This article reports on the effect of shared decision‐making training on the performance of medical oncologists in simulated consultations with a standardized model of a patient with advanced cancer.
Keywords: Shared decision‐making, Physician‐patient relations, Communication, Medical oncology, Palliative care
Abstract
Background.
Systemic treatment for advanced cancer offers uncertain and sometimes limited benefit, while the burden can be high. This study examines the effect of shared decision‐making (SDM) training for medical oncologists on observed SDM in standardized patient assessments.
Materials and Methods.
A randomized controlled trial comparing training with standard practice was conducted. Medical oncologists and oncologists‐in‐training (n = 31) participated in a video‐recorded, standardized patient assessment at baseline (T0) and after 4 months (T1, after training). The training was based on a four‐stage SDM model and consisted of a reader, two group sessions (3.5 hours each), a booster session (1.5 hours), and a consultation card. The primary outcome was observed SDM as assessed with the Observing Patient Involvement scale (OPTION12) coded by observers blinded for arm. Secondary outcomes were observed SDM per stage, communication skills, and oncologists’ satisfaction with communication.
Results.
The training had a significant and large effect on observed SDM in the simulated consultations (Cohen's f = 0.62) and improved observed SDM behavior in all four SDM stages (f = 0.39–0.72). The training improved oncologists’ information provision skills (f = 0.77), skills related to anticipating/responding to emotions (f = 0.42), and their satisfaction with the consultation (f = 0.53).
Conclusion.
Training medical oncologists in SDM about palliative systemic treatment improves their performance in simulated consultations. The next step is to examine the effect of such training on SDM in clinical practice and on patient outcomes.
Implications for Practice.
Systemic treatment for advanced cancer offers uncertain and sometimes limited benefit, while the burden can be high. Hence, applying the premises of shared decision‐making (SDM) is recommended. SDM is increasingly advocated based on the ethical imperative to provide patient‐centered care and the increasing evidence for beneficial patient outcomes. Few studies examined the effectiveness of SDM training in robust designs. This randomized controlled trial demonstrated that SDM training (10 hours) improves oncologists’ performance in consultations with standardized patients. The next step is to examine the effect of training on oncologists’ performance and patient outcomes in clinical practice.
Introduction
Systematic treatment for advanced cancer offers uncertain and often small benefits, while the burden can be high. Hence, treatment decisions depend on patients’ preferences and require shared decision‐making (SDM), an approach whereby physician and patient discuss the benefits and harms of available options and deliberate to reach an agreed‐upon decision [1], [2]. SDM is increasingly advocated based on the ethical imperative to provide patient‐centered care and the evidence for beneficial patient outcomes [3], [4]. Moreover, yet tentatively, SDM in the final period of life may result in increased attention to symptom control and fewer (aggressive) medical interventions [5], [6], [7], [8].
Yet, observational studies consistently show that decision‐making about systemic treatment for advanced cancer often does not meet the standards of SDM [9], [10], [11], [12], [13]. For example, oncologists infrequently discuss the option to refrain from systemic treatment and the potential survival benefits [9], [12], [13]. In addition, a recent study demonstrated that joint deliberation and preference construction is not standard practice [10], particularly not once systemic treatment has started.
SDM requires high‐level communication skills, known to be demanding for clinicians, such as tailoring information provision to the individual patients’ needs [14], [15], dealing with patients’ emotions in response to bad news [16], and coaching patients in constructing a treatment preference. Moreover, SDM conversations are particularly sensitive in the context of dealing with the imminent end of life. Both oncologists and patients have been shown to often prefer to keep a focus on the short term and on “fighting” the cancer, rather than anticipating what is to come. Such focus may enable patients to retain a sense of hope yet may inhibit careful consideration of their wishes and priorities at the end of life [11], [17], [18].
Recent reviews have demonstrated that physician training programs focused on SDM vary widely in format and components with, thus far, little high‐quality research to draw robust conclusions about efficacy [19], [20], [21]. An international environmental scan of SDM training for health professionals [20] noticed a lack of published evaluations of training programs, and a Cochrane review [21] on the effect of SDM interventions only reported on evidence of low or very low quality. To our knowledge, no SDM training program focused on palliative cancer treatment has been formally evaluated in a robust design. Hence, we developed training that addresses medical oncologists’ SDM knowledge, beliefs, and skills in this particular context. This paper reports on the effect of this training on SDM performance of medical oncologists in simulated consultations with a standardized patient with advanced cancer.
Materials and Methods
The paper is written in accordance with the Consolidated Standards of Reporting Trials statement for reporting parallel group randomized trials [22].
Design
This study is part of a four‐arm, parallel, multicenter, randomized, controlled trial (CHOICE; CHOosing treatment together In Cancer at the End of life; Netherlands Trial Registry NTR 5489) [23] investigating the separate and combined effect of skills training for oncologists and a patient communication aid on SDM. The trial examines the effect of the training in simulated consultations as well as the effect of both interventions in real life. This paper reports on the effects of the skills training in a simulated setting. Oncologists were randomly allocated (1:1) to either the intervention arm (training) or the control arm (continue standard practice). Participating oncologists engaged in standardized patient assessments (SPA) at baseline (T0) and in a second SPA after a period of 4 months (T1, after training). Oncologists filled out a questionnaire after each SPA.
Setting and Participants
The source population consisted of all medical oncologists treating patients with metastatic or inoperable tumors working at the medical oncology departments of three academic and three nonacademic hospitals in The Netherlands. In The Netherlands, medical oncologists‐in‐training work under supervision yet communicate with patients largely independently. Therefore, oncologists‐in‐training were considered eligible. Wherever we use the term oncologists, we refer to both senior staff and medical oncologists‐in‐training.
Sample Size
The main trial was powered to detect a large effect (Cohen's d = 0.8; Intraclass correlation (ICC) = 0.20, α = .05, β = 0.80) [24] of the training on observed SDM in real‐life consultations [23]. This resulted in a required sample size of 24 oncologists and 192 real‐life clinical encounters. A total of 31 oncologists were eventually included. Sensitivity power analysis in G*power 3.1.9.2 indicated that with 31 oncologists (α = 0.05, β = 0.80), a large time × training interaction effect (Cohen's f ≥ 0.36) [24] could be detected in the simulated consultations.
Recruitment
The medical oncology departments of both academic and nonacademic hospitals were approached through existing networks until at least 30 oncologists were recruited, considering a possible drop out of 25%. Oncologists were informed about the study by the local and the principal investigator, received an information letter, and were asked for written informed consent.
Randomization
An independent methodologist created the randomization lists, and an independent associate performed the randomization. Oncologists were randomized to receive training or to continue their standard practice in blocks of two, stratifying for working experience (staff vs. resident). Oncologists were randomized in groups per hospital to ensure that in each hospital, about half of the participants would receive training. Oncologists were randomized in sets of at least two “of a kind” (either staff members or residents) to prevent predictable allocation. However, for one hospital, randomization did not result in a sufficiently large training group (>2), and one additional participant was recruited in that hospital and singly randomized. Hence, allocation for the final participant was not concealed.
Blinding
Oncologists could not be blinded for their allocation. However, actors in the simulated conversations were blinded for arm allocation, and so were the outcomes assessors who coded SDM from the video‐recorded SPAs.
Oncologist Training
The training was based on a recent model of SDM [2] with four stages: (a) setting the SDM agenda, (b) informing about the options and pros/cons, (c) exploring patients' values and support preference construction, and (d) making or deferring a decision in agreement. The training aimed to address knowledge (i.e., definition, rationale, effect, and stages of SDM), attitude (i.e., awareness of preference‐sensitive decisions, personal barriers, and motivation), and skills (i.e., ability to apply the four stages using high‐quality communication skills). The training was provided in small groups (n = 3–6) by an experienced trainer (medical psychologist) in two sessions of 3.5 hours each with approximately 2 weeks in between. Staff members and oncologists‐in‐training were trained separately to allow for a safe training environment. The training adopted techniques from behavior change theories [25], such as instruction (in a reader and face‐to‐face), modelling (tailor‐made videos illustrating SDM about palliative systemic treatment), and practice (role‐play with professional actors). Moreover, the training explicitly addressed the transfer of skills from a simulated to a clinical setting. That is, in a booster session of 1–1.5 hours preferably 6 weeks after training, participants received face‐to‐face feedback on a video‐recorded encounter from their actual practice, with the opportunity to repeat parts of the conversation in role‐play. These sessions were preferably scheduled in pairs. Lastly, all participants received a consultation room tool: a pocket‐size card presenting the four SDM stages with example phrases to serve as a reminder and to support transfer into practice. In total, the training took 10 hours (8.5 hours of face‐to‐face contact and 1.5 preparatory reading). The training was piloted with five oncologists‐in‐training from two hospitals and was evaluated positively, with a mean satisfaction score of 8 (scale of 1–10). The training was accredited by the Netherlands Association of Internal Medicine.
SPAs
The standardized cases reflected a patient with metastatic gastric (T0) or esophageal cancer (T1), who met with the oncologist to discuss the start of first‐line palliative chemotherapy. The participating oncologists received a simulated medical file, containing the standard medical information available. Both the actor script and the medical file were developed in a multidisciplinary team (medical psychologists and oncologist) and were adjusted based on a pilot study. Two experienced professional male actors were recruited to play both roles (actor A, 52 years; actor B, 58 years). They were educated about SDM and were instructed to act in a standard way and to be rather passive and not overly emotional. They were taught to ask a set of standard questions and apply a limited set of “if then” rules (e.g., to ask a question only in case the oncologists presented a particular piece of information). They were instructed about their treatment preference (and the underlying values) in case a choice was presented to them, which in both cases was to prefer chemotherapy over best supportive care. The SPAs were video recorded (November 2015 to August 2016).
Measurements
Sample Characteristics.
The baseline survey (T0) included oncologists’ gender and age, years of experience (including residency) in medical oncology and receipt of communication skills training (yes/no) during medical school, residency, and posteducation. Oncologists were asked to write down their treatment plan after the SPA. Based on these notes and the video‐recorded SPA, the decision made was categorized into (a) chemotherapy, (b) best supportive care, or (c) deferred. Lastly, the duration of the simulated consultation was registered.
Validity Check.
After each SPA (T0 and T1), oncologists were asked how realistic and how comparable to their clinical practice the simulated consultation was using two items specifically designed for this study with Likert scale responses (1–10).
Primary Outcome.
SDM. The primary outcome was observed SDM as assessed from the video‐recorded consultations using the Observing Patient Involvement scale (OPTION12) [26], [27], [28], a widely used 12‐item scoring instrument of physician communicative behavior associated with SDM. Items are rated on a 5‐point scale (0: not observed; 4: very high standard), and the sum score is transformed to reflect a total out of 100. Next to the general manual, a study‐specific manual was developed. Two blind raters rated the video‐recorded consultations. This coding process consisted of training, calibration to achieve sufficient interrater reliability, and independent coding (see supplemental online Appendix 1 and supplemental online Table 1 for a full description).
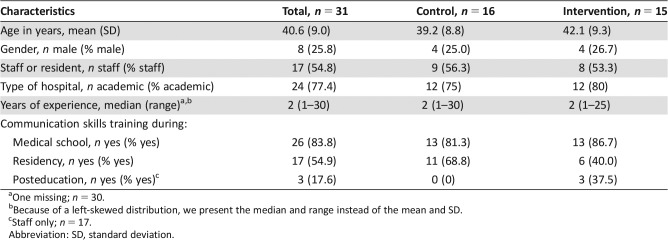
Table 1. Sample characteristics for the total sample and per group.
One missing; n = 30.
Because of a left‐skewed distribution, we present the median and range instead of the mean and SD.
Staff only; n = 17.
Abbreviation: SD, standard deviation.
Secondary Outcomes.
SDM per Stage. Observed SDM was also assessed with the 4SDM, a self‐developed instrument based on the 4‐stage SDM model [2], which provides a score for each of the stages (supplemental online Appendix 2). The 4SDM has eight items (two for each stage), which are coded on a 4‐point scale (0: not observed; 3: observed and of high quality). The 4SDM has a study‐specific manual. Coding was done by the same raters as for the OPTION12, but the raters did not rate both the OPTION12 and the 4SDM for the same encounter (supplemental online Appendix 1).
Communication Skills. Observed communication skills were assessed with two purposefully developed items: one assessing the quality of information giving (skills like inviting questions, structuring information, summarizing) and one assessing the quality of responding to or anticipating patients’ emotions (skills like showing empathy, silences, reflections). Both items were rated on a 5‐point scale ranging from 0 “not or hardly visible” to 4 “very frequently,” with a score of 2 representing “sufficient.” Coding was done by the same raters as for the OPTION12, for all encounters (supplemental online Appendix 1).
Satisfaction with Communication. To assess oncologists' satisfaction (T0 and T1) with communication in the simulated consultations, the 5‐item Patient Satisfaction Questionnaire [29] was used, in a version for oncologists [30]. One additional item about satisfaction with patient involvement in decision‐making was added. Responses were given on Visual Analogue Scales (0–100).
Statistical Analysis
Baseline sample characteristics were checked for imbalances across groups. Between‐group differences on characteristics of the SPAs (e.g., duration, decision made) were tested with the appropriate univariate statistics (t test, Fisher's exact test). The effect of the training was assessed by General Lineair Model (GLM) for repeated measure with time (within subjects), condition (between subjects), and time × condition (effect of the training) as independent variables. Separate analyses were conducted for observed SDM (OPTION12 and 4SDM), the substeps of SDM (4SDM subscales), two types of communication skills, and oncologists’ satisfaction. Cohen's f will be presented as a measure of effect size (f = 0.1 small effect, f = 0.25 medium effect, and f = 0.4 large effect) [24].
Results
Sample Characteristics
Of the 36 oncologists invited at six departments, 31 consented and were included (86%), of whom 15 were allocated to the intervention and 16 to the control condition. On average, participants were 41 years of age and had 7 years of experience in oncology (Table 1). Approximately half were staff members (54.8%) and a quarter were male (25.8%). Most received communication skills training during medical school (83.8%), half during (current) residency (54.9%), and some posteducation (17.6% of post‐training staff). Untrained and trained oncologists did not differ meaningfully (nor statistically significantly) on any of these characteristics.
Intervention and SPA Characteristics
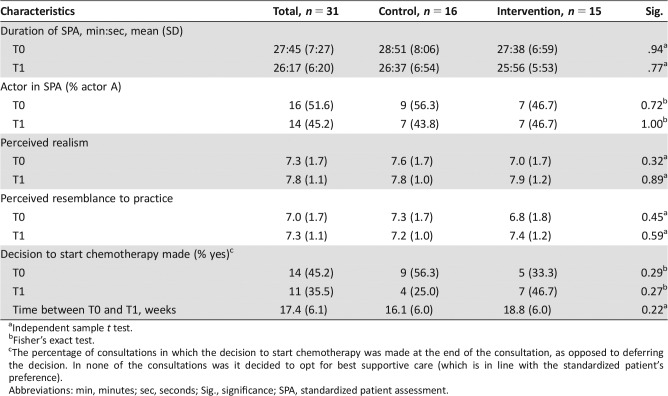
See supplemental online Appendix 3 for information on intervention fidelity. The SPAs took an average of 28 minutes at T0 and 26 minutes at T1 (Table 2). Oncologists met with actor A in 52% (T0) and 45.2% (T1) of the SPAs. Oncologists perceived the consultation as realistic and felt they resembled their personal clinical practice (average ratings ≥7). These ratings were higher at T1 than at T0. The fact that the patient did not bring a companion was often perceived as unrealistic. In all consultations, it was decided to start chemotherapy instead of best supportive care (in line with the preferences of the standardized patient) or to defer the decision to a second consultation. On average, the time between T0 and T1 was 17 weeks. SPAs of untrained and trained oncologists did not significantly differ on any of these characteristics.
Table 2. Characteristics of the standardized patient assessments for the total sample and per group.
Independent sample t test.
Fisher's exact test.
The percentage of consultations in which the decision to start chemotherapy was made at the end of the consultation, as opposed to deferring the decision. In none of the consultations was it decided to opt for best supportive care (which is in line with the standardized patient's preference).
Abbreviations: min, minutes; sec, seconds; Sig., significance; SPA, standardized patient assessment.
Primary Outcome
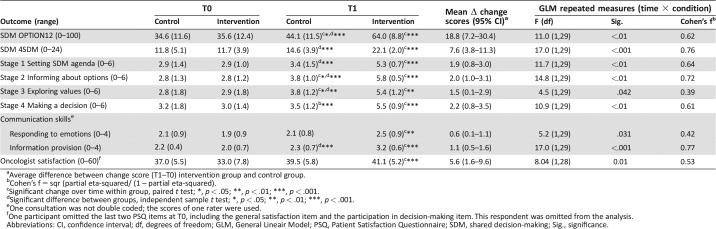
The control group did not differ from the trained group on observed SDM at baseline (Table 3). Both groups significantly improved over time, yet the improvement in the trained group was significantly larger (large effect, f = 0.62). The trained group demonstrated significantly more SDM at T1 than the control group.
Table 3. Means and standard deviations per group at T0 and T1 on all outcomes.
Average difference between change score (T1–T0) intervention group and control group.
Cohen's f = sqr (partial eta‐squared/ (1 – partial eta‐squared).
Significant change over time within group, paired t test; *, p < .05; **, p < .01; ***, p < .001.
Significant difference between groups, independent sample t test; *, p < .05; **, p < .01; ***, p < .001.
One consultation was not double coded; the scores of one rater were used.
One participant omitted the last two PSQ items at T0, including the general satisfaction item and the participation in decision‐making item. This respondent was omitted from the analysis.
Abbreviations: CI, confidence interval; df, degrees of freedom; GLM, General Lineair Model; PSQ, Patient Satisfaction Questionnaire; SDM, shared decision‐making; Sig., significance.
Secondary Outcomes
The training improved SDM in all four stages. All effects were large, except for the moderate effect for SDM stage 3 (i.e., exploring values). The training also significantly improved communication skills (i.e., information provision skills and responsiveness to emotions). The training also significantly improved oncologists' satisfaction with the consultation, although this effect seemed mainly due to the nonsignificant (p = .12) yet substantial (0.6 standard deviation) lower scores of the trained group at baseline (T0). At T1, there was no difference in satisfaction.
Discussion
This randomized, controlled trial demonstrated a strong effect of training on SDM about palliative chemotherapy in consultations with a standardized patient. Additional explorative analyses show that skills improved for all four stages of SDM: (a) setting the SDM agenda, (b) informing about the options and pros/cons, (c) exploring patients’ values and support preference construction, and (d) making or deferring a decision in agreement. Moreover, trained oncologists demonstrated improved communication skills with respect to information provision and responding to and anticipating patient emotions.
The large effect of the training was achieved despite relatively high SDM scores already before training, when compared with studies in real life, mostly curative settings [26]. A recent observational study similarly assessing SDM in simulated consultations on advanced cancer reported a mean score still lower but more comparable to our scores [31]. The simulated nature might have enhanced oncologists’ performance: The standardized patient was not overly assertive or emotional, and context factors that may complicate communication (such as the presence of a companion) were eliminated. Also, the preference‐sensitive nature of a decision about palliative treatment may have been more self‐evident than in the curative settings, triggering SDM behavior. Indeed, in a previous observational study, we also found that communication about values and preferences was more frequent in consultations in palliative oncology [10] than in studies in a curative cancer setting. [32], [33]
Nonetheless, it may be argued that even after training, SDM is suboptimal, as the average OPTION score was still far from the maximum score of 100. This may, however, result from the structure of the OPTION12, which includes items assessing behavior not specific to SDM (e.g., checking comprehension, inviting questions), of which some are highly idealistic (i.e., assessing patients’ preferences for different information formats) [34]. Indeed, post‐training scores on the 4SDM, focusing on SDM behavior only, did approach the top end after training.
The training had the largest effect on information provision skills and the smallest effect on communication about patients’ values and emotions. Whereas the first set of skills is mostly—although not exclusively—about sending messages, the latter requires a more receptive and exploring attitude and may therefore be more difficult to teach and to integrate into one's behavior. Still, for these complex skills, a moderate to large effect of training was also demonstrated. Exploring patients’ preferences and jointly constructing a preference lies at the core of SDM. Hence, the finding that such exchange is improved by training is promising. Yet, the fact that it turned out to be the skill that improved the least does raise the question of to what extent this effect will be sustained in clinical practice and how the training needs to be adjusted to allow for more substantial improvements.
This study has some limitations. First, on the basis of the current phase of the trial, we can conclude that the training improves the required skills for SDM, but no conclusions can yet be drawn about SDM in real‐life clinical consultations. Second, we randomized oncologists before the baseline assessment. As there were no baseline differences on observed SDM and skills, we are confident that this approach did not affect their performance in the SPAs. Yet, it may have affected their personal evaluation. Oncologists assigned to the training were less satisfied about their performance at baseline than oncologists assigned to the control condition, possibly because they were anticipating feedback in the training some weeks later. Third, we included both staff oncologists and oncologists‐in‐training. It is possible that the training was more or only effective in one of these groups, yet the sample size is too small to make the comparison.
The next step in this trial is to examine whether the effect of the training extends to real‐life clinical practice on the long term as well as to patient outcomes [23]. Moreover, we will examine whether preparing patients for SDM with a communication aid makes it easier for oncologists to bring learned skills into practice. Indeed, it has been suggested that interventions to improve communication are most effective if they target both doctors and patients [21], [35]. Future research should address the dose‐response relation for skills training. A blended training, combining e‐learning with less intensive face‐to‐face training, can possibly yield an effect that is not inferior to the 10 hours of training. Finally, the use of virtual patients in communication skills e‐training is promising and deserves further exploration [36], [37].
Conclusion
Reflection and feedback on communication skills in a group of peers is rare for practicing medical staff. Yet, we found that SDM skills training was not only effective but also well appreciated, despite the considerable time and effort requested from participants. This may show that the taught skills and strategies to present and jointly deliberate supports medical oncologists in providing patient‐centered care. This study proves that training medical oncologists in SDM about palliative systemic treatment is feasible and significantly improves their skills.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We would like to thank Mart Calff for her involvement as an experienced and highly qualified professional trainer. Thanks to Marius Schalkwijk and Hans van Dijk for the consistent and careful acting in the SPAs and to all actors involved in the training sessions. Furthermore, we would like to express our gratitude to Leah Waterman for observing and categorizing the decisions made in the SPAs, to Marcel Dijkgraaf for developing the randomization lists, and to Kirsten Douma for randomizing the oncologists. We are grateful to all secretaries in the six hospitals for supporting us in organizing the training as well as the SPAs. And we would like to acknowledge all 31 oncologists for their willingness to participate in this study and their efforts to make it successful. This project was supported by a grant from the Dutch Cancer Society (UVA 2013–5949). Trial Registry: Netherlands Trial Registry 5489 (prospective, September 15, 2015).
Author Contributions
Conception/design: Inge Henselmans, Hanneke W.M. van Laarhoven, Hanneke C.J.M. de Haes, Filip Y.F.L. de Vos, Ellen M.A. Smets
Provision of study material or patients: Hanneke W.M. van Laarhoven, Petronella B. Ottevanger, Serge E. Dohmen, Geert‐Jan Creemers, Dirkje W. Sommeijer, Filip Y.F.L. de Vos
Collection and/or assembly of data: Inge Henselmans, Pomme E.A. van Maarschalkerweerd
Data analysis and interpretation: Inge Henselmans, Hanneke W.M. van Laarhoven, Meltem Tokat, Ellen G. Engelhardt, Pomme E.A. van Maarschalkerweerd, Marleen Kunneman, Ellen M.A. Smets
Manuscript writing: Inge Henselmans, Hanneke W.M. van Laarhoven, Hanneke C.J.M. de Haes, Meltem Tokat, Ellen G. Engelhardt, Pomme E.A. van Maarschalkerweerd, Marleen Kunneman, Petronella B. Ottevanger, Serge E. Dohmen, Geert‐Jan Creemers, Dirkje W. Sommeijer, Filip Y.F.L. de Vos, Ellen M.A. Smets
Final approval of manuscript: Inge Henselmans, Hanneke W.M. van Laarhoven, Hanneke C.J.M. de Haes, Meltem Tokat, Ellen G. Engelhardt, Pomme E.A. van Maarschalkerweerd, Marleen Kunneman, Petronella B. Ottevanger, Serge E. Dohmen, Geert‐Jan Creemers, Dirkje W. Sommeijer, Filip Y.F.L. de Vos, Ellen M.A. Smets
Disclosures
Hanneke W.M. van Laarhoven: Bristol‐Myers Squibb, Eli Lilly and Company, Nordic Pharma (C/A), Bayer, Bristol‐Myers Squibb, Celgene, Janssen, Eli Lilly and Company, Nordic Pharma, Philips, Roche (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681–692. [DOI] [PubMed] [Google Scholar]
- 2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns 2015;98:1172–1179. [DOI] [PubMed] [Google Scholar]
- 3. Kehl KL, Landrum MB, Arora NK et al. Association of actual and preferred decision roles with patient‐reported quality of care. Shared decision making in cancer care. JAMA Oncol 2015;1:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making 2015;35:114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stacey D, Bennett CL, Barry MJ et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011:CD001431. [DOI] [PubMed] [Google Scholar]
- 6. Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA 1998;279:1709–1714. [DOI] [PubMed] [Google Scholar]
- 7. Wright AA, Zhang B, Ray A et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang B, Wright AA, Huskamp HA et al. Health care costs in the last week of life: Associations with end‐of‐life conversations. Arch Intern Med 2009;169:480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Audrey S, Abel J, Blazeby JM et al. What oncologists tell patients about survival benefits of palliative chemotherapy and implications for informed consent: Qualitative study. BMJ 2008;337:a752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henselmans I, Van Laarhoven HW, Van der Vloodt J et al. Shared decision making about palliative chemotherapy: A qualitative observation of talk about patients' preferences. Palliat Med 2017;31:625–633. [DOI] [PubMed] [Google Scholar]
- 11. Buiting HM, Rurup ML, Wijsbek H et al. Understanding provision of chemotherapy to patients with end stage cancer: Qualitative interview study. BMJ 2011;342:d1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gattellari M, Voigt KJ, Butow PN et al. When the treatment goal is not cure: Are cancer patients equipped to make informed decisions? J Clin Oncol 2002;20:503–513. [DOI] [PubMed] [Google Scholar]
- 13. Koedoot CG, Oort FJ, de Haan RJ et al. The content and amount of information given by medical oncologists when telling patients patients with advanced cancer what their treatment options are: Palliative chemotherapy and watchful‐waiting. Eur J Cancer 2004;40:225–235. [DOI] [PubMed] [Google Scholar]
- 14. Douma KF, Koning CC, de Haes HC et al. Do radiation oncologists tailor information to patients needs? And, if so, does it affect patients? Acta Oncol 2012;51:512–520. [DOI] [PubMed] [Google Scholar]
- 15. Gamble K. Communication and information: The experience of radiotherapy patients. Eur J Cancer Care 1998;7:153–161. [DOI] [PubMed] [Google Scholar]
- 16. Baile WF, Buckman R, Lenzi R et al. SPIKES‐A six‐step protocol for delivering bad news: Application to the patient with cancer. The Oncologist 2000;5:302–311. [DOI] [PubMed] [Google Scholar]
- 17. The AM, Hak T, Koeter G et al. Collusion in doctor‐patient communication about imminent death: An ethnographic study. BMJ 2000;321:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Haes HC, Koedoot N. Patient centered decision making in palliative cancer treatment: A world of paradoxes. Patient Educ Couns 2003;50:43–49. [DOI] [PubMed] [Google Scholar]
- 19. Legare F, Politi MC, Drolet R et al. Training health professionals in shared decision‐making: An international environmental scan. Patient Educ Couns 2012;88:159–169. [DOI] [PubMed] [Google Scholar]
- 20. Diouf NT, Menear M, Robitaille H et al. Training health professionals in shared decision making: Update of an international environmental scan. Patient Educ Couns 2016;99:1753–1758. [DOI] [PubMed] [Google Scholar]
- 21. Legare F, Stacey D, Turcotte S et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2014:CD006732. [DOI] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D et al. Consort 2010 statement: Updated guidelines for reporting parallel group randomised trials. Int J Surg 2011;9:672–677. [DOI] [PubMed] [Google Scholar]
- 23. Henselmans I, Smets EMA, de Haes JCJM et al. A randomized controlled trial of a skills training for oncologists and a communication aid for patients to stimulate shared decision making about palliative systemic treatment (CHOICE): Study protocol. BMC Cancer 2018;18:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 25. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379–387. [DOI] [PubMed] [Google Scholar]
- 26. Couet N, Desroches S, Robitaille H et al. Assessments of the extent to which health‐care providers involve patients in decision making: A systematic review of studies using the OPTION instrument. Health Expect 2015;18:542–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elwyn G, Hutchings H, Edwards A et al. The option scale: Measuring the extent that clinicians involve patients in decision‐making tasks. Health Expect 2005;8:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elwyn G, Edwards A, Wensing M et al. Shared decision making: Developing the OPTION scale for measuring patient involvement. Qual Saf Health Care 2003;12:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ong LM, Visser MR, Lammes FB et al. Doctor‐patient communication and cancer patients' quality of life and satisfaction. Patient Educ Couns 2000;41:145–156. [DOI] [PubMed] [Google Scholar]
- 30. Zandbelt LC, Smets EM, Oort FJ et al. Satisfaction with the outpatient encounter: A comparison of patients' and physicians' views. J Gen Intern Med 2004;19:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libert Y, Canivet D, Menard C et al. Predictors of physicians' communication performance in a decision‐making encounter with a simulated advanced‐stage cancer patient: A longitudinal study. Patient Educ Couns 2017;100:1672–1679. [DOI] [PubMed] [Google Scholar]
- 32. Pieterse AH, Henselmans I, de Haes JCJM et al. Shared decision making: Prostate cancer patients' appraisal of treatment alternatives and oncologists' eliciting and responding behavior, an explorative study. Patient Educ Couns 2012;85:e251–e259. [DOI] [PubMed] [Google Scholar]
- 33. Kunneman M, Marijnen CA, Baas‐Thijssen MC et al. Considering patient values and treatment preferences enhances patient involvement in rectal cancer treatment decision making. Radiother Oncol 2015;117:338–342. [DOI] [PubMed] [Google Scholar]
- 34. Elwyn G, Tsulukidze M, Edwards A et al. Using a 'talk' model of shared decision making to propose an observation‐based measure: Observer OPTION 5 item. Patient Educ Couns 2013;93:265–271. [DOI] [PubMed] [Google Scholar]
- 35. Walczak A, Butow PN, Bu S et al. A systematic review of evidence for end‐of‐life communication interventions: Who do they target, how are they structured and do they work? Patient Educ Couns 2016;99:3–16. [DOI] [PubMed] [Google Scholar]
- 36. Fleming M, Olsen D, Stathes H et al. Virtual reality skills training for health care professionals in alcohol screening and brief intervention. J Am Board Fam Med 2009;22:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Triola M, Feldman H, Kalet AL et al. A randomized trial of teaching clinical skills using virtual and live standardized patients. J Gen Intern Med 2006;21:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]