INI‐1 deficient carcinoma is characterized by loss of the SMARCB1 gene. Standard treatments options are currently unavailable for this rare and aggressive cancer. This report describes the case of a patient with INI‐1 deficient carcinoma and the course of treatment resulting in disease stabilization.
Abstract
Integrase interactor 1 (INI‐1)‐deficient carcinoma is a rare cancer characterized by the loss of the SWItch/Sucrose Non‐Fermentable‐related matrix‐associated actin‐dependent regulator of chromatin subfamily B member 1 gene (SMARCB1) and tends to follow an aggressive clinical course. There is no currently available standard therapy option, although a few promising treatment strategies, including enhancer of zeste homolog 2 (EZH2) inhibition, are under active investigation. This report describes a 30‐year‐old woman with INI‐1‐deficient carcinoma who progressed on combination chemotherapy and an EZH2 inhibitor. Next‐generation‐sequencing‐based targeted cancer‐related gene assay confirmed SMARCB1 loss and revealed other mutations in breast cancer 1 gene and checkpoint kinase 2 gene, which may have impacted her clinical course. After discussion at the molecular tumor board, she was offered alisertib, an aurora A kinase inhibitor, on a single‐patient expanded‐use program and achieved prolonged disease stabilization. Aurora A kinase inhibition may have an important role in the management of patients with INI‐1‐deficient tumors, warranting further evaluation in clinical studies.
Key Points.
Loss of the SWItch/Sucrose Non‐Fermentable‐related matrix‐associated actin‐dependent regulator of chromatin subfamily B member 1 gene (SMARCB1), which encodes integrase interactor 1 (INI‐1), is associated with various mesenchymal malignancies, but a few carcinomas with rhabdoid features have been recently described as a distinct entity.
INI‐1‐deficient carcinoma can be very aggressive, and there is no known treatment option available.
There are encouraging preliminary data with an enhancer of zeste homolog 2 inhibitor, tazematostat, in INI‐1‐deficient malignancies, including INI‐1‐deficient carcinomas.
Loss of INI‐1 can activate aurora A kinase (AurkA), and inhibition of AurkA by alisertib could be a viable option and warrants further investigation in this cancer.
Clinical genomic profiling can confirm diagnosis of molecularly defined malignancy and provide insights on therapeutic options.
Patient Story
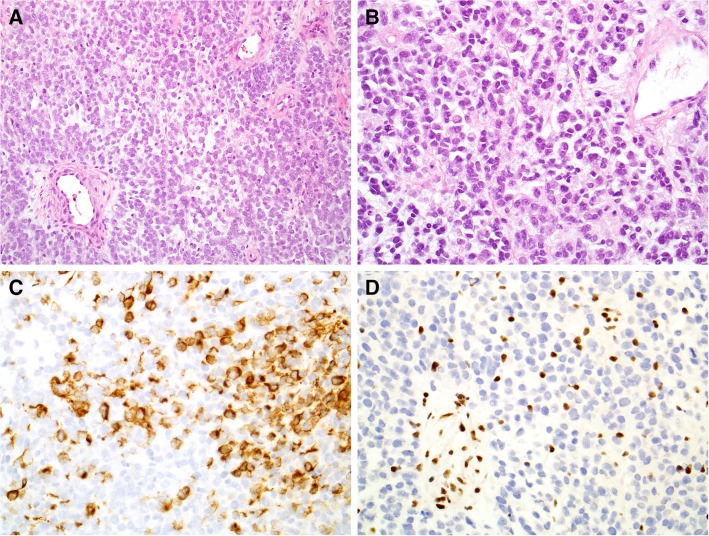
A 30‐year‐old never‐smoker female with no significant medical history initially presented with dry cough and hemoptysis. Her family history was also not significant for cancer, other than colon cancer of her paternal grandfather at age 70. Computed tomography (CT) imaging showed a 4.9‐cm suprahilar mass in her left lung with mediastinal lymphadenopathy and suspicious bone lesions. Subsequent biopsy with mediastinoscopy showed poorly differentiated non‐small cell carcinoma with rhabdoid features and occasional psammoma bodies. Immunostaining revealed that the tumor was positive for AE1/AE3, Cam5.2, p40, and PAX‐8, focally positive for calponin, and negative for TTF‐1, chromogranin, synaptophysin, NUT‐1, CD99, CD5, cKIT, and S100. Brahma‐related gene 1 (encoded by the WI/SNF‐related matrix‐associated actin‐dependent regulator of chromatin subfamily A member 4 gene [SMARCA4]) immunostaining showed nuclear retention, and integrase interactor 1 (INI‐1; encoded by the SWItch/Sucrose Non‐Fermentable [SWI/SNF]‐related matrix‐associated actin‐dependent regulator of chromatin subfamily B member 1 gene [SMARCB1]) staining showed loss of nuclear expression (Fig. 1). Because of the cytokeratin positivity, rhabdoid features, and loss of INI‐1 expression, the tumor was classified as an INI‐1‐deficient carcinoma. Positron emission tomography (PET)‐CT revealed hypermetabolic precarinal and prevascular lymphadenopathy along with hypermetabolic foci in the patient's sixth rib and lumbar vertebral bodies consistent with metastatic bone disease. CT‐guided biopsy of the L1 vertebra confirmed the presence of metastatic poorly differentiated carcinoma. The patient received three cycles of docetaxel, cisplatin, and 5‐FU (TPF) for rapidly progressing disease and achieved partial response. She chose to enroll in a phase II clinical trial of the enhancer of zeste homolog 2 (EZH2) inhibitor tazemetostat (NCT02601950). Unfortunately, 4 months later, her disease progressed, causing complete atelectasis of left upper lobe. Additional cycles of TPF were attempted, but she progressed rapidly through the treatment. Subsequently, she underwent surgical debulking of the tumor in her lung followed by radiotherapy for palliation of respiratory symptoms. A next‐generation‐sequencing‐based genomic profiling test in a Clinical Laboratory Improvement Amendments‐certified laboratory (Foundation Medicine Inc, Cambridge, MA) was performed to get guidance on next line of treatment, and the result showed loss of SMARCB1, which encodes INI‐1, breast cancer 1 gene (BRCA1) mutation (C61G), and checkpoint kinase 2 gene (CHEK2) truncation at intron 2.
Figure 1.
Histopathology of INI‐1 deficient carcinoma. H&E sections showed sheets of monotonous high‐grade malignant cells (A, ×20) that demonstrated a rhabdoid appearance with eccentric eosinophilic cytoplasm (B, ×40). Immunostains showed positivity for low‐molecular‐weight cytokeratin cocktail CAM5.2 (C, ×40) and loss of integrase interactor 1 expression (D, ×40).
INI‐1‐Deficient Carcinoma
SMARCB1 encodes INI‐1, which is a core subunit protein of the SWI/SNF ATP‐dependent chromatin remodeling complex [1]. SMARCB1 is a known tumor suppressor gene and has been implicated in chromatin remodeling and transcription regulation [2]. INI‐1 is ubiquitously expressed in the nuclei of all normal cells, and loss of expression of INI‐1 in the nuclei has been a wide range of epithelial and mesenchymal tumors. Loss of SMARBC1 is the defining genetic alteration seen in epithelioid sarcomas [3], rhabdoid tumors of childhood [4], and SMARCB1‐deficient sinonasal carcinomas [5]. However, INI‐1 loss can also be seen in a less well‐defined cohort of other poorly differentiated carcinomas that tend to have basaloid or rhabdoid morphology and follow an aggressive course; these carcinomas have been reported at a wide range of anatomic sites including the kidney [6] and gastrointestinal tract [7]. INI‐1 loss specifically has not been well characterized in lung cancers. A series of 316 patients with non‐small cell lung cancer demonstrated no cases with INI‐1 loss while revealing infrequent losses in other SWI/SNF complex proteins SMARCA2 and SMARCA4 [8]. Lung tumors with basaloid or rhabdoid morphology have not undergone systematic analysis for INI‐1 alterations [9]. Differentiation between these tumor types requires consideration of not only INI‐1 loss but a wide range of other clinicopathologic features. No systemic chemotherapy has yet demonstrated significant benefit for patients with metastatic INI‐1‐deficient carcinomas. A recent report suggested activity of an EZH2 inhibitor, tazemetostat, in INI‐1‐deficient tumors of pediatric patients [10], and the agent is being investigated in adult patients with various INI‐1‐deficient tumors as well [11]. However, there is no established therapy for this rare, aggressive disease yet.
Molecular Findings and Implications
The case was discussed at our institution's molecular tumor board. Interestingly, clinical genomic profiling of our patient's tumor revealed the presence of a pathogenic C61G mutation of the BRCA1 gene, which affects the ubiquitin ligase function of the really interesting new gene (RING) domain and reduces heterodimerization of BRCA1 and BRCA1‐associated RING domain protein 1 [12]. Unlike other deleterious BRCA1 mutations, this missense mutation was not associated with sensitivity to cisplatin or poly (ADP‐ribose) polymerase inhibitors in a preclinical model [13]. The variant allele fraction of the BRCA1 mutation was 48%, which is suggestive of a heterozygous germline variant, and the patients test results were positive for the germline mutation of BRCA1 C61G (NantOmics, Culver City, CA). Retention of a normal BRCA1 allele and the nature of the mutation affecting the RING domain may explain why our patient did respond to initial platinum‐based chemotherapy but quickly developed resistance later. The tumor also harbored a truncation in intron 2 of CHEK2, which is predicted to be inactivating, and has been reported to be associated with increased risk of breast cancer [14]. CHEK2 encodes for checkpoint kinase 2, a serine/threonine kinase that plays a key role in the DNA damage response [15].
SWI/SNF and Polycomb complexes have antagonistic development roles, and INI‐1 loss leads to upregulation of EZH2, leading to increased histone H3 on lysine 27‐trimethylation in INI‐1‐deficient tumors and upregulation of stem‐cell‐associated programs [16], which are consistent with the dedifferentiated phenotype of these cancers. INI‐1‐deficient tumors are shown to be sensitive to EZH2 inhibition in preclinical studies [17], and early‐phase clinical trials demonstrated promising activity of an EZH2 inhibitor in patients with these tumors. Preliminary data showed that tazemetostat led to partial response in 4 and stable disease in 2 out of 31 patients to date, with 13 patients still on treatment [11]. The only known mechanism implicated in the development of resistance to EZH2 inhibitors is the development of secondary mutations [18], but preclinical studies evaluating other possible mechanisms are ongoing.
Recent studies have highlighted other oncogenic signaling pathways that are de‐repressed in the setting of INI‐1 deficiency, including MYC and aurora A kinase (AurkA) [19]. AurkA regulates the formation and stability of the mitotic spindle during the cell cycle, is implicated in p53 degradation, and is upregulated in a variety of malignancies, representing a promising therapeutic target [20]. Microarray data show that re‐expression of INI‐1 in rhabdoid tumors leads to downregulation of AurkA [21]. Lee et al. showed that SWI/SNF associates with the promoter of AURKA, gene encoding AurkA, repressing its expression particularly in rhabdoid tumor cells, whereas knockdown of AURKA induces mitotic arrest and apoptosis in these cells but not in normal cells [19]. Of note, non‐small cell lung cancer cells harboring mutations of SMARCA4, another critical component of the SWI/SNF complex, are particularly sensitive to AurkA inhibition [22]. Figure 2 represents the oncogenic signaling involving SWI/SNF complex in cancer cells providing targeted therapy opportunities.
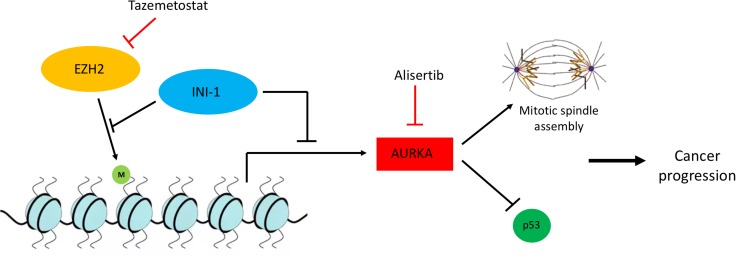
Figure 2.
Oncogenic signaling involving INI‐1 creates novel therapeutic opportunities. INI‐1 as part of the SWItch/Sucrose Non‐Fermentable complex and EZH2 as part of the PRC2 complex have antagonistic roles in the methylation of histones and gene expression. Particularly, EZH2 induces H3K27 trimethylation, whereas INI‐1 inhibits this activity. In the setting of INI‐1 deficiency, EZH2 activity is upregulated, rendering these tumors particularly sensitive to EZH2 inhibitors such as tazemetostat. Similarly, INI‐1 suppresses the expression of AurkA, which is implicated in the stability of the mitotic spindle and the degradation of p53, promoting cancer progression. Thus, INI‐1‐deficient tumors are also sensitive to AurkA inhibition by agents such as alisertib. Abbreviations: AurkA, Aurora kinase A; EZH2, enhancer of zeste homolog 2; INI‐1, integrase interactor 1.
Given these encouraging preclinical findings, AurkA inhibition has been introduced to the clinical setting for patients with INI‐1‐deficient tumors. Alisertib is a novel AurkA inhibitor, having been evaluated in early‐phase clinical trials for nongenomically selected malignancies such as sarcomas [23]. Wetmore et al. reported the use of alisertib in four children with recurrent or progressive atypical teratoid/rhabdoid tumors with INI‐1 deficiency, demonstrating stable disease or disease regression in all four patients, with two patients having stable disease regression for 1 and 2 years on therapy [24]. Based on these findings, the board recommended to use an AurkA inhibitor such as alisertib.
Patient Update
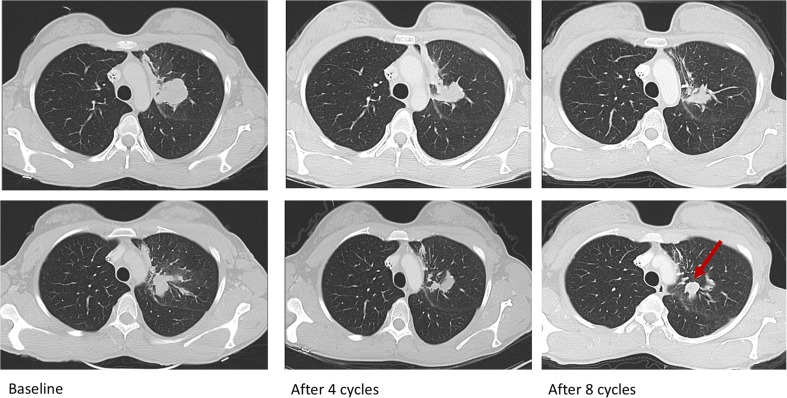
Alisertib use was reviewed and approved by the institutional review board, after being granted a single‐patient expanded use by the U.S. Food and Drug Administration. The patient started alisertib 50 mg twice daily on days 1–7 every 21 days after signing the informed consent. After six cycles, her left upper lobe lesion decreased by 25%, and the rest of the disease remained stable. Her disease progressed with a new enhancing lung nodule after eight cycles, but her index lesion and the rest of the lesions remained stable (Fig. 3). She chose to stay on the treatment given the lack of therapeutic options. After 10 cycles, the new lesion continued to grow and the treatment was held for palliative radiation. She received two additional cycles after radiation, while looking for other treatment options, but her disease continued to progress. She tolerated the treatment well except for oral ulcers between days 7 and 10 of each cycle and two treatment delays from nonfebrile neutropenia. Her progression‐free survival was 6 months.
Figure 3.
Serial scans of the patient demonstrating prolonged stable disease to alisertib, followed by progression of disease with appearance of a new lesion (arrow).
Conclusion
This case illustrates the potential role of an ArukA inhibitor for INI‐1‐deficient carcinoma even after progression on another promising targeted therapy modality, EZH2 inhibition. Our patient started on alisertib and remained stable for 6 months, which was not expected at the time of treatment initiation, given her rapid progression on combination chemotherapy and the EZH2 inhibitor, tazemetostat. She tolerated the treatment well, without significant side effects, and was maintained on the agent for 7.4 months when she was found to have a confirmed disease progression in imaging. The BRCA1‐Chk2 axis can suppress AurkA signaling, although loss of their activity leads to upregulation of AurkA and downstream signaling [25]. Our patient had a pathogenic BRCA1 missense mutation and a CHEK2 inactivating truncation, which could therefore have contributed to her tumor being particularly sensitive to AurkA inhibition by alisertib. This case illustrates the use of pathologic and genomic information in order to provide a personalized treatment offering significant clinical benefit to a patient with a rare and aggressive type of cancer. Finally, the course of our patient, along with preclinical data, supports that AurkA inhibition may have an important role in the management of patients with INI‐1‐deficient tumors, warranting further evaluation in clinical studies.
Glossary of Genomic Terms and Nomenclature
INI‐1: integrase interactor 1.
SMARCB1: SWI/SNF‐related matrix‐associated actin‐dependent regulator of chromatin subfamily B member 1 gene.
BRG1: brahma‐related gene 1.
SMARCA4: SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 4 gene.
SWI/SNF: SWItch/Sucrose Non‐Fermentable.
EZH2: enhancer of zeste homolog 2.
PARP: poly (ADP‐ribose) polymerase.
RING: really interesting new gene.
BRCA1: breast cancer 1 gene.
CHEK2: checkpoint kinase 2 gene.
AurkA: Aurora kinase A.
BARD1: BRCA1‐associated RING domain protein 1.
H3K27: histone H3 on lysine 27.
Acknowledgments
Alisertib was donated by Millennium Pharmaceuticals/Takeda Company. H.K. is currently affiliated with the Department of Medicine, Division of Hematology/Oncology, University of California, San Francisco, CA. J.L. is currently affiliated with Janssen Research and Development; however, work on this article was performed as a full‐time faculty member at Johns Hopkins University, prior to employment with Janssen.
Author Contributions
Conception/design: Theodoros Karantanos, Lisa Rooper, Hyunseok Kang
Provision of study material or patients: Lisa Rooper, Cheng Ting Lin, Pawla Wenga, Sarah Sagorsky, Hyunseok Kang
Collection and/or assembly of data: Theodoros Karantanos, Lisa Rooper, Cheng Ting Lin, Pawla Wenga, Sarah Sagorsky, Josh Lauring, Hyunseok Kang
Data analysis and interpretation: Theodoros Karantanos, Lisa Rooper, Youme Kang, Cheng Ting Lin, Josh Lauring, Hyunseok Kang
Manuscript writing: Theodoros Karantanos, Youme Kang, Hyunseok Kang
Final approval of manuscript: Theodoros Karantanos, Lisa Rooper, Youme Kang, Cheng Ting Lin, Pawla Wenga, Sarah Sagorsky, Josh Lauring, Hyunseok Kang
Disclosures
Josh Lauring: Janssen Research and Development (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
References
- 1.Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci 2017;108:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donner LR, Wainwright LM, Zhang F et al. Mutation of the INI1 gene in composite rhabdoid tumor of the endometrium. Hum Pathol 2007;38:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal‐type epithelioid sarcoma. Am J Surg Pathol 2009;33:542–550. [DOI] [PubMed] [Google Scholar]
- 4.Kim KH, Roberts CW. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet 2014;207:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI‐1)‐deficient carcinomas of the sinonasal tract. Am J Surg Pathol 2014;38:1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agaimy A, Cheng L, Egevad L et al. Rhabdoid and undifferentiated phenotype in renal cell carcinoma: Analysis of 32 cases indicating a distinctive common pathway of dedifferentiation frequently associated with SWI/SNF complex deficiency. Am J Surg Pathol 2017;41:253–262. [DOI] [PubMed] [Google Scholar]
- 7.Agaimy A, Daum O, Markl B et al. SWI/SNF complex‐deficient undifferentiated/rhabdoid carcinomas of the gastrointestinal tract: A series of 13 cases highlighting mutually exclusive loss of SMARCA4 and SMARCA2 and frequent co‐inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol 2016;40:544–553. [DOI] [PubMed] [Google Scholar]
- 8.Herpel E, Rieker RJ, Dienemann H et al. SMARCA4 and SMARCA2 deficiency in non‐small cell lung cancer: Immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol 2017;26:47–51. [DOI] [PubMed] [Google Scholar]
- 9.Tamboli P, Toprani TH, Amin MB et al. Carcinoma of lung with rhabdoid features. Hum Pathol 2004;35:8–13. [DOI] [PubMed] [Google Scholar]
- 10.Chi S, Fouladi M, Shukla N et al. Abstract A175: Phase 1 study of the EZH2 inhibitor, tazemetostat, in children with relapsed or refractory INI1‐negative tumors including rhabdoid tumors, epithelioid sarcoma, chordoma, and synovial sarcoma. Mol Cancer Ther 2018;17:A175–A175. [Google Scholar]
- 11.Gounder MM, Stacchiotti S, Schöffski P et al. Phase 2 multicenter study of the EZH2 inhibitor tazemetostat in adults with INI1 negative epithelioid sarcoma (NCT02601950). J Clin Oncol 2017;35:11058–11058. [Google Scholar]
- 12.Hashizume R, Fukuda M, Maeda I et al. The ring heterodimer BRCA1‐BARD1 is a ubiquitin ligase inactivated by a breast cancer‐derived mutation. J Biol Chem 2001;276:14537–14540. [DOI] [PubMed] [Google Scholar]
- 13.Drost R, Bouwman P, Rottenberg S et al. BRCA1 ring function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell 2011;20:797–809. [DOI] [PubMed] [Google Scholar]
- 14.Decker B, Allen J, Luccarini C et al. Rare, protein‐truncating variants in ATM, CHEK2 and PALB2, but not XRCC2, are associated with increased breast cancer risks. J Med Genet 2017;54:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pommier Y, Sordet O, Rao VA et al. Targeting CHK2 kinase: Molecular interaction maps and therapeutic rationale. Curr Pharm Des 2005;11:2855–2872. [DOI] [PubMed] [Google Scholar]
- 16.Wilson BG, Wang X, Shen X et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 2010;18:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen JK, Cote GM, Gao Y et al. Targeting EZH2‐mediated methylation of H3K27 inhibits proliferation and migration of synovial sarcoma in vitro. Sci Rep 2016;6:25239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibaja V, Shen F, Harari J et al. Development of secondary mutations in wild‐type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors. Oncogene 2016;35:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Cimica V, Ramachandra N et al. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res 2011;71:3225–3235. [DOI] [PubMed] [Google Scholar]
- 20.Nikonova AS, Astsaturov I, Serebriiskii IG et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci 2013;70:661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morozov A, Lee SJ, Zhang ZK et al. INI1 induces interferon signaling and spindle checkpoint in rhabdoid tumors. Clin Cancer Res 2007;13:4721–4730. [DOI] [PubMed] [Google Scholar]
- 22.Tagal V, Wei S, Zhang W et al. SMARCA4–inactivating mutations increase sensitivity to Aurora kinase A inhibitor VX‐680 in non‐small cell lung cancers. Nat Commun 2017;8:14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickson MA, Mahoney MR, Tap WD et al. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol 2016;27:1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetmore C, Boyett J, Li S et al. Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol 2015;17:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ertych N, Stolz A, Valerius O et al. CHK2–BRCA1 tumor‐suppressor axis restrains oncogenic Aurora‐A kinase to ensure proper mitotic microtubule assembly. Proc Natl Acad Sci U S A 2016;113:1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]