This article reports the results of an open label phase I clinical study that assessed the safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of TRC105 when given concurrently with axitinib to adult patients with metastatic renal cell carcinoma who progressed following prior treatment with at least one VEGFR TKI.
Keywords: Endoglin, CD105, TRC105, Axitinib, Renal cell carcinoma
Abstract
Background.
TRC105 is an IgG1 endoglin monoclonal antibody that potentiates VEGF inhibitors in preclinical models. We assessed safety, pharmacokinetics, and antitumor activity of TRC105 in combination with axitinib in patients with metastatic renal cell carcinoma (mRCC).
Subjects, Materials, and Methods.
Heavily pretreated mRCC patients were treated with TRC105 weekly (8 mg/kg and then 10 mg/kg) in combination with axitinib (initially at 5 mg b.i.d. and then escalated per patient tolerance to a maximum of 10 mg b.i.d.) until disease progression or unacceptable toxicity using a standard 3 + 3 phase I design.
Results.
Eighteen patients (median number of prior therapies = 3) were treated. TRC105 dose escalation proceeded to 10 mg/kg weekly without dose‐limiting toxicity. Adverse event characteristics of each drug were not increased in frequency or severity when the two drugs were administered concurrently. TRC105 and axitinib demonstrated preliminary evidence of activity, including partial responses (PR) by RECIST in 29% of patients, and median progression‐free survival (11.3 months). None of the patients with PR had PR to prior first‐line treatment. Lower baseline levels of osteopontin and higher baseline levels of TGF‐β receptor 3 correlated with overall response rate.
Conclusion.
TRC105 at 8 and 10 mg/kg weekly was well tolerated in combination with axitinib, with encouraging evidence of activity in patients with mRCC. A multicenter, randomized phase II trial of TRC105 and axitinib has recently completed enrollment (NCT01806064).
Implications for Practice.
TRC105 is a monoclonal antibody to endoglin (CD105), a receptor densely expressed on proliferating endothelial cells and also on renal cancer stem cells that is implicated as a mediator of resistance to inhibitors of the VEGF pathway. In this Phase I trial, TRC105 combined safely with axitinib at the recommended single agent doses of each drug in patients with renal cell carcinoma. The combination demonstrated durable activity in a VEGF inhibitor‐refractory population and modulated several angiogenic biomarkers. A randomized Phase II trial testing TRC105 in combination with axitinib in clear cell renal cell carcinoma has completed accrual.
Introduction
Angiogenesis is a complex process that is regulated by multiple pathways [1]. Approved antiangiogenic drugs that primarily target the VEGF pathway have revolutionized the treatment of renal cell carcinoma (RCC). These agents include axitinib, sorafenib, sunitinib, pazopanib, and cabozantinib [2]. Inhibition of complementary, non‐VEGF driven angiogenic pathways is an alternative strategy that may improve antitumor activity and limit resistance to VEGF inhibitors.
Axitinib was approved in a trial of patients who failed one systemic treatment for advanced RCC [3]. Although there was an improvement in progression‐free survival (PFS) versus sorafenib (median PFS [mPFS] of 6.7 months with axitinib vs. 4.7 months with sorafenib; hazard ratio [HR] = 0.67), many patients had not received prior therapy directed at the VEGF receptor. The results in patients who had failed prior sunitinib were more modest (mPFS of 4.8 with axitinib vs. 3.4 months with sorafenib; HR = 0.74). It is clear that further improvement in outcomes will depend upon addressing targets other than those in the VEGF pathway. Beyond the VEGF axis, angiogenesis is dependent upon multiple growth factors and stromal elements [4]. Upregulated non‐VEGF pathways may include the IL‐6, TGF‐β, PDGF, bFGF, c‐Met, and angiopoietin axes. In particular, the TGF‐β axis, including the soluble bone morphogenic proteins (BMP), and the endoglin and ALK1 receptors, may provide an escape pathway for tumor angiogenesis. Notably, a recent study that examined putative escape pathways following VEGF inhibition indicated the endoglin ligand TGF‐β was the most significantly upregulated angiogenic factor examined [5]. Genetic knock‐down and knock‐out models also implicate endoglin as a pathway of VEGF resistance: The introduction of the endoglin heterozygous genotype resensitizes spontaneous tumors to large and small molecule VEGF inhibitors, as does the conditional deletion of endoglin in endothelium [6]. Collectively, these data suggest that targeting non‐VEGF angiogenic factors in general, and TGF‐β family members in particular, may complement the activity of VEGF inhibitors.
Endoglin (CD105) is a homodimeric TGF‐β coreceptor expressed on proliferating vascular endothelium in solid tumors [7], [8]. Endoglin is selectively expressed at high density on proliferating endothelial cells and is up‐regulated by hypoxia through the induction of hypoxia‐inducible factor‐1‐α [8], [9]. Endoglin is essential for normal vascular development [10], and loss of endoglin expression is associated with the Osler‐Weber‐Rendu syndrome, a disease characterized by ectatic blood vessel formation that is associated with improved survival for a composite of patients with breast, prostate, colorectal, and lung cancer, that was most notable in breast cancer patients, suggesting that targeting endoglin may have beneficial clinical effects [11]. In patients with solid tumors, including renal cell carcinoma, high tumor microvessel density as assessed by endoglin immunohistochemistry is correlated with poor prognosis [12], [13]. Further endoglin expression has been demonstrated on human renal cancer stem cells isolated from nephrectomy specimens, and is associated with a tumor initiating function [14].
TRC105 (carotuximab; TRACON Pharmaceuticals, San Diego, CA) is a chimeric IgG1 antibody that binds human endoglin with high avidity, competitively inhibits BMP ligand binding required for endothelial signal transduction, induces antibody‐dependent cellular cytotoxicity of proliferating human vascular endothelial cells and endoglin‐expressing tumor cells, and inhibits angiogenesis in response to VEGF and fibroblast growth factor [15]. TRC105 potentiates VEGF inhibitors in preclinical models of angiogenesis and in tumor xenografts, and was well tolerated at 10 mg/kg every week and 15 mg/kg every 2 weeks as a single agent in a phase I trial, with a safety profile that was distinct from that of VEGF inhibitors, that included the lack of hypertension and proteinuria [16].
Here we report the results of an open label phase I clinical study that assessed the safety, tolerability, pharmacokinetics, and pharmacodynamic and antitumor activity of TRC105 when given concurrently with axitinib to adult patients with metastatic renal cell carcinoma who progressed following prior treatment with at least one VEGFR tyrosine kinase inhibitor (TKI).
Subjects, Materials, and Methods
Patient Eligibility
Patients with histologically proven, metastatic, previously treated RCC were eligible. Further inclusion criteria were an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function as demonstrated by an absolute neutrophil count ≥1,500 cells/μL, hemoglobin ≥9 g/dL, platelets ≥100,000/μL, prothrombin time or international normalized ratio within normal limits, creatinine ≤1.5 times the upper limit of normal (ULN), bilirubin ≤1.5 mg/dL, and aspartate and alanine transaminases ≤2.5 times the ULN (or ≤5 times the ULN in patients with liver metastases). Exclusion criteria included untreated central nervous system metastases, thromboembolic disease, clinically significant ascites or pleural effusions, uncontrolled hypertension, a history of hemorrhage, or unhealed surgical wounds within 30 days of study entry. All patients signed an institutional review board‐approved informed consent form prior to undertaking study‐related procedures. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and all applicable local regulatory requirements and laws.
Study Design and Treatments
This was a multicenter, open‐label, nonrandomized, phase Ib, dose‐finding study of TRC105 in combination with axitinib in patients with metastatic RCC who progressed following prior treatment with at least one VEGFR TKI (NCT01806064). TRC105 dose was escalated in two serial cohorts of patients with an initial axitinib dose of 5 mg p.o. b.i.d. using a standard 3 + 3 design whereby if one of the initial three patients in a cohort developed dose‐limiting toxicity (DLT), the cohort was expanded to six patients. Dose‐limiting toxicity was defined as any grade ≥3 hematologic or nonhematologic adverse event related to TRC105 occurring within the first 4 weeks of dosing. Intrapatient dose escalation was not permitted. Patients were allowed to dose reduce and continue treatment for adverse events that resolved to grade 1 or baseline, and were allowed to dose escalate axitinib per its commercial dosing guidelines to 7 mg twice daily starting with week 5 and 10 mg twice daily starting with week 9, in the absence of significant toxicity. Dose expansion was planned at the top dose level to a maximum of 15 patients.
TRC105 was administered weekly, with the initial dose divided over 2 days, by which 3 mg/kg was given on Cycle 1 Day 1 and the balance of the first weekly dose (5 or 7 mg/kg) was given on Cycle 1 Day 4. Premedication included acetaminophen 650 mg, diphenhydramine 50 mg (or similar H1 receptor antagonist), famotidine 20 mg (or similar H2 receptor antagonist), and methylprednisolone 50 mg. Methylprednisolone was tapered and discontinued within the first weeks of dosing.
Safety Assessments
Safety was evaluated at baseline, at regular intervals during treatment, and for 28 days after completing study therapy and included physical examination, hematology, serum chemistry, urinalysis, and adverse events graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.3.
Pharmacokinetics and Immunogenicity
Serum samples for assessment of TRC105 trough concentrations were collected during the 2nd month of dosing. TRC105 concentrations were determined using a validated enzyme‐linked immunosorbent assay (ELISA) with a limit of quantitation of 200 ng/mL.
Serum for assessment of human anti‐drug antibody (ADA) formation to TRC105 was collected prior to dosing, 4 weeks thereafter, and at end of study and approximately 4 weeks following the last dose of TRC105. ADA titers were determined by validated ELISA.
Biomarker Assessment
Double spun, platelet‐poor plasma‐EDTA samples were collected from patients at baseline and on‐treatment. After processing, samples were immediately frozen, shipped to Duke University Medical Center, and stored at −80 °C until use. All biomarkers were measured using the CiraScan multiplex platform (Aushon Biosystems, Inc., Billerica, MA) except for BMP‐9 and TGF‐β R3 (R&D Systems, Inc., Minneapolis, MN), as described previously [17].
Evaluation of Tumor Response
Tumor responses were evaluated using computed tomography (CT) or magnetic resonance imaging per RECIST 1.1 [18] and modified Choi criteria [19]. Evaluations were performed at 2‐month intervals or earlier if disease progression was suspected.
Statistical Analysis
The safety population included all patients who received at least a portion of the initial TRC105 infusion. The evaluable population for determination of response included all patients with a baseline and a follow‐up radiographic assessment for response at designated time points (e.g., 2 months and 4 months). Descriptive statistics (means, medians, standard deviations, and ranges for continuous data and percentages for categorical data) were used to summarize patient characteristics, treatment administration, safety, efficacy, and pharmacokinetic parameters. Efficacy, including response, was evaluated by RECIST 1.1. PFS was defined as the time from date of informed consent to date of progression by RECIST 1.1 or death, and median PFS was estimated by the method of Kaplan and Meier [20]. For biomarker analyses, univariate Cox proportional hazards analyses were performed for each biomarker for PFS. Differential biomarker expression between responders (patients with partial responses) and nonresponders (patients with stable disease or progressive disease) were compared by Wilcoxon signed rank test.
Results
Maximum Tolerated Dose, Dose‐Limiting Toxicity, and Disposition
Between September 2013 and July 2014, 18 patients with metastatic RCC (Table 1) were enrolled at five sites in the U.S. and treated with escalating weekly doses of TRC105 at 8 or 10 mg/kg together with axitinib. Dose escalation proceeded without the development of DLT, such that 3 patients were treated with 8 mg/kg of TRC105 and 15 patients were then treated with 10 mg/kg of TRC105 weekly.
Table 1. Baseline patient characteristics (n = 18).
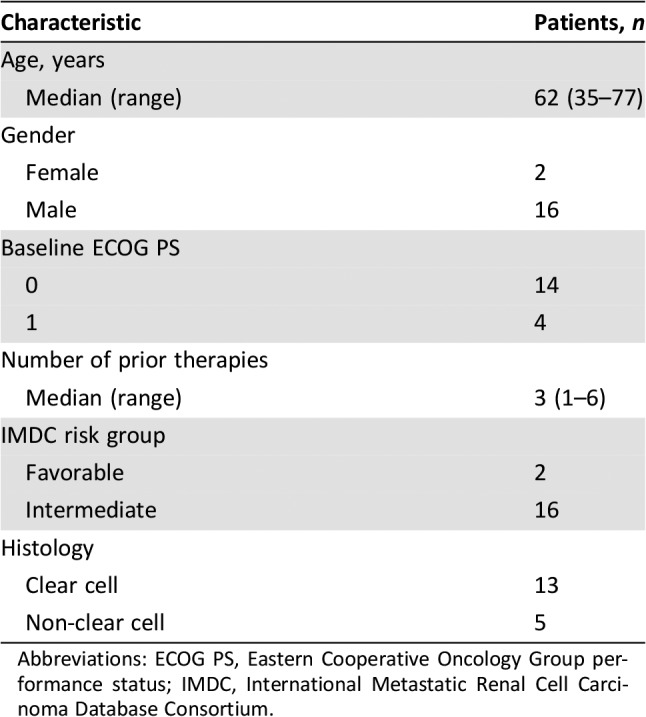
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium.
Twelve patients progressed by RECIST; four patients progressed based on clinical criteria: one due to multiorgan failure related to the rapid growth of hepatic metastases, one due to ascites requiring large‐volume paracentesis, one due to growth of bone metastases, and one for fatigue and progressive disease by CT scan that did not meet RECIST‐defined progression. One patient discontinued for a serious adverse event of suspected event of grade 2 delayed infusion reaction, and one patient withdrew consent within the initial week of dosing.
Safety and Tolerability
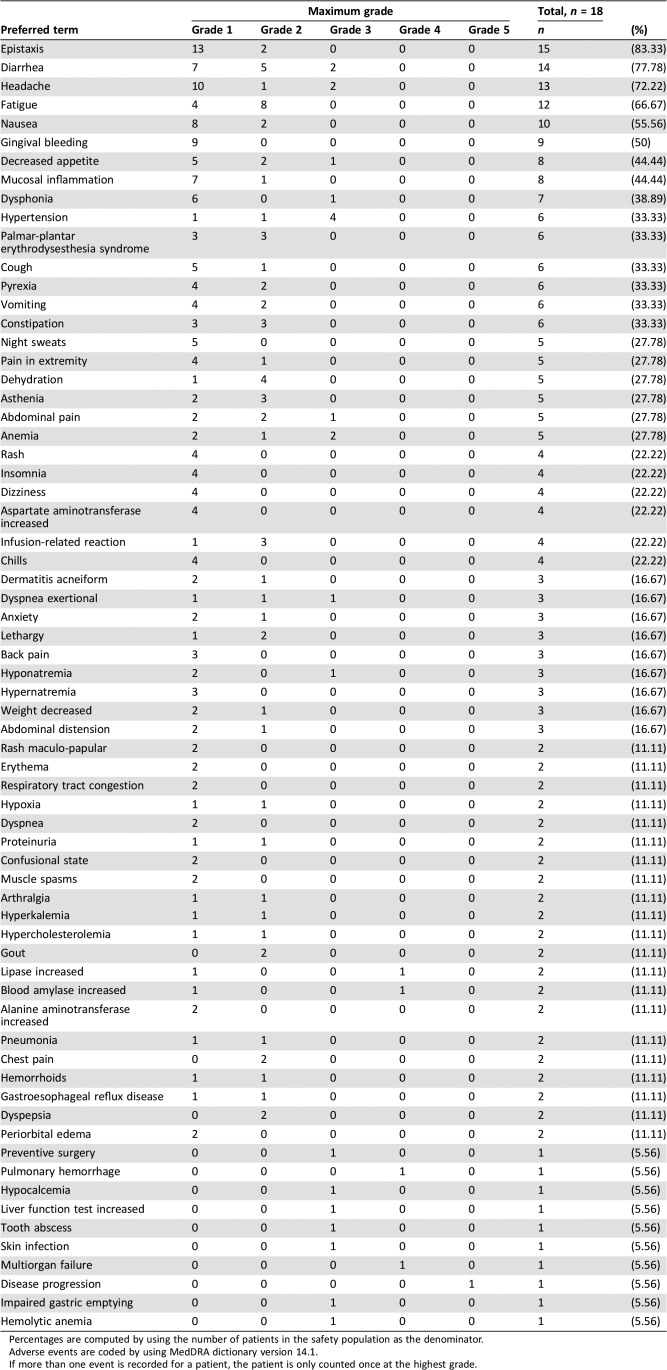
A total of 18 patients received TRC105 and axitinib across the two cohorts without the development of DLT, and 15 patients received the two drugs at their recommended single‐agent doses. The two drugs were well tolerated together and adverse events were similar regardless of the TRC105 dose level (Table 2). Most adverse events were graded as 1 or 2, and grade 4 and 5 suspected adverse reactions were not observed. Recurrent grade 3 suspected adverse reactions were limited to anemia (the dose‐limiting toxicity of TRC105 as a single agent; two patients) and first‐dose headache, a known toxicity of TRC105 treatment (two patients). Anemia prompted transfusion of packed red blood cells in four patients. There were no TRC105 dose reductions. Consistent with other studies of TRC105, headache generally started the evening following initial dosing of both agents, was reduced in frequency and intensity with continued treatment, and was treated with 5‐hydroxytryptophan antagonists, nonsteroidal anti‐inflammatory drugs, and occasionally with narcotics.
Table 2. Most common (n > 1) and all grade 3 and above adverse events.
Percentages are computed by using the number of patients in the safety population as the denominator.
Adverse events are coded by using MedDRA dictionary version 14.1.
If more than one event is recorded for a patient, the patient is only counted once at the highest grade.
At least one sign of the triad of epistaxis, gingival bleeding and telangiectasia, reflecting vascular ectasia characteristic of the Osler‐Weber‐Rendu syndrome, was observed in 16 of the 17 patients who remained on study beyond the 1st week, with onset generally within the initial 4 weeks of dosing.
Two patients experienced serious suspected adverse events. A 53‐year‐old man with a left leg deep venous thrombosis treated with enoxaparin sodium developed worsening left lower leg deep venous thrombosis during the 4th month of dosing that required adjustment of his dose of enoxaprin sodium to improve compliance. A 77‐year‐old man developed symptoms of weakness, fatigue, hiccups, productive cough, fever, and chills 2 days following the TRC105 infusion that responded to hydration and antibiotics. Similar symptoms were reported following prior TRC105 infusions and the event was considered a grade 2 delayed infusion reaction. Serious adverse events considered unrelated to TRC105 treatment included the following: grade 4 multiorgan failure related to the rapid growth of hepatic metastases, grade 1 fever and subsequent grade 3 abdominal pain related to invasion of the sigmoid colon by retroperitoneal tumor, grade 4 bronchopulmonary hemorrhage following endobronchial biopsy, and grade 2 diarrhea.
Adverse events expected with axitinib included diarrhea (14 patients—grade 1 or 2 in 12 cases and grade 3 in 2 cases), fatigue (13 patients—all grade 1 or 2), dysphonia (7 patients), stomatitis (6 patients—all grade 1 or 2), and palmar plantar erythrodysthesia (6 patients—all grade 1 or 2) and were reported at frequencies similar to studies of axitinib given as a single agent [3]. Axitinib dose reduction below 5 mg twice daily occurred in three patients due to anorexia, diarrhea, palmar plantar erythrodysthesia, fatigue, and/or epistaxis.
Pharmacokinetics and Immunogenicity
TRC105 was measurable above the target concentration shown to maximally inhibit endothelial cell signaling of 25 ug/mL in all patients by the time of 5th dose, with a mean concentration at 10 mg/kg dose level of 84.9 ug/mL. ADAs were detected at the end of study in 1 of 12 patients without evidence of an ADA at baseline.
Antitumor Activity
The combination of TRC105 and axitinib was active, including among patients with advanced refractory cancer who had progressed on prior VEGFR TKI treatment. Seventeen patients who had progressed following prior treatment with a prior VEGFR TKI had measurable disease at baseline and received at least one follow‐up scan and were therefore evaluable for the primary outcome of overall response rate by RECIST. Five of the eighteen patients enrolled had non‐clear cell renal cell carcinoma. Two patients were favorable risk and 16 were intermediate risk by International Metastatic Renal Cell Carcinoma Database Consortium criteria [21].
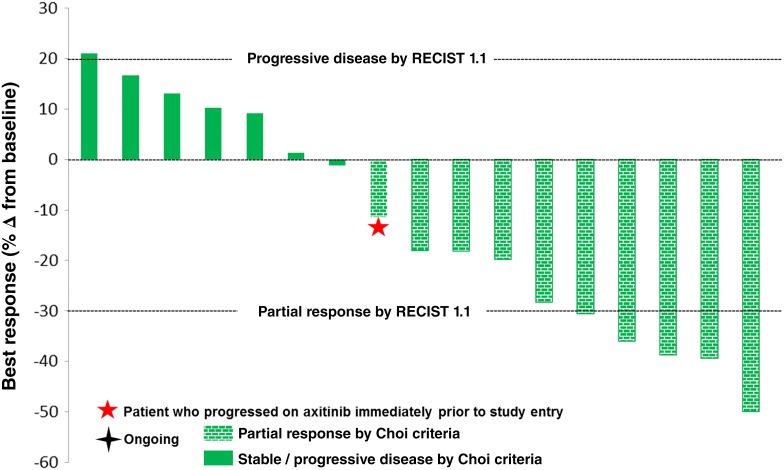
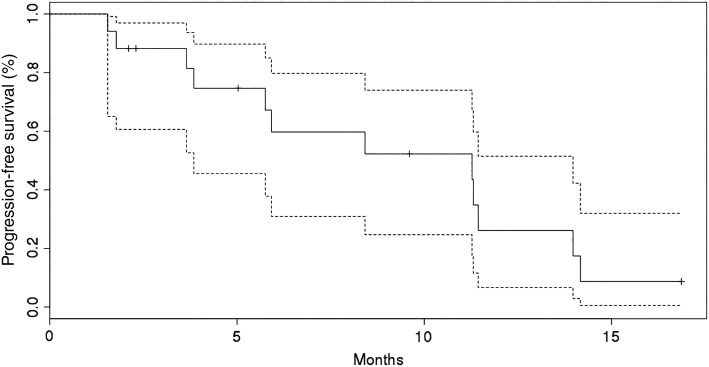
Seventeen patients were evaluable for response by RECIST, of whom ten had a best response of stable disease and five (29%) had a partial response (Fig. 1). Partial responses by RECIST were noted in four patients with clear cell histology and one patient with unclassified histology. None of the five patients with partial response by RECIST responded to initial treatment with sunitinib or pazopanib (Table 3). Partial response by modified Choi criteria (10% or greater reduction in target lesions) was noted in 10 patients. The sole patient treated with prior axitinib immediately prior to study enrollment demonstrated a tumor reduction of 11% in target lesions and remained on study for 6 months prior to progression by RECIST. Two of seven patients who received prior programmed death receptor inhibitor treatment (with nivolumab) and who also progressed on VEGFR TKI treatment had partial responses following treatment with TRC105 and axitinib. Median PFS was 11.3 months in all 18 patients and was 11.3 months in the 13 patients with clear cell histology (Fig. 2).
Figure 1.
Waterfall plot of best response of measurable lesions by RECIST 1.1.
Table 3. Treatment history of patients with a partial response by RECIST 1.1.
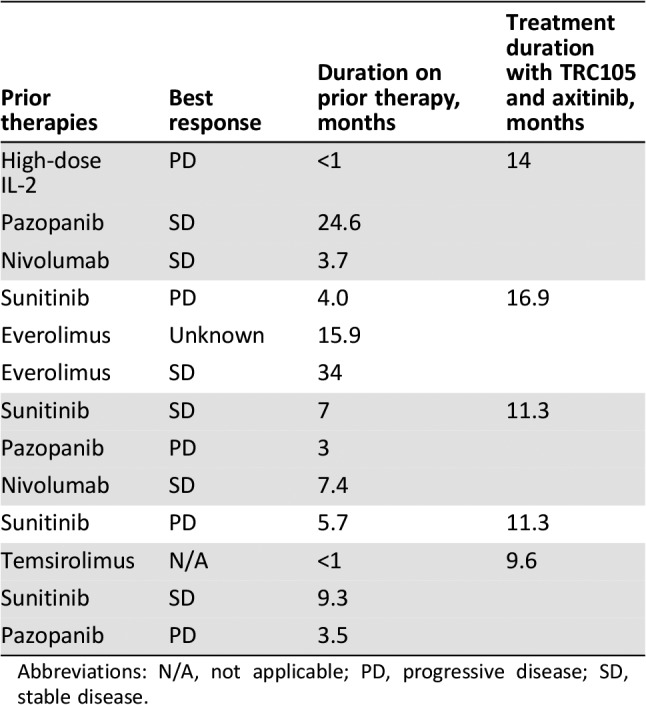
Abbreviations: N/A, not applicable; PD, progressive disease; SD, stable disease.
Figure 2.
Kaplan‐Meier estimates of progression‐free survival including 95% confidence intervals.
Biomarker Assessment
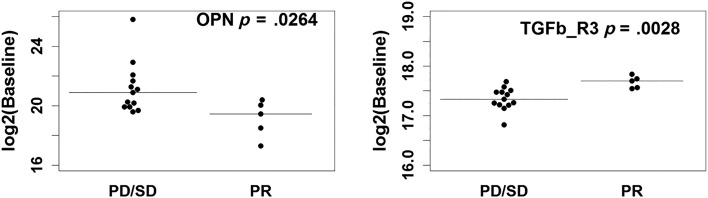
We assessed 22 soluble angiogenic and TGF‐β related biomarkers at baseline and on‐treatment. Baseline biomarkers were associated with both PFS and overall response rate (ORR) to investigate prognostic impact (Tables 4 and 5). Survival analyses indicated higher baseline Ang‐2 and VEGF‐R2 expression were associated with favorable PFS (HR = 0.47, p = .0017 and HR = 0.13, p = .0168, respectively; Fig. 3). Baseline biomarker levels in the five patients who demonstrated partial response by RECIST were compared to nonresponders (stable and progressive disease by RECIST). Lower baseline concentrations of osteopontin and higher baseline concentrations of TGFβ‐R3 were observed in responding patients (Fig. 3).
Table 4. Baseline biomarker level correlation with progression‐free survival.
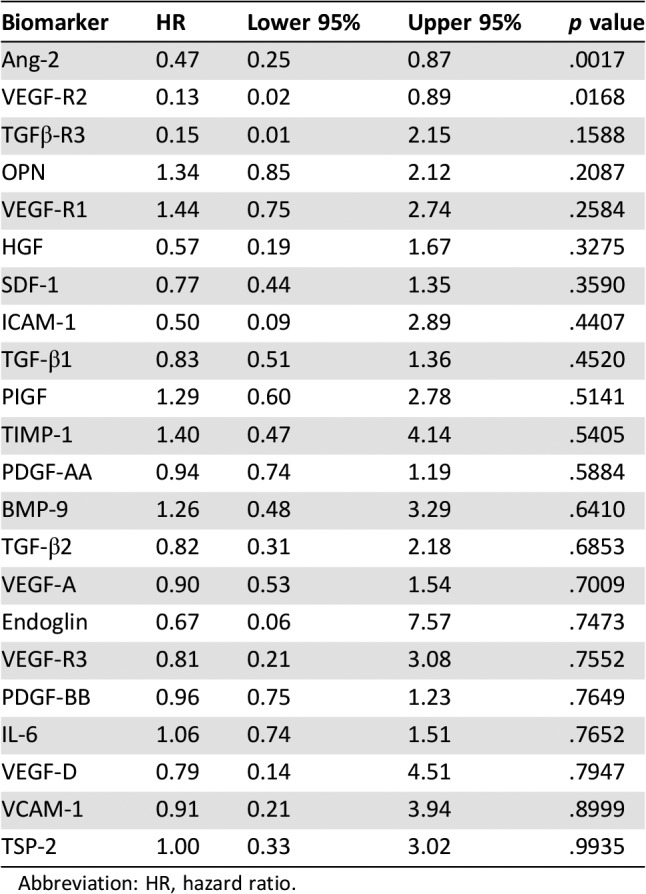
Abbreviation: HR, hazard ratio.
Table 5. Biomarker baseline level correlation with overall response rate by RECIST.
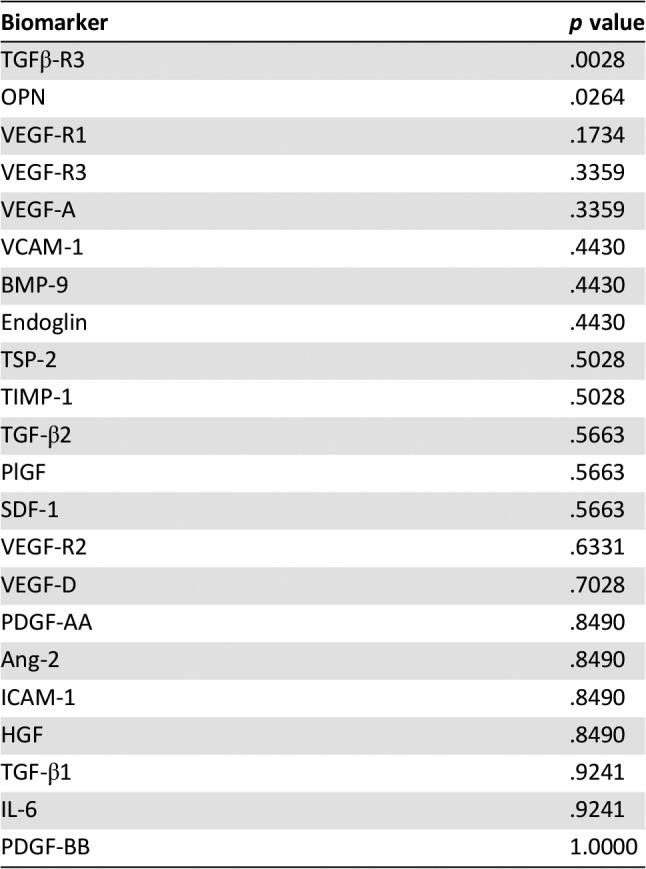
Figure 3.
Baseline expression level of OPN and TGFβ‐R3 between responders (PR by RECIST) and nonresponders (patient with best response of progressive disease or stable disease by RECIST), with median indicated by hash line. Abbreviations: PD, progressive disease; PR, partial response; SD, stable disease.
In addition, changes in these biomarkers were evaluated during treatment. Four weeks following initiation of treatment, soluble endoglin, PlGF, VCAM‐1, and VEGF‐A levels increased after treatment in all patients, whereas Ang‐2, VEGFR2, and VEGFR3 decreased after treatment in all patients (signed rank analysis; p < .0001). However, there was no statistically significant association between PFS and the change in any biomarker between baseline and week 4 of treatment with TRC105 and axitinib (p < .05).
Discussion
The trial of TRC105 with axitinib in patients with renal cell carcinoma was remarkable for the fact that both drugs could be administered at their recommended single‐agent doses without increasing the toxicities of the individual drugs. Notably, the partial response rate by RECIST was higher and PFS was longer than expected with axitinib as single agent.
TRC105, when combined with bevacizumab (bev), also demonstrated activity in patients who had progressed following prior bev therapy. However, a randomized trial of bev and TRC105 in patients with all types of renal cell carcinoma histologies (non‐clear cell: 22%) failed to demonstrate a survival advantage versus single‐agent bevacizumab [22]. Notably, patients in that trial were highly refractory patients who may have progressed following three prior VEGF inhibitors and represent a population of patients in whom bevacizumab is not of proven clinical benefit. In contrast, the fully enrolled randomized phase II trial of axitinib and TRC105 enrolled patients who have failed a single VEGF inhibitor, which is a patient population in whom axitinib has proven clinical activity.
Telangiectasia and associated epistaxis or gingival bleeding were observed routinely in patients dosed with TRC105 and axitinib. These reversible signs and symptoms are expected pharmacologic effects of TRC105 binding to the endoglin receptor to interrupt BMP9 binding and resemble characteristics of the Osler‐Weber‐Rendu syndrome, a syndrome of endoglin heterozygosity associated with superior cancer survival [11]. Epistaxis or gingival bleeding are observed routinely in trials of TRC105 given as a single agent and concurrently with VEGF inhibitors, and provide confirmation that patients have been administered TRC105 doses required to inhibit endoglin function. In contrast, dalantercept, a ligand trap for BMP9 that reported negative data in a trial of clear cell renal cell carcinoma in combination with axitinib, caused epistaxis or gingival bleeding in a minority of patients when dosed with axitinib [23], which may indicate that most patients did not achieve doses of the drug required for pharmacologic inhibition of the endoglin pathway.
Conclusion
Defining populations responsive to the combination of TRC105 and VEGF inhibitors is an area of active research. The combination of TRC105 and VEGF inhibitors has demonstrated preliminary signs of activity in angiosarcoma patients, including durable complete responses, and a phase III trial is enrolling at this time. A robust response rate was also reported in patients with hepatocellular carcinoma treated with TRC105 and sorafenib [24].
Soluble biomarker expression was assessed in these patients at both baseline and during the course of treatment. Two baseline biomarkers, TGFβ‐R3 and osteopontin, correlated with ORR in an exploratory evaluation of 22 soluble markers. Notably, soluble TGFβ‐R3 binds TGF‐β and acts as potent inhibitor of TGF‐β binding to membrane receptors that initiate TGF‐β signal transduction, including the TGF‐β coreceptor endoglin. Importantly, the univariate analyses of biomarker correlations with ORR and PFS in single‐arm studies were exploratory, and the potential prognostic and predictive value of TGFβ‐R3 and osteopontin will be assessed in the ongoing randomized phase II trial of TRC105 and axitinib.
Acknowledgments
We express our appreciation to the patients who participated in this investigational study and to the study staff. This work was presented in part at the following conferences: 2015 GU ASCO Annual Meeting, IKCS 2015, and 2016 ESMO. Trial registry number: NCT01806064. This research was supported by TRACON Pharmaceuticals, Inc., San Diego, CA.
Footnotes
Editor's Note: See the related commentary, “Targeting Endoglin to Treat Metastatic Renal Cell Carcinoma: Lessons from Osler‐Weber‐Rendu Syndrome,” by Andrew W. Hahn et al., on page 143 of this issue.
Author Contributions
Provision of study material or patients: Toni K. Choueiri, M. Dror Michaelson, Edwin M. Posadas, Guru P. Sonpavde, David F. McDermott, Andrew B. Nixon, Yingmiao Liu, Zhenhua Yuan, Ben K. Seon, Meghara Walsh, Manoj A. Jivani, Bonne J. Adams, Charles P. Theuer
Collection and/or assembly of data: Toni K. Choueiri, M. Dror Michaelson, Edwin M. Posadas, Guru P. Sonpavde, David F. McDermott, Andrew B. Nixon, Yingmiao Liu, Zhenhua Yuan, Ben K. Seon, Meghara Walsh, Manoj A. Jivani, Bonne J. Adams, Charles P. Theuer
Data analysis and interpretation: Toni K. Choueiri, M. Dror Michaelson, Edwin M. Posadas, Guru P. Sonpavde, David F. McDermott, Andrew B. Nixon, Yingmiao Liu, Zhenhua Yuan, Manoj A. Jivani, Bonne J. Adams, Charles P. Theuer
Manuscript writing: Toni K. Choueiri, M. Dror Michaelson, Edwin M. Posadas, Guru P. Sonpavde, David F. McDermott, Andrew B. Nixon, Yingmiao Liu, Bonne J. Adams, Charles P. Theuer
Final approval of manuscript: Toni K. Choueiri
Disclosures
Toni K. Choueiri: TRACON Pharmaceuticals, Inc. (RF [institution]); Andrew B. Nixon: TRACON Pharmaceuticals, Inc. (RF [for biomarker analyses]); Ben K. Seon: TRACON Pharmaceuticals, Inc. (IP [inventor of TRC105]); Manoj A. Jivani: TRACON Pharmaceuticals, Inc. (E, OI); Bonne J. Adams: TRACON Pharmaceuticals, Inc. (E, OI); Charles P. Theuer: TRACON Pharmaceuticals, Inc. (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873–887. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med 2017;376:354–366. [DOI] [PubMed] [Google Scholar]
- 3.Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomized phase 3 trial. Lancet 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK. VEGF inhibitors in renal cell carcinoma: Current therapies and future perspectives. Curr Clin Pharmacol 2011;6:164–168. [DOI] [PubMed] [Google Scholar]
- 5.Sennino B, Ishiguro‐Oonuma T, Wei Y et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c‐Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012;2:270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderberg C, Cunha SI, Zhai Z et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J Exp Med 2013;210:563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seon BK, Haba A, Matsuno F et al. Endoglin‐targeted cancer therapy. Curr Drug Deliv 2011;8:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen L, Gordon M, Robert F et al. Endoglin for targeted cancer treatment. Curr Oncol Rep 2014;16:365. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez‐Elsner T, Botella LM, Velasco B et al. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor‐beta pathways. J Biol Chem 2002;277:43799–43808. [DOI] [PubMed] [Google Scholar]
- 10.Li DY, Sorensen LK, Brooke BS et al. Defective angiogenesis in mice lacking endoglin. Science 1999;284:1534–1537. [DOI] [PubMed] [Google Scholar]
- 11.Duarte C, Murray K, Lucas F et al. Improved survival outcomes in cancer patients with hereditary hemorrhagic telangiectasia. Cancer Epi Bio Prev 2014;23:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duff SE, Li C, Garland JM et al. CD105 is important for angiogenesis: evidence and potential applications. FASEB J 2003;17:984–992. [DOI] [PubMed] [Google Scholar]
- 13.Dallas NA, Samuel S, Xia L et al. Endoglin (CD105): A marker of tumor vasculature and potential target for therapy. Clin Cancer Res 2008;14:1931–1937. [DOI] [PubMed] [Google Scholar]
- 14.Bussolati B, Bruno S, Grange C et al. Identification of a tumor‐initiating stem cell population in human renal carcinomas. FASEB J 2008;22:3696–3705. [DOI] [PubMed] [Google Scholar]
- 15.Nolan‐Stevaux O, Zhong W, Culp S et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti‐endoglin antibodies. PLoS One 2012;7:e50920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen L, Hurwitz H, Wong M et al. A phase I first‐in‐human study of TRC105 (anti‐endoglin antibody) in patients with advanced cancer. Clin Cancer Res 2012;18:4820–4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Starr MD, Bulusu A et al. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med 2013;2:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors [in French]. Bull Cancer 2000; 87:881–886. [PubMed] [Google Scholar]
- 19.Choi HC, Charnsangavej C, Faria SC et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clinical Oncol 2007; 25:1753–1759. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–481. [Google Scholar]
- 21.Heng DY, Xie W, Regan MM et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: Results from a large, multicenter study. J Clin Oncol 2009; 27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 22.Dorff TB, Longmate JA, Pal SK et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 2017; 123:4566–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voss MH, Bhatt RS, Plimack ER et al. The DART study: Results from the dose‐escalation and expansion cohorts evaluating the combination of dalantercept plus axitinib in advanced renal cell carcinoma. Clin Cancer Res 2017; 23:3557–3565. [DOI] [PubMed] [Google Scholar]
- 24.Duffy AG, Ma C, Ulahannan SV et al. Phase I and preliminary phase II study of TRC105 in combination with sorafenib in hepatocellular carcinoma. Clin Cancer Res 2017; 23:4633–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]