Abstract
Lessons Learned.
The findings of this prospective, single‐arm, phase II study showed that neoadjuvant erlotinib was well tolerated and might improve the radical resection rate in patients with stage IIIA‐N2 epidermal growth factor receptor mutation‐positive non‐small cell lung cancer (NSCLC).
Erlotinib shows promise as a neoadjuvant therapy option in this patient population.
Next‐generation sequencing may be useful for predicting outcomes with preoperative tyrosine kinase inhibitors (TKIs) in patients with NSCLC.
Large‐scale randomized controlled trials investigating the role of TKIs in perioperative therapy, combining neoadjuvant and adjuvant treatments to enhance personalized therapy for patients in this precision medicine era, are warranted.
Background.
Information on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) as neoadjuvant therapy in non‐small cell lung cancer (NSCLC) is scarce. We evaluated whether neoadjuvant erlotinib improves operability and survival in patients with stage IIIA‐N2 EGFR mutation‐positive NSCLC.
Methods.
We conducted a prospective, single‐arm, phase II study. Patients received erlotinib 150 mg per day for 56 days in the neoadjuvant period. The primary endpoint was the radical resection rate.
Results.
Nineteen patients were included in the final analysis. After erlotinib treatment, 14 patients underwent surgery. The radical resection rate was 68.4% (13/19) with a 21.1% (4/19) rate of pathological downstaging. The objective response rate was 42.1%; 89.5% (17/19) of patients achieved disease control, with a 10.3‐month median disease‐free survival among patients who underwent surgery. Among all 19 patients who received neoadjuvant therapy, median progression‐free survival (PFS) and overall survival were 11.2 and 51.6 months, respectively. Adverse events (AEs) occurred in 36.8% (7/19) of patients, with the most common AE being rash (26.3%); 15.8% experienced grade 3/4 AEs. Quality of life (QoL) improvements were observed after treatment with erlotinib for almost all QoL assessments. Effects of TP53 mutation on prognosis were evaluated in eight patients with adequate tissue samples. Next‐generation sequencing revealed that most patients had a TP53 gene mutation (7/8) in addition to an EGFR mutation. No TP53 mutation, or very low abundance, was associated with longer PFS (36 and 38 months, respectively), whereas high abundance was associated with short PFS (8 months).
Conclusion.
Neoadjuvant erlotinib was well tolerated and may improve the radical resection rate in this patient population. Next‐generation sequencing may predict outcomes with preoperative TKIs.
Abstract
经验获取
• 本次前瞻性单组 II 期研究结果显示,埃罗替尼新辅助治疗在 IIIA‐N2 期表皮生长因子受体突变阳性非小细胞肺癌 (NSCLC) 患者中的耐受性良好,可以提高根治性切除率。
• 在此患者群体中,埃罗替尼显示出了作为新辅助治疗选择的希望。
• 对于预测 NSCLC 患者的手术前酪氨酸激酶抑制剂 (TKI) 结果而言,下一代测序可能十分有用。
• 为了在这个精准医疗的时代提高患者的个性化治疗水平,开展旨在调查 TKI 在围术期治疗(新辅助治疗与辅助治疗相结合)中作用的大规模随机对照试验很有必要。
摘要
背景。关于表皮生长因子受体 (EGFR) 酪氨酸激酶抑制剂 (TKI) 作为非小细胞肺癌 (NSCLC) 的新辅助治疗的信息十分匮乏。我们对埃罗替尼新辅助治疗能否改进 IIIA‐N2 期 EGFR 突变阳性 NSCLC 患者的可手术性和生存期进行了评估。
方法。我们进行了一项前瞻性单组 II 期研究。在新辅助治疗期间,患者每天服用埃罗替尼 150 mg,连续给药 56 天。主要终点是根治性切除率。
结果。最终分析中包含 19 名患者。在埃罗替尼治疗之后,14 名患者接受手术。根治性切除率为 68.4% (13/19),病理性降级率为 21.1% (4/19)。客观反应率为 42.1%;89.5% (17/19) 的患者病情得到控制,在接受手术的患者中,中位无病生存期为 10.3 个月。在所有接受新辅助治疗的 19 名患者中,中位无进展生存期 (PFS) 和总体生存期分别为 11.2 个月和 51.6 个月。36.8% (7/19) 的患者出现不良反应 (AE),最常见的 AE 为皮疹 (26.3%);15.8% 的患者出现 3/4 级 AE。对于几乎所有的生存质量 (QoL) 评估,在治疗后均观察到了 QoL 改善。我们针对 8 名具有适当组织样本的患者评估了 TP53 突变对预后的影响。下一代测序显示,除 EGFR 突变之外,大多数患者均有 TP53 基因突变 (7/8)。无 TP53 突变或极低丰度与较长的 PFS(分别为 36 个月和 38 个月)相关,而高丰度与短 PFS(8 个月)相关。
结论。埃罗替尼新辅助治疗在此患者群体中的耐受性良好,可以提高根治性切除率。下一代测序可以预测手术前 TKI 的结果。
Discussion
Patients with stage IIIA‐N2 NSCLC have a poor prognosis, especially those with EGFR mutations. Sufficient clinical benefit cannot be achieved with chemoradiotherapy without serious safety concerns. EGFR TKI therapy has shown good efficacy and favorable tolerability in patients with EGFR mutation‐positive NSCLC. The TKI erlotinib is approved for first‐line treatment of EGFR mutation‐positive NSCLC; however, its usefulness as neoadjuvant therapy in patients with resectable EGFR mutation‐positive NSCLC remains unclear.
We performed a single‐arm trial to evaluate whether neoadjuvant erlotinib improves operability and survival in patients with stage IIIA‐N2 EGFR mutation‐positive NSCLC. The primary endpoint was the radical resection rate, and the estimated sample size was 30 cases. Of 44 patients diagnosed with stage IIIA‐N2 NSCLC, 25 with EGFR mutation‐positive disease were enrolled. Subsequent retesting with improved sequencing technologies reduced the sample size to 19 patients after an additional 6 patients were excluded (4 were negative for EGFR mutation by amplification‐refractory mutation system polymerase chain reaction sequencing and 2 by next‐generation sequencing [NGS]).
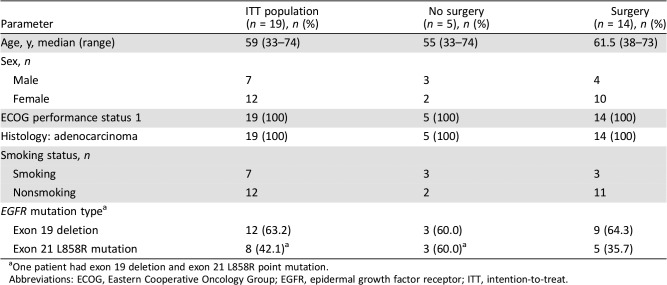
Most patients were female (12/19, 63.2%), the median age was 59 (range, 33–74) years, and all patients had an Eastern Cooperative Oncology Group performance status of 1 and adenocarcinoma histology. After erlotinib treatment, 73.7% of patients (14/19) underwent surgery; the radical resection rate was 68.4% (13/19). Most patients who underwent surgery benefited from neoadjuvant treatment prior to surgical resection and achieved disease control of the target lesion. The objective response rate was 42.1% (8/19) with a 21.1% (4/19) rate of clinical downstaging to T0–3N0M0; 89.5% of patients (17/19) achieved disease control after neoadjuvant therapy (Figure 1). In patients who underwent surgery, postoperative pathology showed that seven patients (50.0%) achieved partial response, seven (50.0%) achieved stable disease, and no patients had progressive disease. Among patients who underwent surgery, median disease‐free survival calculated from the date of surgery and the date of neoadjuvant therapy was 10.3 months and 12.1 months, respectively. Among all patients who received neoadjuvant therapy, the median PFS and overall survival were 11.2 and 51.6 months, respectively.
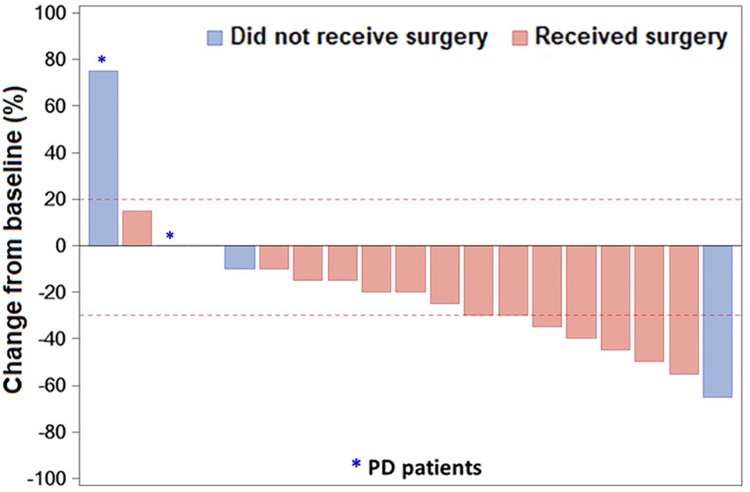
Figure 1.
Waterfall plot of response to erlotinib neoadjuvant therapy. Bars show data from individual patients. Negative values suggest tumor shrinkage and positive values suggest PD; the dashed lines show the thresholds for a partial response (shrinkage by 30%) or for progressive disease (growth by 20%) according to RECIST criteria.
Abbreviation: PD, progressive disease.
To the best of our knowledge, this is the first study to evaluate the role of neoadjuvant EGFR TKI therapy with erlotinib in patients with stage IIIA‐N2 EGFR mutation‐positive NSCLC. Our findings are encouraging in view of the poor prognosis and limited treatment options available for patients with stage IIIA‐N2 NSCLC.
Trial Information
- Disease
Lung cancer – NSCLC
- Stage of Disease/Treatment
Neoadjuvant
- Prior Therapy
None
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Radical resection rate
- Secondary Endpoint
Pathological complete remission
- Secondary Endpoint
Objective response rate
- Secondary Endpoint
Disease‐free survival
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Quality of life
- Secondary Endpoint
Safety
- Secondary Endpoint
Biomarkers (exploratory)
- Additional Details of Endpoints or Study Design
- Patients
- Patients who were at least 18 years of age with stage IIIA‐N2 NSCLC, a confirmed activating mutation of EGFR, and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 were eligible for this study. Tissue confirmation of N2 disease was required. Clinical and pathological stages of NSCLC were assessed in accordance with the American Joint Committee on Cancer 7th edition TNM staging system. Diagnoses were histologically demonstrated in every case and confirmed from medical history, chest computed tomography (CT) scan, whole‐body positron emission tomography scan (if not possible, abdomen CT scan and bone single‐photon emission computed tomography were employed instead), or endobronchial ultrasound transbronchial needle aspiration examination. EGFR mutation testing was done by polymerase chain reaction (PCR)‐based direct sequencing at screening and confirmed by amplification‐refractory mutation system PCR after enrollment. Prior to study entry, all patients were evaluated by a multidisciplinary team including a medical oncologist, thoracic surgeon, radiologists, and endoscopists.
- Study Design and Treatment
- This was a prospective, single‐arm, phase II clinical trial conducted at Shanghai Chest Hospital in Shanghai, People's Republic of China. Patients were evaluated for progressive disease (PD) after the first treatment cycle (4 weeks). Patients without PD continued with the next treatment cycle, whereas those with PD were managed with standard chemotherapy or chemoradiotherapy. Patients were evaluated by a surgical team to determine whether the tumor was resectable: a second chest CT scan was performed 56 days after erlotinib monotherapy to assess benefits from neoadjuvant therapy, but there were no predefined criteria for resectability. Patients who benefited from erlotinib and were determined to have resectable tumors received surgery followed by a platinum‐based chemotherapy regimen. Follow‐up evaluations were performed every 3 months for 2 years, or until death. The criteria for nodal downstaging was based on both imaging and postoperative tissue assessment. Tumor response and progression were assessed according to RECIST version 1.0. AEs were graded by the National Cancer Institute Common Terminology Criteria for AEs version 3.0. This study is registered at clinicaltrials.gov (NCT01217619, ESTERN). The planned sample size was 30 patients (to meet the primary endpoint of the radical resection rate).
- Quality of Life
- Quality of life was evaluated using the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) Scale (version 4). Assessments included the lung cancer subscale (LCS), physical well‐being, social (functional and emotional) well‐being, and the Lung Cancer Symptom Scale. Patients completed questionnaires as part of the FACT‐L Scale before the study began (day 1), in the middle of the treatment period (day 29), and after treatment with erlotinib (day 56). The trial outcome index was evaluated using physical and functional well‐being and LCS subscales.
- Gene Mutation Analysis by Next‐Generation Sequencing
- Eight patients with adequate formalin‐fixed, paraffin‐embedded (FFPE) tumor sections, both prior to commencing erlotinib treatment and after surgery, underwent NGS analysis. In total, 52 genes were screened on the Illumina NextSeq500 sequencing platform (Illumina, Inc., San Diego, CA); seven mutated genes related to NSCLC therapy, and 45 genes related to other cancer treatments. In all samples, more than 97% of regions were covered by >500× with mean depth over 1,200×.
- Tissue DNA Extraction
- DNA was extracted and isolated with the QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA concentration was measured using Qubit dsDNA assay (Thermo Fisher Scientific, Waltham, MA).
- Next‐Generation Sequencing Library Preparation
- Extracted tumor DNA was sheared using the Covaris M220 instrument (Covaris Inc., Woburn, MA), followed by end repair, phosphorylation, and adaptor ligation. Fragments sized 200–400 bp were selected using Agencourt AMPure beads (Beckman Coulter, Brea, CA) followed by hybridization with capture probes baits, hybrid selection with magnetic beads, and PCR amplification. A bioanalyzer high‐sensitivity DNA assay was performed to assess the quality and size of the fragments, and indexed samples were sequenced on the NextSeq500 sequencer with pair‐end reads.
- Sequencing Data Analysis
- The sequencing data in the FASTQ format were mapped to reference sequence hg19 (Human Genome version 19) using BurlistItemPairs‐Wheeler Aligner 0.7.10. Local alignment optimization, variant calling, and annotation were performed using Genome Analysis Toolkit 3.2, MuTect, and VarScan, respectively. DNA translocation analysis was performed using both Tophat2 and Factera 1.4.3. Gene‐level copy number variation was assessed using a t test statistic after normalizing read depths at each region by total reads number and region size, and correcting GC bias using a LOESS model.
- Panel Design
- The panel consisted of all exons and critical introns of 56 NSCLC‐related genes, spanning 330 kb of human genome. All driver genes and genes associated with targeted therapies (not limited to NSCLC) were included in the panel.
- Statistical Methods
- Overall response rate was defined as the proportion of patients who achieved a complete response or a partial response. Disease‐free survival (DFS) was defined as the time from the date of surgery to tumor recurrence or death from any cause, whichever occurred earlier. PFS was defined as the time from the date of neoadjuvant treatment to the first date of disease progression or death. Overall survival was defined as the time from the date of diagnosis to death from any cause. Efficacy analyses were based on the intention‐to‐treat (ITT) population, which comprised enrolled patients with confirmed EGFR mutation who received at least one dose of erlotinib. Therefore, response rate, radical resection rate, PFS, and overall survival were analyzed in the ITT population, and DFS was analyzed in patients who underwent surgery.
- Continuous variables were summarized by mean, standard deviation, median, minimum and maximum, and binary variables were summarized by frequencies and percentages. Student's t test was used to assess change from baseline in quality of life measures, and an analysis of covariance model was used to assess the mean carcinoembryonic antigen differences between patients who did and did not have surgery. All p values were two‐sided. Median DFS and median PFS were calculated using the Kaplan‐Meier method. SAS 9.2 software (SAS Institute, Cary, NC) was used for statistical analyses.
- Investigator's Analysis
- Active and should be pursued further.
Drug Information
- Drug 1
- Generic/Working Name
Erlotinib
- Trade Name
Tarceva
- Company Name
Roche Pharmaceuticals
- Drug Type
Small molecule
- Drug Class
EGFR
- Dose
150 milligrams (mg) per flat dose
- Route
Oral (po)
- Schedule of Administration
150 mg per day for two 4‐week cycles
Patient Characteristics
- Number of Patients, Male
7
- Number of Patients, Female
12
- Stage
IIIA
- Age
Median (range): 59 (33–74) years
- Number of Prior Systemic Therapies
Median (range): 0 (treatement‐naive)
- Performance Status: ECOG
-
0 —
1 — 19
2 —
3 —
Unknown —
- Other
Smoking/nonsmoking: 7/12
- Cancer Types or Histologic Subtypes
-
Histology (adenocarcinoma), n = 19 (100%)
EGFR mutation (exon 19 deletion), n = 12 (63%)
EGFR mutation (exon 21 L858R mutation), n = 8 (42%)
Primary Assessment Method
- Number of Patients Screened
155
- Number of Patients Enrolled
19
- Number of Patients Evaluable for Toxicity
19
- Number of Patients Evaluated for Efficacy
19
- Evaluation Method
RECIST version 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 8 (42%)
- Response Assessment SD
n = 9 (47%)
- Response Assessment PD
n = 2 (11%)
- Response Assessment OTHER
n = 0 (0%)
- (Median) Duration Assessments PFS
11 months
- (Median) Duration Assessments OS
52 months
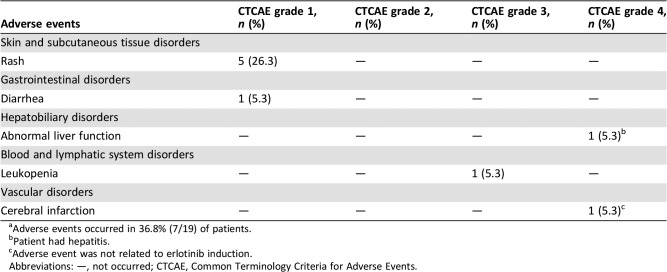
Adverse Events
- See Table 5.
Table 5. Adverse events during erlotinib therapya .
Adverse events occurred in 36.8% (7/19) of patients.
Patient had hepatitis.
Adverse event was not related to erlotinib induction.
Abbreviations: —, not occurred; CTCAE, Common Terminology Criteria for Adverse Events.
Serious Adverse Events
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
Non‐small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases and more than 15% of patients with NSCLC present with locally advanced clinical stage IIIA‐N2 disease [1]. Patients with stage IIIA‐N2 NSCLC whose tumors harbor epidermal growth factor receptor (EGFR) mutations are particularly challenging to treat and have a poor prognosis despite preoperative chemoradiotherapy [2]. Several phase III randomized controlled trials evaluated EGFR tyrosine kinase inhibitor (TKI) therapy in patients with EGFR mutation‐positive NSCLC and demonstrated good efficacy and a favorable tolerability profile for EGFR TKI therapy in these patients [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Erlotinib was one of the first TKIs approved for the initial treatment of EGFR mutation‐positive NSCLC. In this setting, first‐line erlotinib therapy demonstrated superior efficacy over standard chemotherapy, with a more favorable tolerability profile, without the life‐threatening toxicities associated with chemotherapies [11], [13], [14]. However, there is no consensus on the optimal treatment strategy for patients with stage IIIA‐N2 NSCLC, including those with EGFR mutation‐positive disease. The efficacy and tolerability of preoperative EGFR TKI therapy, although promising, requires further research. Therefore, this clinical trial aimed to evaluate whether erlotinib, as a neoadjuvant therapy, improves operability and overall survival (OS) in patients with stage IIIA‐N2 EGFR mutation‐positive NSCLC.
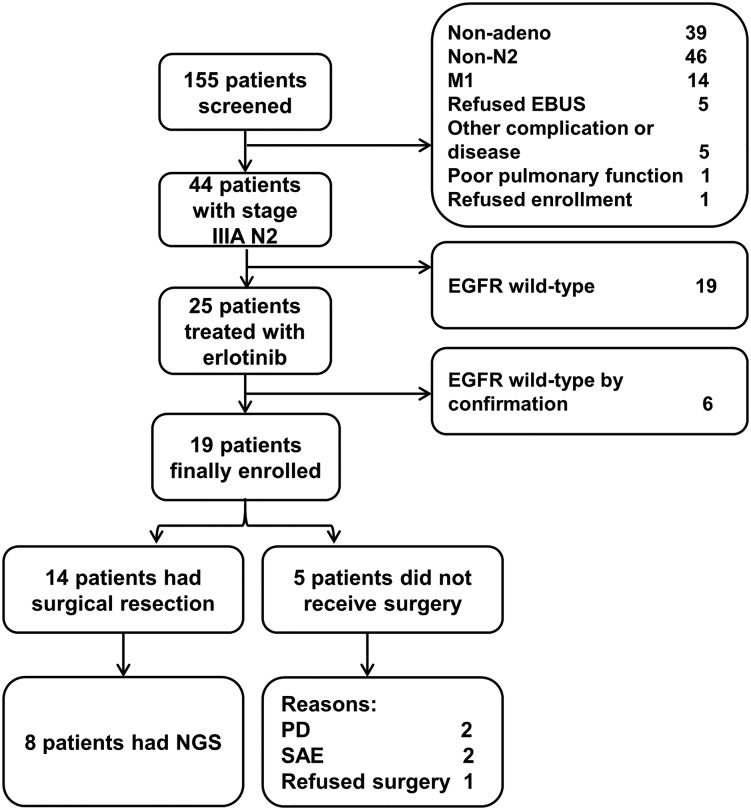
In total, 155 patients were screened from July 2011 to June 2014; 44 were diagnosed with stage IIIA‐N2 NSCLC. Of these 44 patients, 25 patients with EGFR mutation‐positive disease were enrolled. Next‐generation sequencing (NGS) was repeated during the 3‐year recruitment period, during which four patients were found to be negative for EGFR mutation (amplification‐refractory mutation system polymerase chain reaction sequencing) and removed from the study. Further NGS analysis reduced our sample to 19 patients with activating EGFR mutations eligible for inclusion in the study (Fig. 1). Most patients (73.7%; 14/19) underwent surgical resection. Baseline demographic and clinical characteristics are shown in Table 1.
Table 1. Baseline demographic and clinical characteristics.
One patient had exon 19 deletion and exon 21 L858R point mutation.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ITT, intention‐to‐treat.
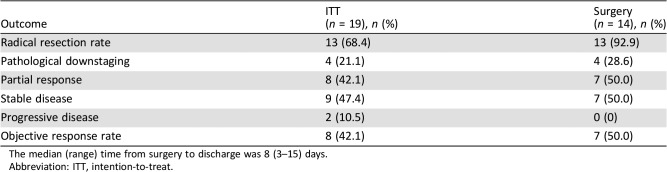
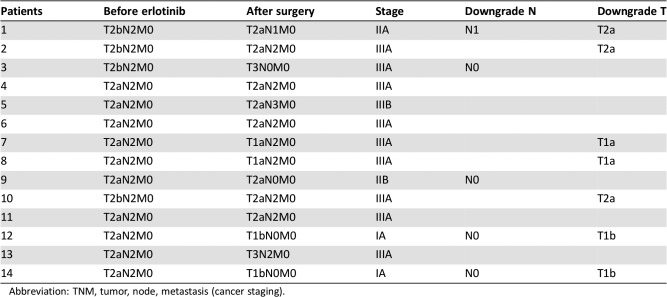
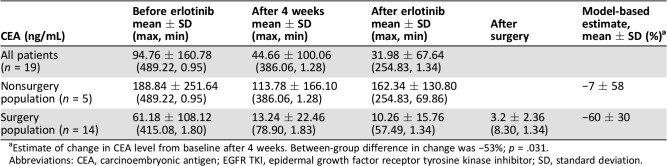
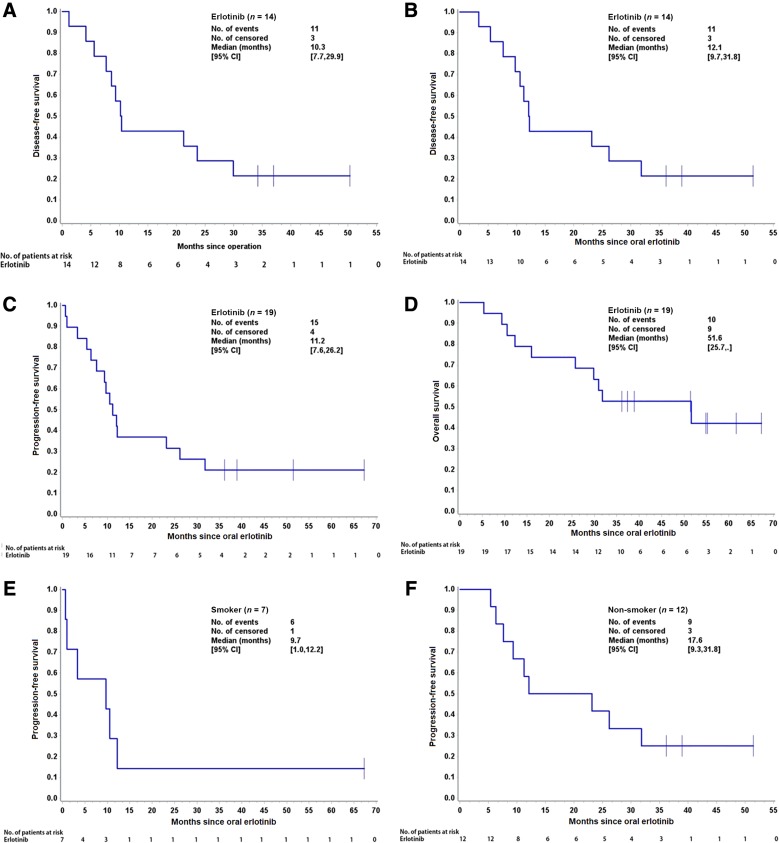
The tumor was resectable in 14 of 19 patients who received neoadjuvant erlotinib, and the radical resection rate in the overall population was 68.4% (13/19; Table 2). Among surgical patients, 35.7% of patients (5/14) had pathological downstaging from N2 to N1/N0, and 50% (7/14) experienced a reduction in tumor size (Table 3). The disease control rate was 89.5% (17/19) after neoadjuvant therapy. A waterfall plot of response to erlotinib neoadjuvant therapy is shown in Figure 2. There was a greater change from baseline in carcinoembryonic antigen level among patients who underwent surgery versus those who did not (Table 4). At the end of follow‐up, most patients received platinum‐based adjuvant chemotherapy (maximum six cycles, mean three cycles); no patients received postoperative radiotherapy. The Kaplan‐Meier curves of disease‐free survival, progression‐free survival (PFS), and OS are shown in Figure 3.
Table 2. Response and radical resection rates.
The median (range) time from surgery to discharge was 8 (3–15) days.
Abbreviation: ITT, intention‐to‐treat.
Table 3. TNM stage list for patients who underwent surgical resection.
Abbreviation: TNM, tumor, node, metastasis (cancer staging).
Figure 2.
Patient flowchart.
Abbreviations: EBUS, endobronchial ultrasound; EGFR, epidermal growth factor receptor; NGS, next‐generation sequencing; PD, progressive disease; SAE, serious adverse event.
Table 4. Trend of serum CEA level before and after neoadjuvant erlotinib treatment.
Estimate of change in CEA level from baseline after 4 weeks. Between‐group difference in change was −53%; p = .031.
Abbreviations: CEA, carcinoembryonic antigen; EGFR TKI, epidermal growth factor receptor tyrosine kinase inhibitor; SD, standard deviation.
Figure 3.
Kaplan‐Meier curves of disease‐free survival (DFS), progression‐free survival (PFS), and overall survival (OS). (A): DFS for 14 patients who underwent surgery (calculated from the date of oral EGFR tyrosine kinase inhibitor to the first date of disease progression). (B): The DFS time of 14 patients who had surgery (calculated from the date of operation to the first date of disease progression). (C): PFS for all 19 patients. (D): OS for all 19 patients. (E): PFS for patients who were smokers. (F): PFS for patients who were nonsmokers.
Abbreviation: CI, confidence interval.
To date, the selection of an optimal treatment regimen for patients with stage IIIA‐N2 NSCLC remains controversial, and there is no standard of care for these patients. Our results suggest that neoadjuvant erlotinib improved the likelihood of surgery and was associated with a good resection rate as shown by the high rate of prospective downstaging in patients with stage IIIA‐N2 NSCLC. In a study by Noh et al., 52.3% of patients with stage IIIA‐N2 NSCLC experienced progressive disease with concurrent chemoradiotherapy, and 7 of 65 patients died from pulmonary radiation therapy‐related adverse events (AEs). In this study, the authors reported a resection rate of 86.4% (456/528) among patients judged as medically operable [15]. Dy et al. found that chemotherapy achieved a relatively positive induction response and a radical resection rate of 94% among patients with early‐stage IB to IIIA NSCLC who underwent surgery [16]. A small study of patients with resectable IIIA‐N2 NSCLC that compared erlotinib versus gemcitabine/carboplatin as neoadjuvant therapy reported response rates of 58.3% versus 25.0% (p = .18), and 54.2% (13/24) patients underwent surgical resection [17]. Compared with these studies, our study demonstrated a strong disease control rate (89.5%) and downstage tumor rate (21.1%). Of note, among the 14 patients who underwent surgery, all but one (93%, 13/14) underwent radical resection. Importantly, patients achieved a median PFS of 11.2 months and median OS of 51.6 months. Taken together, these findings support the use of erlotinib prior to surgery to improve the radical resection rate and long‐term survival of patients with IIIA‐N2 NSCLC.
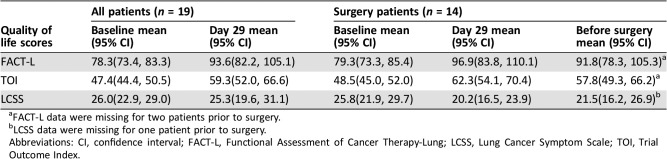
Erlotinib as neoadjuvant therapy was well tolerated. AEs occurred in 36.8% (7/19) of patients (Table 5). No patients died from treatment‐related AEs, and only one patient had a grade 4 AE; these findings are comparable with those of a previous study [13]. A comparison of Functional Assessment of Cancer Therapy‐Lung and Lung Cancer Symptom Scale scores before and after erlotinib treatment demonstrated improved quality of life after treatment with neoadjuvant erlotinib (Table 6).
Table 6. Quality of life in all patients and in patients who underwent surgery.
FACT‐L data were missing for two patients prior to surgery.
LCSS data were missing for one patient prior to surgery.
Abbreviations: CI, confidence interval; FACT‐L, Functional Assessment of Cancer Therapy‐Lung; LCSS, Lung Cancer Symptom Scale; TOI, Trial Outcome Index.
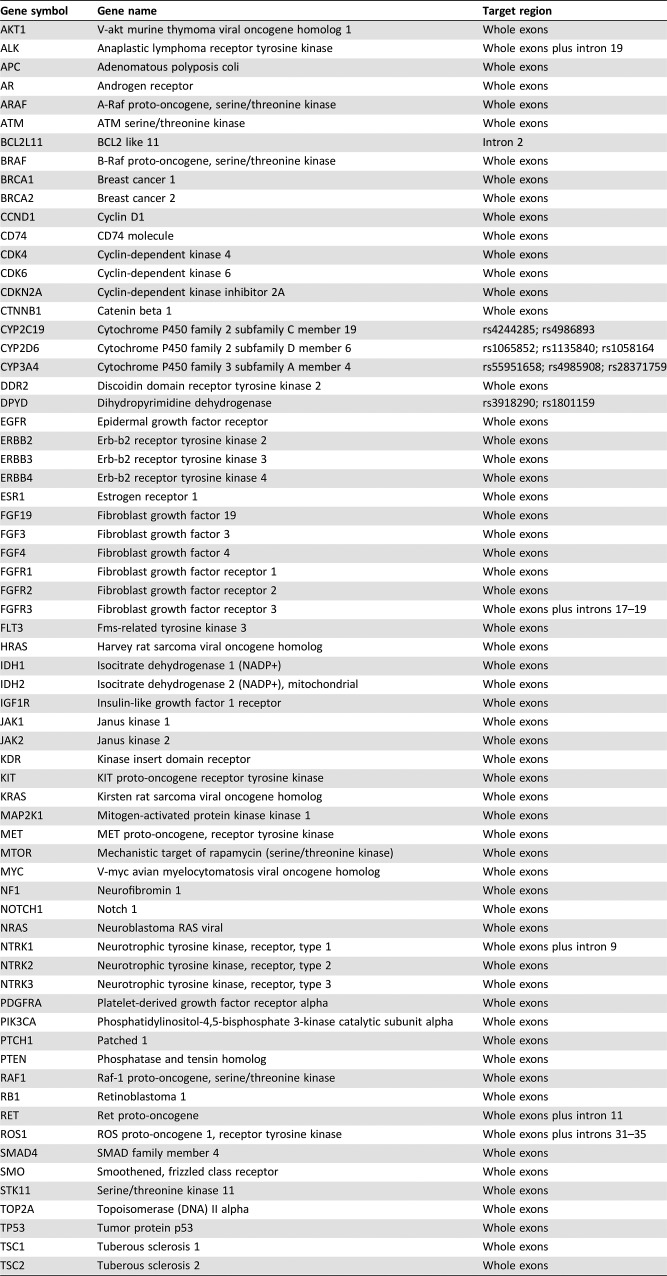
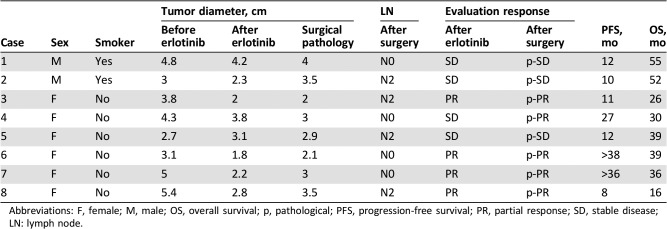
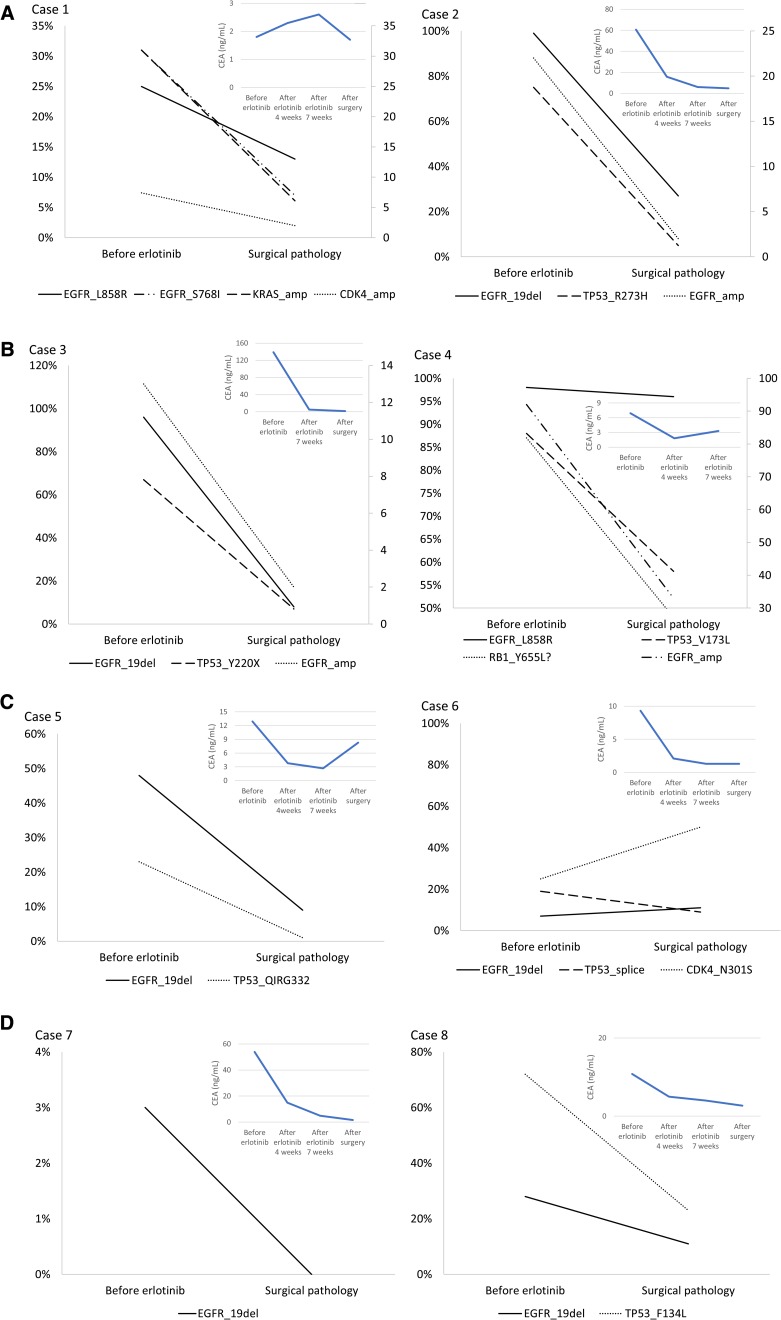
Adequate tissue samples from before neoadjuvant therapy and after surgery were available for eight patients only. Tumor samples for these patients were analyzed for 68 genes by NGS (Tables 7 and 8 and Fig. 4). Patients who benefited most from neoadjuvant erlotinib therapy (cases 6 and 7), with PFS lasting over 36 months, had no TP53 mutation or very low TP53 abundance. Conversely, the patient with the greatest TP53 abundance (case 8) had limited benefits in PFS (8 months) and OS (16 months). These findings indicate that additional biomarkers may play an important role in predicting response to TKI therapy in the neoadjuvant setting. These results are consistent with, and supportive of, previous findings [18], suggesting that TP53 gene mutation is independently associated with shorter OS in NSCLC.
Table 7. List of 68 genes in next‐generation sequencing analysis.
Table 8. Clinical information of next‐generation sequencing analysis.
Abbreviations: F, female; M, male; OS, overall survival; p, pathological; PFS, progression‐free survival; PR, partial response; SD, stable disease; LN: lymph node.
Figure 4.
Next‐generation sequencing analysis of mutation abundance and CEA level for eight cases (A)–(D).
Abbreviations: CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor.
We acknowledge that the small sample size of our study represents a major limitation. We recruited a specific subset of patients with NSCLC who, in addition to having stage IIIA‐N2 disease, were positive for activating EGFR mutations. Our center is one of the largest for clinical lung cancer research in China, yet we encountered challenges with recruiting patients. Nevertheless, we believe that the findings from this study raise important clinical questions and will be helpful for informing larger controlled trials in the future.
In our study, neoadjuvant erlotinib was well tolerated and improved the rate of radical resection in patients with stage IIIA‐N2 EGFR mutation‐positive NSCLC. NGS analysis allowed us to identify markers that may help predict the clinical course of IIIA‐N2 NSCLC. If of predictive value, markers of TP53 abundance could be used to guide preoperative application of TKIs. Given our findings and those of other small studies, we look forward to large‐scale randomized controlled trials investigating the role of TKIs in perioperative therapy, combining neoadjuvant and adjuvant treatments to enhance personalized therapy for patients in this precision medicine era.
Figures and Tables
Acknowledgments
The authors thank all patients who participated in this trial and their families, as well as the investigators, study coordinators, operation staff, and the whole project team who worked on this study. We also thank Michelle Belanger, M.D., of Edanz Medical Writing for medical writing assistance, funded by Shanghai Roche Pharmaceuticals Ltd.
Footnotes
ClinicalTrials.gov Identifier: NCT01217619
Sponsor(s): Shanghai Roche Pharmaceuticals Limited, China Leading Talent Project Funding 2013, Shanghai Health and Family Plan Commission Research Projects, 201440032
Principal Investigator: Baohui Han
IRB Approved: Yes
Disclosures
Baohui Han: Boehringer Ingelheim (C/A), AstraZeneca (speaker's bureau). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.National Cancer Institute, National Institutes of Health . Surveillance, Epidemiology and End Results (SEER) [database]. Available at https://seer.cancer.gov/about/.
- 2.Ahn HK, Choi YL, Han JH et al. Epidermal growth factor receptor mutation and treatment outcome of mediastinoscopic N2 positive non‐small cell lung cancer patients treated with neoadjuvant chemoradiotherapy followed by surgery. Lung Cancer 2013;79:300–306. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Feng J, Zhou C et al. Quality of life (QoL) analyses from OPTIMAL (CTONG‐0802), a phase III, randomised, open‐label study of first‐line erlotinib versus chemotherapy in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (NSCLC). Ann Oncol 2013;24:1615–1622. [DOI] [PubMed] [Google Scholar]
- 4.Costa C, Molina MA, Drozdowskyj A et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR‐mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001–2010. [DOI] [PubMed] [Google Scholar]
- 5.Fukuoka M, Wu YL, Thongprasert S et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866–2874. [DOI] [PubMed] [Google Scholar]
- 6.Han JY, Park K, Kim SW et al. First‐SIGNAL: First‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–1128. [DOI] [PubMed] [Google Scholar]
- 7.Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. New Engl J Med 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T, Morita S, Yatabe Y et al.; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 9.Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 10.Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Zhou C, Liam CK et al. First‐line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer: Analyses from the phase III, randomized, open‐label, ENSURE study. Ann Oncol 2015;26:1883–1889. [DOI] [PubMed] [Google Scholar]
- 12.Yang JJ, Zhou Q, Yan HH et al. A randomized controlled trial of erlotinib versus gefitinib in advanced non‐small‐cell lung cancer harboring EGFR mutations (CTONG0901). J Thorac Oncol 2015;(suppl 2)10:16.13A. [Google Scholar]
- 13.Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 15.Noh JM, Ahn YC, Lee H et al. Definitive bimodality concurrent chemoradiotherapy in patients with inoperable N2‐positive stage IIIA non‐small cell lung cancer. Cancer Res Treat 2015;47:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dy GK, Bogner PN, Tan W et al. Phase II study of perioperative chemotherapy with cisplatin and pemetrexed in non‐small‐cell lung cancer. J Thorac Oncol 2014;9:222–230. [DOI] [PubMed] [Google Scholar]
- 17.Zhong W, Yang X, Yan H et al. Phase II study of biomarker‐guided neoadjuvant treatment strategy for IIIA‐N2 non‐small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol 2015;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina‐Vila MA, Bertran‐Alamillo J, Gascó A et al. Nondisruptive p53 mutations are associated with shorter survival in patients with advanced non‐small cell lung cancer. Clin Cancer Res 2014;20:4647–4659. [DOI] [PubMed] [Google Scholar]