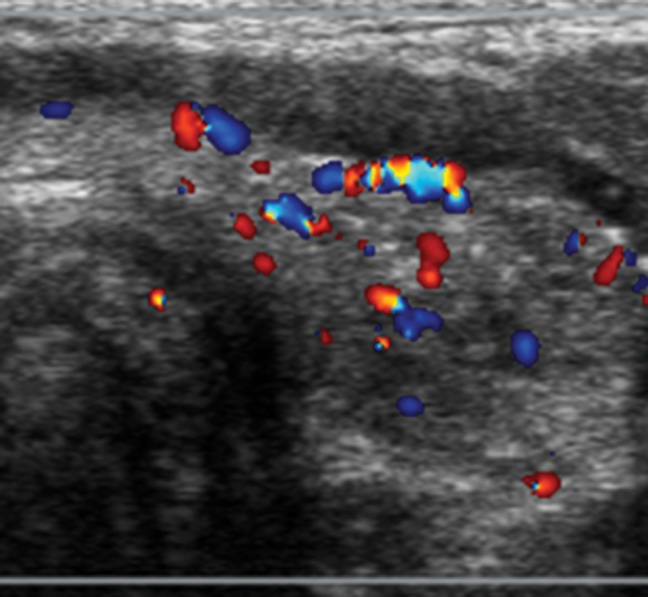
Papillary thyroid cancer in a 16-year-old girl. (a) Transverse gray-scale US image of the left lobe of the thyroid shows a poorly defined hypoechoic solid mass (arrowheads) with associated speckled calcifications consistent with biopsy-proven papillary cancer. (b) Corresponding color Doppler US image shows minimal flow within the lesion.
Individual nodules that underwent more than one FNA were included in the study only once. In these patients, the images obtained at the time of diagnostic aspiration or those obtained closest to surgery were used for the study.
The decision to perform FNA was based on criteria similar to the current American Thyroid Association Management Guidelines for Children with Thyroid Nodules, namely for nodules 1 cm in diameter or larger or for smaller nodules with suspicious sonographic features (1). FNA was performed by one of three pediatric endocrinologists (S.A.H., A.J.W., J.R.S.), with US guidance from the radiologist. Four to six aspirations with a 25-gauge needle were performed per nodule. If the initial FNA results were nondiagnostic, reaspiration was performed one or more times until a diagnostic aspirate was obtained or until the patient underwent surgical resection. FNA specimens were collected in CytoLyt (Cytyc, Marlborough, Mass). Two slides were later prepared by using ThinPrep 2000 (Cytyc) and were stained with a modified Papanicolaou procedure. When possible, a cell block was prepared via sedimentation and was stained with hematoxylin-eosin. Diagnostic FNA results were classified as benign, Hashimoto thyroiditis, atypical (atypical cells of undetermined significance), suspicious for papillary carcinoma, positive for papillary carcinoma, positive for diffuse sclerosing papillary carcinoma, suggestive of follicular neoplasm, or suggestive of Hürthle cell neoplasm. Specimens were considered nondiagnostic if there was insufficient cellular material (fewer than six groups of cells containing more than 10 cells each) without cellular atypia. Repeat FNA was performed in atypical or nondiagnostic nodules. For nodules with atypia at repeat FNA, surgery was recommended. Patients with FNA results suspicious for or positive for papillary carcinoma or follicular neoplasm were referred for surgery. When surgery was performed, the final pathologic diagnosis was recorded.
All nodules were classified as benign or malignant based on the final pathology results after surgery or FNA. Nodules were deemed benign if the initial FNA result was nondiagnostic but the nodule (a) decreased in size over time with at least 1 year of follow-up or (b) showed increased activity at thyroid scintigraphy. In patients in whom final surgical pathology revealed an incidental papillary microcarcinoma distinct from the biopsied nodule, only the nodule pathology results were used for analysis. Nodules were excluded from analysis if no final diagnosis was available due to lack of adequate follow-up.
Results were recorded and analyzed on both a per-patient basis and a per-nodule basis. To determine which demographic and sonographic features were associated with thyroid cancer, Fisher exact and χ2 analyses were used for categorical variables, and the Student t test and logistic regression analyses were used for continuous variables. P < .05 was indicative of a significant difference. The Bonferroni correction was applied for multiple comparisons. Interobserver variability between the two readers was analyzed for each sonographic characteristic by using Cohen κ analysis, with a κ value of 0.21–0.40 indicating fair agreement; a κ value of 0.41–0.60, moderate agreement; a κ value of 0.61–0.80, very strong agreement; and a a κ value of 0.81–0.99, near perfect agreement. Software programs (QuickCalcs, GraphPad Software, https://www.graphpad.com/quickcalcs/; www.vassarstats.net; and Systat, version 9, IBM SPSS Statistics, Armonk, NY) were used for statistical analyses.
Results
In our series, 404 nodules in 314 patients fulfilled the entry criteria. Malignancy was diagnosed in 74 patients (23.6%). Three patients had two separate malignant nodules, for a total of 77 malignant nodules in 74 patients. Of the 404 nodules, 327 were benign (80.9%) and 77 were malignant (19.1%), including 55 papillary carcinomas (71.4%), 13 diffuse sclerosing papillary carcinomas (16.9%), seven follicular carcinomas (9.0%), and two medullary carcinomas (2.6%). In all three patients with two separate cancers, both cancers were papillary. Three patients had both benign and malignant nodules aspirated. An incidental papillary microcarcinoma distinct from the index nodule was identified at surgical pathology in 12 of the 184 patients who underwent surgery.
Clinical characteristics of our patients are reported in Table 1. Most patients were female (260 of 314 [82.8%]). Malignancy rate was slightly higher in boys (15 of 54 [27.8%]) than in girls (59 of 260 [22.7%]), but this difference was not significant (P = .64). The odds ratio for sex as a predictor of malignancy was 1.3 (95% confidence interval: 0.68, 2.54). However, boys with papillary thyroid cancer were more likely than girls to have the diffuse sclerosing variant (five of 12 [41.7%] vs eight of 56 [14.3%], P = .04). Patient age ranged from 2 to 18 years (mean age, 14.9 years). There was no significant association between patient age and diagnosis of cancer (P = .36). The odds ratio for age as a predictor of malignancy was 0.96 (95% confidence interval: 0.88, 1.05). The youngest patient with thyroid cancer was 9 years old. The rate of cancer in prepubertal boys was higher than that in postpubertal boys (four of 11 [36.4%] vs 11 of 43 [25.6%]), but the difference was not significant (P = .48). The rate of cancer in prepubertal girls was lower than that in postpubertal girls (two of 17 [11.8%] vs 57 of 243 [23.5%]); however, this difference also was not significant (P = .56).
Table 1:
Clinical and Demographic Characteristics of the Patient Population
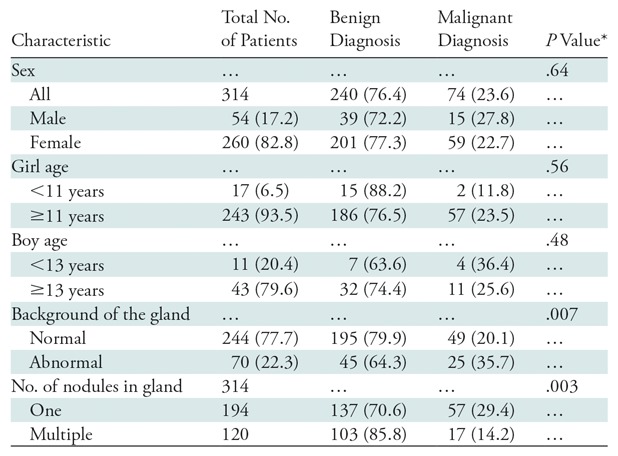
Note.–Data are number of patients, and data in parentheses are percentages.
*P < .01 for significance after Bonferroni correction.
A total of 194 of 314 patients (61.8%) had a solitary thyroid nodule, and 120 (38.2%) had multiple thyroid nodules. The cancer rate in patients with a solitary nodule was significantly higher than that in patients with multiple nodules (57 of 194 [29.4%] vs 17 of 120 [14.2%], P = .003). A total of 244 patients (77.7%) had normal background echotexture, and 70 (22.3%) had abnormal echotexture. A diagnosis of cancer was significantly more likely in patients with an abnormal background appearance of the gland (25 of 70 [35.7%]) than in patients with a normal background appearance (49 of 244 [20.1%], P = .01).
Nodule characteristics are reported in Table 2. Among the 404 nodules, there was no significant difference in malignancy rates between the pre- and postpubertal groups (P >.99). Nodule location was evenly divided between the right lobe (207 of 404 [51.2%]) and the left lobe (170 of 404 [42.1%]), with 26 nodules (6.4%) located in the isthmus. One cancer involved the entire gland. Maximum nodule diameter ranged from 6 to 78 mm, with a mean diameter of 22.4 mm ± 12.1 (standard deviation). The majority of nodules (388 of 399 [96.0%]) measured 10 mm or more. Five malignant nodules could not be measured. All of these were enlarged glands filled with speckled calcifications, and all proved to be the diffuse sclerosing variant of papillary carcinoma. Logistic regression analysis revealed a significant increase in malignancy rate as nodule size increased (P = .019). When we compared nodules with a maximum diameter of 30 mm or more to those with a diameter of less than 30 mm, the former were more likely to be malignant (26 of 96 [27.1%] vs 46 of 303 [18.6%], P = .04), but this difference was not significant. Nodule shape was assessed for the characteristic of taller-than-wide shape. Nineteen of 44 (43.2%) taller-than-wide nodules were malignant as compared with 53 of 355 (14.9%) nodules that were not taller than wide (P < .001). Solid nodules were more likely to be malignant (58 of 139 [41.7%]) than were slightly cystic nodules (<50% cystic, 15 of 158 [9.5%]) or nodules that were at least 50% cystic (four of 107 [3.7%]) (P < .001). Among the 301 nodules that were less than 50% cystic, the majority were isoechoic (n = 108 [35.9%]) or hypoechoic (n = 148 [49.2%]). Hypo- or very hypoechoic nodules were more likely to be malignant than were iso- or hyperechoic nodules (46 of 158 [29.1%] vs 27 of 123 [22.0%]; P = .04), but this difference was not significant. Although infrequent, very hypoechoic nodules had an extremely high rate of malignancy of 70.0% (seven of 10 nodules). Nodules without smooth well-defined margins were much more likely to be malignant that were nodules with smooth margins (46 of 65 [70.8%] vs 31 of 339 [9.1%]; P < .001).
Table 2:
Clinical and Sonographic Characteristics of Thyroid Nodules
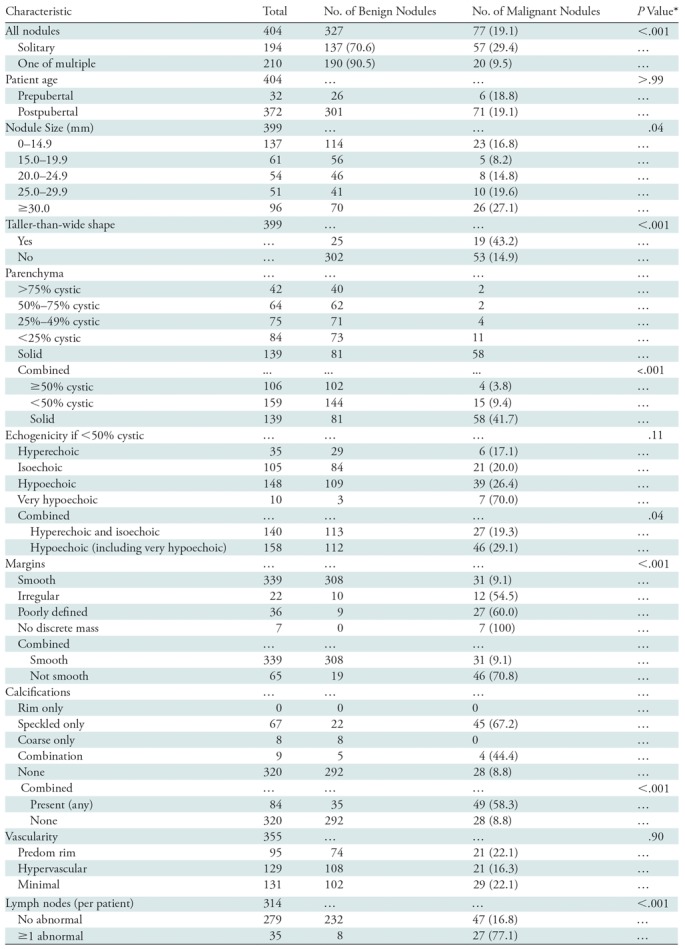
*P < .0046 indicated a significant difference after Bonferroni correction.
With respect to nodule calcifications, the presence of speckled calcifications alone or in combination with coarse calcifications was highly correlated with malignancy (49 of 84 [58.3%] vs 28 of 320 [8.8%], P < .001). Nine nodules had a combination of calcification types: seven contained both coarse and speckled calcifications (four malignant, three benign), and two contained coarse and rim calcifications (both benign). Color Doppler US was performed in 355 nodules. There was no significant difference in the color Doppler US pattern between benign and malignant nodules. Identification of at least one sonographically abnormal lymph node was highly associated with malignancy, although abnormal nodes were found in only 35 patients (27 of whom had a malignancy).
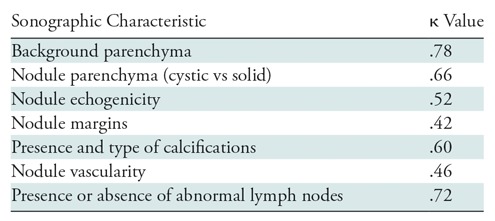
Interobserver reliability (Table 3) was very strong in assessing the background parenchyma of the thyroid and the cystic or solid nature of each nodule, as well as in the identification of abnormal lymph nodes. There was moderately good agreement between readers when assessing nodule echogenicity, nodule margins, presence and type of calcifications, and nodule vascularity.
Table 3:
Interobserver Reliability
Discussion
To our knowledge, this study is the largest series published to date to assess the sonographic appearance of and malignancy risk in pediatric thyroid nodules, with over 300 patients and over 400 nodules. The incidence of thyroid cancer of nearly 25% in our patient population was consistent with other reports of a 19%–26% rate of malignancy in pediatric patients with thyroid nodules (1,9,10,12) and enabled us to confirm the higher rate of malignancy in children as compared with that in adults (8).
We found that most of the sonographic characteristics of a nodule associated with increased risk of cancer in adults (speckled calcifications, irregular margins, taller-than-wide shape, and abnormal lymph nodes) (16–23,25) also convey a higher risk of malignancy in children. Hypoechoic echotexture was associated with malignancy but was not significant after correction for multiple comparisons. We also found that, as in adults, solitary nodules were more likely to be malignant than were nodules that were one of multiple nodules, as were solid nodules. Unlike adults in whom size is not associated with increased malignancy risk (8), in our series, the malignancy rate increased with increasing nodule size.
Similar to several studies in adults (26,27), we found a higher risk of malignancy in children with an abnormal background sonographic appearance as compared with that in patients with normal background gland echotexture. With regards to color Doppler analysis, our findings were similar to those reported in adults (28), namely that color Doppler findings are not a useful differentiating characteristic in the identification of thyroid cancer. The nodules in our cohort that were categorized as hypervascular had a lower rate of malignancy than did those with other blood flow patterns, although the difference was not significant.
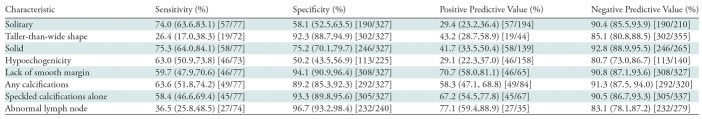
Previous smaller series investigating sonographic characteristics of thyroid nodules that enable prediction of malignancy in pediatric patients have yielded mostly similar results for gray-scale findings but not for color Doppler characteristics. One study reported that microcalcifications, hypoechoic echogenicity, intranodular hypervascularity, and abnormal lymph nodes were each independent predictors of malignancy, with hypervascularity of the nodule and microcalcifications being the strongest predictors (12). Another study reported that the combination of large size (>35 mm), microcalcifications, and irregular margins yielded the best predictive model for malignancy (24). In a study of children exposed to radiation, irregular margins, subcapsular location, and hypervascularity were identified as the most reliable predictors of malignancy in lesions smaller than 15 mm, while for lesions larger than 15 mm, the only reliable criterion for malignancy was hypoechoic echotexture (11). Despite the findings in our current study that certain sonographic characteristics are more likely to be identified in thyroid carcinomas than in benign nodules, the sensitivity and positive predictive value of each characteristic was only moderately good for prediction of thyroid cancer (Table 4). Certain characteristics, including taller-than-wide shape, the presence of calcifications, and the presence of abnormal lymph nodes, show fairly high specificity but only moderate sensitivity for malignancy. Not surprisingly, this result is similar to what has been found in adults, namely that sonographic evaluation alone is insufficient to enable differentiation of benign from malignant thyroid nodules in pediatric patients, and US-guided FNA remains critical in the care of these children (17,20–23).
Table 4:
Test Characteristics of Sonographic Features Associated with Malignancy in Pediatric Thyroid Nodules*
Note.—Data in parentheses are 95% confidence intervals, and data in brackets are numerators and denominators used to calculate the percentages.
As in adults, most of the patients in our cohort were female; however, unlike in adults (8), the rate of cancer per patient and per nodule was similar between the sexes. We also found no difference in cancer rate per patient or per nodule based on patient age or pubertal status.
Also, as in adults, the majority of malignancies in our series were papillary carcinomas. Interestingly, in our cohort, a higher proportion of these (19.1%) were the diffuse sclerosing variant of papillary carcinoma, as compared with the rate of 1%–5% found among adult patients with papillary cancers (Erik K. Alexander, MD, oral communication, February 5, 2017) (29). This aggressive variant of papillary cancer is known to occur at a younger age than classic papillary cancer (30). It has also been reported to affect female patients more often than male patients (31), and this was true in our cohort. It should be noted, however, that among patients in our study in whom papillary cancer was diagnosed, the proportion of cancers presenting as the diffuse sclerosing variant was higher in male patients than in female patients (41% vs 14%).
The interobserver reliability between radiologists in our study was moderate to very strong. Agreement was very strong for identification of abnormal lymph nodes, assessment of nodule parenchyma, and assessment of the background of the gland. The lowest agreement was for nodule margins. One possible explanation for this may be the difficulty associated with establishing clear definitions for each possible margin appearance, with overlap between the categories of irregular and poorly defined margins, rather than the differences in the reader’s perception of the margins.
This study was limited by the small number of prepubertal patients (<10% of our cohort), as well as by its retrospective design. The retrospective image review particularly limited the assessment of lymph nodes, as there might have been abnormal nodes that were not imaged at the time of sonography. There could be selection bias due to the referral nature of our clinic design; however, because we used a consistent approach similar to the American Thyroid Association guidelines to determine which nodule should be biopsied, this should be minimized. In addition, we did not assess whether there might be a combination of nodule characteristics that could indicate a high risk of malignancy in children.
In conclusion, to our knowledge, this study presents the largest and most detailed series of sonographic evaluation of thyroid nodules in pediatric patients to date. We found that the rate of cancer in thyroid nodules is higher in the pediatric population than in adults. The sonographic characteristics of malignancies in children that are similar to those in adults include larger size, solid composition, taller-than-wide shape, speckled calcifications, poorly defined margins, and abnormal lymph nodes. Consideration for FNA of any thyroid nodule in the pediatric patient is warranted, particularly for lesions with suspicious sonographic characteristics or in patients with abnormal lymph nodes. Sampling of enlarged thyroid glands with innumerable calcifications is also indicated to diagnose or exclude the diffuse sclerosing variant of papillary cancer.
Summary
In this pediatric population, the rate of cancer in thyroid nodules was higher than that reported in adults. Consideration for fine-needle aspiration of nodules with suspicious sonographic characteristics or in patients with abnormal lymph nodes is warranted.
Implications for Patient Care
■ In the pediatric population with thyroid nodules, larger size, solid parenchyma, speckled calcifications, lack of smooth margins, taller-than-wide shape, and abnormal lymph nodes are characteristics that increase the risk of malignancy.
■ In pediatric patients with thyroid nodules, age and sex do not change the risk of malignancy.
■ The cancer rate in patients with a solitary nodule was higher than that in patients with multiple nodules.
■ The diffuse sclerosing variant of papillary thyroid cancer is uncommon in adults but accounts for a large percentage (19.1%) of papillary thyroid cancers in pediatric patients.
Received June 7, 2017; revision requested August 3; revision received February 21, 2018; accepted March 5.
Disclosures of Conflicts of Interest: D.M.R. disclosed no relevant relationships. C.B.B. disclosed no relevant relationships. P.M.D. disclosed no relevant relationships. H.E.P. disclosed no relevant relationships. S.A.H. disclosed no relevant relationships. E.A. disclosed no relevant relationships. A.J.W. disclosed no relevant relationships. J.R.S. disclosed no relevant relationships. C.E.C. disclosed no relevant relationships. M.C.F. disclosed no relevant relationships.
Abbreviation:
- FNA
- fine-needle aspiration
References
- 1.Francis GL, Waguespack SG, Bauer AJ, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015;25(7):716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rallison ML, Dobyns BM, Keating FR, Jr, Rall JE, Tyler FH. Thyroid nodularity in children. JAMA 1975;233(10):1069–1072. [PubMed] [Google Scholar]
- 3.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009;39(8):699–706. [DOI] [PubMed] [Google Scholar]
- 5.Hayashida N, Imaizumi M, Shimura H, et al. Thyroid ultrasound findings in children from three Japanese prefectures: Aomori, Yamanashi and Nagasaki. PLoS One 2013;8(12):e83220. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005;237(3):794–800. [DOI] [PubMed] [Google Scholar]
- 7.Hegedüs L. Clinical practice. the thyroid nodule. N Engl J Med 2004;351(17):1764–1771. [DOI] [PubMed] [Google Scholar]
- 8.Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 2006;91(9):3411–3417. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Ly S, Castroneves LA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab 2013;98(8):3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer 2006;13(2):427–453. [DOI] [PubMed] [Google Scholar]
- 11.Lyshchik A, Drozd V, Demidchik Y, Reiners C. Diagnosis of thyroid cancer in children: value of gray-scale and power Doppler US. Radiology 2005;235(2):604–613. [DOI] [PubMed] [Google Scholar]
- 12.Mussa A, De Andrea M, Motta M, Mormile A, Palestini N, Corrias A. Predictors of malignancy in children with thyroid nodules. J Pediatr 2015;167(4):886–892.e1. [DOI] [PubMed] [Google Scholar]
- 13.Lubin JH, Adams MJ, Shore R, et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab 2017;102(7):2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veiga LHS, Holmberg E, Anderson H, et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res 2016;185(5):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sklar C, Whitton J, Mertens A, et al. Abnormalities of the thyroid in survivors of Hodgkin’s disease: data from the Childhood Cancer Survivor study. J Clin Endocrinol Metab 2000;85(9):3227–3232. [DOI] [PubMed] [Google Scholar]
- 16.Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB, Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med 2003;22(10):1083–1090. [DOI] [PubMed] [Google Scholar]
- 17.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med 2004;23(11):1455–1464. [DOI] [PubMed] [Google Scholar]
- 18.Khoo ML, Asa SL, Witterick IJ, Freeman JL. Thyroid calcification and its association with thyroid carcinoma. Head Neck 2002;24(7):651–655. [DOI] [PubMed] [Google Scholar]
- 19.Nam-Goong IS, Kim HY, Gong G, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60(1):21–28. [DOI] [PubMed] [Google Scholar]
- 20.Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002;87(5):1941–1946. [DOI] [PubMed] [Google Scholar]
- 21.Moon HJ, Kwak JY, Kim EK, Kim MJ. A taller-than-wide shape in thyroid nodules in transverse and longitudinal ultrasonographic planes and the prediction of malignancy. Thyroid 2011;21(11):1249–1253. [DOI] [PubMed] [Google Scholar]
- 22.Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 2002;178(3):687–691. [DOI] [PubMed] [Google Scholar]
- 23.Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation—multicenter retrospective study. Radiology 2008;247(3):762–770. [DOI] [PubMed] [Google Scholar]
- 24.Koltin D, O’Gorman CS, Murphy A, et al. Pediatric thyroid nodules: ultrasonographic characteristics and inter-observer variability in prediction of malignancy. J Pediatr Endocrinol Metab 2016;29(7):789–794. [DOI] [PubMed] [Google Scholar]
- 25.Remonti LR, Kramer CK, Leitão CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 2015;25(5):538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gul K, Dirikoc A, Kiyak G, et al. The association between thyroid carcinoma and Hashimoto’s thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid 2010;20(8):873–878. [DOI] [PubMed] [Google Scholar]
- 27.Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto’s thyroiditis. Eur Surg Res 2010;45(3-4):333–337. [DOI] [PubMed] [Google Scholar]
- 28.Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med 2003;22(2):127–131; quiz 132–134. [DOI] [PubMed] [Google Scholar]
- 29.Chow SM, Chan JK, Law SC, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: clinical features and outcome. Eur J Surg Oncol 2003;29(5):446–449. [DOI] [PubMed] [Google Scholar]
- 30.Koo JS, Hong S, Park CS. Diffuse sclerosing variant is a major subtype of papillary thyroid carcinoma in the young. Thyroid 2009;19(11):1225–1231. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Xia D, Lin P, Gao L, Li G, Zhang W. Sonographic findings of the diffuse sclerosing variant of papillary carcinoma of the thyroid. J Ultrasound Med 2010;29(8):1223–1226. [DOI] [PubMed] [Google Scholar]