This article reviews data collected in the Japanese Adverse Drug Event Report (JADER) database, maintained by a Japanese regulatory agency. Drugs suspected of causing Stevens‐Johnson syndrome or toxic epidermal necrolysis were identified, and profiles potentially associated with anti‐cancer agents were analyzed.
Keywords: Stevens‐Johnson syndrome, Toxic epidermal necrolysis, Skin toxicity, Cancer, Treatment
Abstract
Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are potentially life‐threatening cutaneous and mucosal adverse reactions to drugs. Nevertheless, the connection to anticancer agents remains unclear. To provide insight into the association of such adverse reactions with anticancer agents, we analyzed the profile of anticancer agent‐induced SJS and TEN in the Japanese population. Of the 9,738 SJS/TEN events recorded in a database of spontaneous reporting data, 485 (5%, further categorized as SJS, 384 events, 79%; TEN, 101 events, 21%) were identified as anticancer agent‐induced, and 53 of these (11%) were fatal. Multivariate logistic regression analyses indicated that, compared with patients using other drugs, those using anticancer drugs had lower incident risk of death (hazard ratio [HR], 0.592; p = .0006), longer median time to onset of SJS/TEN (18 vs. 11 days; p < .0001; multivariate Cox regression: HR, 0.66; p < .0001), and a higher likelihood of developing SJS/TEN later than 70 days after initiation of the suspected causal agent (15% vs. 7%; p < .0001), highlighting the need for vigilance and continuous monitoring for SJS/TEN in patients treated with anticancer agents.
Implications for Practice.
Life‐threatening skin toxicities induced by anti‐cancer agents indicated significantly lower incident risk of death and longer time to onset of symptoms than for those induced by other drugs.
Introduction
Stevens‐Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are characterized by high fever, with cutaneous and mucosal blisters [1], [2]. The reported incidence varies from 1.2 to 6 and from 0.4 to 1.2 cases per million patient‐years for SJS and TEN, respectively [3]. Despite their rare occurrence, both SJS and TEN often accompany complications involving other organs such as the liver, kidney, gastrointestinal tract, and respiratory tract [4]. Overall mortality remains significant, ranging from 1% to 12% and from 14.8% to 46% for SJS and TEN, respectively [5], [6], [7], [8]. Therefore, SJS and TEN are considered potentially life‐threatening skin toxicities with significant impact on public health [[5], [6], [9]].
Although many casual factors, such as infection, vaccination, and chemical exposure, are known to cause SJS/TEN [10], [11], drug exposure remains the most common cause [12], and over 200 drugs have been reported to be associated with SJS or TEN [13]. Frequently suspected drugs include sulfonamide antibiotics, anticonvulsants, nonsteroidal anti‐inflammatory drugs, allopurinol, and corticosteroids [14], [15], [16]. Although anticancer agent‐induced SJS and TEN have also been reported, there have been few systematic analyses of the reported cases [17]. Given the increasing incidence of cancer and the development of new treatments, the demand for cancer pharmaceuticals is expected to more than double over the next 10 years [18]. Consequently, it is of great relevance to understand anticancer agent‐induced conditions, including life‐threatening skin toxicities.
To achieve this aim, we reviewed the spontaneous reporting data collected in the Japanese Adverse Drug Event Report (JADER) database, maintained by the Pharmaceuticals and Medical Devices Agency (PMDA), which is a Japanese regulatory agency. The JADER database contains information on adverse drug reactions, organized according to four categories: demographics, drugs, drug‐induced adverse reactions, and disease. Using this database, we identified drugs suspected of causing SJS or TEN and analyzed the spontaneous reporting data‐based profile of SJS and TEN potentially associated with anticancer agents.
Materials and Methods
This study was approved by institutional review board and human research ethics committee. We downloaded data from the PMDA website (http://www.info.pmda.go.jp/fukusayoudb/CsvDownload.jsp) and collected the following information: report identification number, age, sex, skin toxicity type (SJS/TEN), time to onset of skin toxicity symptoms, outcome, and names of the drugs and their indication (reason for use). Among the suspected causal agents, anticancer agents were identified based on the name and indication of the drug. According to the Japanese guidelines for TEN management, patients with skin and/or mucous membrane detachment over 10% of the body surface area were diagnosed as having TEN [19]. In the present study, patients with epidermal necrolysis over 10%–30% of the body surface area were also considered to have SJS/TEN, because the definitions of SJS and TEN overlap for such cases. A total of 289,494 reports were filed to the JADER database between April 2004 and December 2013 (based on the identification number). Among the reports filed in this period, 9,738 were identified to describe SJS/TEN events. The frequencies and descriptive statistics of relevant demographic and clinical variables were summarized. Using the t test and Fisher's exact test, respectively, the distribution of values for continuous and discrete variables were compared between patients receiving anticancer agents and those receiving other drugs. Of the 9,738 records of SJS/TEN events identified, only 8,921 contained all data of interest and were eligible for inclusion into our multivariate regression analysis. The time to onset of symptoms of life‐threatening skin toxicities was compared between the two groups (i.e., patients receiving anticancer agents vs. those receiving other drugs) using multivariate Cox regression analysis adjusted for age and sex. The relationship between death and potential risk factors, including age, sex, and time to onset of SJS/TEN, was examined based on the results of the multivariate logistic regression analysis. A two‐sided p < .05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Patient Demographics
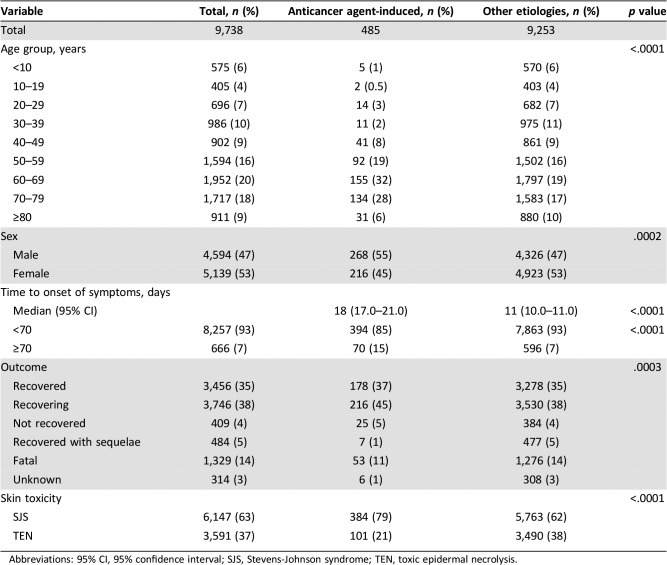
We collected clinical information pertaining to 9,738 events of drug‐induced life‐threatening skin toxicity (SJS/TEN; Table 1). A total of 485 events (5%) were identified as being anticancer agent‐induced (anticancer agent group), with 384 (79%) and 101 (21%) patients presenting with SJS and TEN, respectively. Among the 9,253 patients with skin toxicity of other etiologies (non‐anticancer agent group), the incidence of SJS and TEN was 62% and 38%, respectively. Of the 1,329 SJS/TEN events (14%) that were fatal, 53 events were associated with anticancer agent‐induced toxicity (53/485, 11%).
Table 1. Clinical information regarding 9,738 events of drug‐induced life‐threatening skin toxicity.
Abbreviations: 95% CI, 95% confidence interval; SJS, Stevens‐Johnson syndrome; TEN, toxic epidermal necrolysis.
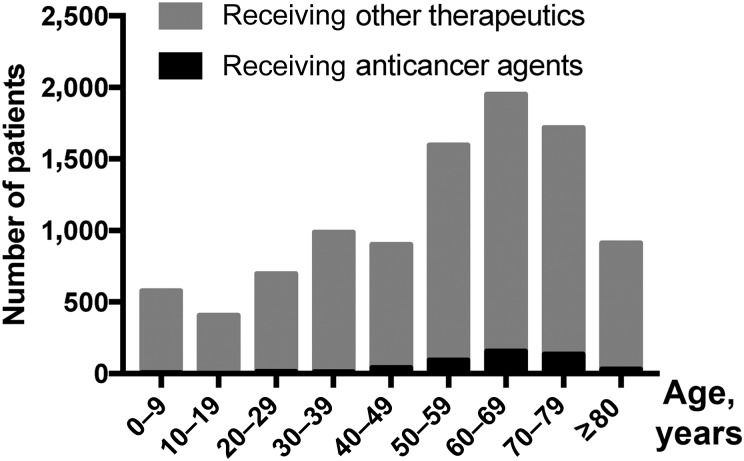
In terms of affected age groups, the highest prevalence of drug‐induced skin toxicity was noted among patients aged 50–79 years, and this trend did not differ between the anticancer and non‐anticancer agent groups. On the other hand, in patients younger than 50 years or older than 80 years, SJS/TEN was less likely to be induced by anticancer agents than by other drugs (Fig. 1). As for anticancer agent‐induced SJS/TEN, 78% of adverse reactions occurred in patients aged between 50 and 79 years, and only 6% occurred in patients older than 80 years.
Figure 1.
Relationship between age and the prevalence of life‐threatening skin toxicities. The cohort included patients with Stevens‐Johnson syndrome or toxic epidermal necrolysis (n = 9,738). Age groups are defined in 10‐year bands or age of more than 80 years. The highest prevalence of drug‐induced skin toxicity was noted among patients aged 50–79 years, both among patients receiving anticancer agents and among those receiving non‐anticancer therapeutics. In this cohort of patients with drug‐induced life‐threatening skin toxicity, an anticancer agent was identified as the causative drug in 5.8%, 7.9%, and 7.8% of patients aged 50–59, 60–69, and 70–79 years, respectively, compared with only 3.4% and 2.0% of patients aged above 80 years or below 50 years, respectively.
Finally, there was a slight predominance of male patients in the anticancer agent group (male vs. female patients, 55% vs. 45%), whereas the opposite was noted in the overall cohort and in the non‐anticancer agent group (47% vs. 53%, respectively, in both cases).
Association Between Patient Demographics and SJS/TEN Outcomes
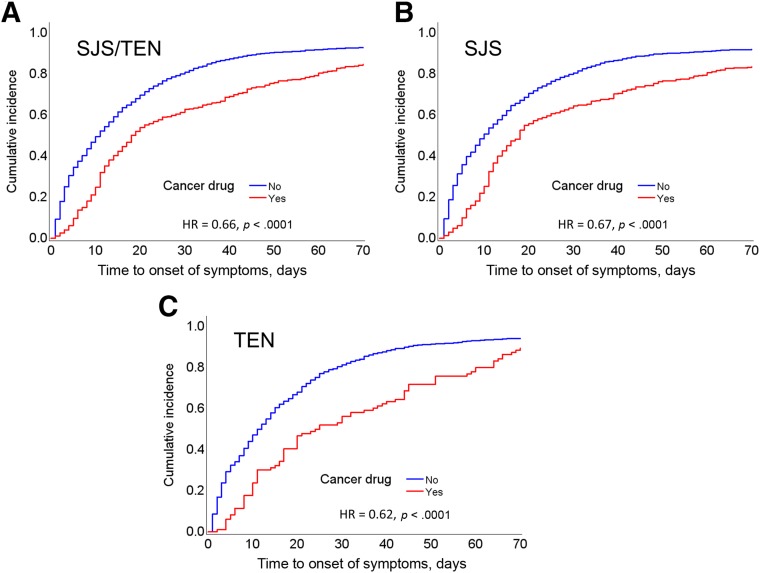
The median interval of time from first drug intake to symptom onset was significantly longer in the anticancer than in the non‐anticancer agent group (18 vs. 11 days, p < .0001). The trend remained statistically significant on multivariate Cox regression analysis (hazard ratio [HR], 0.66; p < .0001; Fig. 2A) and was valid for the overall cohort as well as for the subcohorts (SJS, Fig. 2B; TEN, Fig. 2C). Moreover, the proportion of patients who developed SJS/TEN later than 70 days after initiation of the suspected causal agent was significantly higher in the anticancer than in the non‐anticancer agent group (15% vs. 7%, p < .0001; Table 1).
Figure 2.
Time interval between first drug intake and onset of symptoms of skin toxicity. Data are provided for the overall cohort showing drug‐induced skin toxicity (A), as well as for the subcohort with SJS (B) and that with TEN (C). The cumulative incidence curve was obtained using Cox proportional‐hazards regression analysis. In each cohort, the anticancer agent group showed significantly longer time to onset than that noted in the non‐anticancer agent group. Abbreviations: HR, hazard ratio; SJS, Stevens‐Johnson syndrome; TEN, toxic epidermal necrolysis.
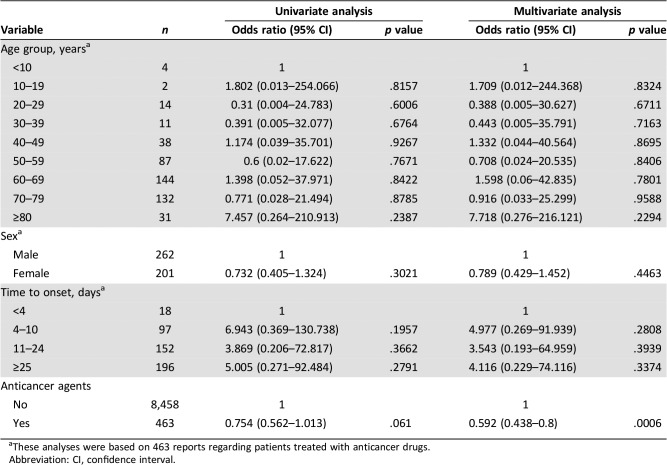
Multivariate logistic regression analysis showed that the incident risk of death was significantly lower in the anticancer than in the non‐anticancer agent group (HR, 0.592; p = .0006; Table 2). Additionally, this risk tended to be lower in female patients than in male patients (HR, 0.78), as well as in patients for whom the median time interval between the first drug intake and the onset of symptoms was less than 4 days. However, these trends were not statistically significant (Table 2). Finally, the prevalence of death was higher for TEN than for SJS in every age group (Fig. 3A; TEN: 28/99 cases, 28.3%; SJS: 29/371 cases, 7.8%; overall, p < .001).
Table 2. Prognostic factors for anticancer drug‐induced skin toxicity.
These analyses were based on 463 reports regarding patients treated with anticancer drugs.
Abbreviation: CI, confidence interval.
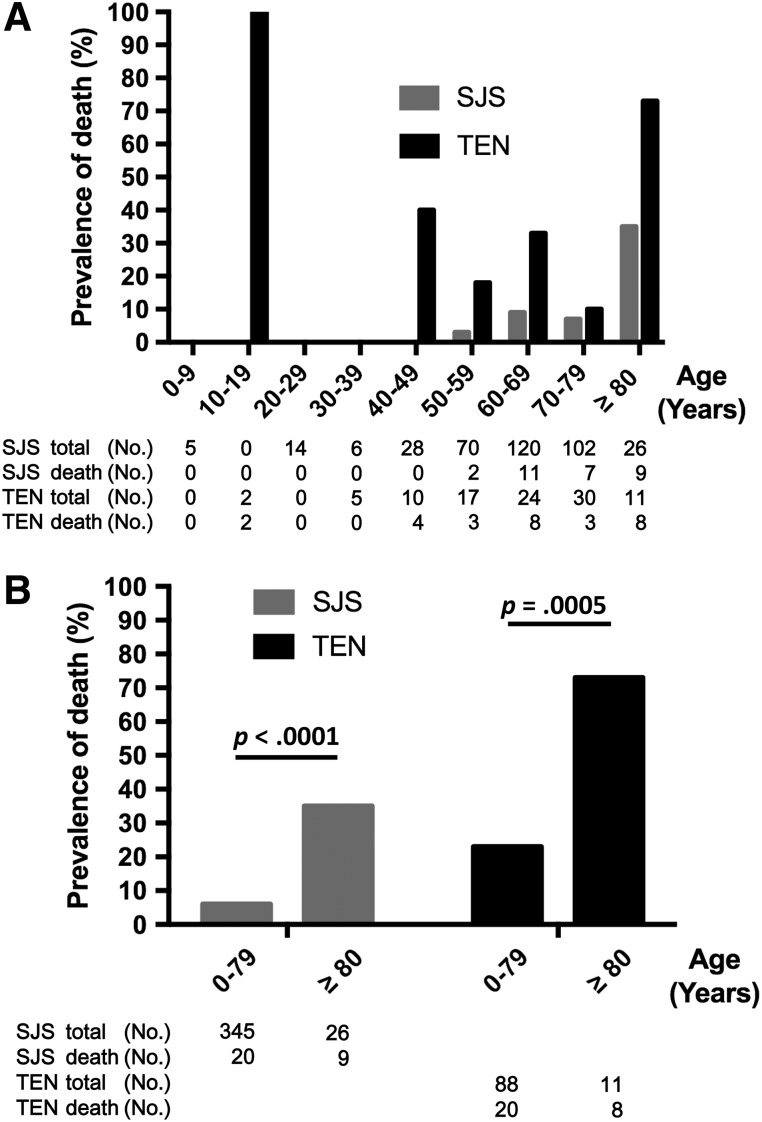
Figure 3.
Relationship between age and prevalence of death in patients with anticancer agent‐induced skin toxicity. Data are provided separately for SJS and TEN. Age groups are defined in 10‐year bands, with the last band covering patients aged above 80 years. The prevalence of death was higher among patients with TEN than among those with SJS for each age group (A). The death rate in patients aged above 80 years was significantly higher than that noted in those aged below 80 years, regardless of the type of skin toxicity (B). Abbreviations: SJS, Stevens‐Johnson syndrome; TEN, toxic epidermal necrolysis.
Although the incidence of SJS/TEN among patients older than 80 years was only 6% (Table 1), the death rate in this age group was significantly higher than that noted in patients younger than 80 years, regardless of the type of skin toxicity (SJS: 35% vs. 5.8%, p < .0001; TEN: 73% vs. 23%, p = .0005; Fig. 3B).
Drug Characteristics Potentially Associated with SJS/TEN
The list of anticancer drugs potentially associated with SJS/TEN is provided in supplemental online Table 1. Information regarding the treatment duration for each drug is also included. Among the conventional cytotoxic agents and novel targeted agents reviewed, the highest number of SJS/TEN events was noted for tegafur‐uracil (n = 76), followed by interferon‐alpha and sorafenib tosylate.
Discussion
Our finding that the time to onset of symptoms was significantly longer for events induced by anticancer agents than for those induced by other drugs indicates the need for increased vigilance and continuous monitoring for SJS and TEN in patients treated using anticancer agents. Although the algorithm for assessment of drug causality in SJS/TEN (ALDEN) suggests that late events are unlikely to be caused by the long‐term use of the drug [20], every suspected causative drug should be withdrawn when SJS or TEN occurs, as early withdrawal of the causative drug is known to be associated with improved prognosis [5]. Therefore, when SJS/TEN occurs in a patient treated with an anticancer agent, the drug should be withdrawn or the treatment should be adapted, even when such changes in the cancer treatment protocol might lead to disease progression. However, it might sometimes be difficult to stop the treatment of patients with cancer, especially when there is reduced likelihood that the anticancer agent is indeed a causal agent. As no ALDEN‐related data were included in the present study, further studies are warranted to determine whether or not ALDEN can also be applied to SJS/TEN induced by anticancer agents.
We found that the incidence of SJS/TEN associated with anticancer agents was 1.24 times higher in male patients than in female patients, whereas the incidence of SJS/TEN associated with other drugs was 1.14 times higher in female patients than in male patients (p = .0002; Table 1). One possible reason for this discrepancy may related to sex‐specific differences in the incidence of cancer, with the prevalence estimated to be 1.4 times higher in male patients than in female patients [21]. In other words, it is more likely that patients receiving treatment with anticancer agents are male. Moreover, the age group of 50–79 years accounted for 78% of the incidence of SJS/TEN associated with anticancer agents recorded over the course of the studied period (2004–2013), which is consistent with the estimated contribution of this age group to the overall cancer incidence in Japan (70%) [21]. These findings suggest that the incidence of SJS/TEN might increase with the number of patients with cancer because such patients are treated with anticancer drugs, which may act as causal agents for SJS/TEN.
Meanwhile, we found that the incidence of SJS/TEN in patients with cancer older than 80 years accounted for only 6% of the overall incidence of SJS/TEN among patients treated with anticancer agents; this is lower than the incidence of cancer among individuals aged above 80 years, which is estimated to account for approximately 25% of the overall incidence of cancer [21]. It is well recognized that treatment with anticancer agents is occasionally associated with severe side effects [22], and advanced age is considered a risk factor for increased toxicity [23] and mortality [24], which is consistent with the findings of the current study (Fig. 3A and 3B). Moreover, the lack of evidence‐based information and the fear of toxicity associated with anticancer treatment in elderly patients is expected to negatively impact on the oncologist's decision to refer elderly patients for treatment with anticancer agents [25]. Hence, the proportion of patients treated with anticancer agents might not correlate with the higher incidence of cancer seen in patients older than 80 years of age. Therefore, the lower incidence of drug‐induced SJS/TEN in elderly patients (≥80 years) treated with anticancer agents may be due to a reduced chance for such patients to be indicated for treatment with anticancer agents. However, more information from prospective studies is needed for accurate evaluation of the factors underlying the prevalence of SJS/TEN associated with anticancer agents in elderly patients.
The present finding that the incident risk of death was significantly lower in the anticancer than in the non‐anticancer agent group might be related to the relative incidence of SJS compared with that of TEN, which was higher in the anticancer than in the non‐anticancer agent group and which corresponds to the relative death rate associated with life‐threatening skin toxicities, which is higher for TEN than for SJS [[5], [6], [9], [26]]. In addition, as it is already known that early withdrawal of the causative drug is associated with better prognosis [5], it is likely that, compared with patients treated with other drugs, patients treated with anticancer agents were monitored more carefully for drug‐related side effects, leading to early diagnosis of life‐threatening skin toxicities, early withdrawal of the causative drug, and early initiation of treatments for SJS/TEN. Furthermore, as advanced age is considered a risk factor for increased mortality [24], our observation that elderly patients (≥80 years) in the anticancer agent group had lower incidence of drug‐induced SJS/TEN (6%) may suggest that younger patients with cancer were more likely to be indicated for treatment with anticancer agents and thus be included in the anticancer agent group. Moreover, because patients in poor general condition should not receive anticancer agents, it is possible that patients in the anticancer agent group had a relatively good general condition. However, information regarding the patients’ general condition was not included in the spontaneous reporting data evaluated in the current study, and thus we could not exclude the effect of the clinical background and disease severity on the incidence of SJS/TEN.
In addition to the selection bias intrinsic to any retrospective study design, there are several limitations and potential biases related to using data from the JADER database, which collects spontaneous reporting data via a passive reporting system and does not implement any validation process. First, there is no clear indication of the total patient population for which these toxicity reports were generated. Therefore, the results of this study should be interpreted carefully. Second, patients undergoing treatment with anticancer agents are often exposed to other drugs mainly used to treat cancer symptoms (e.g., pain medication) or side effects of treatment (e.g., antiemetic agents, antibiotic drugs), but also to drugs commonly associated with SJS/TEN, such as allopurinol, sulfonamide antibiotics, anticonvulsants, and corticosteroids. Furthermore, multiple suspected drugs may be listed per adverse event registered in the JADER database. Third, patients with cancer often have comorbidities and multiorgan involvement by the underlying disease, which add to the overall complexity of each individual case. Therefore, when a patient dies after developing SJS/TEN, the cause of death may be SJS/TEN, cancer itself, a comorbidity, or a combination of causes. Finally, the JADER reports were not sufficiently detailed (e.g., they did not include clinical, laboratory, or histological data) to facilitate an accurate diagnosis of SJS or TEN; this aspect is relevant because there are numerous other conditions presenting with blisters and skin detachment, which should be excluded as far as possible. In the specific setting of cancer and anticancer treatment, such conditions include paraneoplastic pemphigus, bullous pemphigoid, generalized bullous fixed drug eruption, erythema and detachment of the skin associated with doxorubicin and similar anticancer drugs, and extensive drug eruptions associated with targeted therapies. Taking these limitations into account, further population‐based studies with clear and detailed information for diagnosis are warranted to improve our understanding of anticancer drug causality in life‐threatening skin toxicities.
Despite these limitations, the present study reviewed almost 10,000 SJS/TEN events, of which more than 5% were identified as anticancer agent‐associated, representing the largest systematic investigation regarding the association of anticancer agents with life‐threatening skin toxicities such as SJS or TEN. Thus, we believe that our findings carry more statistical power than those of previous studies. Importantly, although SJS/TEN associated with anticancer agents had significantly lower incident risk of death compared with that noted for SJS/TEN induced by other drugs, the death rate remains very high in patients older than 80 years. Our findings indicate that further attention is required when monitoring such patients for skin toxicities.
Conclusion
Higher vigilance and continuous monitoring for SJS and TEN should be applied in patients treated with anticancer agents, as the time to onset of skin toxicity symptoms is longer in these patients. Additionally, the high death rate of patients older than 80 years of age indicates that particular attention is required in their monitoring.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors are thankful to Editage for writing assistance.
Contributed equally
Footnotes
For Further Reading: Adriana T. Lopez, Larisa Geskin. A Case of Nivolumab‐Induced Bullous Pemphigoid: Review of Dermatologic Toxicity Associated with Programmed Cell Death Protein‐1/Programmed Death Ligand‐1 Inhibitors and Recommendations for Diagnosis and Management. The Oncologist 2018;23:1119–1126.
Abstract: Immunotherapy has emerged as a highly effective treatment for numerous cancers. Use of checkpoint inhibitors against various molecules including programmed cell death protein‐1 (PD‐1), programmed death ligand‐1 (PD‐L1), and cytotoxic T‐lymphocyte‐associated protein‐4 have become widespread in clinical practice. Compared with conventional chemotherapy, immunotherapy is associated with a unique set of immune reactions known collectively as immune‐related adverse events (irAEs). Of known irAEs, cutaneous toxicity is among the most frequently observed in patients treated with immunotherapy. Although often mild, dermatologic toxicity can occasionally be high grade and potentially life‐threatening. In this article, we report a case of PD‐1 inhibitor‐induced bullous pemphigoid—a serious adverse event that has been increasingly observed with use of PD‐1/PD‐L1 inhibitors. We will also review diagnosis and management of low‐grade cutaneous irAEs and bullous disease with checkpoint inhibitors.
Key Points.
- PD‐1/PD‐L1 inhibitor‐induced bullous pemphigoid (BP) is a rare but potentially serious dermatologic toxicity associated with checkpoint inhibitors.
- In patients with pruritus or rash that is refractory to topical steroids, physicians should have a greater index of suspicion for higher-grade cutaneous immune‐related adverse events.
- There is no standardized treatment algorithm for management of PD‐1/PD‐L1 inhibitor‐induced BP, but patients frequently require topical and systemic steroids.
Author Contributions
Conception/design: Kan Yonemori, Akihiro Hirakawa
Provision of study material or patients: Ryota Tanaka, Akihiro Hirakawa
Collection and/or assembly of data: Ryota Tanaka
Data analysis and interpretation: Akihiro Hirakawa, Fumie Kinoshita, Yumiko Kobayashi
Manuscript writing: Ryota Tanaka, Naoya Yamazaki, Manabu Fujimoto, Kenji Tamura, Yasuhiro Fujiwara, Kan Yonemori, Akihiro Hirakawa
Final approval of manuscript: Ryota Tanaka, Kan Yonemori, Akihiro Hirakawa, Fumie Kinoshita, Yumiko Kobayashi, Naoya Yamazaki, Manabu Fujimoto, Kenji Tamura, Yasuhiro Fujiwara
Disclosures
Naoya Yamazaki: Ono, Bristol‐Myers Squibb, Novartis (RF, SAB, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lyell A. Toxic epidermal necrolysis: An eruption resembling scalding of the skin. Br J Dermatol 1956;68:355–361. [DOI] [PubMed] [Google Scholar]
- 2.Auquier‐Dunant A, Mockenhaupt M, Naldi L et al. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens‐Johnson syndrome, and toxic epidermal necrolysis: Results of an international prospective study. Arch Dermatol 2002;138:1019–1024. [DOI] [PubMed] [Google Scholar]
- 3.Wolkenstein P, Revuz J. Drug‐induced severe skin reactions. Incidence, management and prevention. Drug Saf 1995;13:56–68. [DOI] [PubMed] [Google Scholar]
- 4.Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens‐Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): The spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol 2017;177:924–935. [DOI] [PubMed] [Google Scholar]
- 5.Garcia‐Doval I, LeCleach L, Bocquet H et al. Toxic epidermal necrolysis and Stevens‐Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000;136:323–327. [DOI] [PubMed] [Google Scholar]
- 6.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272–1285. [DOI] [PubMed] [Google Scholar]
- 7.Hsu DY, Brieva J, Silverberg NB et al. Morbidity and mortality of Stevens‐Johnson syndrome and toxic epidermal necrolysis in United States adults. JInvest Dermatol 2016;136:1387–1397. [DOI] [PubMed] [Google Scholar]
- 8.Sekula P, Dunant A, Mockenhaupt M et al. Comprehensive survival analysis of a cohort of patients with Stevens‐Johnson syndrome and toxic epidermal necrolysis. JInvest Dermatol 2013;133:1197–1204. [DOI] [PubMed] [Google Scholar]
- 9.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Chem Immunol Allergy 2012;97:1–17. [DOI] [PubMed] [Google Scholar]
- 10.Borchers AT, Lee JL, Naguwa SM et al. Stevens‐Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 2008;7:598–605. [DOI] [PubMed] [Google Scholar]
- 11.Hazin R, Ibrahimi OA, Hazin MI et al. Stevens‐Johnson syndrome: Pathogenesis, diagnosis, and management. Ann Med 2008;40:129–138. [DOI] [PubMed] [Google Scholar]
- 12.Harr T, French LE. Stevens‐Johnson syndrome and toxic epidermal necrolysis. Chem Immunol Allergy 2012;97:149–166. [DOI] [PubMed] [Google Scholar]
- 13.Knowles S, Shear NH. Clinical risk management of Stevens‐Johnson syndrome/toxic epidermal necrolysis spectrum. Dermatol Ther 2009;22:441–451. [DOI] [PubMed] [Google Scholar]
- 14.Roujeau JC, Kelly JP, Naldi L et al. Medication use and the risk of Stevens‐Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995;333:1600–1607. [DOI] [PubMed] [Google Scholar]
- 15.Mockenhaupt M, Viboud C, Dunant A et al. Stevens‐Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR‐study. JInvest Dermatol 2008;128:35–44. [DOI] [PubMed] [Google Scholar]
- 16.Halevy S, Ghislain PD, Mockenhaupt M et al. Allopurinol is the most common cause of Stevens‐Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. JAm Acad Dermatol 2008;58:25–32. [DOI] [PubMed] [Google Scholar]
- 17.Rosen AC, Balagula Y, Raisch DW et al. Life‐threatening dermatologic adverse events in oncology. Anticancer Drugs 2014;25:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoppe‐Tichy T. Current challenges in European oncology pharmacy practice. JOncol Pharm Pract 2010;16:9–18. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita Y, Saeki H. A review of toxic epidermal necrolysis management in Japan. Allergol Int 2017;66:36–41. [DOI] [PubMed] [Google Scholar]
- 20.Sassolas B, Haddad C, Mockenhaupt M et al. ALDEN, an algorithm for assessment of drug causality in Stevens‐Johnson syndrome and toxic epidermal necrolysis: Comparison with case‐control analysis. Clin Pharmacol Ther 2010;88:60–68. [DOI] [PubMed] [Google Scholar]
- 21.Hori M, Matsuda T, Shibata A et al. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population‐based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2015;45:884–891. [DOI] [PubMed] [Google Scholar]
- 22.Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. JClin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein BN, Petrelli NJ, Douglass HO et al. Age and sex are independent predictors of 5‐fluorouracil toxicity. Analysis of a large scale phase III trial. Cancer 1995;75:11–17. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Lindquist K, Segal MR et al. Development and validation of a prognostic index for 4‐year mortality in older adults. JAMA 2006;295:801–808. [DOI] [PubMed] [Google Scholar]
- 25.Foster JA, Salinas GD, Mansell D et al. How does older age influence oncologists’ cancer management? The Oncologist 2010;15:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamane Y, Matsukura S, Watanabe Y et al. Retrospective analysis of Stevens‐Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients–treatment and outcome. Allergol Int 2016;65:74–81. [DOI] [PubMed] [Google Scholar]